Abstract
BACKGROUND
Outcome after renal transplantation in children has been variable. We undertook a retrospective study of our experience over the past five years.
STUDY DESIGN
From January 1, 1988, to October 15, 1992, 60 renal transplantations were performed upon 59 children at the Children’s Hospital of Pittsburgh. Twenty-eight (47 percent) of the kidneys were from cadaveric donors, and 32 (53 percent) were from living donors. The recipients ranged in age from 0.8 to 17.4 years, with a mean of 9.8 ± 4.8 years. Forty-six (77 percent) recipients were undergoing a first transplant, while 14 (23 percent) received a second or third transplant. Eight (13 percent) of the patients were sensitized, with a panel reactive antibody of more than 40 percent. Eleven of the 14 patients undergoing retransplantation and seven of the eight patients who were sensitized received kidneys from cadaveric donors. Thirty-three (55 percent) patients received cyclosporine-based immunosuppression, and 27 (45 percent) received FK506 as the primary immunosuppressive agent.
RESULTS
The median follow-up period was 36 months, with a range of six to 63 months. The one- and four-year actuarial patient survival rate was 100 and 98 percent. The one- and four-year actuarial graft survival rate was 98 and 83 percent. For living donor recipients, the one- and four-year actuarial patient survival rate was 100 and 100 percent; for cadaveric recipients, it was 100 and 96 percent. Corresponding one- and four-year actuarial graft survival rates were 100 and 95 percent for the living donor recipients and 96 and 69 percent for the cadaveric recipients. Patients on cyclosporine had a one- and four-year patient survival rate of 100 and 97 percent, and patients on FK506 had a one- and three-year patient survival rate of 100 and 100 percent. Corresponding one- and four-year actuarial graft survival rates were 100 and 85 percent in the cyclosporine group, while one- and three-year actuarial graft survival rates were 96 and 84 percent in the FK506 group.
The mean serum creatinine level was 1.24 ± 0.64 mg per dL; the blood urea nitrogen level was 26 ± 13 mg per dL. The incidence of rejection was 47 percent; 75 percent of the rejections were steroid-responsive. The incidence of cytomegalovirus was 10 percent. The incidence of post-transplant lymphoproliferative disorder was 8 percent. None of the patients on cyclosporine were able to be taken off prednisone; 56 percent of the patients receiving FK506 were taken off prednisone successfully. Early growth and development data suggest that the patients receiving FK506 off prednisone had significant gains in growth.
CONCLUSIONS
These results support the idea that renal transplantation is a successful therapy for end-stage renal disease in children. They also illustrate the potential benefits of a new immunosuppressive agent, FK506.
The results after renal transplantation in pediatric patients have been variable, with some centers describing fair results and others reporting excellent outcomes (1, 2). A recent multicenter report, combining data from most of the major pediatric centers in the United States of America, showed one-year patient survival rates of 96 and 92 percent for living related and cadaveric recipients, and one-year graft survival rates of 88 and 71 percent, respectively (3). We reviewed our own experience during a five-year period with both living related and cadaveric transplantation and examined the effect of different immunosuppressive agents, specifically FK506.
MATERIALS AND METHODS
Between January 1, 1988 and October 15, 1992, 60 consecutive patients underwent renal transplantation only at the Children’s Hospital of Pittsburgh (Table I). Children undergoing concomitant or previous hepatic transplantation were excluded from analysis. Thirty-two (53 percent) patients received kidneys from living donors. These were parents in 30 instances, one grandmother, and one adoptive father. Twenty-eight (47 percent) patients received cadaveric kidneys. The mean recipient age was 9.8 ± 4.8 years, with a range of 0.8 to 17.4 years. Five (8 percent) recipients were less than 2 years of age; 12 (20 percent) were less than 5. Forty-six (77 percent) recipients were undergoing a first transplant, while 14 (23 percent) received a second or third transplant. Eight (13 percent) patients were sensitized, with a panel reactive antibody (PRA) of more than 40 percent. Eleven of the 14 patients undergoing retransplantation and seven of the eight patients who were sensitized received kidneys from cadaveric donors. Thirty-three (55 percent) patients received cyclosporine-based immunosuppression, and 27 (45 percent) received FK506 as the primary immunosuppressive agent. The causes of end-stage renal disease are listed in Table II.
TABLE I.
RECIPIENT CHARACTERISTICS
| No. | Percent | |
|---|---|---|
| Number of patients | 59 | |
| Number of grafts | 60 | |
| Living donor | 32 | 53 |
| Cadaveric donor | 28 | 47 |
| Mean recipient age, y | 98±48 | |
| Recipients <2 y | 5 | 8 |
| Recipients <5 Y | 12 | 20 |
| Retransplantations | 14 | 23 |
| PRA >40 percent | 8 | 13 |
| Cyclosporine | 33 | 55 |
| FK506 | 27 | 45 |
y, Year, and PRA, panel reactive antibody.
TABLE II.
CAUSES OF END-STAGE RENAL DISEASE
| No. | Percent | |
|---|---|---|
| Glomerulonephritis | 9 | 15 |
| Obstructive uropathy | 9 | 15 |
| Congenital hypoplasia | 5 | 8 |
| Hemolytic uremic syndrome | 4 | 7 |
| Polycystic kidney disease | 4 | 7 |
| Prune belly syndrome | 4 | 7 |
| Reflux | 3 | 5 |
| Focal segmental glomerulosclerosis | 3 | 5 |
| Congenital dysplasia | 2 | 3 |
| Pyelonephritis | 2 | 3 |
| Other | 10 | 17 |
| Undetermined | 5 | 8 |
Cyclosporine-based immunosuppression in the 20 living related cases was done with induction Minnesota antilymphoblast globulin (MALG), 20 mg per kg per day, for 2 weeks; Imuran®, Burroughs Wellcome, Research Triangle Park, NC (azathioprine) and Deltasone®, Upjohn, Kalamazoo, MI (prednisone) were used from the outset, while Sandimmune®, Sandoz Pharmaceuticals, East Hanover, NJ (cyclosporine) was begun one week postoperatively, at a dose of 5 mg per kg twice daily. In the 13 cadaveric cases treated with cyclosporine, azathioprine was used in 11 patients, induction OKT3 (5 mg per day for two weeks) was used in four cases, and induction MALG was used in one case. In the 27 patients receiving FK506 (12 living donor instances and 15 cadaveric instances), an intravenous dose of 0.1 mg per kg per day was begun postoperatively as a continuous infusion. The oral dose was begun at 0.15 mg per kg twice daily. Azathioprine, 2 to 3 mg per kg per day, was used initially in ten (36 percent) patients, and steroids were given to all patients.
STATISTICAL ANALYSIS
The standard two-sample t test was used to test differences between means, while differences in proportions were tested using Pearson’s chisquare test of association. The Wilcoxon rank sum test, a nonparametric equivalent to the standard two-sample t test, was used for highly skewed data.
Patient survival was calculated from the date of renal transplantation until death, and graft survival was calculated from the date of renal transplantation until graft failure, retransplantation, or patient death. Survival curves were generated using the Kaplan-Meier (product-limit) method and were compared using the generalized Wilcoxon (Breslow) test. All tests were two tailed. A p value less of than 0.05 was considered statistically significant.
RESULTS
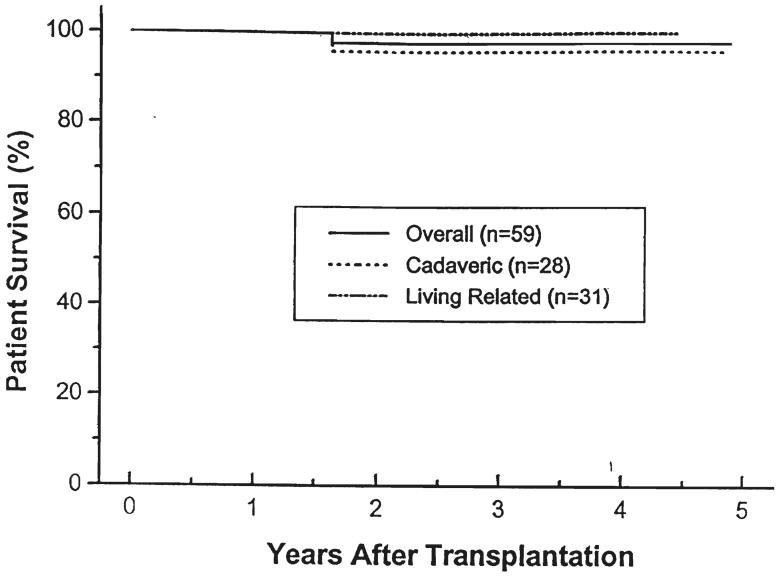
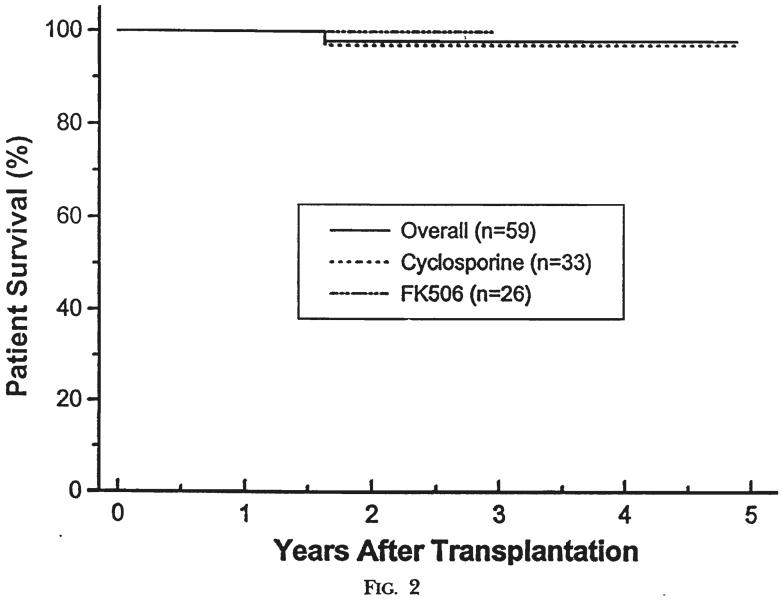
The median follow-up period was 36 months (range of six to 63 months). The one- and four-year actuarial patient survival rate was 100 and 98 percent. One male, 5.7 years old at the time of a second cadaveric transplant under cyclosporine, died with a functioning kidney 19 months postoperatively, as a result of complications related to idiopathic hypertrophic subaortic stenosis. In cadaveric recipients, the one- and four-year actuarial patient survival rate was l00 and 96 percent; in living donor instances, the one- and four-year actuarial patient survival rate was 100 and 100 percent. Patients treated with cyclosporine had a one- and four-year actuarial patient survival rate of 100 and 97 percent. Patients treated with FK506 had a one- and three-year actuarial patient survival rate of 100 and 100 percent, respectively (Figs. 1 and 2).
Fig 1.
Patient survival. Cadaveric versus living related donors.
Fig. 2.
Patient survival. Patients treated with cyclosporine versus FK506.
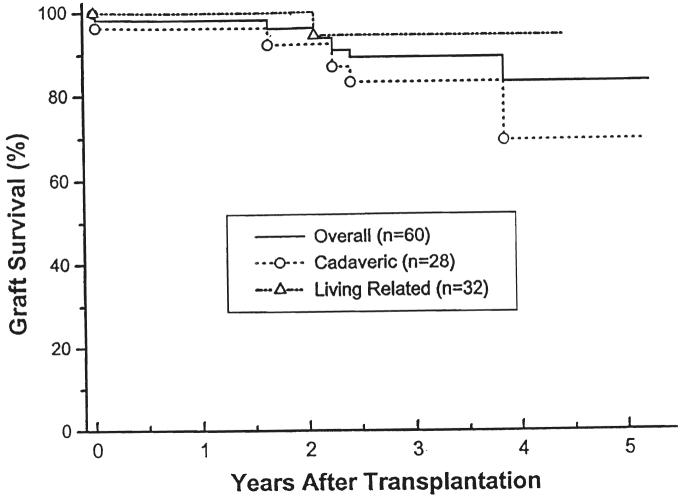
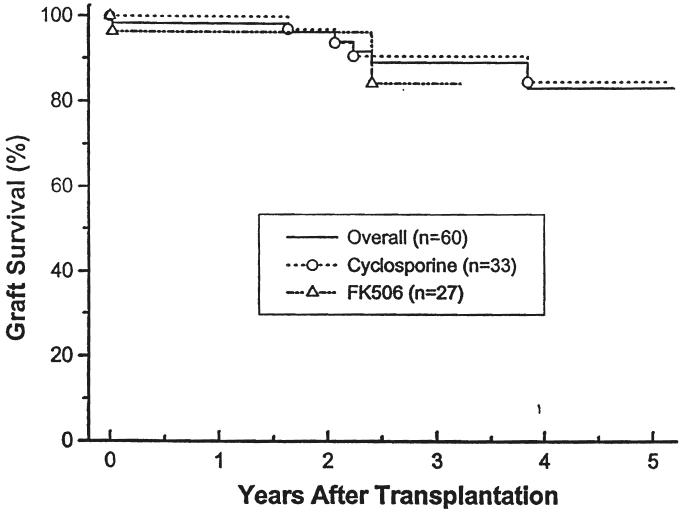
The overall one- and four-year actuarial graft survival rate was 98 and 83 percent; 100 and 95 percent in the living related patients and 96 and 69 percent in the cadaveric group. Patients receiving cyclosporine had a one- and four-year actuarial graft survival rate of 100 and 85 percent. (Fig. 3). Patients treated with FK506 had a shorter follow-up period. Their one- and three-year actuarial graft survival rates were 96 and 84 percent (Fig. 4). In addition to the one late mortality, five additional patients have lost grafts: two to noncompliance 1.8 and 3.2 years after transplantation (both being treated with cyclosporine), one to recurrent hemolytic uremic syndrome within the first week after transplantation (treated with FK506; the first graft of this child treated with cyclosporine was also lost to recurrent hemolytic uremic syndrome in the first postoperative week), one to recurrent focal segmental glomerulosclerosis 3.8 years post-transplantation (treated with cyclosporine initially}, and one to recurrent membranoproliferative glomerulonephritis 2.4 years post-transplantation (treated with FK506; the first transplant for the patient was also lost to recurrent disease, treated with cyclosporine therapy).
Fig. 3.
Graft survival. Cadaveric versus living related donors.
Fig. 4.
Graft survival. Patients treated with cyclosporine versus FK506.
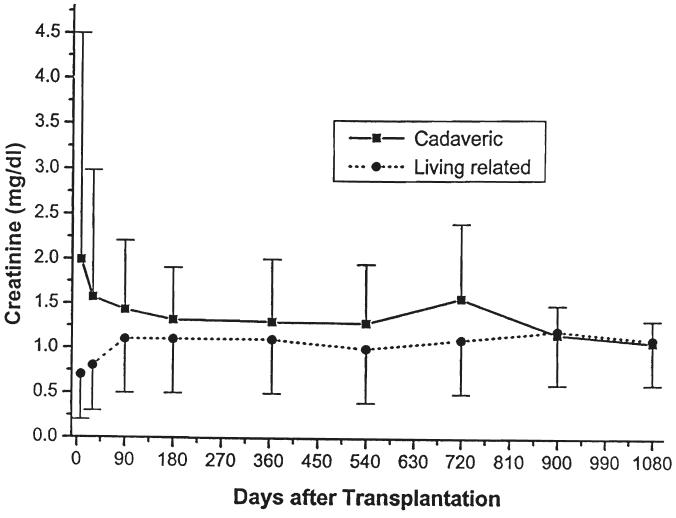
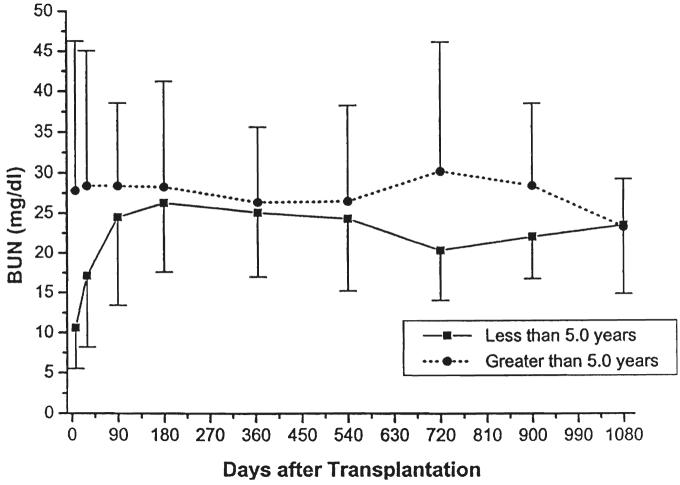
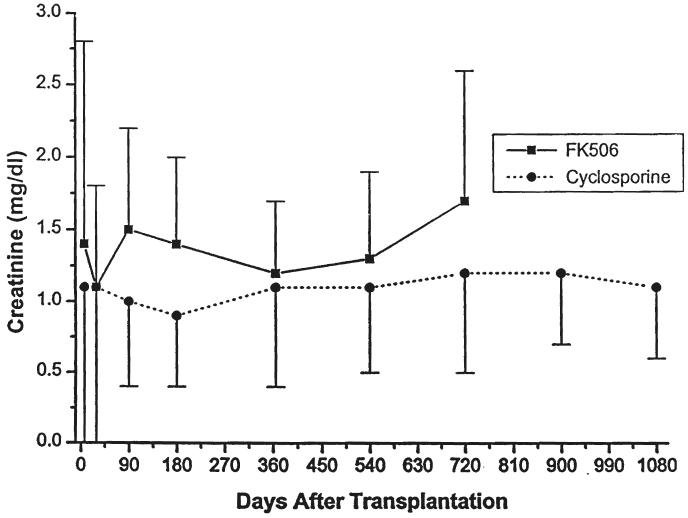
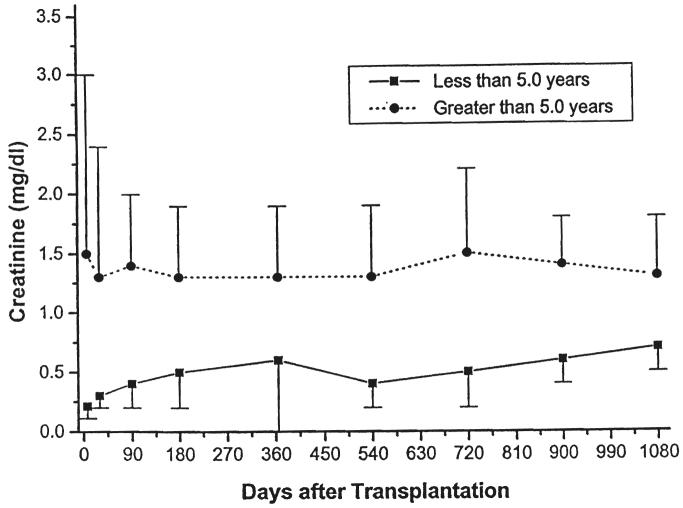
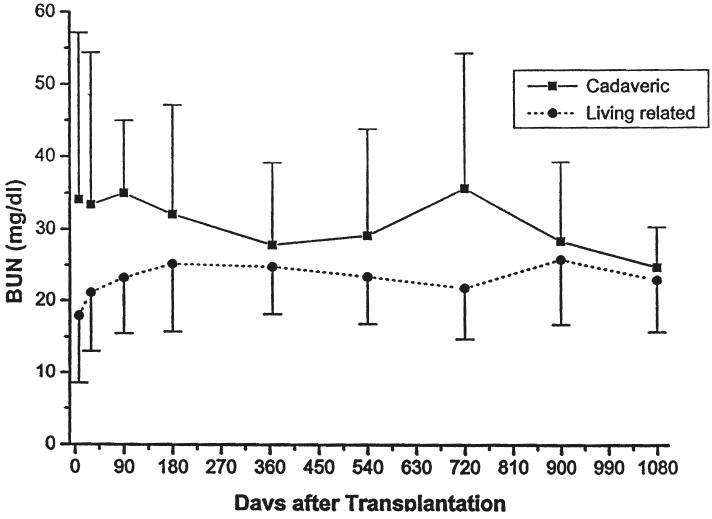
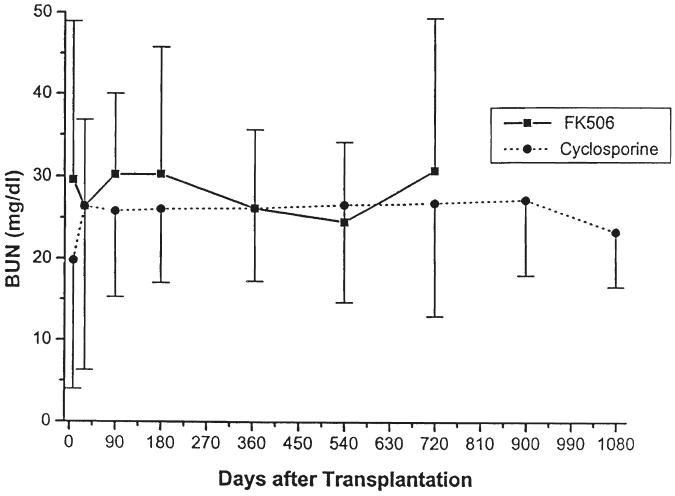
Parameters of renal function over time, stratified by type of transplant (cadaveric or living donor), immunosuppressive agent (cyclosporine or FK506), or age (less than or greater than five years of age at the time of the transplant) are given in Table III and in Figures 5-10. The mean serum creatinine level (representing the most recent value) for all patients was 1.24 ±0.64 mg per dL. The calculated creatinine clearance was 38 ± 26 mL per minute (4, 5). The mean blood urea nitrogen level was 26 ± 13 mg per dL. No differences were seen between living donor or cadaveric recipients, or between patients treated with cyclosporine or FK506, for any of these values. The mean serum creatinine level for recipients less than the age of five years was significantly lower than for the patients five years or older, but the calculated creatinine clearances were not significantly different.
TABLE III.
RENAL FUNCTION AFTER TRANSPLANTATION
| Serum creatinine, _ mg/dL_ | Creatinine clearance, mL/min/ _1.73 m2_ | BUN, _mg/dL_ | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Overall | 1.24 | 0.64 | 66.2 | 19.2 | 26 | 13.00 |
| Baseline immunosuppression | ||||||
| Cyclosporine | 1.22 | 0.60 | 61.5 | 16.8 | 25 | 11.56 |
| FK506 | 1.26 | 0.70 | 71.7 | 20.6 | 27 | 14.50 |
| Donor relationship | ||||||
| Cadaveric | 1.41 | 0.76 | 63.0 | 23.3 | 32 | 16.00 |
| Living | 1.10 | 0.51 | 68.5 | 15.5 | 22 | 8.00 |
| Age at transplant | ||||||
| <5 Y | 0.56 | 0.28* | 73.0 | 18.8 | 22 | 8.00 |
| >5 Y | 1.42 | 0.62* | 64.7 | 19.2 | 28 | 14.00 |
P<0.0001.
BUN, Blood urea nitrogen; SD, standard deviation, and y, year.
Fig. 5.
Serum creatinine. Cadaveric versus living related donors.
Fig. 10.
Serum blood urea nitrogen. Recipients less than five years of age versus those five years or older.
The incidence of rejection in the living related group was 31 percent (ten of 32 patients); in the cadaveric group, it was 64 percent (18 of 28 patients) (p=0.021). Patients receiving cyclosporine had an incidence of rejection of 39 percent (13 of 33 patients); 15 percent (five of 33 patients) required OKT3. In patients receiving FK506, rejection was seen in 56 percent of the patients (15 of 27 patients); 7 percent (two of 27 patients) required OKT3 (p=0.293).
The acute tubular necrosis rate was 5 percent: zero percent in the living related group and 11 percent in the cadaveric group (p=0.058). Two patients required dialysis in the postoperative period, one each on cyclosporine and FK506.
Fifteen (56 percent) patients receiving FK506 were successfully taken off prednisone. Three additional patients were taken off prednisone but experienced late rejection and took steroids. No patient on cyclosporinc had prednisone permanently halted, although one patient was off for nearly 15 months (p<0.00001).
Cytomegalovirus (CMV) infection was seen in six patients (10 percent) and was treated with Cytovene®, Syntex Laboratories, Inc., Palo Alto, CA (gancyclovir). There were three (11 percent) patients receiving kidneys from cadavers and three (9 percent) patients receiving kidneys from living related donors. Of the six patients with CMV, one (3 percent) was treated with cyclosporine, and five (19 percent) were treated with FK506 (p=0.047). Five of these six were seronegative recipients of seropositive kidneys. No patient had CMV pneumonia, and none required admission to the intensive care unit.
There were five (8 percent) instances of post-transplant lymphoproliferative disorder (PTLD), three (11 percent) in cadaveric recipients, two (6 percent) in living donor recipients, one (3 percent) in a patient treated with cyclosporine, and four (15 percent) in patients treated with FK506 (p=0.100). All patients had resolution of the PTLD with cessation or diminution of immunosuppression, and all kept their graft. There was one additional instance of adenopathy with pulmonary nodules that did not show PTLD on biopsy, but that did respond to a reduction in immunosuppression.
There were three patients converted from cyclosporine to FK506, one for rejection and two for proteinuria. The child with rejection was successfully rescued, and one child with proteinuria had a significant decrease in protein excretion. The other patient eventually lost the kidney to recurrent focal segmental glomerulosclerosis.
There was one arterial pseudoaneurysm that required operative repair, one arterial stenosis that responded to balloon dilatation, and one arterial leak that required repair. There were two ureteral complications, one leak requiring repair and one stenosis requiring revision of the ureteroneocystostomy. The technical complication rate was thus 8 percent.
DISCUSSION
The patients in this group were not selected. While most were good risk, low PRA patients, there were also some high risk patients. Several patients weighed less than 10 kg. Some patients, particularly those with a high PRA, had been referred from other centers for retransplantation.
In general, all groups of patients did reasonably well, regardless of the source of the kidney or the immunosuppressive agent. The living donor patients had less rejection and seem to have had better long-term graft survival rates. The patients receiving FK506 required less prednisone. The ability to discontinue prednisone in many of the patients receiving FK506 has important implications for long-term growth after transplantation, and there is preliminary evidence suggesting improved growth in the patients receiving FK506 who are not taking steroids (6). Our experience with adult transplantation has indicated that there is a learning curve associated with using FK506 in renal transplantation (7). While patient and graft survival rates were comparable in the pediatric patients receiving cyclosporine and FK506, there were more CMV infections and a trend toward more PTLD in the FK506 group. Fortunately, these complications all resolved with reduction in the immunosuppression and appropriate antiviral therapy.
We believe that there are several factors that have contributed to these results. The patients were maintained in optimal medical condition by their nephrologists before transplantation. There was a low initial acute tubular necrosis rate, even in the cadaveric group. Only two patients required dialysis after transplantation. Lessons learned from our much larger experience with adult renal transplantation have had a significant role—pediatric transplant patients account for only about 7 percent of the total number of renal transplants performed at the University of Pittsburgh.
Our data suggest that renal transplantation is a successful therapy for end-stage renal disease in children. Further work should accentuate ways of limiting the long-term side effects of chronic immunosuppression. Newer agents, such as FK506, with its steroid-eliminating capability, may be useful in achieving that goal.
Fig. 6.
Serum creatinine. Patients treated with cyclosporine versus FK506.
Fig. 7.
Serum creatinine. Recipients less than five years of age versus those five years of age or older.
Fig. 8.
Serum blood urea nitrogen. Cadaveric versus living related donors.
Fig 9.
Serum blood urea nitrogen. Patients treated with cyclosporine versus FK506.
REFERENCES
- 1.Fine RN, Ettenger RB. Renal transplantation in children. In: Morris PJ, editor. Kidney Transplantation: Principles and Practice 1988. 3rd. ed. W. B. Saunders Co.; Philadelphia: 1988. pp. 635–691. [Google Scholar]
- 2.Almond PS, Matas AJ, Gillingham K, et al. Pediatric renal transplants—results with sequential immunosuppression. Transplantation. 1992;53:46–51. doi: 10.1097/00007890-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Alexander SR, Arbus GS, Butt KMH, et al. The 1989 Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr. Nephrol. 1990;4:542–553. doi: 10.1007/BF00869842. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GJ, Haycock GB, Edelman CM, Jr., Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 5.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J. Pediatr. 1985;106:522–526. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 6.Ellis D, Shapiro R, Gilboa N, et al. Renal function and growth after renal transplantation in children: a comparison of FK506 and cyclosporine regimens. Pediatr, Nephrol. 1994;8:193–200. doi: 10.1007/BF00865477. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro R, Jordan M, Scantlebury V, et al. A randomized trial of FK506/prednisone vs. FK506/azathioprine/prednisone after renal transplantation—preliminary report. Transplant. Proc. 1993;25:669–672. [PMC free article] [PubMed] [Google Scholar]