Summary
The expression of LH receptor mRNA shows significant changes during different physiological states of the ovary. Previous studies from our laboratory have identified a post-transcriptional mechanism by which LH receptor mRNA is regulated following preovulatory LH surge or in response to hCG administration. A specific binding protein, identified as mevalonate kinase, binds to the open reading frame of LH receptor mRNA. The protein binding site is localized to nucleotides 203–220 of the LH receptor mRNA and exhibits a high degree of specificity. The expression levels of the protein show an inverse relationship to the LH receptor mRNA levels. The hCG-induced down-regulation of LH receptor mRNA can be mimicked by increasing the intracellular levels of cyclic AMP by a phosphodiesterase inhibitor. An in vitro mRNA decay assay showed that addition of the binding protein to the decay system caused accelerated LH receptor mRNA decay. Our results therefore show that LH receptor mRNA expression in the ovary is regulated post-transcriptionally by altering the rate of mRNA degradation by a specific mRNA binding protein.
Introduction
The ovary is a unique organ, as in most mammals its structure and function undergo continuous change during the reproductive life span. In rodent and human, luteinizing hormone (LH) plays a critical role of stimulating androgen biosynthesis by the theca-interstitial cells during follicular development. The androgens serve as substrate for estrogen biosynthesis by the granulosa cells (Bjersing 1967). Follicular growth is stimulated mainly by FSH, and possibly augmented by estradiol and insulin/IGF-1 system (Hirshfield 1991, Zeleznik 2004). The growing follicles acquire LH receptors by the combined actions of FSH and estradiol. In most mammals, ovulation occurs in response to the preovulatory LH surge when the oocyte reaches the metaphase of the second meiotic division (Baker 1972). Dramatic changes occur in the follicle following ovulation that involve the transformation of granulosa cells to lutein cells resulting in the formation of corpus luteum within the cortex of the ovary. During this period of transition, a refractory period of LH responsiveness occurs that is characterized by a transient loss of LH receptors and uncoupling of the LH receptor from the cognate G protein-coupled responsive system (Hunzicker-Dunn, et al. 1979, Menon, et al. 2004). This is followed by the full recovery from down-regulation by increasing the expression of LH receptors, which peaks around the mid-point of the luteal phase to support the steroidogenic function of the ovary in order to make the endometrium receptive for the implantation of the blastocyst. If implantation of the blastocyst does not occur, the LH receptors decline as the corpus luteum undergoes atresia. Thus, the expression of LH receptor shows dramatic changes during the ovarian cycle in response to the changing hormonal milieu, principally the changes in the levels of FSH and LH. While FSH along with other paracrine factors is known to regulate the development of the primary follicles to preantral and antral stages, the LH receptor makes its appearance in significant amounts as a result of FSH stimulation (Menon, et al. 2005, Zeleznik 2004). Since FSH acts through the interaction with its receptors localized on plasma membranes, followed by the activation of adenylate cyclase (Channing, et al. 1980, Zeleznik 2004), it is conceivable that the induction of the LH receptor by FSH involves cyclic AMP responsive transcriptional activation. However, other mechanisms for the induction of LH receptors might also operate in these cells. As the ovary acquires LH receptors (Zeleznik, et al. 1974), their expression does not remain at a constant level; instead it changes throughout the ovarian cycle. It should be noted that this occurs, at least in part, due to changes in the circulating levels of LH. In this study, we have focused our attention on the molecular mechanism involved in the changes in LH receptor expression during different physiologic states of the ovary.
LH receptor mRNA expression during ligand-induced down-regulation
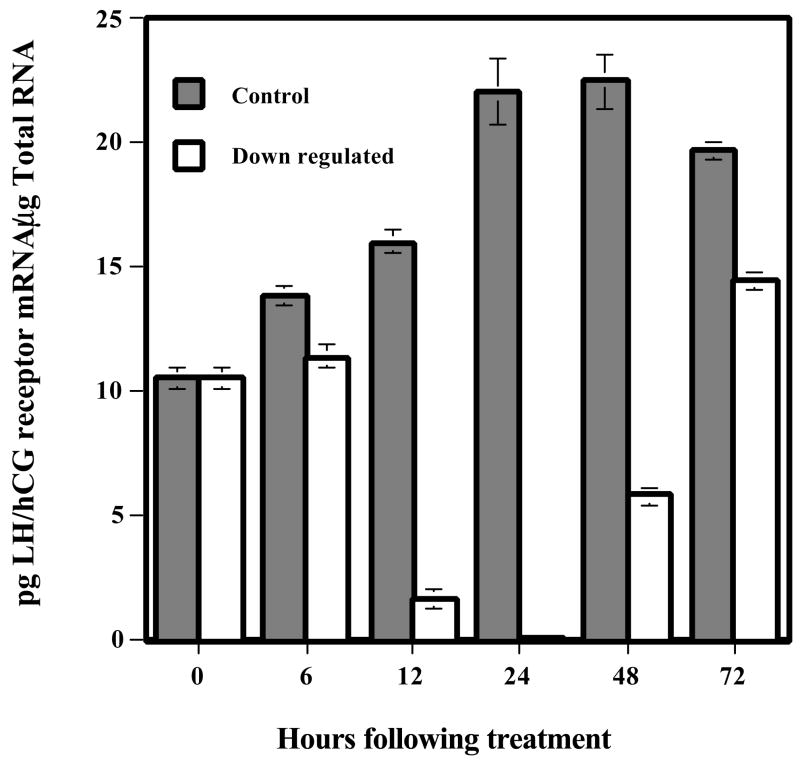
Previous studies have shown that in the rat, preovulatory LH surge produces a rapid decline in LH receptor expression as well as an uncoupling of LH receptor from the cognate G proteins (Hoffman, et al. 1991, Lapolt, et al. 1990, Lu, et al. 1993). The loss of LH receptor from the cell surface may be explained on the basis of the rapid endocytosis of the LH-receptor complex, causing its depletion. The steady state level of receptor expression is the balance between the rate at which the receptor is trafficked to the cell surface and the rate of its endocytosis. The loss of steady state levels of receptor expression could also be due to a temporary pause in synthesis by decreased transcription, or an increase in degradation of mRNA. To examine these possibilities, we used pseudopregnant rats as a model system. Immature rats were treated with 50 IU PMSG followed by 25 IU hCG 56 h later to induce pseudopregnancy. On day 4 of pseudopregnancy, one set of animals was injected with 50 IU hCG, and the control set received saline. Ovaries were collected at different time intervals up to 72 h (Hoffman, et al. 1991, Lu, et al. 1993, Peegel, et al. 1994) and were processed for Northern blot analysis for the expression of LH receptor mRNA, for in situ hybridization analysis in tissue sections to visualize the changes in mRNA expression, and for solution hybridization assay to quantitate changes in mRNA levels. The results showed that within 6 h of hCG injection, there was a steady decline in the expression levels of LH receptor mRNA that reached almost non-detectable levels by 24 h (Hoffman, et al. 1991, Lu, et al. 1993, Peegel, et al. 1994). This was followed by a gradual recovery to control levels by 72 h, as shown in the solution hybridization analysis data presented in Fig 1. The loss of mRNA was rather unexpected, since the decrease of cell surface receptor expression was thought to occur through rapid internalization of the ligand-bound receptor. In the control samples an increase in LHR mRNA expression was seen during the time-course of the experiment, since our previous studies have shown that in pseudopregnant rat ovary the expression of LHR mRNA increased up to day 8 (Hoffman, et al. 1991). The in situ hybridization pattern confirmed the Northern blot data and further suggested that the same corpora lutea that were depleted of the receptor mRNA reacquired the receptor (Peegel et al., 1994). The loss of LH receptor mRNA was specific, as other LH/hCG responsive mRNAs such as Cytochrome p450scc remained at a level significantly higher than the saline treated controls (Hoffman, et al. 1991). The loss of LH receptor mRNA occured through post-transcriptional mechanisms as the transcription rate, determined by nuclear run on assays, remained identical in hCG treated group compared to the control. In fact, the nuclei isolated from the hCG treated ovaries was found to incorporate 3H uridine into total RNA at much higher levels than that seen in control ovaries (Lu, et al. 1993). Thus, we concluded that the loss of steady state LH receptor mRNA seen in the hCG treated group was due to increased mRNA degradation rather than a decrease in the rate of its synthesis.
Fig. 1.
LH/hCG-R mRNA levels during down-regulation time course. RNA was extracted from day 5 pseudopregnant control and down-regulated ovaries at 0, 6, 12, 24, 48, and 72 h after hCG injection. LH/hCG receptor levels were measured by solution hybridization assay. Total RNA (1–40 μg) was incubated with 1 × 104 cpm 35S-labeled LH/hCG receptor antisense RNA. Single stranded RNA was digested by treatment with RNase, and the double stranded RNA was recovered by trichloroacetic acid precipitation. The concentration of LH/hCG receptor mRNA was extrapolated from a standard curve, generated using increasing concentrations of the unlabeled sense strand (0–200 pg), which exhibited reproducible linearity. (Modified from Peegel, H. et al, 1994, Table 1 with permission from Endocrinology)
Accelerated LH receptor mRNA degradation as a regulatory mechanism
It is now well recognized that the expression of specific, highly regulated mRNAs is regulated, at least in part, at the level of mRNA degradation. There are several examples where the mRNA degradation rate controls the steady state levels of mRNA expression (Ross 1995). In almost all instances where the mRNA expression is regulated by controlling its degradation, the changes in the stability of the mRNA have been shown to result from the binding of specific proteins to specific sequences and/or structures of the mRNA. The specific regions that the mRNA binding proteins interact with may be localized either on the 5′ untranslated region, the coding region or the 3′ untranslated region of the mRNA (Ross 1995). c-Fos, c-Myc, tropoelastin, thymidylate synthase and dihydrofolate reductase are some of the RNAs having regulatory elements for trans-acting factors in the coding region (Bernstein, et al. 1992, Chen, et al. 1992, Herrick, et al. 1994, Lemm, et al. 2002, Prokipcak, et al. 1994, Shyu, et al. 1991, Shyu, et al. 1989, Zang, et al. 1999). In general, the steady state levels of the mRNA expression are regulated by either increasing or decreasing the degradation rate resulting from the interaction with specific RNA binding proteins.
We first examined whether an mRNA binding protein that specifically recognizes LH receptor mRNA exists in the rat ovary. Furthermore, we sought to determine if the binding activity shows any correlation with LH receptor mRNA expression during LH/hCG induced receptor down regulation. Our initial studies indicated that the rat ovarian cytosolic fraction (100,000×g supernatant) isolated from LH receptor down-regulated ovaries showed the presence of an mRNA binding protein that specifically recognized the open reading frame of the LH receptor mRNA, using RNA electrophoretic mobility gel shift assay (REMSA) (Kash, et al. 1998). Two RNP complexes were identified, one prominent band corresponding to 50 kDa and a second less intense band of 45kDa (Fig. 2). Since hCG injection produced a 3-fold increase in the 50 kDa RNP complex with no remarkable change in the intensity of the 45kDa band, further studies focused on the larger RNP complex.
Fig. 2.
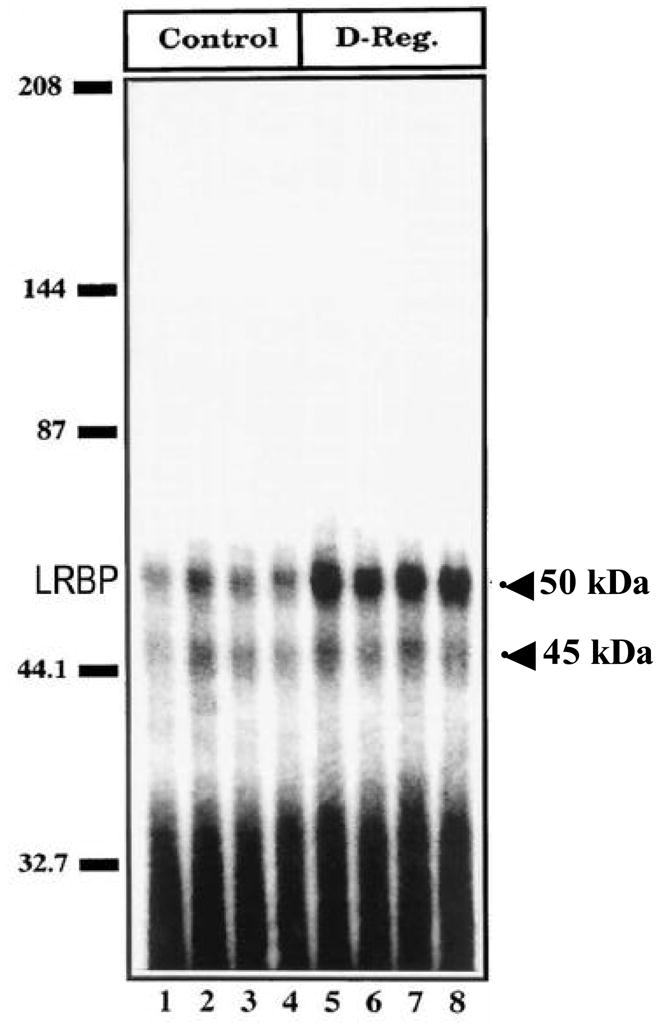
LH/hCG receptor mRNA binding activity of LRBP (luteinizing hormone receptor binding protein) during hCG-induced down-regulation: autoradiogram of RNA gel mobility shift analysis (REMSA). Ovaries were collected from day 5 pseudopregnant, saline-injected (Control), and hCG-injected (12h) rats (D-Reg.). Cytosolic S100 fractions were prepared from the control and hCG down-regulated ovaries. RNA binding reactions were carried out in replicates of four by incubating 1 × 105 cpm of 32P-labeled LHR:102–282 RNA with 50 μg of S100 from the saline-treated control (lanes 1–4) or hCG down-regulated (lanes 5–8) ovaries. (Modified from Kash, J.C. and Menon, K.M.J., 1998, Fig. 6 with permission from the Journal of Biological Chemistry)
Properties of the LH receptor mRNA binding protein
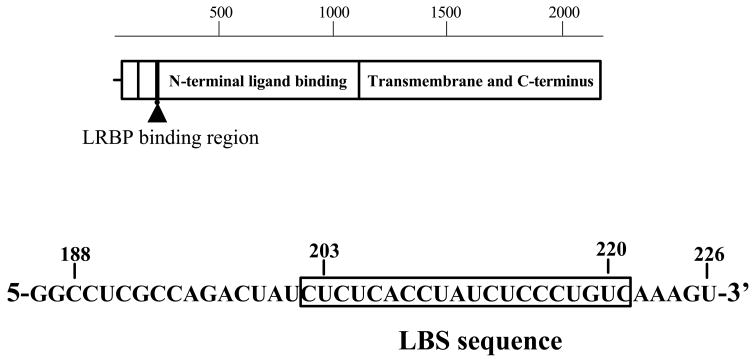
The binding of the mRNA binding protein for RNP complex formation revealed specificity towards a region corresponding to the amino terminal portion of the LH receptor corresponding to nucleotides 102–282 (Kash, et al. 1998). The RNP complex formation was more remarkable in the ovarian tissue from hCG treated animals with very little or no change in non-target tissues (Kash, et al. 1998). Further truncation of this region showed that the contact site resided between nucleotides 188–228 (Kash, et al. 1999). The contact site was identified by hydroxy-radical RNA foot-printing assay and revealed a region corresponding to nucleotides 203–220 consisting of a bipartite polypyrimidine rich sequence, (UCUCX7-UCUCCCU) (Fig. 3) (Kash, et al. 1999). Mutation of the C residues within the bipartite sequence revealed that all C residues participated in binding to the protein (Kash, et al. 1999). The contact site was further confirmed by performing RNA electrophoretic mobility shift assays. The binding was specific, since the ability for RNP complex formation using 32P labeled LH receptor mRNA probe (nucleotides 203–220) was not abolished when non-radioactive nucleotides corresponding to other regions of the mRNA were added to the RNA electrophoretic mobility shift assay reaction to compete with the radioactive mRNA probe (Kash, et al. 1999). The initial attempts of purification of the binding protein showed that the protein was retained on a strong cationic resin and could be eluted with 150 mM KCl.
Fig. 3.
Diagram of the LRBP RNA binding site. Diagram of the LH/hCG receptor mRNA open reading frame showing the position of the LRBP binding site (LBS). The sequence of the LHR:188–226 is also shown. (Modified from Kash, J.C. and Menon, K.M.J., 1999, Fig. 8 with permission from Biochemistry)
Physiological regulation of LH receptor mRNA binding protein
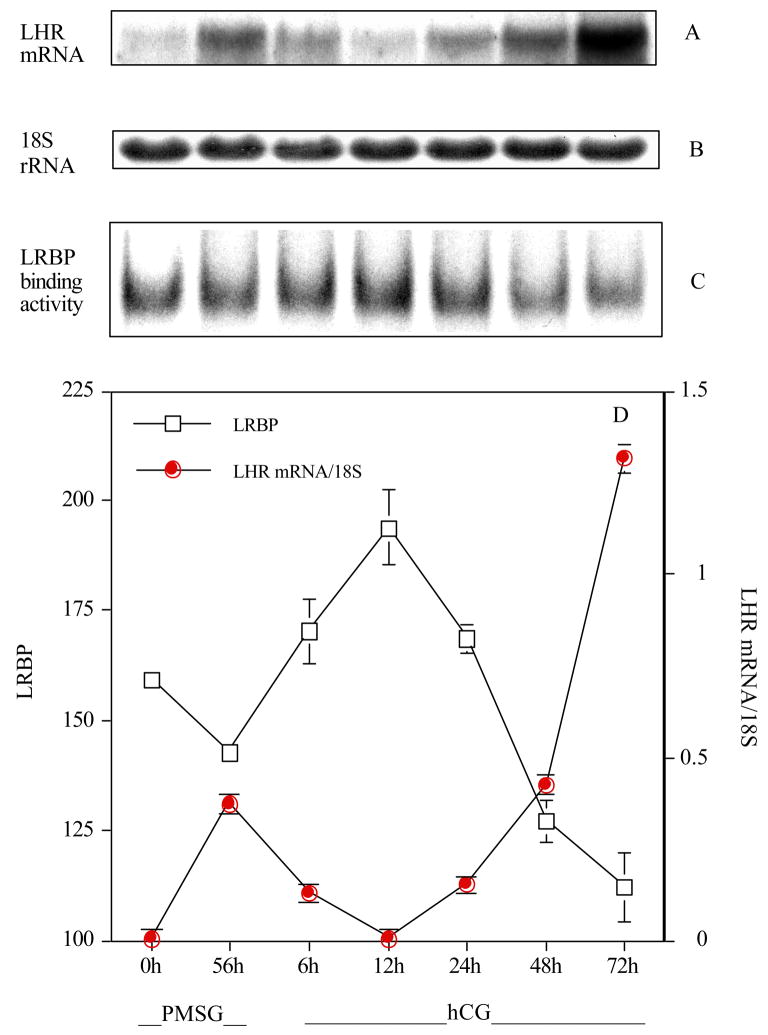
After establishing that the ovarian LH receptor mRNA binding protein was highly specific for recognition of LH receptor mRNA and that the binding activity was induced in response to treatment with hCG, further studies were carried out to determine if the expression of the binding activity has any relationship to tissue levels of LH receptor mRNA (Nair, et al. 2002). To examine this, the levels of expression of the binding protein were examined during follicle maturation, ovulation and luteinzation. Immature rats were treated with 50 IU of PMSG to induce follicle maturation and ovaries were removed at 0 and 56 h later. A treatment with 25 IU of hCG was instituted at 56 h after treatment with PMSG and the ovaries were removed at 6, 12, 24, 48 and 72 h. The ovaries were processed for LH receptor mRNA expression by Northern blot analysis, and the ability of the S-100 fractions to form RNP complexes was determined by electrophoretic mobility gel shift assay. The results showed an increase in the steady state levels of the LH receptor mRNA following treatment with PMSG (Fig. 4). As expected, the expression levels of LH receptor mRNA showed a rapid decline following hCG injection up to 12 h, followed by an increase, reaching highest level of expression by 72 h. The expression pattern of the LH receptor mRNA binding activity showed an inverse relationship to LH receptor mRNA expression levels. When the LH receptor mRNA expression level was high, the RNA binding protein activity was at the lowest level. Conversely, the LH receptor mRNA binding activity was high when the mRNA levels began to fall. Thus, LH receptor mRNA binding protein activity appears to be inversely related to the mRNA level, consistent with its role as an endogenous regulator of LH receptor mRNA expression.
Fig. 4.
LH/hCG receptor mRNA expression and RNA binding activity of LRBP during PMSG and hCG-induced regulation of LH/hCG receptor expression in the ovary. Twenty-one-day old rats were injected with PMSG at 0 h and ovaries were collected immediately and 56 h post injection. Rats were then injected with hCG 56 h post PMSG administration and ovaries were collected at 6, 12, 24, 48 and 72 h intervals. RNA electrophoretic mobility shift analysis (REMSA) was performed for all time intervals with radiolabeled LH/hCG receptor mRNA and 50 μg of S100 fractions prepared from ovaries (C). LH/hCG receptor mRNA levels were obtained by Northern blot analysis (A). The Northern blot was normalized using 18S rRNA (B). The 6.7 kb LH/hCG receptor mRNA transcript and REMSA bands were quantitated by densitometry (D). (Modified from Nair, A.K. et al, 2002, Fig. 5 with permission from the Journal of Biological Chemistry)
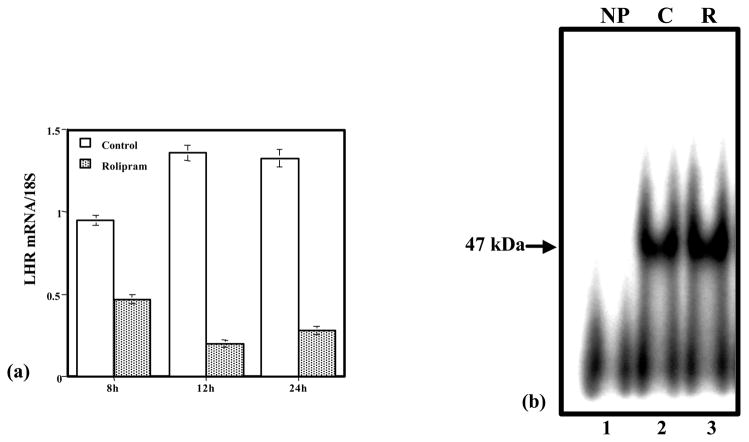
In the studies described so far, down regulation of the LH receptor mRNA was obtained by treating rats with hCG. Since the immediate response of the corpus luteum upon hCG treatment is to increase the production of cyclic AMP, the involvement of cyclic AMP in down- regulation was presumed. To actually show the involvement of cyclic AMP, pseudopregnant rats were treated with rolipram, an inhibitor of type 2 phosphodiesterase (Peegel, et al. 2005). The increase in cyclic AMP production by the ovaries was then assayed. Treatment with multiple doses of rolipram showed a significant increase in cyclic AMP levels (Peegel, et al. 2005). Chronic elevation of cyclic AMP resulted in the down- regulation of LH receptor mRNA similar to that produced by the treatment with hCG (Fig. 5a). The ovaries were then processed for REMSA in order to determine whether the RNA binding activity was increased when LH receptor mRNA was down regulated. The results showed that there was a significant increase in the RNA binding activity in ovarian S100 fractions at time intervals when the LH receptor mRNA expression was down regulated (Fig. 5b). These results provide evidence for the role of cyclic AMP in inducing the RNA binding protein activity.
Fig. 5.
Down-regulation of the LH receptor mRNA following rolipram treatment. (a) Northern blot of LH receptor mRNA was performed from ovaries of vehicle-injected (C) or rolipram-injected (R) rats at 8, 12 and 24 h after the first injection of vehicle or the PDE inhibitor. Total RNA was isolated from the ovaries and 25 μg RNA were run in 1.2% agarose-formaldehyde gels and subsequently transferred to nitrocellulose membranes. The densitrometric scans of the bands of the 6.7 kb transcript of LHR were standardized to the bands for the 18S rRNA. (b) Activity of the LH receptor mRNA binding protein in ovaries of control and rolipram-treated rats. The autoradiograph is a representative RNA electrophoretic mobility shift assay (REMSA) conducted with 1 × 105 cpm of 32P-labeled RNA, the LRBP binding sequence (LBS-188–228), and incubated with no protein (NP): lane 1, which is the (−) control, or with 10 μg of cytosolic S100 fractions of ovaries from vehicle-injected control animals (C): lane 2, which is the (+) control, or from ovaries of rolipram-treated animals (R): lane 3, 8 h after the administration of the PDE inhibitor. The samples were then treated with RNase T1 to eliminate unprotected RNA and were then resolved by electrophoresis on a 5% native polyacrylamide gel. The samples were subsequently visualized by autoradiography. (Modified from Peegel, H. et al, 2005, Fig. 5 with permission from Molecular and Cellular Endocrinology)
In vitro mRNA degradation by the LH receptor mRNA binding protein
After establishing a relationship between the expression of LH receptor mRNA and the RNA binding protein under in vivo conditions, we sought to determine if the RNA binding protein is able to degrade LH receptor mRNA under in vitro conditions. A cell free mRNA decay assay was developed by following a procedure described by Ross and colleagues (Ross 1993). The assay essentially determines the ability of ribosomes to degrade exogenous RNA in the presence and absence of the RNA binding protein. Although all mRNAs are prone to degradation, the rate of degradation varies depending on the cellular environment. Some mRNAs are more labile than others (Ross 1995), a property conferred by the presence of appropriate trans-acting factors that interact with specific structures or sequences present in the mRNA. Initially, we showed that the rate of decay of LH receptor mRNA was very rapid by ribosomes isolated from the ovaries of rats treated with hCG, compared to the degradation of LH receptor mRNA by ribosomes isolated from ovaries of saline treated control animals (Nair, et al. 2002). We then showed that the rate of decay of exogenously added ovarian RNA by ribosomes isolated from saline treated rats was accelerated by the addition of a partially purified ovarian LH receptor mRNA binding protein (Nair, et al. 2002). The rate of LH receptor mRNA decay was not affected by unrelated proteins added to the reaction mixture (unpublished data). These experiments showed that the LH receptor mRNA binding protein plays a role in LH receptor mRNA degradation.
Purification of the LH receptor mRNA binding protein
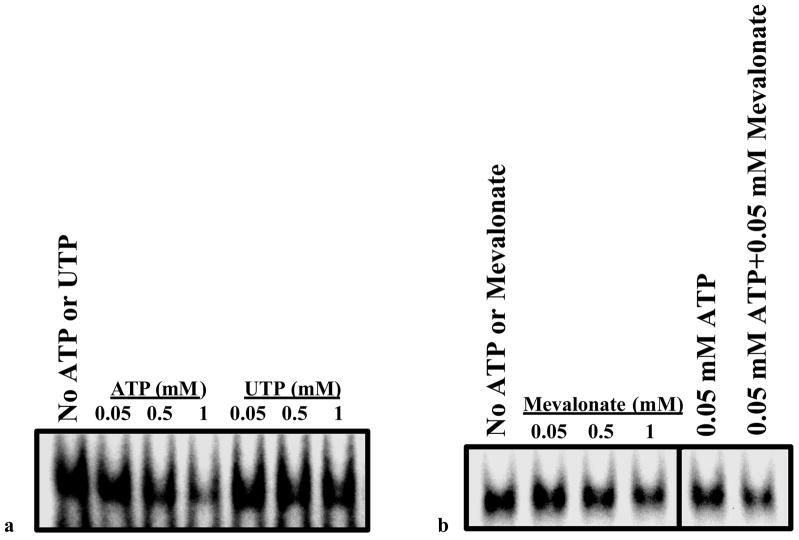
After establishing the existence of a specific LH receptor mRNA binding protein in the cytosolic fraction of the corpus luteum and its association with LH receptor down regulation, attempts were made to purify the protein. Although the RNA binding protein was retained on sepharose columns containing covalently-linked fragments of LH receptor mRNA, the elution of the binding protein was not successful. Therefore, conventional techniques were sought to purify the binding protein from the 100,000×g supernatants prepared from corpora lutea from rats treated with hCG to down regulate the receptor. The supernatants were subjected to chromatography on a strong cation exchange resin (Macro-Prep high S support) and eluted with 150 mM KCl. The eluates were concentrated and subjected to SDS-PAGE. The LH receptor mRNA binding activity associated with the protein band on the gel was identified by an overlay assay (Northwestern blot) using 32P labeled LH receptor mRNA fragment (203–220) as the probe. After extensive standardization of the assay, the protein band corresponding to the band that showed the RNA binding activity was cut, eluted and renatured. The eluted protein was electrophoresed again to determine the purity of the preparation (Fig. 6). The electrophoretically homogeneous protein band was then subjected to amino terminal analysis as well as MS-MALDI analysis to establish its identity. Both analyses revealed the purified protein to be mevalonate kinase (Nair and Menon 2004). Mevalonate kinase was then cloned from rat ovary and overexpressed in 293 T cells (Nair and Menon 2004). The recombinant protein was tested for its ability to bind LH receptor mRNA. The results showed a concentration dependent binding to LH receptor mRNA fragment (Nair and Menon 2004). The binding exhibited all the characteristics of the expected LH receptor mRNA binding protein with respect to specificity for binding to the previously identified contact site (nucleotides 203–220), competition by unlabeled LH receptor mRNA fragment (203–220), dependence on C residues in the ligand binding site and immunoreactivity of the recombinant protein similar to that seen for the electrophoretically purified rat mevalonate kinase, using Western blot. Furthermore, since mevalonate kinase is known to have two binding sites, one for ATP and a second site for mevalonate, the involvement of these sites in LH receptor mRNA binding activity was then determined. It was seen that the binding of the 32P labeled LH receptor mRNA binding sequence to mevalonate kinase was inhibited by ATP and mevalonate (Nair and Menon 2004). The inhibitory effect was even more pronounced in the presence of both ATP and mevalonate (Fig. 7). These results clearly confirm the identity of the RNA binding protein as mevalonate kinase.
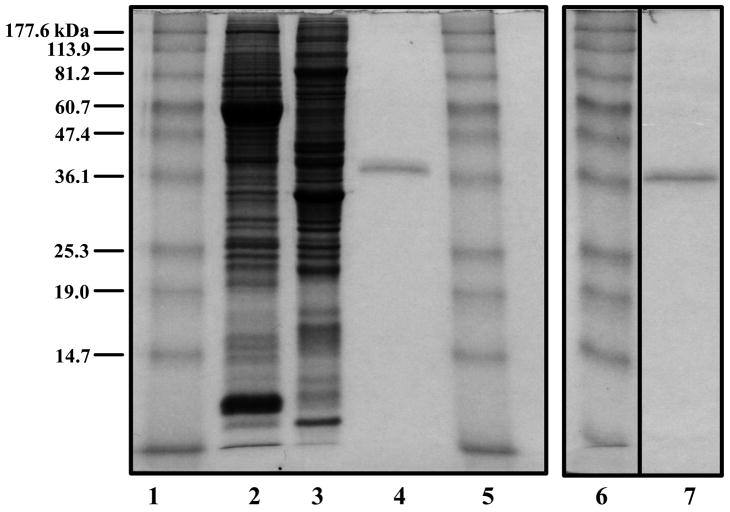
Fig. 6.
Analysis of LRBP purification. Rat ovarian cytosolic proteins (S100-20 μg) (lane 2), partially purified LRBP (20 μg) (lane 3), and two gel-extracted LRBP preparations (1 μg/lane), preparation 1 (lane 4) and preparation 2 (lane 7), were separated on 10% SDS-PAGE and stained with Coomassie Brilliant Blue. (Lanes 1, 5 and 6, prestained standard protein markers (kDa) from Invitrogen.). (Modified from Nair, A.K. and Menon, K.M.J., 2004, Fig. 2 with permission from the Journal of Biological Chemistry)
Fig. 7.
Binding of recombinant mevalonate kinase to LBS: Competition with ATP and mevalonate. 10 μg of cytosolic protein fractions (S100) prepared from 293 cells 48 h after transfection with rat mevalonate kinase cDNA were used for REMSA. ATP and UTP (Magnesium salts) and mevalonate were added to the binding reaction in the concentrations indicated. (Modified from Nair, A.K. and Menon, K.M.J., 2004, Fig. 9 with permission from the Journal of Biological Chemistry).
The relationship of LH receptor mRNA expression to cholesterol metabolism
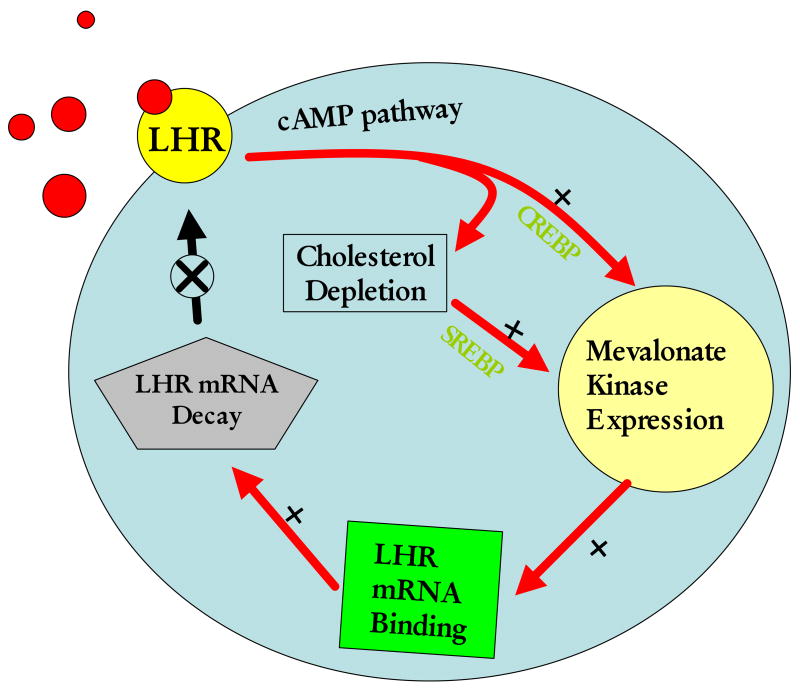
Mevalonate kinase is a metabolic enzyme involved in cholesterol biosynthesis which catalyzes the conversion of mevalonate to phosphomevalonate, the precursor of cholesterol and many natural products. The relationship between LH receptor expression and cholesterol biosynthesis might appear surprising. However, from a functional point of view, they in fact bear a close relationship. For instance, a major function of LH in the corpus luteum is to stimulate steroidogenesis which involves the conversion of cholesterol to steroids. The resultant decrease in the intracellular cholesterol has been shown to cause a proteolytic cleavage of the precursor of the sterol element binding protein (SREBP) to an active SREBP, a transcription factor that stimulates the expression of a group of genes that contain sterol response element (SRE) in the promoter region (Goldstein et al. 2006). A number of enzymes involved in cholesterol biosynthesis including mevalonate kinase, HMG CoA reductase, HMG CoA synthase and farnesyl pyrophosphate synthase are induced in response to cholesterol depletion as they all contain SREs (Shimano 2001). Since the corpus luteum remains refractory with respect to steroidogenesis up to 48 h after hCG-induced down-regulation, it is conceivable that during this period, mevalonate kinase serves as an LH receptor mRNA binding protein. This leads to the degradation of LH receptor mRNA, culminating in the transient loss of LH receptors from the cell surface. Thus, it appears that mevalonate kinase plays a role in the feedback regulation of LH receptor by regulating mRNA stability. In fact, we have shown that mevalonate kinase expression is regulated by LH in the corpus luteum both at the mRNA level as well as at the protein level (Wang and Menon 2005). During LH/hCG -induced down-regulation, mevalonate kinase expression was induced in the corpus luteum. The induction of expression of other SRE containing genes such as HMG CoA reductase, farnesyl pyrophosphate synthase and LDL receptor were also seen during this phase (Wang and Menon 2005). These results suggest that mevalonate kinase expression plays a regulatory role in LH receptor mRNA expression in the corpus luteum. Based on these observations, we present a model for the regulation of LH receptor down-regulation in the ovary (Fig 8). According to this model, activation of LH/hCG receptor causes an increase in the intracellular cAMP levels and subsequent elevation in steroidogenesis. The increase in steroid hormone biosynthesis leads to a depletion in endogenous cholesterol level. This triggers the transcriptional induction of all the genes associated with cholesterol synthesis, including MVK. It is also possible that cAMP may increase the expression of sterol metabolizing enzymes including MVK through transcriptional activation. MVK then binds to LH receptor mRNA leading to its increased degradation and thereby causing the down-regulation of its cell surface expression.
Fig 8.
A model for the post-transcriptional regulation of LH receptor in the ovary. Binding of LH or hCG activates cAMP signal cascade and results in steroid hormone biosynthesis followed by a depletion of ovarian cholesterol. This triggers an increase in transcription of genes associated with synthesis and transport of plasma cholesterol. Mevalonate kinase (MVK), one of the enzymes involved in cholesterol biosynthesis at this time binds to the LH receptor mRNA and leads to its degradation and subsequent down-regulation of receptor expression on the cell surface.
The notion that mevalonate kinase, a metabolic enzyme, acts as an mRNA binding protein is consistent with a similar role of several other metabolic enzymes that have been recently described (Hentze 1994, Menon et al. 2005). The iron response element binding protein, which plays a role in iron homeostasis in cells, is an enzyme in the citric acid cycle (Klausner, et al. 1993). Similarly, thymidylate synthase (Chu, et al. 1999), dihydrofolate reductase(Chu, et al. 1993), glyceraldehyde-3-phosphate dehydrogenase (Nagy and Rigby 1995), and lactate dehydrogenase (Pioli, et al. 2002) all have been shown to serve as RNA binding proteins that regulate different aspects of RNA metabolism. In the case of mevalonate kinase, structural studies have shown that it belongs to a family of ATP binding proteins containing a conserved glycine motif (Fu, et al. 2002, Yang, et al. 2002). Members of this group include galactokinase, homoserine kinase, mevalonate kinase and phosphomevalonate kinase. These proteins share a common fold, a left handed β-α-β loop known as the ribosomal protein S5 domain 2 like fold. This fold is similar to that found in proteins that interact with nucleic acids including elongation factor G and ribonuclease P (Zhou, et al. 2000). Thus from a structural point, it is conceivable that mevonate kinase can serve as an LH receptor mRNA binding protein to regulate cellular expression of LH receptor under select conditions. This is the first example of the feedback regulation of a G protein-coupled receptor by a metabolic enzyme by controlling the mRNA stability. This mechanism may be advantageous for the cell to meet its short-term needs in a continuously changing hormonal environment to regulate the expression of an mRNA by controlling its rate of degradation without the need to reassemble the complex transcriptional machinery.
Footnotes
Supported by NIH grant R37 HD 06656.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker TG. Oogenesis and ovulation. Cambridge: Cambridge University Press; 1972. [Google Scholar]
- Bernstein PL, Herrick DJ, Prokipcak RD, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992;6:642–654. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- Bjersing L. On the morphology and endocrine function of granulosa cells of ovarian follicles and corpora lutea. Acta Endocrinol (cophen) 1967;125(suppl):1–25. doi: 10.1530/acta.0.056s0005. [DOI] [PubMed] [Google Scholar]
- Channing CP, Schaerf FW, Anderson LD, Tsafriri A. Ovarian follicular and luteal physiology. Int Rev Physiol. 1980;22:117–201. [PubMed] [Google Scholar]
- Chen CY, You Y, Shyu AB. Two cellular protiens bind specifically to a purine-rich sequence necessary for the destabilization function of a c-fos protein-coding region determinant of mRNA instability. Mol Cell Biol. 1992;12:5748–5757. doi: 10.1128/mcb.12.12.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E, Schmitz J, Ju J, Copur S. An imunoprecipitation-RNA:rPCR method for the in vivo isolation of ribonucleoprotein complexes. In: Haynes SR, editor. RNA-Protein intraction protocols. Humana Press Inc.; NJ: 1999. pp. 265–274. [DOI] [PubMed] [Google Scholar]
- Fu Z, Wang M, Potter D, Miziorko HM, Kim JP. The structure of a binary complex between a mamalian Mevalonate Kinase and ATP. J Biol Chem. 2002;277:18134–18142. doi: 10.1074/jbc.M200912200. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Hentze MW. Enzymes as RNA-binding proteins: a role for (di)nucleotide-binding domains? Trends Biochem Sci. 1994;19:101–3. doi: 10.1016/0968-0004(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Herrick DJ, Ross J. The half-life of c-myc mRNA in growing and serum-stimulated cells: influence of the coding and 3′ untranslated regions and role of ribosome translocation. Mol Cell Biol. 1994;14:2119–2128. doi: 10.1128/mcb.14.3.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991:388–393. doi: 10.1210/endo-128-1-388. [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Jungmann R, Derda D, Birnbaumer L. LH-induced desensitization of the adenylyl cyclase system in ovarian follicles. Adv Exp Med Biol. 1979;112:27–44. doi: 10.1007/978-1-4684-3474-3_3. [DOI] [PubMed] [Google Scholar]
- Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mRNA binding during receptor down-regulation. J Biol Chem. 1998;273:10658–10664. doi: 10.1074/jbc.273.17.10658. [DOI] [PubMed] [Google Scholar]
- Kash JC, Menon KMJ. Sequence-specific binding of a hormonally regulated mRNA binding protein to cytidine-rich sequences in the lutropin receptor open reading frame. Biochemistry. 1999:16889–16897. doi: 10.1021/bi9915770. [DOI] [PubMed] [Google Scholar]
- Lapolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin-induced up-and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990:3277–3279. doi: 10.1210/endo-126-6-3277. [DOI] [PubMed] [Google Scholar]
- Lemm I, Ross R. Regulation of c-myc mRNA decay by translational pausing in a coding region instability determinant. Mol Cell Biol. 2002;22:3959–3969. doi: 10.1128/MCB.22.12.3959-3969.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DL, Peegel H, Mosier SM, Menon KMJ. Loss of lutropin/human choriogonadotropin receptor messenger ribonucleic acid during ligand-induced down-regulation occurs post-transcriptionally. Endocrinology. 1993:235–240. doi: 10.1210/endo.132.1.8419125. [DOI] [PubMed] [Google Scholar]
- Menon KMJ, Clouser CL, Nair AK. Gonadotropin Receptors: Role of Post-translational Modifications and Post-transcriptional Regulation. Endocrine. 2005;26:249–258. doi: 10.1385/ENDO:26:3:249. [DOI] [PubMed] [Google Scholar]
- Menon KMJ, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: A perspective. Biol Reprod. 2004;70:861–866. doi: 10.1095/biolreprod.103.024471. [DOI] [PubMed] [Google Scholar]
- Nagy E, Rigby WF. Glyceraldehyde-3 phosphate dehydrogenase selectively binds AU-rich RNA in the NAD(+)-binding region (Rossmann fold) J Biol Chem. 1995;270:2755–2753. doi: 10.1074/jbc.270.6.2755. [DOI] [PubMed] [Google Scholar]
- Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002:21468–21473. doi: 10.1074/jbc.M111653200. [DOI] [PubMed] [Google Scholar]
- Nair A, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA fron the ovary. J Biol Chem. 2004;279:14937–14944. doi: 10.1074/jbc.M309484200. [DOI] [PubMed] [Google Scholar]
- Peegel H, Randolph J, Jr, Midgley AR, Jr, Menon KMJ. In situ hybridizatin of luteinizing hormone/human chorionic gonadotropin receptor messenger ribonucleic acid during hormone-induced down-regulation and the subsequent recovery in rat corpus luteum. Endocrinology. 1994:1044–1051. doi: 10.1210/endo.135.3.8070346. [DOI] [PubMed] [Google Scholar]
- Peegel H, Towns R, Nair A, Menon KMJ. A novel mechanism for the modulation of luteinizing hormone receptor mRNA expression in the rat ovary. Mol Cell Endocrinol. 2005;233:65–72. doi: 10.1016/j.mce.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Pioli PA, Hamilton BJ, Connolly JE, Brewer G, Rigby WF. Lactate dehydrogenase is an AU-rich element-binding protein that directly interacts with AUF1. J Biol Chem. 2002;277:35738–35745. doi: 10.1074/jbc.M204002200. [DOI] [PubMed] [Google Scholar]
- Port JD, Huang LY, Malbon CC. B-Adrenergic agonists that down-regulate receptor mRNA up-regulate a Mr 3500 protein(s) that selectively binds to B-adrenergic receptor mRNAs. J Biol Chem. 1992:24108–24108. [PubMed] [Google Scholar]
- Prokipack RD, Herrick D, Ross R. Purification and properties of a protein that binds to the C-terminal coding region of human c-myc mRNA. J Biol Chem. 1994;269:9261–9269. [PubMed] [Google Scholar]
- Ross J. RNA-protein interaction protocols. Humana Press. 1993:459–476. [Google Scholar]
- Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs AB. Messenger RNA degradation in eukaryotes. Cell. 1993:413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Shyu AB, Greenberg ME, Belasco JG. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA dedcay. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40:439–452. doi: 10.1016/s0163-7827(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Wang L, Menon KMJ. Regulation of luteinizing hormone/chorionic gonadotropin receptor messenger ribonucleic acid expression in the rat ovary: Relationship to cholesterol metabolism. Endocrinology. 2005;146:423–431. doi: 10.1210/en.2004-0805. [DOI] [PubMed] [Google Scholar]
- Yang D, Shipman LW, Rossener CA, Scott AI, Sacchettini JC. Structure of the metanococcus jannaschii Mevalonate Kinase, a member of the GHMP kinase superfamily. J Biol Chem. 2002;277:9462–9467. doi: 10.1074/jbc.M110787200. [DOI] [PubMed] [Google Scholar]
- Zang M, Pierce R, Wachi H, Mecham R, Parks W. An Open Reading Frame Element Mediates Posttranscriptional Regulation of Tropoelastin and Responsiveness to Transforming Growth Factor β1. Mol Cell Biol. 1999;19:7314–7326. doi: 10.1128/mcb.19.11.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik A, Midgley A, Jr, Reichert L., Jr Granulosa cell maturation in the rat: increased binding of human chorionic gonadotropin following treatment with follicle-stimulating hormone in vivo. Endocrinology. 1974;95:818–825. doi: 10.1210/endo-95-3-818. [DOI] [PubMed] [Google Scholar]
- Zeleznik A. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31. doi: 10.1186/1477-7827-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Daugherty M, Grishin NV, Osterman AL, Zhang H. Structure and mechanism of homoserine kinase: prototype for the GHMP kinase superfamily. Structure Fold Des. 2000:1247–1257. doi: 10.1016/s0969-2126(00)00533-5. [DOI] [PubMed] [Google Scholar]
- Zhou T, Daugherty M, Grishin NV, Osterman AL, Zhang H. Structure and mechanism of homoserine kinase: prototype for the GHMP kinase superfamily. Structure Fold Des. 2000:1247–1257. doi: 10.1016/s0969-2126(00)00533-5. [DOI] [PubMed] [Google Scholar]