Abstract
Background
Tacrolimus (formerly FK 506) was first used clinically in 1989 to successfully replace cyclosporine in hepatic transplant recipients who were experiencing intractable rejection or as the baseline drug from the time of operation. After extensive pilot experience, an institutional review board-mandated clinical trial comparing cyclosporine with tacrolimus was performed.
Study Design
From February 16, 1990 to December 26, 1991, 154 patients were recruited. The competing drugs were combined with equal induction doses of prednisone in both arms of the study for the first 81 patients and with subsequently higher doses of prednisone in the remaining 35 patients who received cyclosporine and were entered into the trial. Drug crossover was permitted for lack of efficacy or adverse events. End points were rejection confirmed by biopsy and treatment failure leading to retransplantation or death.
Results
Seventy-nine patients were randomized to the tacrolimus arm and 75 to the cyclosporine arm during 1990 and 1991. All patients were available for follow-up throughout the trial, which terminated on May 30, 1995. The mean duration of follow-up was four years. Patients randomized to the tacrolimus arm were less likely to experience acute rejection than were those receiving cyclosporine, with 36.2 percent of the patients receiving tacrolimus and 16.8 percent of the patients receiving cyclosporine showing freedom from rejection at one year (p=0.003, likelihood ratio test). Survival of patients over the course of the study was virtually the same in the two groups.
Conclusions
Tacrolimus was more effective than cyclosporine in preventing acute rejection.
Although a properly designed and executed randomized controlled trial (RCT) is the best way to determine the effectiveness of competing therapies, such studies are underrepresented in the surgical literature (1, 2). A partial explanation has been provided by Solomon and McLeod (3), who found that even in an ideal clinical research setting, only 40 percent of questions involving a surgical procedure could have been answered by an RCT. Although Solomon and McLeod support RCTs whenever such studies are feasible, they stated that “… if a new treatment is shown to result in a dramatic improvement in outcome in uncontrolled, immediate historic controlled trials or nonrandomized controlled trials, an RCT may be unnecessary or even unethical” (3). On the other hand, undue adherence to such lines of reasoning has hampered therapeutic progress by allowing inappropriate or ineffective therapy, or both, to become institutionalized on the basis of case series and retrospective studies.
We report herein the long-term results from and dilemmas posed by an RCT that has been inundated by the foregoing controversies. The trial comparing the immunosuppressants tacrolimus (FK 506, Prograf™, Fujisawa USA, Inc., Deerfield, IL) and cyclosporine (Sandimmune®, Sandoz Pharmaceuticals, East Hanover, NJ) for hepatic transplantation was required as a condition for continuing the clinical development of tacrolimus. However, the drug already had been shown to systematically rescue hepatic allografts from otherwise intractable rejection (4, 5) and to cause no unique or unexpected toxicity compared with cyclosporine when used as long-term therapy (5, 6). In addition, improved survival of patients and grafts with reduced dependence on adjuvant adrenal corticosteroids relative to historical controls had been observed in almost 200 recipients treated with tacrolimus from the time of hepatic or other organ transplantation (7–10).
Two design features made the Pittsburgh RCT reported here conceptually different from the European (11) and American (12) multicenter trials that were started later. In this single-center trial, tacrolimus and cyclosporine were compared “head to head, ” with all other treatment factors (including corticosteroids) balanced at baseline (13). In addition, both competing drugs were used flexibly, with rapidly adjustable doses, rather than with the limited dose maneuverability that was a feature of the multicenter trials. Consequently, problems with overdosing of tacrolimus and consequent side effects that were characteristic of the subsequent multicenter trials on both sides of the Atlantic were avoided.
Materials and Methods
Inclusion and exclusion criteria
All male and female patients aged 16 to 60 years referred to the University of Pittsburgh for hepatic transplantation were considered to be potential candidates for randomization. Patients were excluded if they were hepatitis B virus carriers, had cancer, were undergoing transplantation of multiple organs, had serum creatinine levels greater than 2 mg percent, had active infection, were in stage 4 coma (unconscious and ventilator dependent), had clinically significant heart or lung disease, or had had previous hepatic hilar reconstructive or portal venous bypass procedures. Pregnant or nursing women were also excluded.
Details of randomization
Informed consent was obtained in advance for all patients, and patients were randomized four hours after full revascularization. Treatment assignment (using sealed-envelope implementation) was determined by a computer-programmed block randomization technique (block size of eight) to assure that the treatment groups remained reasonably balanced. Randomization was aborted if the operation was technically unsatisfactory or if inadequate bile production or intraoperative biochemical blood studies (such as documentation of lactic acidosis) suggested substandard graft function. Once randomization was performed, continuous intravenous (IV) infusion of tacrolimus or cyclosporine was started within two hours. The surgeons did not know the randomization status during the donor search at operation or in the early intraoperative phase.
Determination of rejection of the hepatic transplant
Postoperative ultrasound studies of the hepatic vascular structure and bile ducts were routinely performed. When findings on ultrasound were equivocal, a cholangiogram and an arteriogram were performed. Protocol biopsies were obtained after approximately ten postoperative days, before discharge from the hospital, and at two months. Additional biopsies were obtained when indicated by clinical events.
Clinical criteria for rejection
Findings that prompted a work-up and biopsy of the graft included fever greater than 38.3 degrees C without an identifiable source of infection, diminished output of bile or altered character of the bile (if a T tube was present), and formation of ascites. If total bilirubin, serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase, alkaline phosphatase or γ-glutamyl transpeptidase measurements were abnormal before the work-up to determine rejection, findings greater than 1.5 times the previous week's value were considered suspicious. When the baseline values were normal, elevations exceeding double the upper limit of normal triggered further work-up.
Histopathologic criteria
No diagnosis of rejection was accepted without verification by biopsy. Findings consistent with acute rejection (14, 15) were a predominantly mononuclear (50 to 60 percent) portal tract infiltrate intermixed with polymorphonuclear cells and eosinophils, localization of the inflammatory cells around and beneath the swollen endothelium of portal capillaries and small veins, and/or infiltration and damage of the epithelium of small bile ductules. The stigmata of chronic rejection were arteriopathy, ductopenia, and fibrosis (14–16).
Rejection is a diagnosis of exclusion (14–16). Therefore, important negative findings were absence of findings that suggested hepatitis (i.e., panlobular inflammations, piecemeal necrosis, disarray with ballooning and spotty individual hepatocyte necrosis, and prominent lymphohistiocyte infiltration of the hepatic lobule with inflammatory cell destruction of hepatocytes), and absence of stigmata indicating obstruction of the bile duct, including cholangiolar proliferation.
Details of immunosuppression
Tacrolimus
The starting IV dose of 0.1 mg/kg/day was given by constant 24-hour infusion with no IV overlap when oral medications were started. A new physician's order was required each day as long as the patient was in the hospital. During the period of constant IV infusion, dose adjustments were considered between normal ordering times if changes in the pattern of urine output, evidence of neurotoxicity, or other events generated suspicion of drug toxicity (7, 8, 17–19). The characteristic side effects of tacrolimus determined the maximum dose, and the occurrence of rejection established the minimum dose.
Same-day plasma concentrations of tacrolimus (20) were measured in an on-site monoclonal immunoassay laboratory (21), which was the only one with this capability outside of Japan at the time. When the results were correlated with the observations about efficacy and toxicity, the appropriate 12-hour plasma trough concentration was quickly deduced in the same way that previously had allowed development of the efficient use of cyclosporine with prednisone (22). The target 12-hour plasma level of tacrolimus was 1 to 2 ng/mL, above which the incidence of nephrotoxicity, neurotoxicity, and diabetogenicity progressively rose.
Trough concentrations greater than 5 ng/mL were not permitted. However, when there was evidence of rejection, tacrolimus doses were increased to allow trough levels in the 2 to 5 ng/mL range, providing this caused little or no toxicity. Conversely, doses were reduced if toxicity was present, even if trough levels were already low. Patients were not discharged from the hospital until oral dosages were stable, at which time the usual plasma trough levels were near 1 ng/mL. In the months that followed, these plasma concentrations and the oral doses were allowed to drift down.
Cyclosporine
A sliding scale strategy was used for dosing, beginning with a 24-hour IV infusion of 4 mg/kg/day and an optional oral starting dose of 8 mg/kg cyclosporine every 12 hours. In the first month, 12-hour whole blood trough concentrations were targeted in the 800 to 1,500 ng/mL range (23). From the second month onward, those concentrations were permitted to fall to 600 to 800 ng/mL or lower if toxicity occurred but there was no evidence of rejection. The similarity of side effects between cyclosporine and tacrolimus, as well as the fact that doses were driven by the same three interrelated factors—rejection, toxicity, and trough levels—greatly facilitated management and outcome comparisons between the competing drugs.
Prednisone
In the first 81 cases (tacrolimus, n=41, cyclosporine, n=40), methylprednisolone, 20 mg, was given daily until oral therapy was started with prednisone, 20 mg/day (Fig. 1). A dose reduction to 10 mg/day was allowed on both arms of the study at two weeks and a further reduction to 5 mg/day at the end of the month if there had been no clinical or histopathologic evidence of rejection. Corticosteroids were discontinued at the physician's discretion after this time.
Fig. 1.
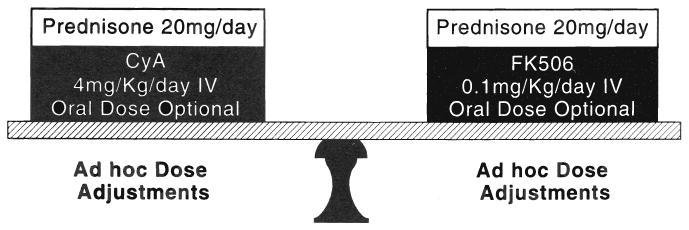
The experimental design of the Pittsburgh randomized trial. All treatment variables were equal except for the competing drugs (cyclosporine and tacrolimus). CyA, Cyclosporine; IV, intravenous; and FK506, tacrolimus.
Beginning with case 82, methylprednisolone (Depo-medrol®, Upjohn, Kalamazoo, MI) 1 g IV, was given intraoperatively to both groups. However, the two arms of the study were unbalanced by adding a prophylactic five-day burst of postoperative methylprednisolone (previously used only to treat rejection) beginning on postoperative day one, for subsequent patients randomized to receive cyclosporine (n=35), but not to those randomized to receive tacrolimus (n=38). The additional induction dose for the cyclosporine-treated patients was a direct response to the high rejection rates observed in that arm of the study.
Conditions for crossover
Treatment failure was defined as the inability of the initial immunosuppression therapy plus an orderly sequence of secondary therapy (when necessary) to control rejection confirmed by biopsy (Fig. 2). In the event of rejection a 1-g bolus of methylprednisolone, which could be repeated once, was given. If no response was obtained within 48 hours, a five-day course of IV OKT3 (10 mL/day) was given. If no improvement or a relapse occurred, a five-day course of tapering corticosteroids was given, starting with methylprednisolone, 200 mg, with 40-mg decrements to a baseline dose of 20 mg/day. If these maneuvers were unsuccessful, crossover from cyclosporine to tacrolimus or vice versa was permitted at the discretion of the attending surgeons.
Fig. 2.
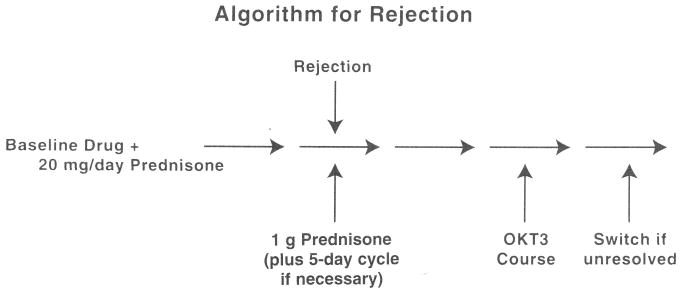
Algorithm used to determine treatment failure, with the consequent option of crossover to the competing immunosuppressant drug. The diagnosis of rejection required proof by biopsy. OKT3, Orthoclone OKT3.
Although crossover to the competing treatment was permitted when drug toxicity occurred, the side effects never directly necessitated this, because toxicity could be easily adjusted by increasing or decreasing the doses of drugs administered. In the appropriate doses, it was known from the preceding pilot experience that the principal factors limiting the dosage of tacrolimus were the same as for cyclosporine: nephrotoxicity (17, 24), diabetogenicity (25), and neurotoxicity (24). It also had been established by comparison with historical controls that flexible regimens for tacrolimus were associated with an equivalent or lower rate of infection than those using cyclosporine (7, 8, 26, 27), could be effective with a reduced cumulative need for prednisone (7, 8, 28), resulted in a lower incidence of hypertension (7–9, 17) and hyperlipidemia (7, 8), and did not cause the cosmetic changes associated with cyclosporine (hirsutism, facial brutalization, and gingival hyperplasia) that precluded blinded trials. When used properly, the total treatment regimen (especially the prednisone component) required to control rejection and minimize toxicity with either tacrolimus or cyclosporine was never identical in any two patients.
Oversight committee
At the request of the clinicians caring for the patients, a multi-institutional patients' rights committee (faculty members at the University of Pittsburgh, Carnegie Mellon University, and Harvard Medical College) was convened to evaluate interim results every three months and make recommendations to the institutional review board (IRB) about the continuation or termination of the trial.
Statistical analysis
A Cox proportional hazards regression model (29) was used to analyze the time-to-event data, with treatment effects tested for statistical significance using a likelihood ratio test. This approach allows for varying lengths of patient follow-up and simultaneous control for the effect of patient baseline confounders on outcome. The end points for statistical analysis were freedom from biopsy-confirmed rejection, retransplantation, and death. Crossover was not considered to be an end point.
Results
Patient characteristics
From February 16, 1990 to December 26, 1991, 154 patients were recruited, of whom 79 patients were randomized to the tacrolimus arm and 75 to the cyclosporine arm of the study. The patient groups that emerged from the intraoperative randomization were similar with respect to baseline characteristics, although there was some imbalance in representation by ABO blood type and indications for transplant (Table I). All patients were available for follow-up throughout the trial, which terminated on May 30, 1995. The mean duration of follow-up was four years. After randomization, 47 patients receiving cyclosporine were crossed over to the tacrolimus arm. Of the crossovers, 70 percent occurred within the first month of randomization and another 28 percent during the first year of follow-up. Seven patients were crossed over before rejection of the transplant, whereas the other 40 were switched to tacrolimus following rejection (Table II). The remaining 28 patients randomized to receive cyclosporine remained in the assigned treatment arm for the duration of the trial, irrespective of intervening episodes of rejection or retransplantation. Only one patient in the tacrolimus arm was crossed over to cyclosporine. This occurred following retransplantation that was preceded by an episode of rejection.
Table I. Baseline Characteristics.
| Characteristics | Tacrolimus, n=79 | CyA, n=75 | CyA to FK, n=47 | CyA only, n=28 |
|---|---|---|---|---|
| Mean age, y | 43.2 | 42.5 | 42.3 | 42.9 |
| Male sex, percent | 62.0 | 61.3 | 55.3 | 71.4 |
| White race, percent | 89.9 | 88.0 | 87.2 | 89.3 |
| Blood type, percent | ||||
| A | 36.7 | 54.7 | 48.9 | 64.3 |
| O | 43.0 | 36.0 | 38.3 | 32.1 |
| B | 13.9 | 5.3 | 8.5 | 0.0 |
| AB | 6.3 | 4.0 | 4.3 | 3.6 |
| Indications for transplant, percent | ||||
| Cirrhosis | ||||
| Alcoholic | 35.4 | 28.0 | 19.2 | 42.9 |
| Chronic post-NANB | 21.5 | 18.7 | 21.3 | 14.3 |
| Cryptogenic | 10.1 | 12.0 | 12.8 | 10.7 |
| Cholestatic disease | ||||
| Primary biliary cirrhosis | 10.1 | 10.7 | 12.8 | 7.1 |
| Sclerosing cholangitis | 8.9 | 8.0 | 6.4 | 10.7 |
| Autoimmune hepatitis | 2.5 | 6.7 | 8.5 | 3.6 |
| Biliary atresia | 2.5 | 1.3 | 0.0 | 3.6 |
| Inborn metabolic disorders | 2.5 | 5.3 | 8.5 | 0.0 |
| Other | 6.3 | 9.3 | 10.6 | 7.1 |
n, Number of patients; CyA, cyclosporine; FK, tacrolimus; y, years; and NANB, non-A, non-B hepatitis.
Table II. Reasons for Crossover from Cyclosporine to Tacrolimus.
| Reason for crossover | Prior to rejection, n=7 | Following rejection, n=40 |
|---|---|---|
| Corticosteroid-resistant rejection | — | 26 |
| Refractory rejection | — | 13 |
| CyA nephrotoxicity | — | 1 |
| Ischemic injury | 5 | — |
| Hemolysis | 1 | — |
| Family insistence | 1 | — |
n, Number of patients, and CyA, cyclosporin.
Primary analysis: freedom from rejection
Cox regression analysis was performed using an indicator variable to represent treatment status and using time to rejection as the outcome variable. The freedom-from-rejection curves (Fig. 3a) showed that patients randomized to the tacrolimus arm were less likely to experience acute rejection than were those receiving cyclosporine (p=0.003, likelihood ratio test). The freedom from rejection at one year was 36.2 percent for patients receiving tacrolimus and 16.8 percent for patients receiving cyclosporine. Confidence limits of 95 percent for the 19.4 percentage point difference in these rates are 6.9 to 31.9 percent. Note that the interpretation of this intent-to-treat analysis is unaffected by the pattern of crossovers, as the vast majority (85 percent) of these occurred after rejection (Table II). A subsequent analysis, which censored the seven (15 percent) patients receiving cyclosporine who were crossed over before occurrence of an acute rejection, yielded similar results. An analysis including age, gender, race, blood type, indications for transplant, and starting dose of prednisone as covariates produced similar results.
Fig. 3.
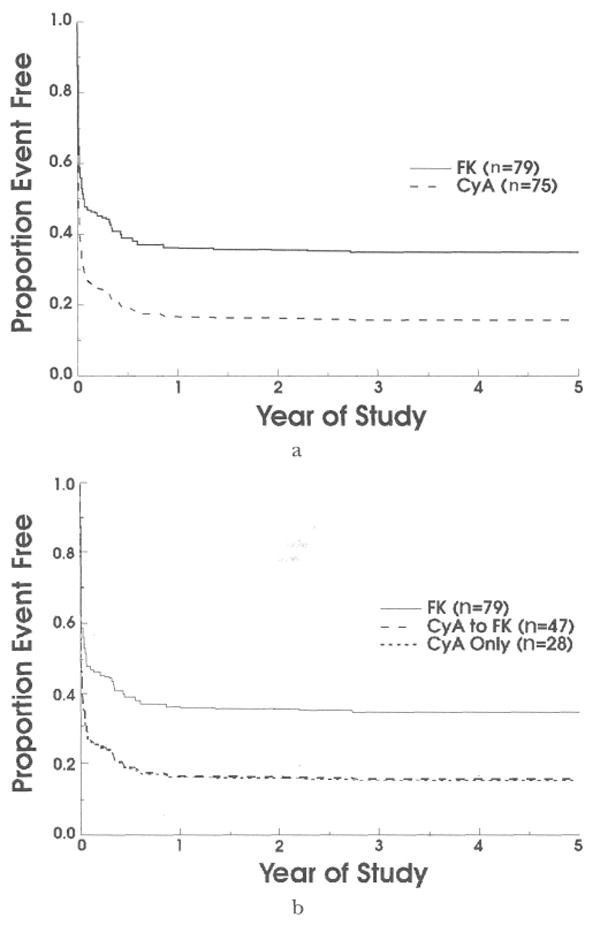
a, Freedom from rejection (acute and chronic), comparing the two randomized treatment arms. b, Freedom from rejection comparing patients randomized to receive tacrolimus with those randomized to receive cyclosporine who were subsequently crossed over, and with those who continued receiving cyclosporine for the duration for the trial. FK, Tacrolimus; CyA, cyclosporine; and n, number.
An assumption underlying the Cox regression analysis is that the hazard rates are proportional. This assumption was examined by plotting the freedom-from-rejection curves on a log-log scale. Because the two curves were parallel, the proportional hazards model can be regarded as appropriate.
Exploratory analyses
The unique study design and pattern of crossovers influenced the framework for a set of exploratory analyses. For these analyses, three groups were compared: the 79 patients randomized to receive tacrolimus, the 47 patients who crossed over from cyclosporine to tacrolimus therapy, and the 28 patients who remained in the cyclosporine arm of the study. The results of these comparisons must be interpreted cautiously because they are not protected by the randomization process.
The baseline characteristics for these three groups are shown in Table I. Figure 3b shows that the freedom-from-rejection curves for patients who were crossed over from cyclosporine to tacrolimus therapy are identical to the rejection curves for those patients who did not cross over. This is reassuring, as the vast majority of crossovers from cyclosporine to tacrolimus therapy occurred after rejection of the transplant. There were no statistically significant differences among the groups with regard to freedom from graft failure (Fig. 4a, p=0.42), defined as a composite outcome of retransplantation or death. When these outcomes were considered separately, there is a striking difference in freedom from retransplantation for patients receiving tacrolimus in comparison with those who continued receiving cyclosporine (Fig. 4b, p=0.02). At three years, freedom from retransplantation for patients receiving tacrolimus was 90.3 percent compared with 70.7 percent for patients continuing to receive cyclosporine (see reasons for retransplantation in Table III). Finally, survival of patients throughout the trial was virtually identical, at approximately 84 percent in each of the three groups (Fig. 4c, p=0.95). Causes of death are listed in Table IV.
Fig. 4.
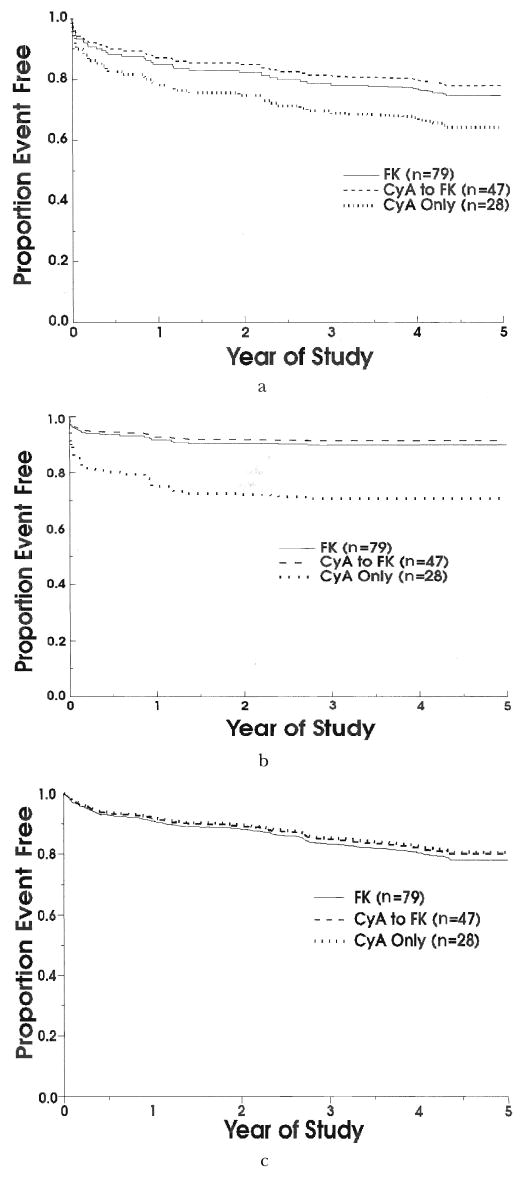
a, Freedom from retransplantation or death. b, Freedom from retransplantation. c, Freedom from death. FK, Tacrolimus; CyA, cyclosporine, and n, number.
Table III. Reasons for Retransplantation.
| Reason | Tacrolimus, n=8 | CyA to FK, n=4 | CyA only, n=8 |
|---|---|---|---|
| Rejection | 1 | 1 | — |
| Primary failure | 1 | — | 4 |
| Recurrent liver disease | 2 | 1 | — |
| Vascular event | 1 | — | 4 |
| Ischemic injury | 3 | 1 | — |
| Infection | — | 1 | — |
n, Number of patients; CyA, cyclosporin; and FK, tacrolimus.
Table IV. Causes of Death.
| Tacrolimus, n=17 | CyA to FK, n=10 | CyA only, n=5 | ||||
|---|---|---|---|---|---|---|
| Cause of death | n | Percent | n | Percent | n | Percent |
| Infection | 8 | 47.1 | 5 | 50.0 | 2 | 40.0 |
| Vascular event | 4 | 23.5 | 2 | 20.0 | 1 | 20.0 |
| Graft failure | 1 | 5.9 | — | — | 1 | 20.0 |
| Hepatic disease | — | — | 3 | 30.0 | — | — |
| Malignancy | 2 | 11.8 | — | — | 1 | 20.0 |
| Multiple organ failure | 1 | 5.9 | — | — | — | — |
| Respiratory failure | 1 | 5.9 | — | — | — | — |
n, Number of patients; CyA, cyclosporin; and FK, tacrolimus.
Adverse events
The adverse events encompassed complications affecting the central nervous, genitourinary, cardiovascular, respiratory, and gastrointestinal systems. Not all adverse events were suspected of being side effects of the treatment drug. Infections, neoplasms, and hematologic abnormalities were treated in the same way. A spectrum of complications not obviously related to drug therapy included technical surgical accidents, worsening of preexisting cardiovascular disease, degenerative disorders, and traumatic accidents.
Two de novo malignancies developed, one in each arm of the study: one patient in the cyclosporine group died of a squamous cell carcinoma of the larynx after 4.4 years and one patient in the tacrolimus group died after 2.3 years of a disseminated squamous cell carcinoma of the oropharynx. Neither patient had been crossed over to the other treatment.
Discussion
The results of this study indicate that tacrolimus is a more potent and satisfactory immunosuppressant than is cyclosporine for combination therapy with prednisone and other adjuvant agents. As seen in Figure 3, patients randomized to the tacrolimus arm were significantly less likely to experience acute rejection than were those receiving cyclosporine. Survival of patients was virtually identical in the two groups.
Historically, renal transplantation was the whole organ procedure with which new immunosuppressive drugs and regimens were evaluated (30–37), and then applied secondarily to the unpaired vital organs. This precedent was broken with the development of tacrolimus, largely because it was demonstrated at the outset that the new agent could rescue hepatic grafts from intractable rejection under cyclosporine-based immunosuppression (4, 5). The consequent demand for tacrolimus prompted two decisions by the United States Food and Drug Administration (FDA): first, to place it on to the developmental “fast track,” and second, to require two multicenter randomized trials. However, randomized rescue trials in which half of the patients facing death or retransplantation would be assigned to a less potent therapeutic option were unappealing. At meetings of the FDA in October and November 1989, randomized European and American multicenter trials were recommended to compare tacrolimus with cyclosporine as the primary immunosuppressant from the time of liver replacement.
Although this decision averted an indefensible rescue trial, it created other issues. By the end of 1989, extensive pilot studies already suggested that the improvement in outcome, including quality of life, after transplantation of a variety of organs was as obvious as when cyclosporine succeeded azathioprine (Imuran®, Glaxo Wellcome Inc., Research Triangle Park, NC) as the baseline immunosuppressant a decade before. On the earlier occasion, recipients of the life-stake organs (liver and heart) had been spared randomization. All were given cyclosporine, and randomized trials were restricted to renal transplant recipients (36) to whom dialysis back-up could be offered.
In the IRB-mandated Pittsburgh trial comparing tacrolimus with cyclosporine, the resulting ethical quandary was dealt with by the experimental design, which had three objectives. The first was to identify at an early time those recipients whose liver graft survival would require high-dose prednisone therapy or other potentially dangerous adjuvant immunosuppression. The second objective was to provide a sensitive trigger for allowing patients prompt access to the more potent baseline drug if they required it. Third, scientifically valid safety and efficacy information was ensured by equalization at the outset of all treatment variables except for the competing drugs. After the first 81 cases, the prednisone doses on the cyclosporine arm of the study had to be increased because of the high incidence of rejection.
The single-center trial reported here was more than half completed before the multicenter trials began in August (12) and September 1990 (11). Although the results in all three studies were generally congruent, the multicenter protocols led to systematic overdosing of tacrolimus. In addition, the unequal use of other immunosuppressants in the competing arms of the study deprived the multicenter investigations of a focused view of the new drug's potential (37).
Finally, a handicap in the multicenter trials was the unavailability of same-day determinations of circulating tacrolimus. Ironically, the results of the University of Pittsburgh randomized trial were ultimately disqualified for inclusion at the FDA advisory hearings because drug monitoring had been done by measuring plasma concentrations of tacrolimus, whereas whole blood determination, which has ten to 20 times higher readout, was eventually adopted as the laboratory standard. However, both technologies are based on the same principle (21), provide results that are highly correlated, and have similar accuracy (38). Consequently, the plasma method, which was the only one available at the beginning of 1990 (and only in Japan and in the Pittsburgh laboratories), provided on-site pharmacokinetic data (7, 8, 17, 19, 20, 24) that remain useful and valid today.
Although the Pittsburgh trial was smaller than the multicenter trials referred to here, it was characterized by a substantially longer follow-up period, and therefore adds further understanding to the relative properties of immunosuppressant agents. The same general conclusions as those from the hepatic transplantation trials have been reported to apply for renal (39), pulmonary (40), and cardiac (41) transplantation.
Acknowledgments
This study was supported in part by Project Grant No. DK 29661 from the National Institutes of Health, Bethesda, MD.
References
- 1.Pollock AV. The rise and fall of the random controlled trial in surgery. Theor Surg. 1989;4:163–170. [Google Scholar]
- 2.Solomon MJ, McLeod RS, Laxamana A, Devore L. Randomized controlled trials in surgery. Surgery. 1994;115:707–712. [PubMed] [Google Scholar]
- 3.Solomon MJ, McLeod RS. Should we be performing more randomized controlled trials evaluating surgical operations? Surgery. 1995;118:459–467. doi: 10.1016/s0039-6060(05)80359-9. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Todo S, Fung J, et al. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung JJ, Todo S, Jain A, et al. Conversion of liver allograft recipients with cyclosporine related complications from cyclosporine to FK 506. Transplant Proc. 1990;22:6–12. [PMC free article] [PubMed] [Google Scholar]
- 6.Fung JJ, Todo S, Tzakis A, et al. Conversion of liver allograft recipients from cyclosporine to FK506-based immunosuppression: benefits and pitfalls. Transplant Proc. 1991;23:14–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK 506. JAMA. 1990;264:63–67. [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armitage JM, Kormos RL, Griffith BP, et al. The clinical trial of FK 506 as primary and rescue immunosuppression in cardiac transplantation. Transplant Proc. 1991;23:1149–1152. [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro R, Jordan M, Fung J, et al. Kidney transplantation under FK 506 immunosuppression. Transplant Proc. 1991;23:920–923. [PMC free article] [PubMed] [Google Scholar]
- 11.The European FK 506 Multicentre Liver Study Group. Neuhaus P, Pichlmayr R, Williams R. Randomised trial comparing tacrolimus (FK 506) and cyclosporin in prevention of liver allograft rejection. Lancet. 1994;344:423–428. [PubMed] [Google Scholar]
- 12.The U.S. Multicenter FK 506 Liver Study Group. Klintmalm G. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 13.Fung J, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK 506 vs cyclosporine. Transplant Proc. 1991;23:2977–2983. [PMC free article] [PubMed] [Google Scholar]
- 14.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, editor. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders Co.; 1969. pp. 464–465. [Google Scholar]
- 15.Starzl TE, Demetris AJ. Current Problems in Surgery Classic. Chicago: Year Book Medical Publishers; 1990. Liver transplantation: a 31 year perspective; pp. 85–99.pp. 101–115. [DOI] [PubMed] [Google Scholar]
- 16.Demetris AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporine to FK 506 immunosuppressive therapy: a clinicopathologic study of 96 patients. Transplantation. 1992;53:1056–1062. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCauley J, Takaya S, Fung J, et al. The question of FK 506 nephrotoxicity after liver transplantation. Transplant Proc. 1991;23:1444–1447. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Fung JJ. Contempo 90: transplantation. JAMA. 1990;263:2686–2687. [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Elmagd K, Fung JJ, Alessiani M, et al. The effect of graft function on FK506 plasma levels, doses, and renal function, with particular reference to the liver. Transplantation. 1991;52:71–77. doi: 10.1097/00007890-199107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkataramanan R, Jain A, Warty VW, et al. Pharmacokinetics of FK 506 following oral administration: a comparison of FK 506 and cyclosporine. Transplant Proc. 1991;23:931–933. [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Kobayashi M, Hashimoto K, et al. A highly sensitive method to assay FK 506 levels in plasma. Transplant Proc. 1987;Suppl 6, 19:23–29. [PubMed] [Google Scholar]
- 22.Starzl TE, Hakala TR, Rosenthal JT, et al. Variable convalescence and therapy after cadaveric renal transplantation under cyclosporin A and steroids. Surg Gynecol Obstet. 1982;154:819–825. [PMC free article] [PubMed] [Google Scholar]
- 23.Plebani M, Paleari CD, Masiero M, et al. Fluorescence polarization immunoassay for cyclosporine. A determination in whole blood. Ther Drug Monit. 1990;12:284–287. doi: 10.1097/00007691-199005000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Starzl TE, Abu-Elmagd K, Tzakis A, et al. Selected topics on FK 506: with special references to rescue of extrahepatic whole organ grafts, transplantation of “forbidden organs,” side effects, mechanisms, and practical pharmacokinetics. Transplant Proc. 1991;23:914–919. [PubMed] [Google Scholar]
- 25.Mieles L, Gordon RD, Mintz D, et al. Glycemia and insulin need following FK 506 rescue therapy in liver transplant recipients. Transplant Proc. 1991;23:949–953. [PMC free article] [PubMed] [Google Scholar]
- 26.Kusne S, Fung J, Alessiani M, et al. Infections during a randomized trial comparing cyclosporine to FK 506 immunosuppression in liver transplantation. Transplant Proc. 1992;24:429–430. [PMC free article] [PubMed] [Google Scholar]
- 27.Alessiani M, Kusne S, Martin M, et al. Infections in adult liver transplant patients under FK506 immunosuppression. Transplant Proc. 1991;23:1501–1503. [PMC free article] [PubMed] [Google Scholar]
- 28.Jain AB, Fung JJ, Todo S, et al. Incidence and treatment of rejection episodes in primary orthotopic liver transplantation under FK506. Transplant Proc. 1991;23:928–930. [PMC free article] [PubMed] [Google Scholar]
- 29.Cox DR, Snell EJ. Analysis of Binary Data. 2nd. London: Chapman and Hall; 1989. [Google Scholar]
- 30.Murray JE, Merrill JP, Dammin GJ, et al. Kidney transplantation in modified recipients. Ann Surg. 1962;156:337–355. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray JE, Merrill JP, Harrison JH, et al. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 32.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 33.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 34.Calne RY, Rolles K, Thiru S, et al. Cyclosporin A initially as the only immunosuppressant in 34 patients of cadaveric organs: 32 kidneys, 2 pancreas, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 35.Starzl TE, Weil R, III, Iwatsuki S, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, editor. The Puzzle People. Pittsburgh: University of Pittsburgh Press; 1992. Letter in a Birmingham Jail; pp. 231–242. [Google Scholar]
- 37.Starzl TE, Donner A, Elasziw M, et al. Randomized trialomania? The multicenter liver transplant trials. Lancet. 1995;346:1346–1350. doi: 10.1016/s0140-6736(95)92349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warty V, Zuckerman S, Venkataramanan R, et al. Tacrolimus analysis: a comparison of different methods and matrices. Ther Drug Monit. 1995;17:159–167. doi: 10.1097/00007691-199504000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gjertson DW, Cecka JM, Terasaki PI. The relative effects of FK 506 and cyclosporine on short- and long-term kidney graft survival. Transplantation. 1995;60:1384–1388. doi: 10.1097/00007890-199560120-00002. [DOI] [PubMed] [Google Scholar]
- 40.Griffith BP, Bando K, Hardesty RL, et al. Prospective randomized trial of FK 506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994;57:848–851. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pham SI, Kormos RL, Hattler BG, et al. A prospective trial of tacrolimus (FK 506) in clinical heart transplantation: intermediate term results. J Thorac Cardiovasc Surg. 1996;111:764–772. doi: 10.1016/s0022-5223(96)70336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


