In previous studies from this, and other, laboratories, the role of the so-called portal hepatotrophic factors in regulating or permitting hepatic regeneration has been evaluated. In a recent investigation in dogs, an additional possibility was evaluated that something in the hepatic remnant after partial hepatectomy could contribute to, or even initiate, its own regrowth (12). It was found that cytosol liver extracts prepared from 48 hour, and especially 72 hour, regenerating liver and infused into the tied-off left portal branch for six hours, shortly after performing a portacaval shunt, induced a delayed proliferative response. The effect was noted in the directly infused left lobes at two days, and even more prominently at three days. The response far exceeded the modest proliferation associated with acute hepatocyte atrophy that is caused by portacaval shunt.
The present study in test dogs with normal hepatic circulation was undertaken to answer four questions. When given intraportally, would the active liver extracts obtained 48 and 72 hours after 72 per cent partial hepatectomy amplify the regeneration following a conventional 44 per cent partial hepatectomy or was this stimulatory effect of liver extracts only noted with the special conditions of portacaval shunt? Would a similar short infusion of high and low dose insulin administered intraportally for six hours only, within the first 12 hours after 44 per cent partial hepatectomy, augment the regenerative response? Hepatic regneration had previously been noted in dogs treated with insulin after portacaval shunt, but the insulin had been given intraportally for four days (13). Would the heightened mitotic rate in the liver fragment following 44 per cent partial hepatectomy allow an inhibitory response to be unmasked when cytosol from normal livers was infused? Such an effect had not been noted in the portacaval shunt experiments, but it was possible that the background mitotic activity was too low to permit detection of down regulation (12). In their work, LaBrecque and Pesch (7), Lavigne and associates (8), and Verly and colleagues (14) had suggested that an inhibitory factory was present in normal, nonregenerating, adult liver. Were the intraperitoneal and intravenous routes of administration of liver cytosol as effective as the direct intraportal route?
METHODS
Test animals
The testing of liver extracts, insulin and control solutions was done in animals which had undergone a 44 per cent partial hepatectomy. Healthy, adult mongrel dogs, weighing between 12 and 29 kilograms, were used. Anesthesia for all procedures and for sacrifice of the animal was with pentobarbital sodium, supplemented with phencyclidine hydrochloride and succinylcholine chloride.
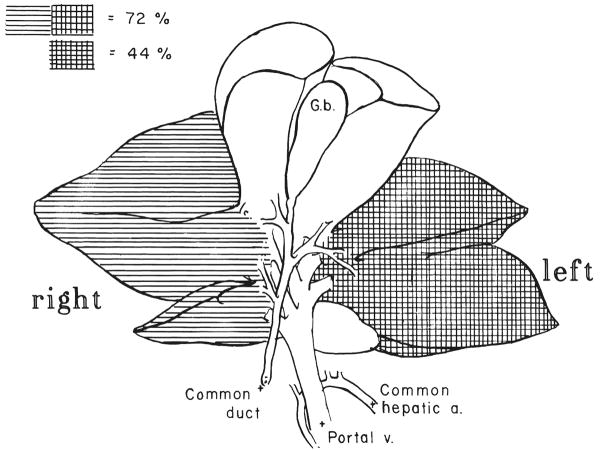
The 44 per cent hepatectomy (Fig. 1) consisted of removal of the two far left lobes of the liver. Approximately 1 liter of dextrose saline solution was administered intravenously during the operation. An additional 500 millimeters of dextrose saline solution was administered subcutaneously on the evening of the operation. The normal regeneration response after this procedure (Table I) was compared with that previously observed in our laboratory (6) and found to be the same.
Fig. 1.
The 44 per cent hepatectomy test model was prepared by resecting the two leftmost lobes of the dog liver. For the 72 per cent hepatectomies used to prepare cytosol liver extract, the two right lobes were also excised. G.b., Gallbladder; a., artery; v., vein.
TABLE I. CHANGES IN RESIDUAL RIGHT LOBES OF LIVER AFTER 44 PER CENT PARTIAL HEPATECTOMY IN DOGS.
| —Experiments— | Time after hepatectomy, hrs. | Labeled hepatocytes per 1,000 hepatocytes, mean ± S.D. | p Value cf. normal controls | Mitoses per 1,000 hepatocytes, mean ± S.D. | p Value cf. normal controls | Cell size, units, mean ± S.D. | p Value cf. normal controls | |
|---|---|---|---|---|---|---|---|---|
| Group | No. | |||||||
| Normal | 10 | 0 | 1.59±0.41 | 0.07±0.03 | 0.174±0.012 | — | ||
| A | 6 | 48 | 6.42±1.31 | <0.001 | 0.62±0.13 | <0.001 | 0.181±0.019 | N.S. |
| B | 6 | 72 | 9.60±0.81 | <0.001 | 0.90±0.11 | <0.001 | 0.192±0.019 | N.S. |
N.S., Not significant.
In those experiments in which portal perfusion was to be performed (Tables II, III and IV), a catheter was inserted through a small proximal colonic branch, and its tip was advanced into the main portal vein until it was just proximal to the hilus. The catheter was led through a subcutaneous tunnel and out the back to a constant infusion pump. After completion of the six hour infusion, the catheter was tied off and encouraged to retract beneath the skin. For intraperitoneal infusion, the catheter was left free in the peritoneal cavity, and for intravenous infusion, the catheter was placed in the subclavian vein through a jugular vein. Dogs were housed in separate cages for the duration of the experiment. From the first postoperative day, standard kennel rations were given daily, and the dogs had free access to water.
TABLE II. EFFECT OF REGENERATING LIVER EXTRACTS ON LIVER REGENERATION TWO DAYS AFTER 44 PER CENT PARTIAL HEPATECTOMY IN DOGS.
| —Experiments— | Treatment | Labeled hepatocytes per 1,000 hepatocytes, mean ± S.D. | p Value cf. unmodified 44 percent hepatectomy | Mitoses per 1,000 hepatocytes, mean ± S.D. | p Value cf. unmodified 44 per cent hepatectomy | Cell size, units, mean ± S.D. | p Value cf. unmodified 44 percent hepatectomy | |
|---|---|---|---|---|---|---|---|---|
| Group | No. | |||||||
| A | 6 | No infusion | 6.42±1.31 | — | 0.62±0.13 | — | 0.181 ±0.26 | — |
| ———SALINE SOLUTION INTRAPORTALLY——— | ||||||||
| 1 | 6 | Saline solution | 6.53±1.06 | N.S. | 0.68±0.15 | N.S. | 0.178±0.019 | N.S. |
| ———LE INTRAPORTALLY——— | ||||||||
| 2 | 6 | Normal liver | 6.28±1.47 | N.S. | 0.58±0.18 | N.S. | 0.171±0.019 | N.S. |
| 3 | 6 | 24 hr. regenerating | 6.71 ±0.85 | N.S. | 0.67±0.13 | N.S. | 0.179±0.018 | N.S. |
| 4 | 6 | 48 hr. regenerating | 8.20±1.64 | N.S. | 0.78±0.21 | N.S. | 0.187±0.030 | N.S. |
| 5 | 6 | 72 hr. regenerating | 9.86±1.86 | =0.004 | 1.03±0.13 | <0.001 | 0.192±0.022 | N.S. |
| ———LE INTRAPERITONEALLY——— | ||||||||
| 6 | 3 | Normal liver | 6.10±1.90 | N.S. | 0.59±0.22 | N.S. | 0.173±0.037 | N.S. |
| 7 | 4 | 24 hr. regenerating | 6.60±1.89 | N.S. | 0.61±0.19 | N.S. | 0.177±0.021 | N.S. |
| 8 | 2 | 48 hr. regenerating | 9.50±1.27 | <0.05 | 0.90±0.13 | <0.05 | 0.186±0.017 | N.S. |
| 9 | 2 | 72 hr. regenerating | 8.10±2.40 | N.S. | 0.79±0.15 | N.S. | 0.183±0.024 | N.S. |
| ———LE INTRAVENOUSLY——— | ||||||||
| 10 | 2 | 48 hr. regenerating | 6.70±0.84 | N.S. | 0.60±0.06 | N.S. | 0.158±0.010 | 0.05<p<0.1 |
| ———INSULIN INTRAPORTALLY——— | ||||||||
| 11 | 4 | 0.16 units/kgm. | 6.55±0.58 | N.S. | 0.66±0.18 | N.S. | 0.180±0.014 | N.S. |
| 12 | 4 | 0.64 units/kgm. | 6.50±0.89 | N.S. | 0.67±0.09 | N.S. | 0.179±0.017 | N.S. |
LE, Liver extract.
N.S., Not significant.
TABLE III. EFFECT OF REGENERATING LIVER EXTRACTS IN LIVER REGENERATION THREE DAYS AFTER 44 PER CENT PARTIAL HEPATECTOMY IN DOGS.
| —Experiments— | Treatment | Labeled hepatocytes per 1,000 hepatocytes, mean ± S.D. | p Value cf. unmodified 44 per cent hepatectomy | Mitoses per 1,000 hepatocytes, mean ± S.D. | p Value cf. unmodified 44 per cent hepatectomy | Cell size, units, mean ± S.D. | p Value cf. unmodified 44 per cent hepatectomy | |
|---|---|---|---|---|---|---|---|---|
| Group | No. | |||||||
| B | 6 | No infusion | 9.60±0.81 | — | 0.90±0.11 | — | 0.192±0.026 | — |
| ———SALINE SOLUTION INTRAPORTALLY——— | ||||||||
| 13 | 6 | Saline solution | 9.47±1.13 | N.S. | 0.88±0.20 | N.S. | 0.189±0.026 | N.S. |
| ———LE INTRAPORTALLY——— | ||||||||
| 14 | 6 | Normal liver | 8.98±1.83 | N.S. | 0.82±0.18 | N.S. | 0.186±0.026 | N.S. |
| 15 | 6 | 24 hr. regenerating | 9.53±0.95 | N.S. | 0.92±0.17 | N.S. | 0.199±0.039 | N.S. |
| 16 | 7 | 48 hr. regenerating | 10.06±1.75 | N.S. | 0.96±0.21 | N.S. | 0.194±0.020 | N.S. |
| 17 | 7 | 72 hr. regenerating | 14.07±3.15 | =0.004 | 1.61±0.31 | <0.001 | 0.198±0.044 | N.S. |
| ———LE INTRAPERITONEALLY——— | ||||||||
| 18 | 2 | Normal liver | 9.00±3.25 | N.S. | 0.88±0.41 | N.S. | 0.187±0.031 | N.S. |
| 19 | 2 | 24 hr. regenerating | 9.25±0.64 | N.S. | 0.85±0.06 | N.S. | 0.180±0.017 | N.S. |
| 20 | 2 | 48 hr. regenerating | 9.55±1.77 | N.S. | 0.85±0.09 | N.S. | 0.190±0.017 | N.S. |
| 21 | 2 | 72 hr. regenerating | 9.35±2.62 | N.S. | 0.85±0.21 | N.S. | 0.203±0.028 | N.S. |
| ———LE INTRAVENOUSLY——— | ||||||||
| 22 | 2 | 48 hr. regenerating | 8.05±0.21 | =0.005 | 0.71±0.12 | 0.05<p<0.1 | 0.196±0.031 | N.S. |
| ———INSULIN INTRAPORTALLY——— | ||||||||
| 23 | 4 | 0.16 units/kgm. | 9.53±0.99 | N.S. | 0.86±0.08 | N.S. | 0.191±0.015 | N.S. |
| 24 | 4 | 0.64 units/kgm. | 9.60±1.25 | N.S. | 0.89±0.11 | N.S. | 0.190±0.023 | N.S. |
LE, Liver extract.
N.S., Not significant.
TABLE IV. EFFECTS OF REGENERATING LIVER EXTRACTS ON LIVER REGENERATION THREE DAYS AFTER INFUSION INTO NORMAL DOG LIVER.
| —Experiments— | Treatment | Labeled hepatocytes per 1,000 hepatocytes, mean ± S.D. | p Value, untreated vs. treated | Mitoses per 1,000 hepatocytes, mean ± S.D. | p Value, untreated vs. treated | Cell size, units, mean ± S.D. | p Value, untreated vs. treated | |
|---|---|---|---|---|---|---|---|---|
| Group | No. | |||||||
| Normal | 10 | Nil | 1.59±0.41 | — | 0.07±0.03 | — | 0.174±0.012 | — |
| ———LE INTRAPORTALLY——— | ||||||||
| 25 | 6 | 72 hr. regenerating | 1.62±0.53 | N.S. | 0.05±0.03 | N.S. | 0.172±0.013 | N.S. |
LE, Liver extract.
N.S., Not significant.
Intraportal or intravenous infusions of the test substance were commenced four to six hours after the completion of the 44 per cent partial hepatectomy and completed within six hours. Intraperitoneal infusion was administered for five minutes, four to six hours after the partial hepatectomy.
The experiments were completed during a nine month period. Almost all of the experiments in the various groups were performed in a preplanned, random order to ensure that variations in technique or types of dog, or other factors, did not influence the results. The 44 per cent hepatectomies were performed between 8:30 and 11:30 am.
Blood samples were taken for serum glutamic-oxalacetic transaminase determinations, preoperatively, preinfusion, immediately postinfusion and at one day postinfusion. In dogs given insulin infusions, blood glucose was measured preoperatively and, again, immediately after and at two hours postinfusion.
Tissue extract donors
The 72 per cent hepatectomies (6) were performed (Fig. 1) between 8 and 10 am. Exactly one, two and three days later, the remaining livers were removed for preparation of cytosol.
The cytosol extracts from regenerating and normal livers were prepared by the method of LaBrecque and Pesch (7) with previously described modifications (12). The excised liver was immediately perfused free of blood with cold 0.9 per cent saline solution. Two hundred grams were ground up, homogenized and submitted to preliminary cold centrifugation to remove gross tissue. The supernatant was decanted and, subsequently, ultracentrifuged at 145,000 times gravity for two hours at 4 degrees C. The uppermost, whitish lipid layer was aspirated and discarded. The clear, reddish-yellow supernatant was removed, avoiding its contamination from the cloudy sediment layer at the bottom. Approximately 90 to 100 milliliters of supernatant were obtained, diluted with cold 0.9 per cent sodium chloride to a volume of 240 milliliters and split into equal aliquots for infusion into two different experimental animals. This extract contained soluble proteins and other cytoplasmic constitutents. By electron microscopy, it was free of organelles, cell membranes, microsomes and virus (12). It did not contain insulin or glucagon (12). Cytosol prepared from three normal dogs had a glutamic-oxalacetic transaminase concentration of 11,433±3,308, S.D., International units per milliliter. The extract was kept on ice before and during infusion on the same day of its preparation. The origin of the extract and the route of its administration are shown for each experimental group in Tables II, III and IV.
Control infusions
Insulin doses of 0.16 and 0.64 unit kilogram were given intraportally in six hours (Tables II and III). The higher total dose, given continuously for four days, has been shown to cause a brisk mitotic increase in dog livers after portacaval shunt (13). The saline solution infusions provided inert controls.
Histopathologic studies
The test dogs were sacrificed under anesthesia exactly 48 (Table II) and 72 hours (Table III) after completion of the 44 per cent partial hepatectomy. Two hours before sacrifice, 0.2 millicurie per kilogram of body weight, CH3–3H, of thymidine, 47 curies per millimole, was administered intravenously. Immediately after sacrifice, a full postmortem examination was performed, including confirmation of the position of the catheter tip.
All experimental end-points were histopathologic, as previously described (6, 9, 10, 12), the observations being recorded without prior knowledge of the experimental groups from which the specimens came. While the dogs were still living, tissues were obtained from a standard location from one of the right lobes of the liver and from one kidney and were fixed in 10 per cent buffered formaldehyde. Other liver samples from the same site were fixed in glutaraldehyde solution, post-fixed in osmic acid and embedded in epoxy resin.
Frozen and paraffin sections were prepared from the formalin-fixed material. Frozen sections were stained for fat, and paraffin sections were stained with hematoxylin and eosin and other special stains. The size of hepatocytes in the mid-lobular zones was measured (9), and the results were expressed in arbitrary size units. The hepatocytes in the middle zone of the liver lobules were also used for measuring in electron micrographs the area of rough endoplasmic reticulum per volume of cytoplasm (6). Hepatocytes and glomerular cells in mitosis were counted.
Other paraffin sections of liver and kidney were dewaxed, dipped in Ilford K2 nuclear emulsion and used for autoradiography. Exposure was for four to eight weeks until counts of the labeled nuclei stopped increasing. With liver sections, only hepatocytes were counted, excluding stromal and other cells. In the kidney samples, only labeled glomerular endothelial, mesangial and epithelial cells were recorded. All statistical analyses were based upon Student’s t test.
RESULTS
Response to 44 per cent partial hepatectomy
The autoradiographic changes noted two and three days after partial hepatectomy were identical (Table I) to those previously demonstrated at this laboratory (6). The number of labeled hepatocytes increased four and six times above the normal base line level at two and three days, respectively. A similar increase in the number of cells in mitosis was noted.
Although the cell size units increased, the changes did not reach statistical significance. The enlargement of the hepatocytes included the nucleus and nucleolus and was accompanied by the accumulation of large numbers of fat globules within the cytoplasm. The amount of both rough and smooth endoplasmic reticulum was increased and maximal at three days. Lysosomes were larger and more numerous. These and other changes were similar to those noted previously (6). The 48 hour, group A, results were used as the base line control data for the experimental groups at two days (Table II), and the 72 hour, group B, results provided the controls for the experimental groups at three days (Table III).
Infusions and the two day regenerating liver
The autoradiographic and mitotic changes characteristic of the 48 hour regenerating dog liver were not significantly altered by a six hour intraportal infusion of 120 milliliters of saline solution, group 1; by cytosol from normal adult liver, group 2, or by cytosol from 24 hour regenerating liver, group 3 (Table II). The lowest autoradiographic counts were obtained with extract from normal liver, group 2, but the reduction was not significant.
Intraportal infusion of extract from the 48 hour regenerating liver produced a noticeable, but nonsignificant, increase in regeneration, group 4, and the 72 hour regenerating liver extract produced a greater and, now significant, increase in thymidine incorporation and mitotic count, group 5. This was not accompanied by a significant increase in cell size (Table II). Electron microscopy of the hepatocytes in groups 4 and 5 showed increases in the average area of both rough and smooth endoplasmic reticulum that were greater than were observed 48 hours after 44 per cent hepatectomy alone. The increases were similar to those found in the untreated 72 hour regenerating dog liver.
Intraperitoneal infusion of normal and 24 hour regenerating liver cytosol, groups 6 and 7, did not modify the response to 44 per cent hepatectomy (Table II). There was an increase in thymidine incorporation and mitotic counts with infusion of the 48 and 72 hour regenerating liver cytosol, groups 8 and 9, but no alteration in cell size. The increased proliferation was significant only in group 8 (Table II). However, in both groups 8 and 9, electron microscopy revealed greater than expected increases in the average area of both rough and smooth endoplasmic reticulum.
Intravenous infusion of 48 hour regenerating liver cytosol, group 10, had no effect other than a slight diminution in cell size. This is reported in Table II.
A six hour intraportal infusion of low and high dose intraportal insulin, groups 11 and 12, had no effect upon labeled hepatocytes or mitotic count, and both cell size and hepatocyte ultrastructure were unaltered compared with those with 44 per cent hepatectomy alone. Neither dosage of insulin caused hypoglycemia.
Infusion and the three day regenerating liver
The autoradiographic and mitotic changes, characteristic of the 72 hour regenerating dog liver, were not altered by a six hour intraportal infusion of 120 milliliters of saline solution, group 13; by cytosol from normal liver, group 14, or by cytosol from 24 hour regenerating liver, group 15 (Table III). The lowest counts were again noted after infusion with normal liver extract, group 14, but the reductions were not significant.
Given intraportally, the 48 hour regenerating liver cytosol produced a trend to increase counts, group 16, which reached significance when 72 hour regenerating liver extract was infused, group 17 (Table III). The cell size remained unchanged when compared with an unaltered three day regenerating liver, although, upon electron microscopy, the average area of both rough and smooth endoplasmic reticulum was greater than was ordinarily observed 72 hours after 44 per cent partial hepatectomy.
Intraperitoneal infusion of normal and regenerating liver cytosol four to six hours after partial hepatectomy, groups 18 to 21, did not result in any noticeable effect. Hepatocyte size was unaltered, and electron microscopy only showed those changes usually encountered at this time after 44 per cent partial hepatectomy.
In group 22 (Table III), intravenous infusion of 48 hour regenerating cytosol caused a slight decrease in labeled hepatocytes and mitotic count.
Six hour intraportal infusion of low and high dose insulin had no effect upon labeled hepatocytes or mitotic count compared with those with 44 per cent hepatectomy alone, and both cell size and hepatocyte ultrastructure were unaltered, groups 23 and 24 (Table III). Neither dose of insulin caused hypoglycemia when blood glucose was measured immediately postinfusion or two hours postinfusion.
Infusion and the normal liver
In group 25, the potent 72 hour regenerating liver extract had no effect after a six hour intraportal infusion into a normal liver, when assessed at three days (Table IV). In addition, electron microscopy showed no changes in cell structure.
Glutamic-oxalacetic transaminase
The results of foregoing histopathologic studies did not reveal necrosis caused by the infused cytosol. However, the cytosol contained so much glutamic-oxalacetic transaminase that its infusion caused a transient transaminasemia (Table V) that was most pronounced after intravenous administration. This artefact was engrafted upon the smaller transaminase increases following 44 per cent hepatectomy that could be seen in the dogs receiving saline solution infusions (Table V).
TABLE V. EFFECT OF INFUSIONS PLUS 44 PER CENT HEPATECTOMY ON SERUM GLUTAMIC-OXALACETIC TRANSANIMASE.
| Experiments, No. | Preoperative | Preinfusion after hepatectomy | Postinfusion | 18 hrs. later | |
|---|---|---|---|---|---|
| Intraportal cytosol* from normal and regenerating livers. | 34 | 21.0±19.7 | 84.2±49 | 810.5± 476.7 | 224.7±107.8 |
| Intravenous cytosol from regenerating livers | 4 | 23.7±18 | 96.3±15.9 | 2475 ±2087 | 225.0±106.5 |
| Intraportal saline solution | 12 | 12.2± 9.4 | 96.2±69.9 | 301.7± 165 | 92.0± 57.8 |
Values are means plus standard deviations.
Cytosol prepared from three normal dog livers contained 11,433±3,330, S.D., International units per milliliter of glutamic-oxalacetic transaminase.
Renal changes
The frequency of mitoses in the glomerular cells and the number of these cells incorporating thymidine showed no significant variation in the various experimental groups of dogs, groups A and B and groups 1 to 25, and did not differ from the findings in the normal dog.
DISCUSSION
In these investigations, the four questions which they were designed to examine were answered. First, as LaBrecque and Pesch (7) had previously demonstrated, it was shown that active liver extracts could augment a regeneration response set in motion by partial hepatectomy. The effect was analogous to that described by us (12) in dogs after portacaval shunt. The disparate procedures of partial hepatectomy and portacaval shunt created conditions of testing which had the common feature of heightened proliferation. Thus, the liver extracts were imposed at a time of unstable hepatocyte growth control and, in both instances, amplified a response that was independently in process. It was perplexing that the active cytosol had no stimulatory effect upon normal nonregenerating livers when given intraportally. Blomqvist (2) and others, whose older work has been reviewed (11), have noted stimulation in normal adult rat livers with intraperitoneal injections of crude extracts from regenerating or weanling livers. Possible explanations are dose or timing of treatment and species variation.
The second question concerned the possible interchangeability of the cytosol liver extract and insulin. Although insulin had been shown to have a potent mitogenic effect when given continuously to dogs for four days after portacaval shunt (13), the cytosol was thought to be free of insulin (12). In the experiments reported herein, a six hour infusion of cytosol had an unequivocal delayed effect which was not identifiable until two days and which did not reach a peak until three days. On the other hand, the six hour insulin infusion had no such effect. The long fuse of the liver extract has been one of the interesting qualities of cytosol and one that could be compatible with a true initiation factor.
The third inquiry was: could cytosol from normal adult dog liver be shown to inhibit regeneration after 44 per cent partial hepatectomy. The presence of nonspecific growth-inhibiting substances in the liver was suggested in early work by Brues and co-workers (4), while the concept of a specific hepatic growth inhibitor in non-regenerating adult liver was supported by the work of LaBrecque and Pesch (7), Lavigne and co-authors (8) and Verly and collaborators (14). We were unable to demonstrate such an inhibition, although a nonsignificant trend with lower mitotic rates was seen in all experiments in which normal liver cytosol was infused intraportally. Thus, the possibility that normal liver might contain a mitotic inhibitor remains an open question. If inhibitors, as well as stimulators, could be found in hepatic tissue, a rational postulate would be that intrinsic regulatory mechanisms first promote and then halt hepatic restoration at the appropriate time. Such a dynamic state has been discussed by Bradbrook and associates (3), Bucher (5), LaBrecque and Pesch (7), Lavigne and colleagues (8), Verly and co-workers (14) and Weinbren (15). The possible involvement of chalones has been emphasized by Bard (1), Lavigne and collaborators (8) and Verly and co-authors (14).
The fourth question examined in this study concerned the most effective route of administration. Cytosols which were active by the intraportal route gave equivocal results when given intraperitoneally and had no demonstrable effect when given into systemic veins.
Some of the intriguing questions about growth-altering substances in regenerating livers were not dealt with in this study. Whether or not such substances influence, or merely reflect, regeneration is not known, although their late appearance as regeneration proceeds might weigh toward the latter possibility. The origin of the substances, their relationship to portal hepatotrophic factors, their species and organ specificity or lack thereof, and their role, if any, in initiating and terminating regeneration are not known. Decisive answers to these last questions will depend upon purification, biochemical characterization and testing of the stimulatory and possibly the inhibitory factors in regenerating liver fragments and in normal liver.
SUMMARY
A cytosol liver extract was prepared from adult dog livers and from liver remnants that had been regenerating for one, two and three days after 72 per cent partial hepatectomy. Given intraportally, the most active of these cytosols did not stimulate proliferation in the livers of normal dogs. However, infused during a six hour period into the portal vein of test group dogs, the cytosol from 48 and, especially, 72 hour regenerating livers augmented the regeneration response ordinarily produced by 44 per cent partial hepatectomy. The effect was delayed. It became identifiable 48 hours after infusion and reached a peak at 72 hours. Neither augmentation nor significant inhibition of the normal regeneration response was produced by cytosol from normal liver and 24 hour regenerating liver or by a six hour infusion of insulin. The amplification effect of active cytosol was equivocal when the infusions were given intraperitoneally and was not demonstrable at all by the intravenous route. In these investigations, it is confirmed that there are growth control factors in regenerating liver but the nature or physiologic significance of the factor or factors has not been clarified.
Acknowledgments
This work was supported by research grants from the Veterans Administration; by Grant Nos. AM-17260 and AM-07772 from the National Institutes of Health, and by Grant Nos. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health, Bethesda, Maryland, and the Medical Research Council, South Africa.
Contributor Information
John Terblanche, CapeTown, South Africa.
Kendrick A. Porter, London, England
John Moore, Denver, Colorado
Lawrence Patzelt, Denver, Colorado
Nobuo Hayashida, Denver, Colorado
References
- 1.Bard JB. A quantitative model of liver regeneration in the rat. J Theor Biol. 1978;73:509. doi: 10.1016/0022-5193(78)90155-8. [DOI] [PubMed] [Google Scholar]
- 2.Blomqvist K. Growth stimulation in the liver and tumor development following intraperitoneal injections of liver homogenates in the rat. Acta Pathol Microbiol Scand. 1957;(Suppl 121):1. [PubMed] [Google Scholar]
- 3.Bradbrook RA, Newcombe RG, Thatcher J, Blumgart LH. The inhibition of the uptake of 3H-thymidine into liver DNA by the intraportal infusion of fresh serum after partial hepatectomy in the rat. Eur. Surg. Res. 1974;6:364. doi: 10.1159/000127743. [DOI] [PubMed] [Google Scholar]
- 4.Brues AM, Subbarow Y, Jackson EB, Aub J. Growth inhibition by substances in the liver. J Exp Med. 1940;71:423. doi: 10.1084/jem.71.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucher NLR. Experimental aspects of hepatic regeneration. N Eng J Med. 1967;277:686. doi: 10.1056/NEJM196709282771306. [DOI] [PubMed] [Google Scholar]
- 6.Francavilla A, Porter KA, Benichou J, et al. Liver regeneration in dogs; morphologic and chemical changes. J Surg Res. 1978;25:409. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol Lond. 1975;248:273. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavigne J, Lalanne M, Thompson JP, Simard A. Specific inhibition of the incorporation of tritiated thymidine in regenerating rat liver by a rat hepatocyte supernatant. Rev Can Biol. 1977;36:27. [PubMed] [Google Scholar]
- 9.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg GynecolObstet. 1973;137:179. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Francavilla A, Porter KA, et al. The effect of splanchnic viscera removal upon canine liver regeneration. Surg Gynecol Obstet. 1978;147:193. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Terblanche J. In: Hepatotrophic substances. In: Progress in Liver Diseases. Popper H, Schaffner F, editors. VI. New York: Grune & Stratton; 1979. p. 135. [PubMed] [Google Scholar]
- 12.Starzl TE, Terblanche J, Porter KA, et al. Growth-stimulating factor in regenerating canine liver. Lancet. 1979;1:127. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete protacaval shunt in dogs. Lancet. 1976;1:821. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 14.Verly WG, Deschamps Y, Pushpathadam J, Desrosiers M. The hepatic Chalone—I, assay method for the hormone and purification of the rabbit liver chalone. Can J Biochem. 1971;49:1376. doi: 10.1139/o71-198. [DOI] [PubMed] [Google Scholar]
- 15.Weinbren K. Regeneration of the liver. Gastroenterology. 1959;37:657. [PubMed] [Google Scholar]