Abstract
BACKGROUND:
Monitoring noninvasive biomarkers of inflammation is an important adjunct in asthma therapy.
OBJECTIVE:
The goal of the present study was to identify airway and alveolar site(s) of inflammation using exhaled nitric oxide (NO) as a marker in asthmatic patients, and to evaluate the NO response to maintenance fluticasone 250 μg/salmeterol 50 μg (F/S) and add-on montelukast 10 mg (M).
METHODS:
Thirty (24 women) nonsmoking, mild to moderate asthmatic patients were studied, mean age (± SD) 43±9 years, treated with F/S for more than one year. All were clinically stable for longer than eight weeks and had not taken oral corticosteroids and/or leukotriene antagonists for eight weeks before the present study. Spirometry, Juniper asthma symptom score, fractional exhaled NO (FENO) 100 mL/s, bronchial NO and alveolar NO concentration (CANO) were measured in a single-blind, nonrandomized crossover study.
PROTOCOL:
Visit 1: baseline F/S; visit 2: after four weeks of F/S plus M; visit 3: after four weeks of S plus M; and visit 4: after four weeks of S only. Values in asthmatic patients were also compared with 34 nonsmoking age-matched healthy controls with normal lung function.
RESULTS:
After 180 μg aerosolized metered dose inhaler albuterol, the forced expiratory volume in 1 s at baseline was 2.6±0.8 L (86%±16% of the predicted value) and the forced expiratory volume in 1 s over the forced vital capacity was 77%±9% (mean ± SD), and was similar at visits 2 to 4. Juniper scores were mildly abnormal at visits 1 to 3, but significantly worse (P=0.03) at visit 4 versus visits 1 to 3. FENO values at visits 1 to 3 were similar but significantly increased (P=0.007) at visit 4. Bronchial NO was higher (P=0.03) at visit 4, versus visits 1 and 2, and was no different at visit 3. Compared with the healthy subjects, FENO and bronchial NO values were abnormal (greater than the normal mean plus 2 SD) in 33% of asthmatic patients at visits 1 to 3. CANO was similar for visits 1 to 4. CANO was abnormal (greater than the normal mean + 2 SD) in 20% of asthmatic patients.
CONCLUSION:
In clinically stable asthmatic patients, despite controller treatment including moderate-dose inhaled corticosteroids and add-on M, 33% of mild to moderate asthmatic patients have ongoing nonsuppressed bronchial sites of increased NO production, compared with healthy control subjects. These controllers have no effect on CANO, which was abnormal in 20% of the asthmatic patients studied. The addition of add-on M to baseline moderate-dose inhaled corticosteroid did not further reduce total exhaled, bronchial and/or alveolar NO production.
Keywords: Asthma, Exhaled nitric oxide, Montelukast, Salmeterol/fluticasone
Abstract
HISTORIQUE :
La surveillance des biomarqueurs non effractifs de l’inflammation est un ajout important à la thérapie de l’asthme.
OBJECTIF :
La présente étude visait à repérer les foyers inflammatoires des voies aériennes et des alvéoles à l’aide de monoxyde d’azote expiré (MAE) comme marqueur des patients asthmatiques, ainsi qu’à évaluer la réponse du monoxyde d’azote (MA) à un traitement d’entretien de 250 μg de fluticasone associés à 50 μg de salmétérol (F-S) et à l’ajout de 10 g de motélukast (M).
MÉTHODOLOGIE :
Trente (24 femmes) patients asthmatique non-fumeurs atteints d’asthme bénin à modéré, d’un âge moyen±ÉT de 43±9 ans et traités par F-S pendant plus d’un an, ont participé à l’étude. Tous étaient cliniquement stables depuis plus de huit semaines et n’avaient pas pris de corticoïdes par voie orale ou d’antagonistes des leucotriènes depuis huit semaines avant la tenue de la présente étude. La spirométrie, l’indice de l’asthme de Juniper, le MAE fractionnel (MAEF) à 100 mL/s, le MA bronchique et la concentration de MA alvéolaire (CMAA) ont été mesurés dans le cadre d’une étude transversale non aléatoire à simple insu.
PROTOCOLE :
Visite 1 : F-S de départ; visite 2 : au bout de quatre semaines de F-S plus M; visite 3 : au bout de quatre semaines de S et de M; visite 4 : au bout de quatre semaines de S seulement. Les valeurs des patients asthmatiques ont également été comparées à celles de 34 sujets témoins non-fumeurs en santé appariés selon l’âge et dont la fonction pulmonaire était normale.
RÉSULTATS :
Après l’administration de 180 μg d’albutérol en aérosol-doseur, le volume expiratoire maximal par seconde en début d’étude s’élevait à 2,6±0,8 L (86 % ±16 % de la valeur prédictive), et le volume expiratoire maximal par seconde par rapport à la capacité vitale forcée était de 77 % ±9 % (moyenne±ÉT) et était similaire aux visites 2 à 4. Les indices de Juniper étaient légèrement anormaux aux visites 1 à 3, mais avaient beaucoup empiré (P=0,03) à la visite 4 par rapport aux visites 1 à 3. Les valeurs de MAEF aux visites 1 à 3 étaient similaires, mais elles avaient augmenté considérablement (P=0,007) à la visite 4. Le MA bronchique était plus élevé (P=0,03) à la visite 4 qu’aux visites 1 et 2 et n’avait pas changé à la visite 3. Par rapport aux sujets en santé, les valeurs de MAEF et de MA bronchique étaient anormales (supérieures à la moyenne normale plus 2 ÉT) chez 33 % des patients asthmatiques aux visites 1 à 3. La CMAA était similaire aux visites 1 à 4, mais était anormale (supérieure à la moyenne normale plus 2 ÉT) chez 20 % des patients asthmatiques.
CONCLUSION :
Chez les patients asthmatiques cliniquement stables, malgré un traitement stabilisateur incluant des corticoïdes en aérosol-doseur et l’ajout de M, 33 % des patients souffrant d’asthme bénin à modéré présentent des foyers bronchiques non supprimés continus de production accrue de MA par rapport aux sujets témoins en santé. Ces médicaments stabilisateurs n’ont aucun effet sur la CMAA, anormale chez 20 % des patients asthmatiques à l’étude. L’ajout de M aux corticoïdes en aérosol-doseur de base ne réduisait pas la production de MAE total, de MA bronchique ou de MA alvéolaire.
We previously emphasized (1) that the measurement of fractional exhaled nitric oxide (FENO) is a relatively simple, sensitive, nonspecific, reproducible noninvasive test (2,3) for monitoring endogenous inflammatory signals, presumably from activated eosinophil-mediated pathological airways in asthma (4,5). It has been reported that the source of nitric oxide (NO) originates from trafficking, infiltrating, residential inflammatory and epithelial lining cells of both the large and small airways (3–6) and alveoli (7–9). This follows upregulation of inducible NO synthase through activated proinflammatory mediators and cytokines. Exhaled NO in asthmatic patients may be reduced using inhaled and oral corticosteroids (7–19). Furthermore, montelukast, when used alone (20–23) or in combination with inhaled corticosteroids as an add-on treatment (24–32), has resulted in further reduction (20–28), variation (29,30) or no further effect (19,31,32) on exhaled NO. However, none of these studies investigated the potential site(s) of bronchial or alveolar inflammation using NO production as the surrogate signal (33).
Our goal was to prospectively evaluate bronchial and alveolar sites of inflammation using NO as the signal and the response following inhaled corticosteroids and add-on montelukast, a cysteinyl leukotriene receptor antagonist.
METHODS
Patient selection
FENO was measured in 31 nonsmoking, clinically stable asthmatic patients (24 women), mean (± SD) age 43±9 years, who were regularly followed in a tertiary referral outpatient clinic for management of mild to severe persistent asthma (34,35). They all took inhaled fluticasone 250 μg/salmeterol 50 μg (F/S) twice a day for longer than a year. Aerosolized albuterol sulfate from a metered dose inhaler (MDI) and/or nebulizer, or a combination aerosolized albuterol sulfate and ipratropium bromide MDI and/or nebulizer, were used as acute relievers. All asthmatic patients were clinically stable for the previous eight weeks at baseline.
The classification of asthma guidelines (34,35) was modified in two ways: to include only treated asthmatic patients, and to determine severity stratification after 180 μg albuterol sulfate using an MDI. ‘Mild’ refers to a forced expiratory volume in 1 s (FEV1) of at least 80% of predicted value, ‘moderate’ refers to an FEV1 of 79% to 61% of the predicted value and ‘severe’ refers to an FEV1 lower than 61% of the predicted value.
All asthmatic patients had previous high-resolution, thin-section computed tomography of the lung with no, or trivial, emphysema present (36).
All asthmatic patients studied gave informed consent for participation. The present study was approved by the Institutional Review Board.
Asthmatic patients were not taking oral corticosteroids or leukotriene inhibitors within eight weeks of the measurement of baseline FENO. Optimal medical therapy was confirmed by evaluating clinical status and serial spirometry obtained during routine outpatient clinic visits, every one to two months over three to five years, in each asthmatic patient, to ensure all baseline values were equal to the best values previously documented.
Patient protocol:
Single-blinded, nonrandomized, fixed-sequence crossover intervention.
Visit 1 (Baseline):All asthmatic patients who were clinically stable for longer than eight weeks were taking F/S twice a day for longer than a year.
Visit 2:After four weeks of taking F/S plus montelukast 10 mg daily (M).
Visit 3:After four weeks of taking M plus S.
Visit 4:After four weeks of taking S only.
Attempts were made to ensure asthmatic patients adhered to their medication schedule through weekly telephone calls and/or office visits.
Normal controls
Normal values for FENO were previously obtained from 34 subjects (13 men), mean age 40±17 years, who were asymptomatic, healthy nonsmokers with normal lung function (1,7).
Measurement of exhaled NO gas exchange
FENO was measured in every asthmatic patient at three separate constant expiratory flow rates: 100 mL/s, 150 mL/s and 200 mL/s, in triplicate. The mean of three values (that were required to be within 10% of each other to be acceptable) was reported using a Sievers NOAi 280 chemiluminescence analyzer (Ionics Instruments, USA) with varying expiratory airflow resistors. Most importantly, Tsoukias and George (33), Tsoukias et al (37) and George et al (38) have shown that the relationship between alveolar NO concentration (CANO) and expiratory flow is linear above a threshold of 50 mL/s. To avoid nasal NO contamination, a mouth pressure greater than 5 cm H2O was used, as previously recommended (39). The NO analyzer was calibrated, at minimum, daily, with a known NO concentration (45 ppm), and before each patient or control subject was tested, with NO-free air. The technique of Tsoukias and George (33,37,38) was used to calculate bronchial NO maximal flux and steady-state (CANO), as previously published (1,7). One of the authors (CFT), solely responsible for obtaining and calculating NO gas exchange values and all other lung function data, was blinded to patient medication and dose.
Other lung function studies
Asthmatic patients, who were clinically stable for at least eight weeks, were instructed to continue all their medications, but to withhold inhaled albuterol sulfate and ipratropium bromide 8 h before testing, and long-acting beta2-agonists 48 h before testing. Techniques for measuring spirometry, thoracic gas volumes and airway resistance using plethysmography and diffusing capacity have been previously published (40,41).
Measurement of the Juniper asthma symptom score
Asthma clinical status was assessed using this relatively simple, previously validated (42) six-questionnaire test.
Statistical methods
Healthy subjects and asthmatic patients were compared using ANOVA on the ranks (nonparametric ANOVA, Kruskal-Wallis test). Alternatively, the Wilcoxon rank sum test was used. Data distribution by visit was tested using the Shapiro Wilk W statistic. If the P value was greater than 0.15, and W was greater than 0.94, then the hypothesis of normality was not rejected. Otherwise, it was rejected, and further analyses were performed using natural log-transformed data. ANOVA for repeated measurements was used to test for differences between different visits (General Linear Model Procedure in SAS, using individual asthmatic patients as the random variable). Pair-wise comparison of asthmatic patients where the general test indicated significant differences were performed using posthoc linear contrasts and Student-Newman-Keuls tests to keep the overall experimental error lower than 0.05. P<0.05 was considered statistically significant. SAS, version 8.02 for Windows, was used for analysis (SAS Institute Inc, USA).
RESULTS
Baseline lung function and NO gas exchange
Results of baseline lung function studies in 30 asthmatic patients, who completed the present study without any missing data, are described in Table 1. An additional patient declined to continue the present study following an asthma exacerbation during the first week. Results of baseline and subsequent NO gas exchange data following drug intervention are described in Table 2 and Figures 1 to 4.
TABLE 1.
Results of lung function in asthmatic patients at baseline
| Age (years) | Number (sex) | FEV1 (L) | FEV1 (%pred) | FVC (L) | FVC (%pred) | FEV1/ FVC (%) | DLCOSB (mL/min/mmHg) | DLCOSB %pred | DL/VA | %pred | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients on F/S | 43±9 | 30 (6 men) | 2.6±0.8 | 86±16 | 3.3±0.8 | 95±17 | 77±9 | 23±6 | 99±25 | 5.2±1.1 | 124±22 |
Lung function results are presented as mean ± SD. %pred Percent predicted; DL DIffusing capacity; DLCOSB Single breathing diffusing capacity for carbon monoxide; F/S Inhaled corticosteroids, fluticasone 250 μg/salmeterol 50 μg twice a day; FEV1 Forced expiratory volume in 1 s; FVC Forced vital capacity; VA Alveolar volume
TABLE 2.
Results of lung function studies and nitric oxide (NO) gas exchange for visits 1 to 4 in asthmatic patients and healthy subjects
| Healthy subjects | Visit 1 F/S | Visit 2 F/S/M | Visit 3 S/M | Visit 4 S | |
|---|---|---|---|---|---|
| FVC (L) | 3.31±0.85 | 3.30±0.82 | 3.25±0.83 | 3.27±0.85 | |
| FVC (%pred) | 86±17 | 86±19 | 83±17 | 84±19 | |
| FEV1 (L) | 2.6±0.8 | 2.6±0.7 | 2.5±0.8 | 2.6±0.8 | |
| FEV1 (%pred) | 86±17 | 86±19 | 83±17 | 84±19 | |
| FEV1/FVC (%) | 77±10 | 78±9 | 77±10 | 77±10 | |
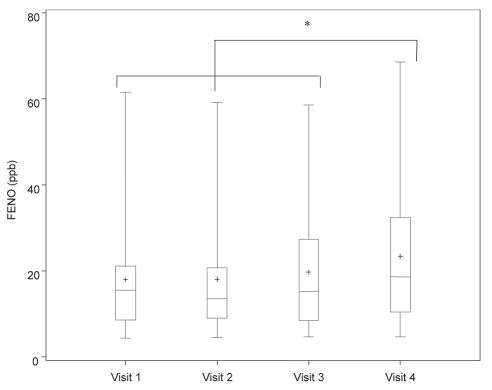
| FENO, 100 mL/s (ppb) | 12±5 | 16 (9–21) | 14 (9–21) | 15 (9–27) | 19 (10–32)* |
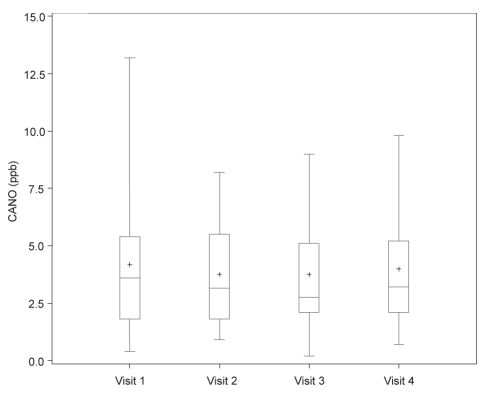
| CANO (ppb) | 3.2±2.0 | 3.6 (1.8–5.4) | 3.2 (1.8–5.5) | 2.8 (2.1–5.1) | 3.2 (2.1–5.2) |
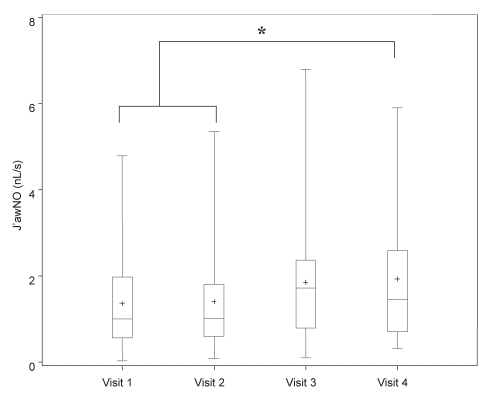
| Airway NO (nL/s) | 0.85±0.55 | 1.0 (0.6–2.0) | 1.0 (0.6–1.8) | 1.7 (0.8–2.4) | 1.5 (0.7–2.6)** |
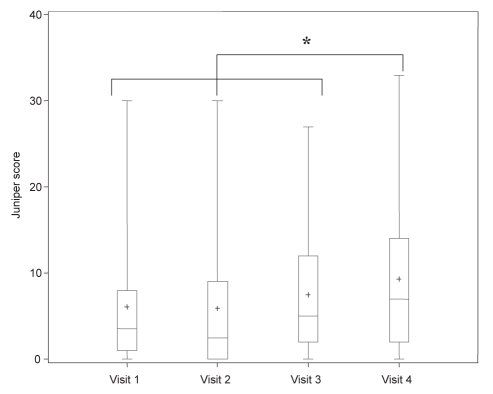
| Juniper score | 3.5 (1–8) | 2.5 (0–9) | 5 (2–12) | 7 (2–14)* |
Results for asthmatic patients are expressed as mean±SD and median (1–3 interquartile range). Results for healthy subjects are expressed as mean ± SD (1,7).
Visit 4 is different from visits 1 to 3, P<0.007;
Visit 4 is different from visits 1 and 2, P=0.03. %pred Percent predicted; CANO Alveolar NO concentration; F Fluticasone 250 μg twice a day; FENO fractional exhaled NO; FEV1 Forced expiratory volume in 1 s; FVC Forced vital capacity; M Montelukast 10 mg once a day; ppb Parts per billion; S Salmeterol 50 μg twice a day
Figure 1).
Measurement of fractional exhaled nitric oxide (FENO) at 100 mL/s, including mean (+), median, first and third interquartile range, and fifth and 95th percentile in asthmatic patients on four different treatment regimens. Visit 1 (baseline): fluticasone 250 μg/salmeterol 50 μg (F/S) twice a day; visit 2: post four weeks F/S and montelukast 10 mg daily (M); visit 3: post four weeks M and S; visit 4: post four weeks S only. *P<0.05 when comparing different treatment regimens. ppb Parts per billion
Figure 4).
Measurement of Juniper asthma symptom score, including mean (+), median, first and third interquartile range, and fifth and 95th percentile in asthmatic patients on four different treatment regimens. Visit 1 (baseline): fluticasone 250 μg/salmeterol 50 μg (F/S) twice a day; visit 2: post four weeks F/S and montelukast 10 mg daily (M); visit 3: post four weeks M and S; visit 4: post four weeks S only. *P<0.05 when comparing different treatment regimens
Spirometry
There were no statistical differences for spirometry in asthmatic patients during visits 1 to 4.
Juniper asthma symptom score
Juniper scores (42) were significantly higher (P=0.03) when taking S alone (visit 4) when compared with the three other regimens (visits 1 to 3) (Table 2).
NO gas exchange
Results of FENO (ppb) at 100 mL/s, CANO (ppb) and bronchial NO maximal flux (nL/s) are described in Table 2 and Figures 1 to 4 for different regimens. Values for FENO were significantly higher (P=0.007) when taking S alone compared with the three other regimens. Values for bronchial NO flux were significantly higher (P=0.03) when taking S alone, compared with F/S and F/S plus M. There was no significant difference for bronchial NO between S plus M, and S alone. Compared with healthy patients, 33% of all asthmatic patients had abnormal FENO (greater than 2 SD from the normal mean) and bronchial NO, despite the use of F/S, F/S plus M, and S plus M. Values for CANO were similarly elevated in all regimens, and abnormal in 20% of the asthmatic patients, when compared with normal values.
DISCUSSION
A novel observation in the present study identified no significant differences among CANO values when comparing F/S, F/S plus M, M plus S and S alone. Moreover, while there were significant differences for bronchial NO suppression for F/S and F/S plus M versus S alone, there were no significant differences between S plus M versus S alone, and between F/S versus F/S plus M. These compartmental differences would be masked if analyses were limited to FENO alone at a single expiratory flow rate.
The results in this single-blinded, nonrandomized crossover study of 30 asthmatic patients were similar to our pilot observations (43) following four- and six-week washout of F in 13 asthmatic patients comparing similar therapeutic interventions. Furthermore, previous studies have noted that the vast majority of changes in FENO following treatment and subsequent washout with inhaled corticosteroids occurred within several days to one week (17,44). Sandrini et al (23) have also previously demonstrated, in a randomized, double-blinded, placebo-controlled crossover design lasting two weeks, M significantly reduced FENO after day 1 of treatment, with maximal effect occuring by day 7. These observations reinforced our efforts to design an efficient and cost-effective study, yet with sufficient washout time to avoid the carryover effects of anti-inflammatory controller medications.
The current bronchial NO results are consistent with bronchial biopsy studies that demonstrate reduced anti-inflammatory changes with montelukast versus inhaled fluticasone (45). Results in the present study noted the inability of inhaled corticosteroids to significantly suppress alveolar NO. Our previous observations using systemic corticosteroids (1), and studies by Berry et al (9), reported that five to 10 days of 30 mg prednisone resulted in the marked reduction of alveolar NO in asthmatic patients. This suggested that bronchoreactive, inducible NO synthase in the distal airways and/or alveoli responds to oral, but not inhaled, corticosteroids. The current NO observations lend support to previously published studies that reported equivocal results for the incidence of clinical exacerbations in asthmatic patients when comparing inhaled fluticasone plus salmeterol versus fluticasone plus montelukast (46,47).
Our observations of both increased bronchial and alveolar NO are consistent with results obtained by Berry et al (9) and van Veen et al (48) in asthmatic patients taking inhaled and oral corticosteroids, with a similar magnitude of expiratory airflow limitation as the present study. Elevated alveolar NO has also been noted in steroid-naïve patients with mild, intermittent asthma, with minimal expiratory airflow limitation and presence of nocturnal symptoms (8). The present results also complement the observations of Mahut et al (49), who noted increased alveolar NO in mildly symptomatic childhood asthmatic patients on inhaled corticosteroids, but with normal or near-normal spirometry. Alternatively, previous studies by Högman et al (50) and Shin et al (51) noted normal values for CANO in adult and childhood asthmatic patients, despite elevated bronchial NO.
The relationship between increased FENO, an inflammatory marker, and persistent expiratory airflow limitation has been further defined. In a recent publication (1), we noted that after taking 180 μg aerosolized albuterol, an FEV1 of 76% of the predicted value or lower, and a FENO 100 mL/s of at least 28 ppb, could stratify clinically stable asthmatic patients at very high risk for future exacerbations requiring oral or parenteral corticosteroids over an 18-month observation period (1).
In addition to elevated alveolar NO, corroborative evidence suggests there may be a concomitant lung parenchymal component in moderate or severe persistent asthma. This includes the presence of inflammatory cells in both bronchoalveolar lavage fluid (52) and in lung parenchyma obtained by fibre optic bronchoscopy transbronchial lung biopsy in nocturnal asthmatic patients (53). Moreover, we recently reported unexpected and unexplained loss of lung elastic recoil properties not due to emphysema in stable, non-smoking, chronic asthmatic patients with persistent, abnormal expiratory airflow limitation (40,41). However, there was no correlation with abnormal NO gas exchange. These physiological observations also challenged the prevailing concept that intrinsic small and large airway remodelling is solely responsible for expiratory airflow limitation in chronic, persistent asthma. Furthermore, Mauad et al (54,55) have noted fragmentation and decreased elastic fibres in subepithelial layers in both large and small airways, but not in alveoli.
The present observations noted increased bronchial and alveolar NO levels in chronic asthmatic patients taking inhaled corticosteroids, with no further NO suppression with the addition of M. This suggests concurrent, separate, incomplete or nonsuppressed proximal and distal airway inflammatory sites in the asthmatic patients studied.
Figure 2).
Measurement of bronchial nitric oxide maximal flux (J’awNO), including mean (+), median, first and third interquartile range, and fifth and 95th percentile in asthmatic patients on four different treatment regimens. Visit 1 (baseline): fluticasone 250 μg/salmeterol 50 μg (F/S) twice a day; visit 2: post four weeks F/S and montelukast 10 mg daily (M); visit 3: post four weeks M and S; visit 4: post four weeks S only. *P<0.05 when comparing different treatment regimens
Figure 3).
Measurement of alveolar nitric oxide concentration (CANO), including mean (+), median, first and third interquartile range, and fifth and 95th percentile in asthmatic patients on four different treatment regimens. Visit 1 (baseline): fluticasone 250 μg/salmeterol 50 μg (F/S) twice a day; visit 2: post four weeks F/S and montelukast 10 mg daily (M); visit 3: post four weeks M and S; visit 4: post four weeks S only. ppb Parts per billion
Acknowledgments
The authors thank Christy Kirkendall and Michelle Curry for patient coordination.
Footnotes
SUPPORT: This study was funded by an investigator-initiated grant from Merck & Co, Inc, Whitehouse Station, New Jersey 08889, USA, to AFG. Merck & Co, Inc, had no input on data collection, analyses or development of the manuscript.
REFERENCES
- 1.Gelb AF, Flynn Taylor C, Shinar CM, Gutierrez C, Zamel N. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest. 2006;129:1492–9. doi: 10.1378/chest.129.6.1492. [DOI] [PubMed] [Google Scholar]
- 2.Kharitonov SA, Gonio F, Kelly C, Meah S, Barnes PJ. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J. 2003;21:433–8. doi: 10.1183/09031936.03.00066903a. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society, European Respiratory Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 4.Silkoff PE, Lent AM, Busacker AA, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. 2005;116:1249–55. doi: 10.1016/j.jaci.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Lim S, Jatakanon A, Meah S, Oates T, Chung KF, Barnes PJ. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in mild to moderately severe asthma. Thorax. 2000;55:184–8. doi: 10.1136/thorax.55.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid Q, Springall DR, Riveros-Moreno V, et al. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–3. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 7.Gelb AF, Flynn Taylor C, Nussbaum E, et al. Alveolar and airway sites of nitric oxide inflammation in treated asthma. Am J Respir Crit Care Med. 2004;170:737–41. doi: 10.1164/rccm.200403-408OC. [DOI] [PubMed] [Google Scholar]
- 8.Lehtimäki L, Kankaanranta H, Saarelainen S, Turjanmaa V, Moilanen E. Inhaled fluticasone decreases bronchial but not alveolar nitric oxide output in asthma. Eur Respir J. 2001;18:635–9. doi: 10.1183/09031936.01.00000201. [DOI] [PubMed] [Google Scholar]
- 9.Berry M, Hargadon B, Morgan A, et al. Alveolar nitric oxide in adults with asthma: Evidence of distal lung inflammation in refractory asthma. Eur Respir J. 2005;25:986–91. doi: 10.1183/09031936.05.00132404. [DOI] [PubMed] [Google Scholar]
- 10.Jones SL, Kittelson J, Cowan JO, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164:738–43. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 11.Leuppi JD, Salome CM, Jenkins CR, et al. Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am J Respir Crit Care Med. 2001;163:406–12. doi: 10.1164/ajrccm.163.2.9912091. [DOI] [PubMed] [Google Scholar]
- 12.Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- 13.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–8. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stirling RG, Kharitonov SA, Campbell D, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Asthma and Allergy Group. Thorax. 1998;53:1030–4. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 16.Covar RA, Szefler SJ, Martin RJ, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003;142:469–75. doi: 10.1067/mpd.2003.187. [DOI] [PubMed] [Google Scholar]
- 17.van Rensen EL, Straathof KC, Veselic-Charvat MA, Zwinderman AH, Bel EH, Sterk PJ. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax. 1999;54:403–8. doi: 10.1136/thx.54.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 19.Green RH, Brightling CE, McKenna S, et al. Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. Eur Respir J. 2006;27:1144–51. doi: 10.1183/09031936.06.00102605. [DOI] [PubMed] [Google Scholar]
- 20.Lee MY, Lai YS, Yang KD, Chen CJ, Hung CH. Effects of montelukast on symptoms and eNO in children with mild to moderate asthma. Pediatr Int. 2005;47:622–6. doi: 10.1111/j.1442-200x.2005.02142.x. [DOI] [PubMed] [Google Scholar]
- 21.Straub DA, Minocchieri S, Moeller A, Hamacher J, Wildhaber JH. The effect of montelukast on exhaled nitric oxide and lung function in asthmatic children 2 to 5 years old. Chest. 2005;127:509–14. doi: 10.1378/chest.127.2.509. [DOI] [PubMed] [Google Scholar]
- 22.Bratton DL, Lanz MJ, Miyazawa N, White CW, Silkoff PE. Exhaled nitric oxide before and after montelukast sodium therapy in school-age children with chronic asthma: A preliminary study. Pediatr Pulmonol. 1999;28:402–7. doi: 10.1002/(sici)1099-0496(199912)28:6<402::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Sandrini A, Ferreira I, Gutierrez C, Jardim JR, Zamel N, Chapman KR. Effect of montelukast on exhaled nitric oxide and nonvolatile markers of inflammation in mild asthma. Chest. 2003;124:1334–40. doi: 10.1378/chest.124.4.1334. [DOI] [PubMed] [Google Scholar]
- 24.Lee DK, Jackson CM, Haggart K, Lipworth BJ. Repeated dosing effects of mediator antagonists in inhaled corticosteroid-treated atopic asthmatic patients. Chest. 2004;125:1372–7. doi: 10.1378/chest.125.4.1372. [DOI] [PubMed] [Google Scholar]
- 25.Biernacki WA, Kharitonov SA, Biernacka HM, Barnes PJ. Effect of montelukast on exhaled leukotrienes and quality of life in asthmatic patients. Chest. 2005;128:1958–63. doi: 10.1378/chest.128.4.1958. [DOI] [PubMed] [Google Scholar]
- 26.Currie GP, Lee DK, Haggart K, Bates CE, Lipworth BJ. Effects of montelukast on surrogate inflammatory markers in corticosteroid-treated patients with asthma. Am J Respir Crit Care Med. 2003;167:1232–8. doi: 10.1164/rccm.200209-1116OC. [DOI] [PubMed] [Google Scholar]
- 27.Ghiro L, Zanconato S, Rampon O, Piovan V, Pasquale MF, Baraldi E. Effect of montelukast added to inhaled corticosteroids on fractional exhaled nitric oxide in asthmatic children. Eur Respir J. 2002;20:630–4. doi: 10.1183/09031936.02.01512002. [DOI] [PubMed] [Google Scholar]
- 28.Laviolette M, Malmstrom K, Lu S, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. Montelukast/Beclomethasone Additivity Group. Am J Resp Crit Care Med. 1999;160:1862–8. doi: 10.1164/ajrccm.160.6.9803042. [DOI] [PubMed] [Google Scholar]
- 29.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Peroni D, Bodini A, Miraglia Del Giudice M, et al. Effect of budesonide and montelukast in asthmatic children exposed to relevant allergens. Allergy. 2005;60:206–21. doi: 10.1111/j.1398-9995.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanniess F, Richter K, Böhme S, Jörres RA, Magnussen H. Montelukast versus fluticasone: Effects on lung function, airway responsiveness and inflammation in moderate asthma. Eur Respir J. 2002;20:853–8. doi: 10.1183/09031936.02.00244602. [DOI] [PubMed] [Google Scholar]
- 33.Tsoukias NM, George SC. A two-compartment model of pulmonary nitric oxide exchange dynamics. J Appl Physiol. 1998;85:653–66. doi: 10.1152/jappl.1998.85.2.653. [DOI] [PubMed] [Google Scholar]
- 34.National Asthmatic Education and Prevention Program Clinical Practice Guidelines. Expert Panel Report 2. Guidelines for the Diagnosis and Management of Asthma. <http://www.nhlbi.nih.gov/guidelines/archives/epr-2/asthgdln_archive.pdf> (Version current at May 15, 2008).
- 35.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention, National Institutes of Health, National Heart, Lung, and Blood Institute, revised 2002<http://www.ginasthma.com/Guidelineitem.asp?l1=2&l2=1&intId=82&archived=1> (Version current at May 15, 2008).
- 36.Gelb AF, Hogg JC, Müller NL, et al. Contribution of emphysema and small airways in COPD. Chest. 1996;109:353–9. doi: 10.1378/chest.109.2.353. [DOI] [PubMed] [Google Scholar]
- 37.Tsoukias NM, Shin HW, Wilson AF, George SC. A single-breath technique with variable flow rate to characterize nitric oxide exchange dynamics in the lungs. J Appl Physiol. 2001;91:477–87. doi: 10.1152/jappl.2001.91.1.477. [DOI] [PubMed] [Google Scholar]
- 38.George SC, Hogman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J Appl Physiol. 2004;96:831–9. doi: 10.1152/japplphysiol.00950.2003. [DOI] [PubMed] [Google Scholar]
- 39.Silkoff PE, McClean PA, Slutsky AS, et al. Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am J Resp Crit Care Med. 1997;155:260–7. doi: 10.1164/ajrccm.155.1.9001322. [DOI] [PubMed] [Google Scholar]
- 40.Gelb AF, Licuanan J, Shinar CM, Zamel N. Unsuspected loss of lung elastic recoil in chronic persistent asthma. Chest. 2002;121:715–21. doi: 10.1378/chest.121.3.715. [DOI] [PubMed] [Google Scholar]
- 41.Gelb AF, Zamel N. Unsuspected pseudophysiologic emphysema in chronic persistent asthma. Am J Respir Crit Care Med. 2000;162:1778–82. doi: 10.1164/ajrccm.162.5.2001037. [DOI] [PubMed] [Google Scholar]
- 42.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 43.Gelb AF, Flynn Taylor C, Epstein JD, Shinar CM, Gutierrez C, Zamel N.Effect of fluticasone/salmeterol and montelukast on total exhaled, bronchial, and alveolar nitric oxide in asthmatics Chest 2004126813S–4S. (Abst) [Google Scholar]
- 44.Kharitonov SA, Donnelly LE, Montuschi P, Corradi M, Collins JV, Barnes PJ. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax. 2002;57:889–96. doi: 10.1136/thorax.57.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overbeek SE, O'Sullivan S, Leman K, Mulder PG, Hoogsteden HC, Prins JB. Effect of montelukast compared with inhaled fluticasone on airway inflammation. Clin Exp Allergy. 2004;34:1388–94. doi: 10.1111/j.1365-2222.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- 46.Bjermer L, Bisgaard H, Bousquet J, et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: One year, double blind, randomised, comparative trial. BMJ. 2003;327:891–7. doi: 10.1136/bmj.327.7420.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ilowite J, Webb R, Friedman B, et al. Addition of montelukast or salmeterol to fluticasone for protection against asthma attacks: A randomized, double-blind, multicenter study. Ann Allergy Asthma Immunol. 2004;92:641–8. doi: 10.1016/S1081-1206(10)61430-5. [DOI] [PubMed] [Google Scholar]
- 48.van Veen IH, Sterk PJ, Schot R, Gauw SA, Rabe KF, Bel EH. Alveolar nitric oxide versus measures of peripheral airway dysfunction in severe asthma. Eur Respir J. 2006;27:951–6. doi: 10.1183/09031936.06.00087905. [DOI] [PubMed] [Google Scholar]
- 49.Mahut B, Delacourt C, Zerah-Lancner F, De Blic J, Harf A, Delclaux C. Increase in alveolar nitric oxide in the presence of symptoms in childhood asthma. Chest. 2004;125:1012–8. doi: 10.1378/chest.125.3.1012. [DOI] [PubMed] [Google Scholar]
- 50.Högman M, Holmkvist T, Wegener T, et al. EExtended NO analysis applied to patients with COPD, allergic asthma and allergic rhinitis. Respir Med. 2002;96:24–30. doi: 10.1053/rmed.2001.1204. [DOI] [PubMed] [Google Scholar]
- 51.Shin HW, Rose-Gottron CM, Cooper DM, Newcomb RL, George SC. Airway diffusing capacity of nitric oxide and steroid therapy in asthma. J Appl Physiol. 2004;96:65–75. doi: 10.1152/japplphysiol.00575.2003. [DOI] [PubMed] [Google Scholar]
- 52.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: Role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–8. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 53.Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996;154:1505–10. doi: 10.1164/ajrccm.154.5.8912772. [DOI] [PubMed] [Google Scholar]
- 54.Mauad T, Xavier AC, Saldiva PH, Dolhnikoff M. Elastosis and fragmentation of fibers of the elastic system in fatal asthma. Am J Respir Crit Care Med. 1999;160:968–75. doi: 10.1164/ajrccm.160.3.9809088. [DOI] [PubMed] [Google Scholar]
- 55.Mauad T, Silva LF, Santos MA, et al. Abnormal alveolar attachments with decreased elastic fiber content in distal lung in fatal asthma. Am J Respir Crit Care Med. 2004;170:857–62. doi: 10.1164/rccm.200403-305OC. [DOI] [PubMed] [Google Scholar]