Summary
Targeting proteases and their activators would retard the invasive ability of cancer cells and, has been shown to induce apoptosis in certain instances. Various methods have been developed to specifically target proteases molecules in an attempt to retard invasion and migration. Of these methods, RNA interference (RNAi) holds great therapeutic potential. RNAi technology is now being used to target specific molecules for use as potential anti-cancer agents. RNAi-mediated silencing is almost catalytic when compared to antisense. Of these targets, the uPAR-uPA system and MMPs holds great promise Targeting uPA/uPAR may provide additive or synergistic treatment benefits if used in combination with conventional therapeutics such as chemotherapy or radiation. Studies point to the fact that specifically targeting MMP-9 or MMP-2 singly or in combination with other proteases could have specific therapeutic implications in the treatment of cancer. In this review we discuss the therapeutic potential of siRNA-mediated targeting of uPAR-uPA system and MMPs as therapeutic agents for the treatment of cancer.
Keywords: uPAR, uPA, MMP, RNAi, siRNA
Introduction
By definition, the term “cancer” encompasses a group of diseases in which cells are aggressive, invasive, and/or metastatic. These three malignant properties of cancers differentiate them from benign tumors, which are self-limited in their growth and do not metastasize. Cancers are caused by abnormalities in the genetic material of the transformed cells; cancer-promoting genetic abnormalities may be inherited or randomly acquired through errors in DNA replication. Interactions between carcinogens and the host genome can explain why only some develop cancer after exposure to known carcinogens.
New aspects of the genetics of cancer pathogenesis, such as DNA methylation and microRNAs, are increasingly being recognized as important aspects in cancer development. Genetic abnormalities found in cancer typically affect two general classes of genes: oncogenes and tumor suppressor genes. Oncogenes are often activated in cancer cells and subsequently give those cells new characteristics, such as uncontrolled cell division, apoptotic inhibition, migration and invasion. Tumor suppressor genes are often downregulated or non-functional in cancer cells, thereby resulting in the loss of normal functions in those cells including accurate DNA replication, cell cycle regulation, orientation, tissue adhesion, and interaction with protective cells of the immune system.
The ability of cancer cells to migrate and invade surrounding tissues is mediated by molecular interactions of receptors with ligands and various proteases. The most common of these proteases are metalloproteases and serine proteases. Urokinase plasminogen activator (uPA), which activates plasminogen to plasmin, is the most common of these proteases. Targeting of these proteases and their activators would retard the invasive ability of cancer cells and, in some cases, has been shown to induce apoptosis. Various methods have been developed to specifically target these molecules in an attempt to retard invasion and migration. Of these methods, RNA interference (RNAi) holds great therapeutic potential.
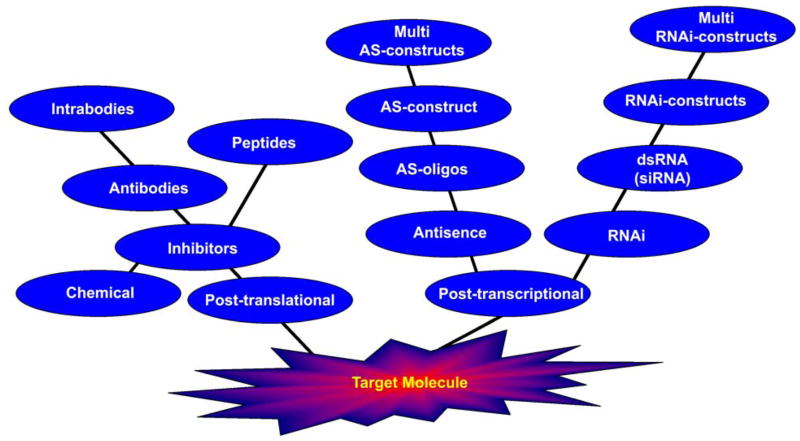
Targeted molecular therapies have evolved to concentrate on either post-transcription or post-translation. Post-translational therapies involve the use of specific chemical inhibitors or antibodies, which block or inhibit the activity of the target molecule. This method interferes only at the final step where the involvement of the target molecule is observed. In contrast, post-transcriptional therapies involve targeting of the molecule before the molecule is formed—in other words, the mRNA of the target molecule. This strategy has evolved from antisense techniques to RNAi and includes the possibility of simultaneously targeting multiple molecules (Fig. 1).
Figure 1. Evolution of targeted molecular therapies.
Two approaches have been used to date: post-translation and post-transcription. The post-translational method deals with direct protein or target molecule inhibitors such as chemical drugs, antibodies and inhibitory peptides. The post-transcriptional method deals with targeting the target molecule precursor mRNA; this method includes antisense and RNAi therapies.
What is RNAi?
RNA interference (RNAi; also called “RNA-mediated interference”) is a mechanism for RNA-guided regulation of gene expression in which double-stranded ribonucleic acid inhibits the expression of genes with complementary nucleotide sequences. Conserved in most eukaryotic organisms, the RNAi pathway is thought to have evolved as a form of innate immunity against viruses. This pathway also plays a major role in regulating development and genome maintenance. The RNAi pathway is initiated by the enzyme dicer, which cleaves double-stranded RNA (dsRNA) to short double-stranded fragments of 20–25 base pairs. One of the two strands of each fragment, known as the guide strand, is then incorporated into the RNA-induced silencing complex (RISC) and base pairs with complementary sequences.
The best-studied outcome of this recognition event is a form of post-transcriptional gene silencing. This occurs when the guide strand base pairs with a messenger RNA (mRNA) molecule and induces degradation of the mRNA by argonaute, the catalytic component of the RISC complex. The short RNA fragments are known as small interfering RNA (siRNA), which are perfectly complementary to the gene to which they are suppressing as they are derived from long dsRNA of that same gene or microRNA (miRNA), which are derived from the intragenic regions or an intron and are thus only partially complementary.
The RNAi pathway has been particularly well studied in certain model organisms such as the nematode worm Caenorhabditis elegans, the fruit fly Drosophila melanogaster, and the flowering plant Arabidopsis thaliana. RNAi technology is only now being used to target specific molecules for use as potential anti-cancer agents. Of these targets, the uPAR system holds great promise.
Advantages of RNAi over antisense therapy
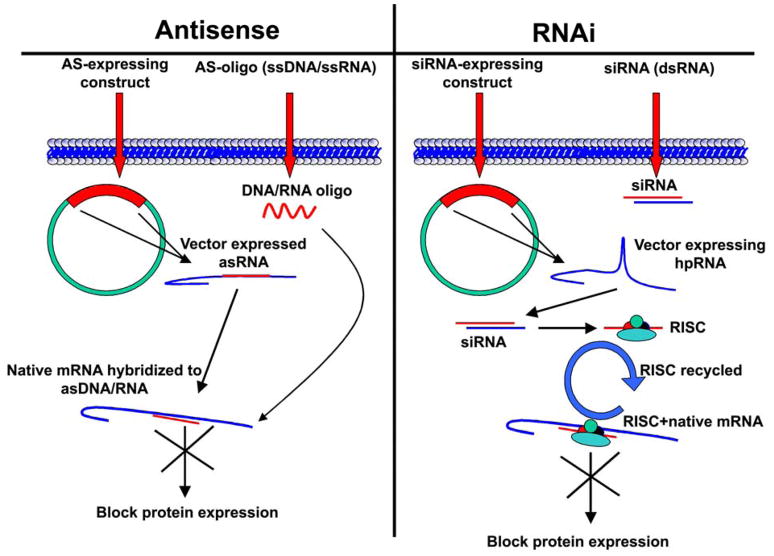
Antisense therapy involves the introduction of single-stranded DNA/RNA complimentary to the target native mRNA in question or introduction of a construct (plasmid or viral) that expresses appropriate RNA sequence complimentary to the target mRNA driven by an appropriate promoter (e.g., CMV). These complimentary DNA/RNA molecules hybridize to the expressed mRNA, thereby blocking the translation of mRNA to proteins. In this case, every molecule of mRNA requires an antisense molecule. Hence, effective silencing is achieved only when equimolar quantities of antisense molecules are present to the corresponding mRNA molecule. In contrast, with RNAi, double-stranded RNA molecules are utilized instead of a single-stranded DNA or RNA molecule. These dsRNA molecules are recycled, thereby effectively silencing multiple mRNA molecules at the same time. RNAi-mediated silencing is almost catalytic when compared to antisense (Fig. 2).
Figure 2. Antisense versus RNAi.
Antisense therapy involves the addition of antisense DNA or RNA molecules to block the target mRNA molecule and involves a 1:1 molar ratio of antisense to mRNA molecules. RNAi involves the use of specific siRNA molecules that complex with proteins to form the RNA-induced silencing complex (RISC). In RNAi, one siRNA molecule is capable of targeting multiple mRNA molecules.
What is the uPA-uPAR system?
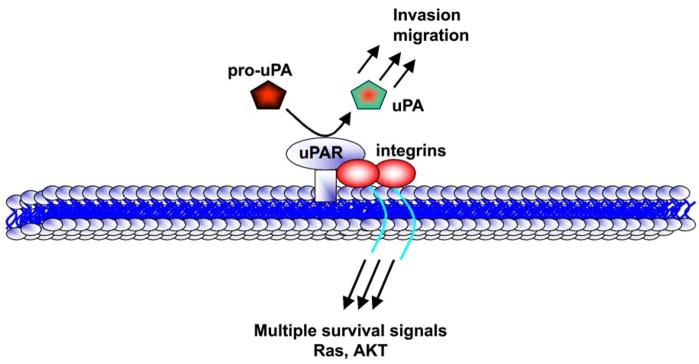
The uPA-uPAR system is involved in a variety of cell functions including extracellular proteolysis, adhesion, proliferation, chemotaxis, neutrophil priming for oxidant production and cytokine release. All of these processes contribute to tumor development, implantation, angiogenesis, inflammation and metastasis (1). The uPA-uPAR system consists of a serine protease uPA, its cell membrane-associated receptor (uPAR), a substrate plasminogen and plasminogen activator inhibitors (PAI-1 and PAI-2) (2, 3). uPA is produced and secreted as an inactive single-chain polypeptide, called pro-uPA, which lacks plasminogen-activating activity. The binding of pro-uPA to uPAR induces its activation (3) which in turn converts plasminogen to the active serine protease plasmin. Plasmin is involved in the degradation of the extracellular matrix (ECM) and basement membranes through direct proteolytic digestion or the activation of other proteases (Fig. 3) including metalloproteases and collagenases. These processes promote tumor invasion and migration (4).
Figure 3. The uPAR system.
The uPAR-uPA system deals with uPA and its receptor uPAR. Pro-uPA is activated to active uPA, which in turn, activates plasminogen to plasmin, thereby activating multiple proteases. uPAR is known to be associated with integrins, which initiate multiple survival intracellular signals in cancer cells.
The binding of uPA to uPAR provides an inducible (Fig. 3), transient and localized cell surface proteolytic activity (5) hereby enabling focused proteolysis of the ECM. Several studies have suggested that uPAR may play a more significant role in the metastatic process (5). Most of the activities of uPA, including its activation by plasmin, are dependent on its binding to uPAR (2, 3). uPAR protein is heavily glycosylated and is covalently linked to the outer layer of the cell membrane via a glycosyl phosphatidylinositol (GPI) anchor with no transmembrane domain (6, 7). uPAR is a >60 kDa glycoprotein, which consists of three cysteine-rich domains (D1, D2 and D3) connected by short linker regions (8). The amino-terminus of the uPAR domain 1 is the primary site for the binding of uPA. However, domains 2 and 3 may be important for high affinity binding of uPA as purified domain 1 has a 1,500-fold lower ligand affinity than the complete tri-domain uPAR (7).
uPAR is a multifunctional protein and is believed to play a role in the regulation of several physiological and pathological conditions that exploit cell adhesion and migration including wound healing, neutrophil recruitment during inflammation as well as tumor invasion and metastasis (8, 9). Several recent studies have shown that the various functions of uPAR are invoked not only by proteolysis but also by intracellular signaling (3, 10–12). uPAR levels have been strongly correlated with metastatic potential and advanced disease as has been demonstrated in tumor samples obtained from patients with colon and breast cancers (13–15). For example, uPAR is overexpressed in invasive breast cancer tissues, but not in normal and benign breast tumors (16). The importance of the uPAR system makes it a potential target for cancer therapy.
Therapeutic RNAi-mediated strategies for targeting the uPAR uPA system
Several approaches have been employed to target uPAR as a means of cancer therapy. These approaches include small molecule and peptide antagonists of the uPA-uPAR interactions as well as the uPAR interactions that are downstream of uPA binding; these include antibiotics, monoclonal antibodies, and antisense technology. To date, antisense technologies used against uPAR include either the classic antisense oligodeoxynucleotides technology, which consists of the injection of antisense DNA strands complementary to uPAR mRNA, or the antisense RNA technology based on cell transfection with a vector capable of expressing the antisense transcript complementary to uPAR mRNA. Research groups investigating antisense RNA technology for downregulation of uPAR in vivo have employed both plasmid and adenovirus constructs for this purpose (17–26).
RNAi has provided new avenues for the treatment of cancer. Small interfering RNAs (siRNAs) are believed to be more potent inhibitors of gene expression with less toxicity (27). Our laboratory had already employed shRNA-based RNAi plasmid system for the downregulation of uPAR in prostate cancer (28), glioma (11, 29–33), and meningioma (34, 35). We have utilized a plasmid construct expressing the same small hairpin RNA (shRNA) to target uPAR. For the above-referenced studies, human glioblastoma cells were intracranially injected into athymic nude mice. Eight to ten days after tumor growth, mice were implanted with mini osmotic pumps with a sustained release of 0.25 μL/h of 150 μg of the shRNA-expressing plasmid construct in a subcutaneous sac with a catheter to the intracranial tumor site. The mice were sacrificed and analyzed at the end of the 5-week follow up period or when the control mice started showing symptoms. We reported a 65% regression of pre-established intracranial tumor growth (23). These findings were further confirmed in our laboratory where we reported a 70% inhibition of pre-established intracranial tumor growth (26). Pulukuri et al. (28) reported that intratumoral injection with a plasmid construct expressing shRNA for uPAR resulted in partial reduction of pre-established orthotopic prostate tumor in athymic male nude mice with no observable secondary tumor.
Downregulation of more than one component involved in tumor invasion and metastasis may possibly have a synergistic or additive effect in impeding tumor dissemination. We have reported that intracranial injection of human glioma cells infected with a bicistronic adenoviral construct capable of simultaneously expressing antisense uPAR and antisense matrix metalloproteinase-9 (MMP-9) resulted in decreased invasiveness and tumorigenicity in mice (26). Further, subcutaneous injections of the bicistronic construct into established tumors caused tumor regression. MMP-9 is involved with metastasis of various types of cancers, though its inhibition has not led to significant improvements in clinical trials as yet. We therefore hypothesized that a dual targeted approach combining MMP-9 downregulation with that of uPAR has the potential for efficient tumor targeting. Indeed, we found that a bicistronic plasmid construct expressing shRNA simultaneously targeting both uPAR and MMP-9 resulted in total regression of pre-established intracerebral tumor growth in mice (Lakka et al., 2005).
We have also shown that RNAi-mediated downregulation of uPAR and cathepsin B reduced glioma cell invasion and angiogenesis in in vivo models (29). In addition, intratumoral injections of these plasmid vectors expressing shRNA for uPAR and cathepsin B resulted in the regression of pre-established intracranial tumor. Similarly, we have also demonstrated that intraperitoneal injection of a bicistronic plasmid construct expressing shRNA for uPA and uPAR caused the regression of pre-established, intracranial tumors in mice (33). Despite our success thus far, it should be noted that the delivery of uPAR downregulation constructs, whether plasmid vectors, adenoviral vectors, or synthetic strands, still needs to be assessed appropriately in human systems.
Homozygous uPAR-deficient mice display normal growth and fertility, do not show histological abnormalities in tissues and do not differ from wild-type mice for spontaneous lysis of experimental pulmonary plasma clot (Dewerchin et al., 1996). This is very similar to what is also noted in uPA-deficient mice (Carmeliet et al., 1994). Thus, the apparent lack of toxicity from inhibiting this proteolytic system makes it an ideal candidate for targeting as a cancer therapeutic agent. The uPA-uPAR plays a very important role in cancer metastasis and may function via a number of signaling pathways. Binding of uPA with its receptor uPAR can activate downstream signaling molecules, including the mitogen-activated protein kinase, signal transducer and activator of transcription (Stat) and the Ras/extracellular signal-regulated kinase pathway, which in turn, lead to cell proliferation, migration, and invasion (3). uPA–uPAR-mediated signaling can upregulate the production of MMPs, which induce ECM degradation and, in turn, tumor invasion and metastasis (3). Since uPA-uPAR and their downstream signaling pathways are implicated in many cancers, including essential cellular functions that contribute to the malignancy of tumor cells, targeting uPA–uPAR-mediated signaling pathways may be promising in the future treatment of metastatic disease. In addition, targeting uPA/uPAR may provide additive or synergistic treatment benefits if used in combination with conventional therapeutics such as chemotherapy or radiation.
Potential for targeting MMPs
Matrix metalloproteinases (MMPs) are a family of structurally related and highly conserved zinc-dependent endopeptidases collectively capable of degrading most components of the basement membrane and ECM. MMP substrates also include a wide variety of proteins, such as chemotactic molecules, adhesion molecules, proteinase inhibitors, cell-surface receptors, blood clotting factors, latent growth factors, and growth factor-binding proteins. Most human MMPs can be divided according to their sequence homology, substrate specificity, and cellular location into the following subclasses: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and others (36). The basic multi-domain structure of MMPs comprises the following: (1) an amino-terminal domain; (2) a catalytic domain; and (3) a carboxy-terminal domain. To date, we know of a minimum of 25 secreted or membrane-bound human MMPs. The expression, secretion, and activity of MMPs in normal tissues are subject to tight control.
Data generated from intensive studies on MMP activities in different cells and tissues, as well as studies from knock-out animals, illustrate the importance of these enzymes in many normal physiologic processes (e.g., embryonic development, bone resorption, angiogenesis, and wound healing) and pathologic processes (rheumatoid arthritis, multiple sclerosis, periodontal disease, and tumor growth and metastasis) (37). MMPs are secreted as pro-MMPs and then activated by sequential cleavage steps (38, 39), which involve the removal of signal peptide and pro-peptide domains or a change in configuration, which activates the enzymes. MMP expression and proteolytic activity are tightly regulated at three stages: gene transcription, pro-enzyme activation and activity of natural inhibitors (tissue inhibitors of metalloproteinase: TIMPs). The balance between production, activation and inhibition prevents excessive proteolysis or inhibition. Several factors like cytokines, growth factors, phorbol esters, cell:cell and cell:matrix interactions are thought to control MMP expression (40). Most MMPs are secreted as inactive zymogens, which may be proteolytically activated by different proteinases such as other MMPs, plasmin, trypsin, chymotrypsin and cathepsins. Several cell types produce MMPs including monocytes, macrophages, neutrophils (41, 42), T-lymphocytes (43), endothelial cells (44), fibroblasts (45) as well as microglia, astrocytes, oligodendrocytes and neurons in the CNS (46–48). In particular, MMP-2 and MMP-9 are secreted by microglia and astrocytes as active forms (49).
MMPs have been shown to regulate tumor cell invasion through their interactions with extracellular matrix components including cell matrix embedded growth factors and cell adhesion molecules (15, 50). The most important of these metalloproteases are MMP-9 and MMP-2, which have shown to be involved in glioma invasion and angiogenesis (51, 52). MMPs are controlled by enzyme activation to produce a functional form and at the level of gene expression (53). There are also other underlying mechanisms that affect mRNA stability, protein secretion, and specific degradation and clearance (53). Growth factors, such as endothelial growth factor (EGF), basic fibroblast growth factor (b-FGF), transforming growth factor (TGF-β1 and β2) and vascular endothelial growth factor (VEGF), have been shown to upregulate MMP-2 and MMP-9 (54). MMP-9 and stromelysin (MMP-3) have been shown to be chiefly transcribed under the influence of various transcription factors commonly found to be involved with cellular stress responses and tissue morphogenesis, including NF-κβ, ETS family members and AP-1 (55). Epidermal growth factor variant subtype III promoted activation of MMP-9, possibly through the activation of MAPK/ERK in glioblastoma (56). We have previously shown that MMP-9 production is induced by cytoskeletal changes involving protein kinase C activation mediated by NF-κβ (57). The mitogen-activated kinase/extracellular signal-regulated kinase (MEK/ERK) signaling pathway is essential for MMP-9 upregulation in astrocytes after PKC induction and TNF-α (cytokine) stimulation. It has been reported that SNB19 cells transfected with dominant negative JNK, MEKK and ERK1 expression vectors decreased MMP-9 expression as well as promoter activity (58). The mt-ERK stable SNB19 cells showed decreased levels of MMP-9 and less invasiveness as compared to parental and vector-transfected stable clones (25). All these studies point to the fact that specifically targeting MMP-9 or MMP-2 singly or in combination with other proteases could have specific therapeutic implications in the treatment of cancer.
RNAi-mediated strategies for targeting MMPs
We have previously demonstrated that inhibition of cathepsin B and MMP-9 gene expression via RNA interference reduced tumor cell invasion, tumor growth and angiogenesis in a glioblastoma cell line (59). We have also demonstrated that specific interference of uPAR and MMP-9 gene expression induced by double-stranded RNA resulted in decreased invasion, tumor growth, and angiogenesis in gliomas (52). Other researchers have silenced MMP-1 to elucidate the mechanism involved in signaling (60). Wyatt et al. concluded that MMP-1 expression is essential for the ability of MDA-231 cells to invade and destroy a collagen matrix. In vivo experiments suggest an important role for MMP-1 in breast tumor growth and have demonstrated the potential of RNAi-mediated targeting of MMP-1 (61). Researchers have also demonstrated that siRNA-mediated blocking of either membrane type-1 MMP (MT1-MMP) or MMP-2 were effective in reducing the hypoxia-induced invasion in MDA-MB-231 and MDA-MB-435 breast carcinoma cell lines (62). Knocking down of MMP-7 by small interfering RNA was shown to suppress lysophosphatidic acid (LPA)-induced invasion in two EOC cell lines (DOV13 and R182). These results show that MMP-7 expression is correlated with EOC invasiveness and LPA-induced MMP-7 secretion/activation and may represent new mechanisms that facilitate ovarian cancer invasion besides the well-known induction of MT1-MMP-mediated pro-MMP-2 activation by LPA (63).
Our studies have reported that the simultaneous targeting of two or more components involved in invasion or migration is significantly more relevant therapeutically than concentrating on one component alone. For example, RNAi-mediated targeting of uPAR and MMP-9 gene expression in the IOMM-lee malignant meningioma cell line inhibited tumor growth, tumor cell invasion and angiogenesis both in vitro and in vivo. Our results show that downregulation of uPAR and MMP-9 leads to a decrease in the activation of some of the important enzymes participating in the MAPK and PI3 kinase pathways, which in turn, might decrease cell survival and proliferation. In addition, we have demonstrated the efficiency of RNAi-mediated targeting of uPAR and MMP-9 in pre-established tumor growth in vivo. We observed a significant regression of pre-established orthotopic tumors upon RNAi-mediated targeting of uPAR and MMP-9 and have also demonstrated that targeting both the proteins simultaneously augmented the therapeutic treatment of human meningiomas (35).
In another study, we introduced small interfering RNA to downregulate the expression of uPAR and MMP-9 in breast cancer cell lines (MDA MB 231 and ZR 75 1). In vitro angiogenesis studies indicated a decrease in the angiogenic and invasive potential of the treated cells. These results suggest a synergistic effect from the simultaneous downregulation of uPAR and MMP-9. We also assessed the levels of phosphorylated forms of MAPK, ERK and AKT signaling pathway molecules and found reduced levels of these molecules in cells treated with the bicistronic construct as compared to the control cells. Furthermore, targeting both uPAR and MMP-9 using RNAi totally regressed orthotopic breast tumors in nude mice, thereby providing evidence that the simultaneous downregulation of uPAR and MMP-9 using RNAi technology may provide an effective tool for breast cancer therapy (64).
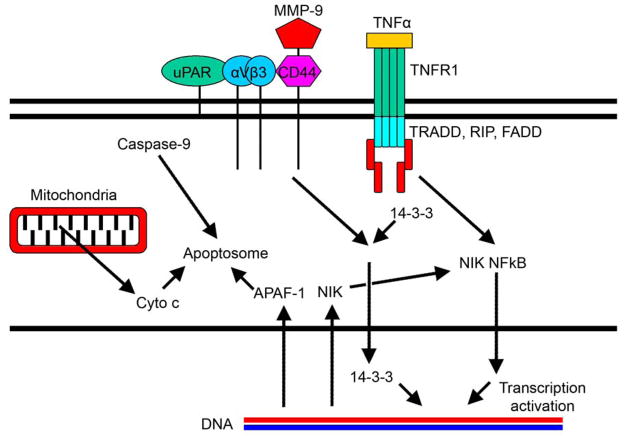
In another study, we have demonstrated that the simultaneous targeting of more than two components is significantly superior to targeting two alone. We have showed that direct intratumoral injections of plasmid DNA expressing hpRNA for uPA, uPAR and MMP-9 significantly regressed pre-established intracranial tumors in nude mice as compared to the controls. In addition, cells treated with RNAi for uPAR, uPA and MMP-9 showed reduced pERK levels when compared to parental and EV/SV-treated SNB19 cells. Our results support the therapeutic potential of RNAi as a method for gene therapy in treating gliomas (30). A brief schematic representation of the possible mechanisms involved in MMP-9 and uPAR targeted RNAi therapy is given in Figure 4.
Figure 4. The uPAR system and its association with MMPs.
The uPAR system and MMPs are involved in multiple intracellular survival and proliferative signaling events.
The main objective of cancer therapy is to arrest tumor invasion and convert it to a controlled, localized disease. Accumulated lines of evidence indicate that MMPs and the uPAR system play an essential role in tumor invasion and metastasis. Therapeutic strategies that can inhibit a broad spectrum of MMPs and the uPAR system may be beneficial for retarding and preventing tumor progression. To achieve this, further investigation and understanding of proteases at the molecular level should play an important role in the future development of new, target-selective treatments. It is sufficiently clear that the simultaneous targeting of multiple systems is more synergistic than additive.
Reference List
- 1.Ge Y, Elghetany MT. Urokinase plasminogen activator receptor (CD87): something old, something new. Lab Hematol. 2003;9:67–71. [PubMed] [Google Scholar]
- 2.Mazar AP, Henkin J, Goldfarb RH. The urokinase plasminogen activator system in cancer: implications for tumor angiogenesis and metastasis. Angiogenesis. 1999;3:15–32. doi: 10.1023/a:1009095825561. [DOI] [PubMed] [Google Scholar]
- 3.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 4.Mazzieri R, Masiero L, Zanetta L, Monea S, Onisto M, Garbisa S, Mignatti P. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 1997;16:2319–2332. doi: 10.1093/emboj/16.9.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y. The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis. Med Res Rev. 2001;21:146–170. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Alfano D, Franco P, Vocca I, Gambi N, Pisa V, Mancini A, Caputi M, Carriero MV, Iaccarino I, Stoppelli MP. The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thromb Haemost. 2005;93:205–211. doi: 10.1160/TH04-09-0592. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y. The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis. Med Res Rev. 2001;21:146–170. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Ploug M. Structure-function relationships in the interaction between the urokinase-type plasminogen activator and its receptor. Curr Pharm Des. 2003;9:1499–1528. doi: 10.2174/1381612033454630. [DOI] [PubMed] [Google Scholar]
- 9.Ploug M. Identification of specific sites involved in ligand binding by photoaffinity labeling of the receptor for the urokinase-type plasminogen activator. Residues located at equivalent positions in uPAR domains I and III participate in the assembly of a composite ligand-binding site. Biochemistry. 1998;37:16494–16505. doi: 10.1021/bi981203r. [DOI] [PubMed] [Google Scholar]
- 10.Gondi CS, Kondraganti S, Dinh D, Olivero W, Gujrati M, Rao JS. RNAi-mediated simultaneous downregulation of uPAR and cathepsin B induces the accumulation of FasL on the cell surface and induces caspase 8-mediated apoptosis in SNB19 human glioma cells. Proceedings 96th American Association for Cancer Research. 2005;46:1269. [Google Scholar]
- 11.Gondi CS, Kandhukuri N, Kondraganti S, Gujrati M, Olivero WC, Dinh DH, Rao JS. Down-regulation of uPAR and cathepsin B retards cofilin dephosphorylation. Int J Oncol. 2006;28:633–639. [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi Y, Lakka SS, Chandrasekar N, Yanamandra N, Gondi CS, Mohanam S, Dinh DH, Olivero WC, Gujrati M, Tamiya T, Ohmoto T, Kouraklis G, Aggarwal B, Rao JS. Down-regulation of integrin alpha(v)beta(3) expression and integrin-mediated signaling in glioma cells by adenovirus-mediated transfer of antisense urokinase-type plasminogen activator receptor (uPAR) and sense p16 genes. J Biol Chem. 2001;276:47171–47177. doi: 10.1074/jbc.M104334200. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre-Ghiso JA, Alonso DF, Farias EF, Gomez DE, Kier Joffe EB. Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur J Biochem. 1999;263:295–304. doi: 10.1046/j.1432-1327.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- 14.Cantero D, Friess H, Deflorin J, Zimmermann A, Brundler MA, Riesle E, Korc M, Buchler MW. Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br J Cancer. 1997;75:388–395. doi: 10.1038/bjc.1997.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger A, Soeltl R, Lutz V, Wilhelm OG, Magdolen V, Rojo EE, Hantzopoulos PA, Graeff H, Gansbacher B, Schmitt M. Reduction of breast carcinoma tumor growth and lung colonization by overexpression of the soluble urokinase-type plasminogen activator receptor (CD87) Cancer Gene Ther. 2000;7:292–299. doi: 10.1038/sj.cgt.7700144. [DOI] [PubMed] [Google Scholar]
- 16.Tan X, Egami H, Nozawa F, Abe M, Baba H. Analysis of the invasion-metastasis mechanism in pancreatic cancer: involvement of plasmin(ogen) cascade proteins in the invasion of pancreatic cancer cells. Int J Oncol. 2006;28:369–374. [PubMed] [Google Scholar]
- 17.Dass CR, Nadesapillai AP, Robin D, Howard ML, Fisher JL, Zhou H, Choong PF. Downregulation of uPAR confirms link in growth and metastasis of osteosarcoma. Clin Exp Metastasis. 2005;22:643–652. doi: 10.1007/s10585-006-9004-3. [DOI] [PubMed] [Google Scholar]
- 18.Dass CR, Choong PF. Biophysical delivery of peptides: Applicability for cancer therapy. Peptides. 2006;27:3479–3488. doi: 10.1016/j.peptides.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y. The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis. Med Res Rev. 2001;21:146–170. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Go Y, Chintala SK, Mohanam S, Gokaslan Z, Venkaiah B, Bjerkvig R, Oka K, Nicolson GL, Sawaya R, Rao JS. Inhibition of in vivo tumorigenicity and invasiveness of a human glioblastoma cell line transfected with antisense uPAR vectors. Clin Exp Metastasis. 1997;15:440–446. doi: 10.1023/a:1018410523635. [DOI] [PubMed] [Google Scholar]
- 21.Kook YH, Adamski J, Zelent A, Ossowski L. The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J. 1994;13:3983–3991. doi: 10.1002/j.1460-2075.1994.tb06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondi CS, Lakka SS, Yanamandra N, Siddique K, Dinh DH, Olivero WC, Gujrati M, Rao JS. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene. 2003;22:5967–5975. doi: 10.1038/sj.onc.1206535. [DOI] [PubMed] [Google Scholar]
- 23.Gondi CS, Lakka SS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Tung CH, Weissleder R, Rao JS. Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Res. 2004;64:4069–4077. doi: 10.1158/0008-5472.CAN-04-1243. [DOI] [PubMed] [Google Scholar]
- 24.Lakka SS, Rajagopal R, Rajan MK, Mohan PM, Adachi Y, Dinh DH, Olivero WC, Gujrati M, Ali-Osman F, Roth JA, Yung WK, Kyritsis AP, Rao JS. Adenovirus-mediated antisense urokinase-type plasminogen activator receptor gene transfer reduces tumor cell invasion and metastasis in non-small cell lung cancer cell lines. Clin Cancer Res. 2001;7:1087–1093. [PubMed] [Google Scholar]
- 25.Lakka SS, Rajan M, Gondi CS, Yanamandra N, Chandrasekar N, Jasti SL, Adachi Y, Siddique K, Gujrati M, Olivero W, Dinh DH, Kouraklis G, Kyritsis AP, Rao JS. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene. 2002;21:8011–8019. doi: 10.1038/sj.onc.1205894. [DOI] [PubMed] [Google Scholar]
- 26.Lakka SS, Gondi CS, Yanamandra N, Dinh DH, Olivero WC, Gujrati M, Rao JS. Synergistic down-regulation of urokinase plasminogen activator receptor and matrix metalloproteinase-9 in SNB19 glioblastoma cells efficiently inhibits glioma cell invasion, angiogenesis, and tumor growth. Cancer Res. 2003;63:2454–2461. [PubMed] [Google Scholar]
- 27.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 28.Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA Interference-directed Knockdown of Urokinase Plasminogen Activator and Urokinase Plasminogen Activator Receptor Inhibits Prostate Cancer Cell Invasion, Survival, and Tumorigenicity in Vivo. J Biol Chem. 2005;280:36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23:8486–8496. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 30.Gondi CS, Lakka SS, Dinh D, Olivero W, Gujrati M, Rao JS. Downregulation of uPA, uPAR and MMP-9 using small, interfering, hairpin RNA (siRNA) inhibits glioma cell invasion, angiogenesis and tumor growth. Neuron Glia Biology. 2004;1:165–176. doi: 10.1017/s1740925x04000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gondi CS, Kandhukuri N, Kondraganti S, Gujrati M, Olivero WC, Dinh DH, Rao JS. RNA interference-mediated simultaneous down-regulation of urokinase-type plasminogen activator receptor and cathepsin B induces caspase-8-mediated apoptosis in SNB19 human glioma cells. Mol Cancer Ther. 2006;5:3197–3208. doi: 10.1158/1535-7163.MCT-05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol. 2007;31:19–27. [PMC free article] [PubMed] [Google Scholar]
- 33.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Intraperitoneal injection of an hpRNA-expressing plasmid targeting uPAR and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res. 2007;13:4051–4060. doi: 10.1158/1078-0432.CCR-06-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondraganti S, Gondi CS, McCutcheon I, Dinh DH, Gujrati M, Rao JS, Olivero W. RNAi-mediated downregulation of urokinase plasminogen activator and its receptor in human meningioma cells inhibits tumor invasion and growth. Int J Oncol. 2006;28:1353–1360. [PMC free article] [PubMed] [Google Scholar]
- 35.Tummalapalli P, Gondi CS, Dinh DH, Gujrati M, Rao JS. RNA interference-mediated targeting of urokinase plasminogen activator receptor and matrix metalloproteinase-9 gene expression in the IOMM-Lee malignant meningioma cell line inhibits tumor growth, tumor cell invasion and angiogenesis. Int J Oncol. 2007;31:5–17. [PMC free article] [PubMed] [Google Scholar]
- 36.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 37.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108:1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 38.Murphy G, Knauper V, Cowell S, Hembry R, Stanton H, Butler G, Freije J, Pendas AM, Lopez-Otin C. Evaluation of some newer matrix metalloproteinases. Ann NY Acad Sci. 1999;878:25–39. doi: 10.1111/j.1749-6632.1999.tb07672.x. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro SD, Fliszar CJ, Broekelmann TJ, Mecham RP, Senior RM, Welgus HG. Activation of the 92-kDa gelatinase by stromelysin and 4-aminophenylmercuric acetate. Differential processing and stabilization of the carboxyl-terminal domain by tissue inhibitor of metalloproteinases (TIMP) J Biol Chem. 1995;270:6351–6356. doi: 10.1074/jbc.270.11.6351. [DOI] [PubMed] [Google Scholar]
- 40.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 41.Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Dano K. 92 kDa type IV collagenase (MMP–9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer. 1996;65:57–62. doi: 10.1002/(SICI)1097-0215(19960103)65:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 42.Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990;86:1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995;154:4379–4389. [PubMed] [Google Scholar]
- 44.Haas TL, Madri JA. Extracellular matrix-driven matrix metalloproteinase production in endothelial cells: implications for angiogenesis. Trends Cardiovasc Med. 1999;9:70–77. doi: 10.1016/s1050-1738(99)00014-6. [DOI] [PubMed] [Google Scholar]
- 45.Unemori EN, Bair MJ, Bauer EA, Amento EP. Stromelysin expression regulates collagenase activation in human fibroblasts. Dissociable control of two metalloproteinases by interferon-gamma. J Biol Chem. 1991;266:23477–23482. [PubMed] [Google Scholar]
- 46.Gottschall PE, Deb S. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. Neuroimmunomodulation. 1996;3:69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- 47.Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Uhm JH, Dooley NP, Oh LY, Yong VW. Oligodendrocytes utilize a matrix metalloproteinase, MMP-9, to extend processes along an astrocyte extracellular matrix. Glia. 1998;22:53–63. doi: 10.1002/(sici)1098-1136(199801)22:1<53::aid-glia5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100:103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 50.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 51.Lakka SS, Rao JS. Role and regulation of proteases in human glioma. In: Lendeckel U, Hooper NM, editors. Proteases in the Brain. Springer; New York: 2005. pp. 151–177. [Google Scholar]
- 52.Lakka SS, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao VH, Sioka C, Rao JS. Specific interference of uPAR and MMP-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth and angiogenesis in gliomas. J Biol Chem. 2005;280:21882–21892. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 53.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rooprai HK, Rucklidge GJ, Panou C, Pilkington GJ. The effects of exogenous growth factors on matrix metalloproteinase secretion by human brain tumour cells. Br J Cancer. 2000;82:52–55. doi: 10.1054/bjoc.1999.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VanMeter TE, Rooprai HK, Kibble MM, Fillmore HL, Broaddus WC, Pilkington GJ. The role of matrix metalloproteinase genes in glioma invasion: co-dependent and interactive proteolysis. J Neurooncol. 2001;53:213–235. doi: 10.1023/a:1012280925031. [DOI] [PubMed] [Google Scholar]
- 56.Choe G, Park JK, Jouben-Steele L, Kremen TJ, Liau LM, Vinters HV, Cloughesy TF, Mischel PS. Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin Cancer Res. 2002;8:2894–2901. [PubMed] [Google Scholar]
- 57.Chintala SK, Sawaya R, Aggarwal BB, Majumder S, Giri DK, Kyritsis AP, Gokaslan ZL, Rao JS. Induction of matrix metalloproteinase-9 requires a polymerized actin cytoskeleton in human malignant glioma cells. J Biol Chem. 1998;273:13545–13551. doi: 10.1074/jbc.273.22.13545. [DOI] [PubMed] [Google Scholar]
- 58.Lakka SS, Jasti SL, Kyritsis AP, Yung WK, Ali-Osman F, Nicolson GL, Rao JS. Regulation of MMP-9 (type IV collagenase) production and invasiveness in gliomas by the extracellular signal-regulated kinase and jun amino-terminal kinase signaling cascades. Clin Exp Metastasis. 2000;18:245–252. doi: 10.1023/a:1006724826083. [DOI] [PubMed] [Google Scholar]
- 59.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 60.Yuan J, Dutton CM, Scully SP. RNAi mediated MMP-1 silencing inhibits human chondrosarcoma invasion. J Orthop Res. 2005;23:1467–1474. doi: 10.1016/j.orthres.2005.04.004.1100230633. [DOI] [PubMed] [Google Scholar]
- 61.Wyatt CA, Geoghegan JC, Brinckerhoff CE. Short hairpin RNA-mediated inhibition of matrix metalloproteinase-1 in MDA-231 cells: effects on matrix destruction and tumor growth. Cancer Res. 2005;65:11101–11108. doi: 10.1158/0008-5472.CAN-05-2446. [DOI] [PubMed] [Google Scholar]
- 62.Munoz-Najar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25:2379–2392. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 63.Wang FQ, Smicun Y, Calluzzo N, Fishman DA. Inhibition of matrilysin expression by antisense or RNA interference decreases lysophosphatidic acid-induced epithelial ovarian cancer invasion. Mol Cancer Res. 2006;4:831–841. doi: 10.1158/1541-7786.MCR-06-0153. [DOI] [PubMed] [Google Scholar]
- 64.Kunigal S, Lakka SS, Gondi CS, Estes N, Rao JS. RNAi-mediated downregulation of urokinase plasminogen activator receptor and matrix metalloprotease-9 in human breast cancer cells results in decreased tumor invasion, angiogenesis and growth. Int J Cancer. 2007;121:2307–2316. doi: 10.1002/ijc.22962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]