Abstract
MCAK, a kinesin related motor protein with microtubule depolymerizing activity, is known to play an important role in spindle assembly and correcting errors in mitotic chromosome alignment. Experiments to determine how cellular levels of the protein are regulated demonstrate that MCAK accumulates during cell cycle progression, reaches a maximum at G2/M phase, and is rapidly degraded by the proteasome during mitosis. Immunofluorescence microscopy further indicates that MCAK largely disappears from kinetochores and spindle poles at the metaphase to anaphase transition. A phosphorylated form of MCAK appears during mitosis and seems to be preferentially degraded, but degradation does not appear to depend on Aurora B, a kinase reported to be involved in regulating the error correcting activity of the protein. These studies indicate that MCAK activity is limited during the latter stages of mitosis by protein degradation, and argue against a role for the protein in anaphase chromosome movement.
Keywords: mitosis, kinesin, proteasome, phosphorylation
Introduction
Mitotic centromere associated kinesin (MCAK) belongs to a large family of motor proteins that transport cargo along microtubules, but has the unusual ability to depolymerize microtubules and regulate microtubule dynamics.1,2 This protein has also been called Kin I and Kif 2c; more recently it has been assigned to the Kin 13 class of kinesin motor proteins.3 Although MCAK and related proteins in other organisms are present in both the cytoplasm and the nuclei of interphase cells, their best characterized functions are in mitosis where they have been reported to participate in anaphase sister chromatid segregation through a “Pac-Man-like” mechanism at chromosome kinetochores as well as promotion of poleward microtubule flux at the spindle poles.4,5 MCAK has also been implicated in correcting misaligned chromosomes during their congression to the metaphase plate.6 The importance of MCAK in ensuring the faithful segregation of chromosomes prior to cytokinesis is consistent with the observation that it is upregulated in various types of cancer and could be involved in causing the aneuploidy that is often associated with this disease.7,8 In agreement with this view, both overexpression and depletion of MCAK in cultured mammalian cells cause defects in mitotic spindle assembly and errors in chromosome segregation.9–11 Thus, it would appear that normal mitotic progression requires relatively precise levels of MCAK.
Additional regulation of MCAK occurs by phosphorylation. For example, phosphorylation of MCAK by Aurora B kinase has been reported to inhibit its microtubule depolymerizing activity.12,13 Multiple Aurora B phosphorylation sites have been reported and attempts are being made to identify which functions are controlled by phosphorylation at specific sites.14,15 A role for phosphorylation in controlling the activity of MCAK in mitosis is further supported by the recent observation of a slower migrating MCAK band on SDS gels.8 The kinase responsible for creation of the slow migrating species has not been identified.
Our laboratory has recently begun to examine how mammalian cells regulate MCAK levels. We demonstrate here that MCAK abundance is low during early G1, increases as cells progress to mitosis, and then falls again at the metaphase to anaphase transition. We further show that inhibition of proteasome activity blocks cells in mitosis and prevents the loss of MCAK from spindle poles and kinetochores at metaphase. Our results are consistent with a role for MCAK in congression of chromosomes to the metaphase plate, but not with a role in anaphase chromosome movement.
Results
Transfected FLAG-MCAK mimics the endogenous protein
We cloned a full length human MCAK cDNA encoding a FLAG epitope tag at the amino terminus into a pTOPneo vector for tetracycline regulated expression, transfected it into CHO tTApur 6.6a cells that stably express a tetracycline regulated transactivator,16 and selected G418 resistant clones. Clones in which at least 90% of the cells exhibited staining with FLAG antibody, produced low levels of the ectopic protein, and were well regulated by tetracycline were chosen for further study. Western blots of one such clone, Clone 2, are shown in Fig. 1. Probing with an antibody to the FLAG tag demonstrated that the protein is produced in the absence, but not in the presence, of tetracycline (Fig. 1A). To determine the amount of protein produced, the same extracts were probed with an antibody that recognizes both endogenous and ectoptic MCAK (Fig. 1A). We estimate from these experiments that induction of FLAG-MCAK produces approximately a 2-fold increase in total MCAK. The cells were also tested to ensure that accumulation of FLAG-MCAK to this level did not interfere with cell growth or normal progression through mitosis (data not shown).
Figure 1.
Synthesis and localization of FLAG-MCAK. A, Western blot analysis of Clone 2, a CHO cell line stably transfected with a cDNA encoding FLAG-MCAK. The cells were grown in the presence (+) or absence (−) of tetracycline and the blot was probed with antibodies to the FLAG epitope tag or to the MCAK protein. An antibody to Actin was included as a gel loading control. B–G, Immunofluorescence localization of endogenous and ectoptic MCAK. Clone 2 cells in interphase (B–D) and prophase (E–G) were stained with antibodies to MCAK (B, E) and FLAG (C, F) as well as the DNA stain DAPI (D, G). Arrows (B, C) indicate the position of the centrosome; arrows (E, F) indicate the positions of the spindle poles. MCAK also localizes to chromosome centromeres in the prophase cells (E, F). Bar (B) = 10 μm.
Immunofluorescence microscopy demonstrated that FLAG-MCAK localizes to the same structures as the endogenous protein. During interphase, antibody to MCAK was found in the nucleus as well as the cytoplasm where it prominently stained the centrosome (arrow, Fig. 1B) and weakly stained the microtubules. Antibody to the FLAG tag gave essentially the same pattern (Fig. 1C). In prophase cells, MCAK staining at the spindle poles (arrows, Fig. 1E) increased as did the staining of interphase microtubules. In addition, staining of the centromeric region of the condensed chromosomes now became evident as a number of bright spots in the nuclear area. Antibody to FLAG again gave a similar pattern in these prophase cells (Fig. 1F). These results for the localization of MCAK in mammalian cells are similar to those that have been reported from many other laboratories. We conclude that the transfected FLAG-MCAK behaves in a similar manner to the endogenous protein and does not cause an observable disruption of MCAK function at a 2-fold level of expression. Because the FLAG antibody gave us a much stronger signal than the antibody to MCAK (e.g., see Fig. 1A), much of the data presented in this study followed the FLAG-tagged MCAK. However, all the results were confirmed in nontransfected cells using the antibody to MCAK to be sure that the endogenous protein behaved in a similar manner.
Degradation of MCAK correlates with the generation time of various cell lines
To determine the stability of FLAG-MCAK, Clone 2 was grown without tetracycline for 1 day to accumulate the ectopic protein and then tetracycline was added back to inhibit further expression. Cells were harvested at various times after tetracycline addition, and western blots of the cell lysates were analyzed for FLAG-MCAK and actin content. Because actin is a stable protein that was not under tetracycline regulation, its abundance remained relatively constant and served as a control over the time course of our experiment. In contrast, FLAG-MCAK decreased continuously and was largely gone by 12 h (Fig. 2A and filled circles, Fig 2B).
Figure 2.
Stability of MCAK. Clone 2 was induced to express FLAG-MCAK (FMCAK) and then treated with tetracycline to stop further transcription. A, Western blot of cell extracts at various times after addition of tetracycline (Tet) probed with antibodies to FLAG and Actin (used as a loading control). B, Quantification of MCAK degradation from western blot data. Solid circles, FLAG-MCAK in Clone 2. For the other samples, untransfected cells were treated with puromycin to stop protein synthesis and cell extracts were probed with an antibody to MCAK at various times after drug addition. Open circles, CHO cells; open squares, HeLa cells; open triangles, MCF-7 cells. MCAK to actin ratios were calculated for each time point and plotted relative to the ratio at zero time set at 100%. Points represent the average of 4 experiments and the error bars represent the standard deviation from the mean.
The limited stability of FLAG-MCAK led us to question whether endogenous MCAK has a similar half-life. To answer this question, we used an MCAK antibody to carry out the same experiment with non-transfected CHO, HeLa and MCF-7 cells, except that in this case cells had to be treated with puromycin to halt protein synthesis. The results (Fig. 2B) demonstrate that endogenous MCAK in CHO cells (open circles, Fig. 2B) disappears as rapidly as transfected FLAG-MCAK (closed circles), but the protein in HeLa (open squares) and MCF-7 (open triangles) cells appears to be more stable. Based on this observation, we reasoned that the stability of MCAK depends on the rate of growth of the various cell lines. In support of this notion, we found that the lifetime of the protein correlated very well with the 14 h, 21 h, and 38 h doubling times we measured when we assayed the growth rates of the 3 cell lines (data not shown).
MCAK is degraded in a cell cycle dependent manner
The correlation between cellular growth rate and MCAK stability in different cell lines suggested that the protein was being degraded in a cell cycle specific manner. To directly test this possibility, Clone 2 was induced to express FLAG-MCAK and synchronized by sequential S-phase and M-phase blocks. After release from the mitotic block, cells were microscopically monitored by DNA staining to determine the distribution of cells at different stages of mitosis. Flow cytometry data demonstrating the efficiency of synchronization is shown in Fig. S1.
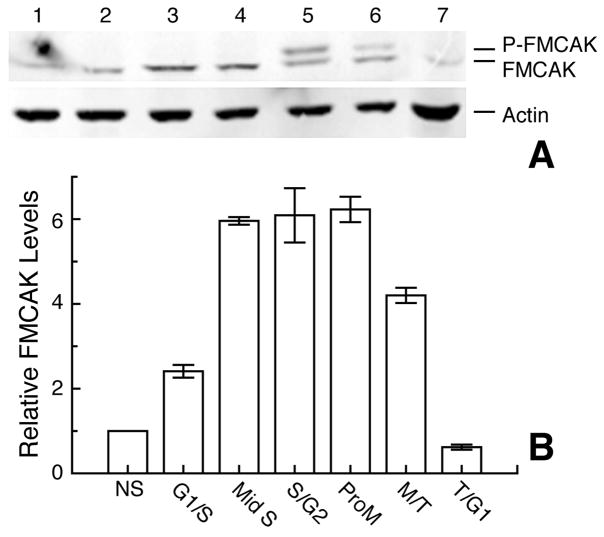
Western blots were used to assess the FLAG-MCAK level relative to actin at various stages of the cell cycle (Fig. 3). Using nonsynchronized cells (Fig. 3A, lane 1) as a reference, we found that FLAG-MCAK levels increased several-fold as cells accumulated in S-phase, G2 and mitosis (lanes 2–5), decreased during mitotic progression (lane 6), and reached their lowest level by telophase or early G1 (lane 7). In agreement with a recent report,8 we observed a slower migrating FLAG-MCAK band during mitosis (lanes 5–7). This upper band likely represents a phosphorylated form of FLAG-MCAK as it can be largely eliminated by phosphatase treatment of mitotic cell extracts (reference 8 and data not shown). FLAG-MCAK/Actin ratios were calculated for each stage of the cell cycle and the data are summarized in Fig. 3B. The figure compares the total relative FLAG-MCAK (upper and lower bands) at all stages of the cell cycle, but it should be noted in the western blots that, during mitosis, the upper band disappeared more rapidly than the lower band suggesting the possibility that a phosphorylated form of FLAG-MCAK might be preferentially degraded. Similar results to these were also obtained in non-transfected cells using an antibody to MCAK (data not shown).
Figure 3.
Cell cycle accumulation of FLAG-MCAK (FMCAK). Clone 2 was synchronized with successive thymidine and nocodazole blocks, and cells from various stages of the cell cycle were analyzed by western blots (A) probed with antibodies to FLAG and Actin (loading control). Samples came from cells that were not synchronized (NS, Lane 1); were blocked in thymidine for 8 h (G1/S, Lane 2), or 12 h (Mid S, Lane 3); were blocked 12 h and released 4 h (S/G2, Lane 4); were released 4 h and then blocked in nocodazole 4 h (ProM, Lane 5); and were released from nocodazole for 20 min (M/T, Lane 6) or 40 min (T/G1, Lane 7). Relative FLAG-MCAK Levels in cells at early S-Phase (G1/S), Mid S-phase (Mid S), late S-phase (S/G2), prometaphase (ProM), metaphase to telophase (M/T), and late telophase to early G1 (T/G1) were calculated from western blots in 3 independent experiments as FLAG-MCAK/Actin ratios and plotted relative to the ratio from unsynchronized cells that was arbitrarily set at 1 (B). The error bars represent standard deviations from the mean. Note that during mitosis a slower migrating band appears that we attribute to a phosphorylated form of FLAG-MCAK (P-FMCAK). Both upper and lower bands were added to get the total FLAG-MCAK during mitosis.
MCAK is degraded at the transition from metaphase to anaphase
Biochemical measurements of MCAK degradation at various stages of the cell cycle are complicated by the fact that cells do not move through mitosis with perfect synchrony. For example, the cell population labeled as M/T in Fig. 3B (lane 6 in Fig. 3A), contained cells that we estimated by chromosome staining to be at the following stages of mitosis: 40% prometaphase, 35% near metaphase, 5% anaphase, 15% telophase and 5% early G1. In an attempt to get a more precise indication of when MCAK is degraded during mitosis, we turned to a microscopic assay in which mitotic cells were stained with an antibody to FLAG-MCAK and photographed using a constant exposure so that cells in different stages of mitosis could be directly compared. As demonstrated in Fig. 4, prophase cells typically had the brightest staining and showed FLAG-MCAK localized to the spindle poles (arrows), kinetochores, and some of the microtubules emanating from the spindle poles (Fig. 4A). Staining remained bright in prometaphase (Fig. 4B) but was significantly weaker at metaphase (Fig. 4C), anaphase (Fig. 4D, E), and telophase (Fig. 4F). Although staining was greatly reduced at these latter stages of mitosis, it was not completely absent as indicated by the enhanced image shown in the inset of Fig. 4C. Note in the enhanced image, however, that kinetochores are barely visible and even the polar staining is only slightly greater than the weak staining normally associated with microtubules. As a control for these experiments, cells were also stained with a CREST antibody that stains kinetochores throughout the cell cycle.17 No decrease in CREST staining during anaphase (Fig. 4H) or telophase (Fig. 4I) compared to prometaphase (Fig. 4G) was noted. Although immunofluorescence does not distinguish between degradation of MCAK and dissociation of MCAK from its binding sites, the fact that the loss of staining occurred at approximately the same time that we measured a decrease in MCAK levels biochemically (see Fig. 3), argues that degradation predominantly occurs at the metaphase to anaphase transition. Immunofluorescence of nontransfected cells using an antibody to endogenous MCAK also indicated a large reduction in kinetochore associated MCAK in anaphase and telophase cells (Fig. S2).
Figure 4.

FLAG-MCAK localization during mitosis. Clone 2 cells at various stages of mitosis were stained with an antibody to FLAG (A–F) or to CREST (G–I) (white) and DAPI (pseudocolored red). A, prophase cell with bright kinetochore and spindle pole (arrows) staining; B, prometaphase; C, metaphase cell with photographically enhanced FLAG-MCAK staining shown in the inset; D, anaphase; E, late anaphase/early telophase; F, late telophase; G, prophase; H, anaphase; I, telophase. Photographs (except inset, panel C) were taken at constant exposure. Bar (A) = 10 μm.
MCAK degradation does not require phosphorylation by Aurora B
The preferential loss of the slower migrating band during mitosis (see Fig. 3A) suggested the possibility that phosphorylation of MCAK triggers its degradation. A number of publications have indicated that Aurora B kinase phosphorylates MCAK during mitosis and regulates its function. To test whether phosphorylation by Aurora B kinase also provides the signal for degradation of MCAK, we first used a cloning efficiency assay to determine that the minimum lethal dose of the Aurora B kinase inhibitor ZM447439 in CHO cells is 0.3 μg/ml. We then synchronized Clone 2 cells with thymidine and released the cells into media with nocodazole in the presence or absence of ZM447439 at concentrations 1.7, 3.3, and 6 times the minimal lethal dose. At all concentrations, addition of ZM447439 had no effect on the ability of the cells to progress up to mitosis, nor did it inhibit production of the upper band in the nocodazole arrested cells (Fig. 5A). The results indicate that kinases other than Aurora B are involved in creating the slower migrating form of MCAK.
Figure 5.
Effect of Aurora B kinase inhibition. Clone 2 cells were synchronized using thymidine, and then nocodazole in the presence (+) or absence (−) of varying concentrations of Aurora B kinase inhibitor ZM447439 (Tocris Bioscience, Ellisville, MO). A, western blot probed with antibodies to FLAG and Actin. FMCAK, FLAG-MCAK; P-FMCAK, phosphorylated FLAGMCAK. B–C, Immunofluorescence of a prophase cell (B) and an aberrant anaphase cell (C) both of which were treated with ZM447439. The cells were stained with antibodies to tubulin (red) and FLAG-MCAK (white) as well as DAPI (blue). Bar (C) = 10 μm.
Although CHO cells could be blocked at prometaphase with nocodazole in the presence of ZM447439, removal of nocodazole in the continued presence of ZM447439 inhibited normal progression through mitosis and so the effects of the inhibitor on MCAK degradation could not be assessed biochemically. Instead we turned to a microscopic assay in which we examined individual cells for the presence of FLAG-MCAK by immunofluorescence. Although the Aurora B kinase inhibitor slowed progression through mitosis and caused chromosome missegregation, all cells that progressed beyond the metaphase stage had greatly reduced FLAG-MCAK staining indicating that the Aurora kinase inhibitor did not prevent degradation of the protein (Fig. 5C). A prophase cell photographed at the same exposure is shown in Fig. 5B to demonstrate that the inhibitor did not prevent the normal localization of FLAG-MCAK at earlier stages of mitosis. Our data therefore argue against involvement of Aurora B kinase in the degradation of MCAK.
Proteasome inhibition stabilizes MCAK at spindle poles and kinetochores
The degradation of MCAK at the metaphase to anaphase transition occurs at about the time the anaphase promoting complex, an E3 ubiquitin ligase, is activated.18 To test whether FLAG-MCAK is degraded by the proteasome, Clone 2 cells were synchronized by successive thymidine and nocodazole blocks and were then released into medium with and without the proteasomal inhibitor MG132. Western blot analysis of cells 40 min after they were released from nocodazole into normal medium showed that FLAG-MCAK was greatly decreased (Fig. 6A, lane 2) relative to cells that were kept in nocodazole (Fig. 6A, lane 1). Cells released for 40 min into MG132-containing medium, on the other hand, retained a high level of the protein (Fig. 6A, lane 3).
Figure 6.

Effect of proteasome inhibition. Clone 2 was induced to express FLAG-MCAK for 24 h and then synchronized by thymidine and nocodazole blocks. Cells blocked in nocodazole (A, lane 1), released 40 min into normal medium (A, lane 2), or released 40 min into medium containing 20 μM MG132 (Peptide Institute INC, Minoh, Japan)(A, lane 3) were analyzed by western blots probed with antibodies to FLAG and Actin. FMCAK, FLAG-MCAK; P-FMCAK, phosphorylated FLAG-MCAK. B–C, Immunofluorescence of metaphase cells in the absence (B) or presence (C) of MG132. The cells were stained with antibodies to tubulin (red) and FLAG (green) as well as DAPI (blue). Arrow, panel C indicates the location of a spindle pole. Bar (B) = 10 μm.
In a second experiment to probe the involvement of the proteasome in degrading MCAK, synchronous CHO cells expressing FLAG-MCAK were released from a nocodazole block into medium containing MG132 for 1 h to accumulate them at metaphase. Comparison of the metaphase cells blocked in MG132 (Fig. 6C) with non-treated metaphase cells (Fig. 6B) demonstrated that the presence of the proteasomal inhibitor stabilized FLAG-MCAK at both the kinetochores and the spindle poles.
Discussion
Mitosis requires the participation of many proteins acting in concert to ensure the faithful segregation of DNA prior to cell division. One of the primary approaches for dissecting this complex process is the spatial and temporal identification of proteins that comprise or interact with the mitotic spindle apparatus. A protein that has received a lot of recent attention in this regard is MCAK, a kinesin-related protein that stimulates microtubule depolymerization.1 Prior studies of interphase cells have indicated that MCAK is associated with the nucleus, the cytoplasm, the microtubule organizing center, and cytoplasmic microtubules where it seems to accumulate at their ends.4,19,20 During mitosis, MCAK and related kinesins have been localized to spindle poles where they may be catalyzing the poleward flux of microtubules, and to the inner centromere region of chromosomes where they may be acting to help correct microtubule attachments to misaligned chromosomes as well as helping to depolymerize kinetochore-linked microtubules during anaphase sister chromatid segregation.4–6 MCAK has also been found in the midbody of telophase and recently divided G1 phase cells.
The data presented here largely bear out these prior localization studies, but differ from them in showing that MCAK staining of spindle poles and centromeric regions of chromosomes decreases markedly at metaphase. This decreased staining at metaphase does not result from the use of transfected FLAG-MCAK because we get similar results with endogenous MCAK in untransfected CHO cells. It is also not cell-specific because we have noted a similar decrease in MCAK using nontransfected HeLa and MCF-7 cells. Further support for the destruction of MCAK during mitosis comes from the observations that both endogenous and transfected proteins disappear from cells with timecourses that mirror the cell doubling times (Fig. 2) and that the protein accumulates throughout the cell cycle until its disappearance during mitosis in synchronized cell populations (Fig. 3).
The cellular signal for degradation is not yet clear, but we favor a model in which phosphorylation of MCAK is involved. This is consistent with our observations that a phosphorylated, slower migrating form of MCAK (upper band) is seen on SDS gels of mitotic extracts, and that this upper band disappears more rapidly than the lower, faster migrating band as cells progress through mitosis. Moreover, we have noted that the upper band is stabilized relative to the lower band when degradation is blocked with the proteasomal inhibitor MG132 (e.g., see Fig. 6B); thus, more rapid loss of the upper band during mitosis is unlikely to result simply from dephosphorylation. A final proof that phosphorylation triggers the degradation of MCAK, however, awaits identification and mutation of the relevant amino acid(s).
Other questions regarding MCAK degradation remain. For example, the kinase responsible for the slower migrating form of MCAK is unknown. ZM447439, an inhibitor of Aurora B kinase, had no effect on the formation of the upper band, nor did it prevent the loss of MCAK staining during the latter stages of mitosis. We therefore propose that a different kinase triggers MCAK degradation. A second question relates to the timing of the signal for degradation. Given that degradation occurs at metaphase, it is tempting to speculate that the critical phosphorylation event occurs at this stage of mitosis. However, there is currently no direct evidence for this and it remains equally likely that phosphorylation occurs earlier but accelerated degradation awaits activation of the anaphase promoting complex. This complex, an E3 ubiquitin ligase, is activated at metaphase and has been implicated in the destruction of several other mitotic proteins.18 The possibility that the signal for MCAK degradation might be acquired early in mitosis is consistent with the observation that phosphorylation and appearance of an MCAK upper band occur at least as early as prometaphase.
Our finding that MCAK is significantly reduced at the metaphase to anaphase transition argues against the view that the protein plays a crucial role in anaphase chromosome movement.21–23 Others have reported that depletion of MCAK in CHO cells causes the appearance of lagging chromosomes during anaphase, a result that has been interpreted as evidence that MCAK aids sister chromatid segregation by destabilizing microtubules attached to the kinetochores.4 It should be noted, however, that lagging chromosomes can arise from alignment errors that occur prior to anaphase24 and thus are not a good marker for defects that are specific for anaphase chromosome movement. Moreover, other studies were unable to confirm a role for MCAK in anaphase movement.6 A role for Kin I-like proteins during anaphase has also been proposed in insects where KLP59C has been reported to be responsible for 60% of the rate of anaphase chromosome movement in Drosophila embryos.5 Similar to the situation in mammalian cells, however, RNAi studies in insect S2 cells did not confirm this observation.25 The involvement of MCAK in anaphase has therefore remained an unsettled issue.
Because we are unable to prepare pure populations of metaphase or anaphase cells, we cannot biochemically measure exactly how much MCAK remains following its degradation at metaphase, but even with imperfect synchrony the decrease appears to be substantial (see Fig. 3). Furthermore, we have visual confirmation of reduced MCAK in the latter stages of mitosis (see Fig. 4), and another recent study has reported reduced MCAK staining in T47D and MCF-7 cells.8 Nevertheless, we cannot rule out the possibility that the small residual amount of MCAK that remains after metaphase is able to catalyze the depolymerization of microtubules and contribute to anaphase chromosome movement. It seems illogical, however, that the cell would target the destruction of MCAK at a time when its need should be greatest. Instead, we favor the idea that the primary activity of MCAK occurs during the events leading up to metaphase.6
The destruction of MCAK in mitosis raises the possibility that its presence during anaphase might interfere with, rather than promote, sister chromatid segregation. Unfortunately, this possibility is not yet directly testable. Inhibitors that prevent MCAK phosphorylation could potentially stabilize the protein, but are likely to be non-specific and may themselves inhibit mitotic progression as we saw in the case of the Aurora B kinase inhibitor ZM447439. To properly address whether MCAK might have deleterious effects in anaphase, it will be necessary to identify sequences in MCAK that are involved its degradation and mutate those sites to stabilize the protein.
Although we cannot yet evaluate whether the specific presence of elevated MCAK during anaphase would be detrimental to anaphase chromosome movement, we can predict that one likely consequence of inhibiting MCAK degradation would be an increased cellular content of the protein throughout mitosis that would be deleterious to mitotic progression. Prior studies have demonstrated that overexpression of MCAK leads to the formation of abnormal spindles and prometaphase arrest.4,10 Our own studies have shown that 2-fold overexpression of MCAK has no effect on mitotic progression or cell growth, but that 6-fold overexpression produces mitotic defects (data not shown). Thus, the ability to degrade and limit MCAK accumulation is an important aspect of its regulation. This conclusion is supported by a recent report that a microtubule depolymerizing kinesin-like protein, possibly related to MCAK, is also degraded in a cell cycle specific manner in Leishmania major suggesting that the process is evolutionarily conserved.26
Materials and Methods
Maintenance of cell lines
Cells were maintained at 37 °C and 5% CO2 in alpha modification of minimum essential medium ( αMEM) supplemented with 5% (CHO cells) or 10% (HeLa and MCF-7 cells) fetal bovine serum (Atlanta Biologicals, Atlanta, GA).
Preparation of constructs
Full length human MCAK cDNA (GenBank Accession No. BC014924) cloned into pCMV-Sport6 was obtained from American Type Culture Collection (Manassas, VA). The cDNA was excised and subcloned into the tetracycline regulated expression vector pTOPneo16 to produce a plasmid pTOP/MCAK. An octapeptide FLAG epitope-encoding sequence was inserted in-frame at the amino terminus of MCAK using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The mutagenic primers used were TTG CTG ACT CTC CGA ATG GAT TAT AAG GAT GAT GAC GAC AAG GCC ATG GAC TCG TCG CTT CAG and it’s reverse complement. The underlined bases indicate the FLAG nucleotide sequence. The final plasmid, pTOP/FLAG-MCAK, was sequenced to be sure there were no errors.
Isolation of stably transfected cell lines
CHO tTA6.6a cells expressing a tetracycline-regulated transactivator16 were seeded into 35-mm tissue culture dishes and transfected with pTOP/FLAG-MCAK using Lipofectamine (Invitrogen, Carlsbad, CA). Following transfection, the cells were grown overnight in the presence of 1 μg/ml tetracycline, trypsinized and replated in 100-mm dishes containing αMEM, 1 μg/ml tetracycline and 2 mg/ml G418. After 10 days the drug resistant cells were trypsinized and stored as a total G418-resistant population. From the stable G418 population about 100 cells were plated on 60 mm dishes and allowed to grow for 7 days to form isolated colonies. The colonies were screened by Western blot analysis for MCAK production, and cell lines with low expression of FLAG-MCAK (≤2-fold increase in total cellular MCAK content) were chosen for further experiments.
Immunofluorescence
Cells were grown on glass coverslips, rinsed briefly in PBS, and fixed directly in methanol. Sometimes they were pre-extracted 1.5 min with microtubule stabilizing buffer (20 mM Tris-HCl, pH 6.8, 0.5% Nonidet P-40, 1 mM MgCl2, 2 mM EGTA, 4 μg/ml paclitaxel) at 4°C, and then fixed in methanol at −20°C for 20 min. The fixed cells were stained with 1:50 dilutions of mouse FLAG-specific antibody M2 (Sigma-Aldrich, St. Louis, MO), a rabbit antibody specific for α-tubulin (gift of Dr. Jeanette Bulinski), rabbit anti-MCAK (Cytoskeleton, Boulder, CO), or mouse α-tubulin antibody DM1 α (Sigma-Aldrich). Goat antimouse and antirabbit antibodies conjugated to Alexa 488 or Alexa 594 (Invitrogen) were also used at 1:50 dilutions. For CREST staining of kinetochores, a human CREST autoimmune serum (gift of Dr. Bill Brinkley) was used at 1:5000 dilution followed by Alexa 594-conjugated goat anti-human IgG at 1:500 dilution.
Cell synchronization
CHO cells were synchronized as previously described27 by incubating them in medium containing 5 mM thymidine overnight, reversing the S-phase block for 4 h, adding 35 ng/ml of nocodazole for 4 h, and shaking off mitotic cells at various time after reversal from nocodazole. Cell cycle profiles of the cells were obtained using an EasyCyte flow cytometer (Guava, Technologies, Hayward, CA). The efficiency of nocodazole-induced prometaphase arrest in isolated mitotic cells was above 95% in all experiments. Progression of the cells through mitosis following removal of nocodazole was assessed by removing an aliqot, staining the cells with 10 μg/ml Hoescht 33342, and observing the DNA organization by immunofluorescence.
Electrophoretic techniques
Cells were lysed in 1% SDS and the proteins were precipitated with 5 volumes of acetone, resuspended in SDS sample buffer (0.0625 M Tris-HCl, pH 6.8, 2.5% SDS, 5% 2-mercaptoethanol, 10% glycerol), fractionated on a 7.5% polyacrylamide SDS minigel, and transferred to PROTRAN nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The membranes were then incubated in 2% milk in PBST (PBS containing 0.05% Tween-20) overnight at 4 °C, washed 3 times in PBST, and incubated in 1:2000 dilutions of rabbit anti-MCAK rabbit or mouse anti-FLAG M2 antibody. A 1:30,000 dilution of actin-specific mouse C4 antibody (Chemicon, Temecula, CA) was also added as a control for sample loading. Antibody incubations were carried out for 1 h at room temperature. Reactive bands were detected with 1:2000 dilutions of Alexa 647-conjugated goat anti-mouse and anti-rabbit IgGs (Invitrogen). The bands were visualized and quantified by capturing fluorescence emission on a STORM 860 imager (Molecular Dynamics Inc., Sunnyvale, CA).
MCAK degradation
Degradation in non synchronized cells was measured by inducing FLAG-MCAK expression in transfected cells by removal of tetracycline overnight and then stopping further transcription of the gene by the readdition of 1 μg/ml tetracycline. For non transfected cells, endogenous protein synthesis was stopped with 7 μg/ml puromycin. In each case, samples were taken at various times after inhibiting further production of MCAK and cell lysates were analyzed by western blots probed with FLAG or MCAK antibodies in combination with an actin antibody used as an internal control. Additional experiments eschewed the use of protein synthesis inhibitors and measured relative MCAK or FLAG-MCAK content in cells at various stages of the cell cycle using synchronized CHO cell populations.
Supplementary Material
Acknowledgments
The authors thank Dr. Bill R. Brinkley for the CREST antibody and Dr. Jeanette Bulinski for the rabbit α-tubulin antibody used in these studies. This work was supported by National Institutes of Health Grant CA85935 (to F. C.).
Abbreviations used
- CHO
Chinese hamster ovary
- MCAK
mitotic centromere associated kinesin
- MEM
minimum essential medium
- DAPI
4′-6-Diamidino-2-phenylindole
References
- 1.Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 2.Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L. A standardized kinesin nomenclature. J Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maney T, Hunter AW, Wagenbach M, Wordeman L. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J Cell Biol. 1998;142:787–801. doi: 10.1083/jcb.142.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers GC, Rogers SL, Schwimmer TA, Ems-McClung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature. 2004;427:364–70. doi: 10.1038/nature02256. [DOI] [PubMed] [Google Scholar]
- 6.Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell. 2004;15:1146–59. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y, Tanaka F, Haraguchi N, Mimori K, Matsumoto T, Inoue H, Yanaga K, Mori M. Clinicopathological and biological significance of mitotic centromere-associated kinesin overexpression in human gastric cancer. Br J Cancer. 2007;97:543–9. doi: 10.1038/sj.bjc.6603905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimo A, Tanikawa C, Nishidate T, Lin ML, Matsuda K, Park JH, Ueki T, Ohta T, Hirata K, Fukuda M, Nakamura Y, Katagiri T. Involvement of kinesin family member 2C/mitotic centromere-associated kinesin overexpression in mammary carcinogenesis. Cancer Sci. 2008;99:62–70. doi: 10.1111/j.1349-7006.2007.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maney T, Wagenbach M, Wordeman L. Molecular dissection of the microtubule depolymerizing activity of mitotic centromere-associated kinesin. J Biol Chem. 2001;276:34753–8. doi: 10.1074/jbc.M106626200. [DOI] [PubMed] [Google Scholar]
- 10.Kline-Smith SL, Walczak CE. The microtubule destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Mol Biol Cell. 2002;13:2718–31. doi: 10.1091/mbc.E01-12-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stout JR, Rizk RS, Kline SL, Walczak CE. Deciphering protein function during mitosis in PtK cells using RNAi. BMC Cell Biol. 2006;7:26. doi: 10.1186/1471-2121-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–68. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 13.Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14:273–86. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–10. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Lan W, Ems-McClung SC, Stukenberg PT, Walczak CE. Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol Biol Cell. 2007;18:3264–76. doi: 10.1091/mbc.E07-01-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A β-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274:23875–82. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- 17.Brenner S, Pepper D, Berns MW, Tan E, Brinkley BR. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981;91:95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang G, Yu H, Kirschner MW. Control of mitotic transitions by the anaphase-promoting complex. Philos Trans R Soc Lond B Biol Sci. 1999;354:1583–90. doi: 10.1098/rstb.1999.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, Howard J. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell. 2003;11:445–57. doi: 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995;128:95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J. Molecular Cell Biology. New York: W. H. Freeman and Company; 2004. p. 973. [Google Scholar]
- 22.Moore A, Wordeman L. The mechanism, function and regulation of depolymerizing kinesins during mitosis. Trends Cell Biol. 2004;14:537–46. doi: 10.1016/j.tcb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Moores CA, Milligan RA. Lucky 13-microtubule depolymerisation by kinesin-13 motors. J Cell Sci. 2006;119:3905–13. doi: 10.1242/jcs.03224. [DOI] [PubMed] [Google Scholar]
- 24.Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153:517–27. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goshima G, Vale RD. The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J Cell Biol. 2003;162:1003–16. doi: 10.1083/jcb.200303022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubessay P, Blaineau C, Bastien P, Tasse L, Van Dijk J, Crobu L, Pages M. Cell cycle-dependent expression regulation by the proteasome pathway and characterization of the nuclear targeting signal of a Leishmania major Kin-13 kinesin. Mol Microbiol. 2006;59:1162–74. doi: 10.1111/j.1365-2958.2005.05013.x. [DOI] [PubMed] [Google Scholar]
- 27.Brady R, Schibler MJ, Cabral F. Isolation of mitotic spindles from mammalian cells. Methods Enzymol. 1986;134:217–25. doi: 10.1016/0076-6879(86)34090-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.