Renal transplantation as originally described by Küss and later refined by Murray and Harrison has proved to be an effective operation from a technical point of view. The donor kidney is placed in the contralateral iliac fossa of the recipient, and thereby the anteroposterior relations of the hilar structures are reversed. The renal vessels are attached to the end and side, respectively, of the hypogastric artery and the external iliac vein. Urinary drainage is provided with ureteroureterostomy or a ureteroneocystostomy.
In children, the use of this simple extraperitoneal operation is inconvenient or impossible if an adult kidney is used, because the large homograft may not fit into the relatively diminutive iliac fossa. In addition, the size of the pelvic vessels may be so small compared with those of the homograft that an unfavorable disparity can create additional hazards.
Recently, these problems have been considered in 3 children who were treated with renal homografts from their mothers. The patients were 8, 6, and 3 years old, and weighed 22.5, 20, and 15 kilograms respectively. A previously undescribed method was employed for transplantation. The present report is concerned with a description of the operative technique. In addition, observations are included on the hemodynamic consequences of revascularizing a large kidney in a host who would have to contribute a disproportionately large fraction of cardiac output to the relatively oversized new organ.
DESCRIPTION OF TECHNIQUE
A midline abdominal incision is used, extending from the xiphoid to the pubis. Splenectomy and left nephrectomy are performed. An incision is then made in the posterior peritoneum to the right of the ascending colon. The ascending colon is reflected to the left as in the preparatory stages of colectomy (Fig. 1). The right kidney is removed.
FIG. 1.
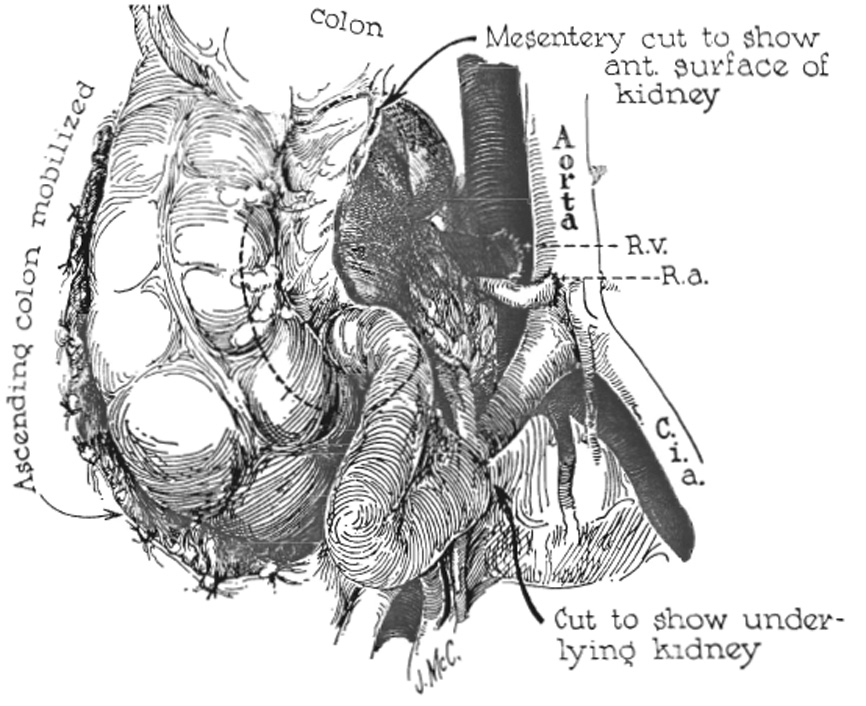
Technique of transplantation. Fixation of the homograft is not required.
The terminal portion of the inferior vena cava is freed circumferentially for a distance of 3 or 4 centimeters. Posteriorly, 2 or 3 tributary lumbar veins require ligation and division. The junction of the aorta with the right common iliac artery is similarly denuded of its covering investments. The bifurcation of the aorta occurs at a slightly higher level than that of the inferior vena cava. Consequently, the right common iliac artery crosses the lower end of the vena cava en route to its location lateral to the right common iliac vein. Just above this point, an anatomic window is present in which lies the mobilized portion of vena cava (Fig. 1).
The technique is essentially the same with use of either the left or right donor kidney. After arrival of the homograft, a segment of the distal portion of the inferior vena cava is isolated between noncrushing clamps and an end-to-side renal venous-inferior vena caval anastomosis is first performed using No. 5-0 silk. The end of the renal artery is attached with No. 6-0 silk to the side of the common iliac artery just below or at the origin of the latter vessel from the aorta (Fig. 1). It is not necessary to occlude the terminal aorta completely at any time, since a proximal vascular clamp can be placed in such a way that flow is not interruped to the left common iliac artery. Distal control is obtained by cross clamping the common iliac artery just above its division. In 2 patients, the renal artery was brought in front of the terminal vena cava. In the other, it was more convenient to bring the renal artery behind. During and immediately after restoration of homograft circulation, particular attention is paid to the recipient's hemodynamic response. No evidence of high output cardiac overload was encountered in the patients in whom the method was used.
The adult organ almost completely fills a child's right paravertebral gutter, extending from the under surface of the liver to the pelvis. By reattaching the blood supply at a level which is lower than normal, but which is considerably higher than that used in the more traditional retroperitoneal operation, the anatomic position of the new kidney is mechanically perfect. The ascending colon is dropped back on the anterior surface of the transplant and no other fixation for the new kidney is provided.
The ureter is brought inferiorly crossing the common iliac artery midway along its course and tunneled retroperitoneally to the base of the bladder (Fig. 1). It is brought through the vesicle wall just superior to the normal entrance of the ureter and implanted using the ureteroneo-cystostomy of Paquin and Marshall.
After operation, the patients are confined to bed for 48 hours, during which time they are allowed to lie only on the back or right side. Ambulation is then commenced. The 3 children on whom this operation was performed have been followed up from 1 week to 4 months. Good renal function was obtained and no adverse consequences of any sort have been encountered which could be attributed to the operative procedure per se.
DISCUSSION
Donor right kidneys were transferred to the right paravertebral gutters of 2 children. With this arrangement, structures of the renal pedicle occupy a normal anteroposterior relationship in the transplant site, in contrast to the more traditional retroperitoneal technique. It was just as easy, however, in the third child to transplant the donor left kidney to the same location in the recipient's right side since the technical details were essentially unchanged.
Although the operation has thus far been employed only for children of 3, 6, and 8 years of age, the capaciousness of the space available for receipt of the homograft is such that an adult organ could probably be fitted into a recipient of even smaller size. Because of the small total body dimensions, the amount of ureter necessary to reach the bladder is no longer than usual despite the fact that the kidney is placed at a higher anatomic position. There is little likelihood that relative stenosis of the 3 anastomoses will result from growth of the recipient patient, since the lumens created are as large from the beginning as will ever be required.
Acknowledgments
Aided by Grants A-6283, A-6344, AI 041542, HE 00735, AM 07772, and OG 27 from the U. S. Public Health Service.
REFERENCES
- 1.Küss R, Teinturier J, Milliez P. Quelques essais de greffes du rein chez 1'homme. Mém. Acad. chir., Par. 1951;77:755. [PubMed] [Google Scholar]
- 2.Murray JE, Harrison JH. Management of 50 patients with kidney transplants including 18 pairs of twins. Am. J. Surg. 1963;105:205. doi: 10.1016/0002-9610(63)90292-7. [DOI] [PubMed] [Google Scholar]
- 3.Paquin AJ. Ureterovesical anastomosis; the description and evaluation of a technique. J. Urol. Balt. 1959;82:573. doi: 10.1016/S0022-5347(17)65934-2. [DOI] [PubMed] [Google Scholar]


