Summary
To understand the genetics of sleep apnea, we evaluated the relationship between the apnea hypopnea index (AHI) and body mass index (BMI) through linkage analysis to identify genetic loci that may influence AHI and BMI jointly and AHI independent of BMI.
Haseman-Elston sibling regression was conducted on AHI, AHI adjusted for BMI and BMI in African-American and European-American pedigrees. A comparison of the magnitude of linkage peaks was used to assess the relationship between AHI and BMI. In EAs, the strongest evidence for linkage to AHI was on 6q23-25 and 10q24-q25, both decreasing after BMI adjustment, suggesting loci with pleiotropic effects. Also, a promising area of linkage to AHI but not BMI was observed on 6p11-q11 near the orexin-2 receptor, suggesting BMI independent pathways. In AAs the strongest evidence of linkage for AHI after adjusting for BMI was on chromosome 8p21.3 with linkage increasing after BMI adjustment and on 8q24.1 with linkage decreasing after BMI adjustment. Novel linkage peaks were also observed in AAs to both BMI and AHI on chromosome 13 near the serotonin-2a receptor. These analyses suggest genetic loci for sleep apnea that operate both independently of BMI and through BMI-related pathways.
Keywords: sleep apnea, body mass index, linkage (genetics)
Introduction
Obstructive sleep apnea (OSA) is a complex disorder characterized by the repeated collapse of the pharyngeal airway during sleep and is associated with snoring, frequent cortical arousals, overnight hypoxemia and daytime hypersomnolence. The major defining metric of OSA is the apnea hypopnea index (AHI) which averages the number of complete and partial airway occlusions that occur hourly during sleep. With a 9 to 24% prevalence in U.S. adults for mild-moderate cases (defined by an AHI ≥ 5) and 2-9% for moderate cases (defined by an AHI ≥ 15), OSA and its co-morbidities (obesity, hypertension, and diabetes) are a high public health burden (Young et al. 2002). Increasing evidence suggests a genetic basis for OSA (Patel & Tishler 2007); however, it is unclear which genetic variants for OSA overlap those for obesity, a factor associated with a 4 to 10 fold increased risk of OSA (Redline & Patel 2007).
Preliminary linkage analyses of OSA have been reported on individuals from the Cleveland Family Study (CFS), a cohort of families selected with and without index cases of sleep apnea. Given a strong association between the AHI and body mass index (BMI), linkage findings were reported for each trait unadjusted and adjusted for the other trait (Palmer et al. 2003;Palmer et al. 2004). Given the modest sample size analyzed and the overall absence of other published linkage data for AHI, we have now included additional family members previously not genotyped and additional new families to double the sample size. This type of analysis is called a confirmation study, because new family members are added to existing families, thus improving familial informativity for linkage with better estimates of allele sharing. Linkage peaks are identified that are common to AHI and BMI, as well as those that are unique to each trait.
The relationship between underlying quantitative trait loci (QTLs) for AHI and BMI was explored through linkage analysis, identifying linkage peaks for AHI, AHI-adjusted for BMI, and BMI. A comparison of the relative peak heights can provide insight into the potential independence (coincident linkage) of two or more underlying QTLs or pleiotropic actions of a single putative gene under each of these peaks. The simultaneous presence of linkage peaks for both BMI and AHI (whether or not the one trait is adjusted for the other) may suggest that an underlying QTL acts on both traits, reflecting pleiotropy. In addition, when adjusting a phenotype for the other trait, such as AHI for BMI, results in no reduction in linkage evidence, this can be interpreted as providing evidence for an underlying QTL acting uniquely on that phenotype. Increased evidence for linkage may result when the covariate adjustment reduces the residual error of the model and improves power to detect linkage in scenarios where an underlying QTL for one trait (e.g., AHI) is not associated with the other trait (e.g., BMI) (Schaid et al. 2003;Zeegers et al. 2004). The latter also may occur when there is heterogeneity in the phenotype, and the adjusted trait reduces this heterogeneity.
The presence of a linkage peak for just one phenotype, such as for BMI in the absence of linkage to the AHI, may suggest that the study is underpowered to detect linkage for the latter trait, perhaps due to measurement error, inappropriate method of analysis, lack of sample size or, more simply, the original BMI peak is a false positive. When one peak is substantially reduced by adjustment for the other covariate, it is impossible to determine whether an underlying QTL is acting uniquely on one trait, or whether the one trait operates in the causal pathway for the other phenotype. For example, a decrease in the linkage peak for a model where AHI is adjusted for BMI may occur when the genetic loci influences BMI, and BMI is in the causal pathway for apnea development.
Methods
Cleveland Family Study
The CFS consists of 2534 individuals, of which 46% were African Americans (AAs), representing 356 families studied on up to 4 occasions over a period of 16 years. Participants were selected from either index or control families as described previously (Redline et al. 1995) and summarized in the online supplement.
Phenotyping
Phenotype exams before the year 2000 were conducted in the homes of study participants, with AHI measured by overnight unattended limited channel polysomnography (Edentrace; Eden Prairie, MN). Study visits after 2000 were conducted in a dedicated facility with AHI determined by 14-channel attended overnight polysomnography (Compumedics E series, Abottsville, AU). In a subsample of 169 study participants, it has been demonstrated that the AHI measured in this manner correlates closely with the AHI derived from the home monitoring approach (intraclass correlation = 0.83) (Redline et al. 2003). Potential phenotypes were obtained from longitudinal AHI measurements using mean, lowest, highest, as well as other longitudinally derived indices. As a result, preliminary heritability analyses were conducted to identify the phenotype that demonstrated the most consistent level of heritability. Based on these analyses (online supplement), we used the lowest AHI as the sleep apnea phenotype. BMI was defined as weight (kg) divided by height-squared (m2) and derived from the same examination as the AHI.
Genotyping and pooling of data
Genotyping data were previously obtained on 592 individuals by the Marshfield Mammalian Genotyping Services with marker screening set 10 containing 375 autosomal microsatellites with an average spacing of 9.1 cM. The results of the linkage scans of these data were previously published (Palmer et al. 2003;Palmer et al. 2004). This analysis includes data from an additional 715 individuals who were later genotyped using Marshfield screening set 13 (including 382 autosomal microsatellites of a similar average spacing and average heterozygosity of 76.1%). The combined data set consists of 634 individuals from 128 AA families and 641 individuals from 109 European American (EA) families. Details related to combining data across the two screening sets and error checking procedures are available in the online supplement.
The proportion of alleles shared identical by descent at each marker and at 2 cM intervals was computed with GENIBD (S.A.G.E. 2006), assuming the Haldane mapping function, for use in Haseman-Elston (HE) regression on the pooled sample. Estimates of the allele frequencies were calculated by maximum likelihood using the FREQ program in S.A.G.E.
Statistical methods
Using SAS (v.9.13), the traits log(AHI +1) and BMI were adjusted separately for the covariates age, age2, and age*sex. Additionally, AHI was adjusted for BMI and non- linear functions of BMI (BMI, BMI2, (BMI)½, BMI3, and log(BMI)) to fully remove any relationship between AHI and BMI. . Residuals were analyzed with HE regression, using a weighted average of the squared sib mean-corrected residual sum and the squared sib difference, applying the best linear unbiased predictor for each sibship for the mean correction. Both full and half sibling pairs were used in analyses of the AAs, while analyses of the EA sample were limited to only full sibling pairs owing to the small number of half sibling pairs. To calculate empirical p-values (pe), permutation testing on full siblings was conducted by permuting up to 100,000 replicates within and across sibships of the same size for any nominal p-value (pn) < 0.001.
Results
Sample Characteristics
More AA individuals, but fewer EA individuals, were genotyped in the second genome scan compared to the first genome scan (Table 1). The addition of new family members resulted in larger families for linkage analysis, evidenced by the larger mean pedigree sizes in both races for the pooled sample. Four hundred twenty-nine AA individuals contributed to the 273 full sibling pairs and 135 half sibling pairs.
Table 1.
Pedigree information from the Cleveland Family Study on individuals with genotype data
| AAs | EAs | |||||
|---|---|---|---|---|---|---|
| Genome Scan 1 | Genome Scan 2 | Pooled* | Genome Scan 1 | Genome Scan 2 | Pooled* | |
| Individuals: | ||||||
| N | 251 | 402 | 634 | 341 | 313 | 641 |
| % of individuals from proband families: | 89% | 89% | 89% | 83% | 68% | 76% |
| Pedigrees: N | 58 | 101 | 128 | 65 | 80 | 109 |
| Mean Size ± S.D. | 4.3 ± 1.9 | 3.8 ± 2.3 | 5.0 ± 2.6 | 5.2 ± 3.0 | 3.9 ± 2.7 | 5.9 ± 3.7 |
| (min,max) | (2,10) | (1,13) | (2,13) | (1,14) | (1,17) | (2,23) |
| Relative Pairs N: | ||||||
| Sibling: | 101 | 131 | 273 | 225 | 143 | 410 |
| Half sib | 38 | 85 | 135 | 7 | 7 | 19 |
The pooled data exclude individuals with missing phenotype information
The sample characteristics are shown in Table 2. The AHI when untransformed was highly skewed, ranging from no events to 134 events per hour in AAs. The mean BMI at the time of the lowest measurement of AHI was 29.7. Approximately 50% of the AA sample was obese, defined as a BMI > 30. Four hundred thirteen EA individuals were analyzed, providing 410 sibling pairs. The EAs were approximately the same ages as the AAs (33.3 years in EAs vs. 31.6 years in AAs) and had a slightly lower mean BMI.
Table 2.
Characteristics of individuals included in Haseman-Elston regression
| African Americans | European Americans | |||
|---|---|---|---|---|
| Value | Range | Value | Range | |
| AHI,geometric mean, (SD) | 5.5 (3.5) | 4.5 (3.4) | ||
| Untransformed | ||||
| AHI, median, (IQR*) | 3.1 (1.0-11.8) | 0-133.8 | 1.9 (0.8,9.5) | 0-174.8 |
| Age (y), mean (SD) | 31.6 (17.1) | 2.6-80.5 | 33.3 (18.2)) | 3.1-83.6 |
| BMI (kg/m2), mean (SD) | 29.7 (9.3) | 12.7-61.6 | 27.8 (8.4) | 13.8-62.5 |
IQR = Interquartile Range
Linkage Results, European Americans
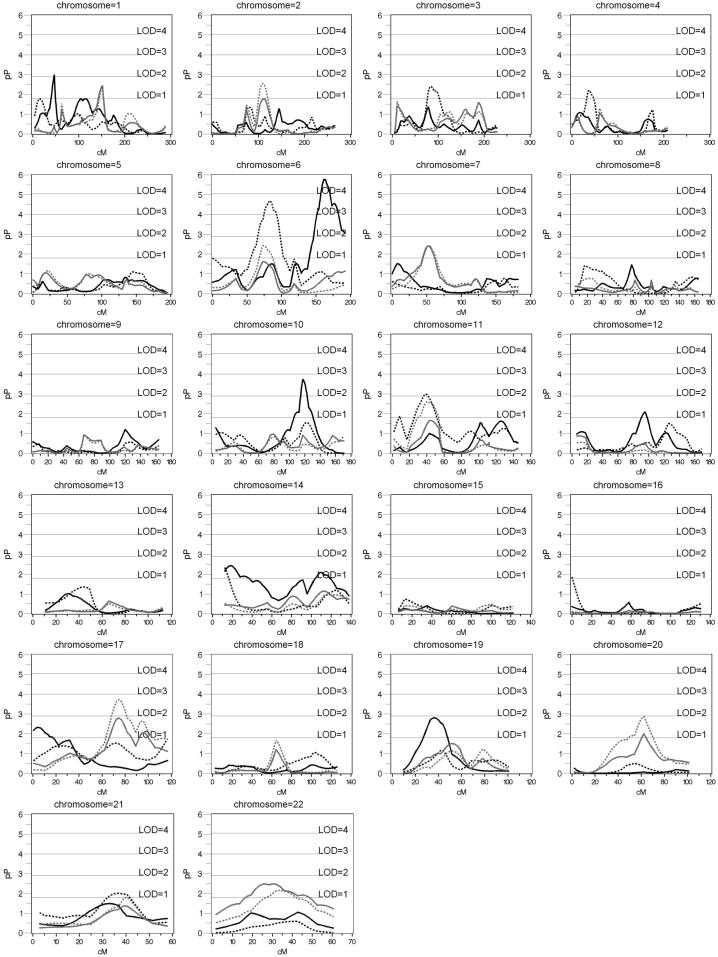
The highest linkage peaks for AHI adjusting only for age and sex were seen on chromosomes 1 at 46 cM (pn=0.0012; LOD=2.0), 6 at 162 cM (pn=<.00001; LOD=4.7), 10 at 122 cM (pn=0.00019; LOD=2.7), and 19 at 35 cM (pn=.00155; LOD=1.9). Adjusting for BMI led to a decrease in the significance of these linkage peaks: to pn=0.13085, for chromosome 1; pn=.09 for chromosome 6 (see Table 3); pn=0.01334 for chromosome 10; and pn= 0.02900 at 39 cM for chromosome 19. None of these AHI peaks had concomitant BMI peaks (p < .001) (Figure 1). Empirical p-values and their LOD scores are presented for nominal p-values < 0.001 in Table 3.
Table 3.
Empirical p-values for AHI and BMI in EAs, calculated for loci with nominal p-values < .001
| AHI | BMI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AHI | BMI | ||||||||
| adjusted for BMI(5) | Adjusted for AHI | ||||||||
| Chr | cM | Nominal | Empirical | Nominal | Empirical | Nominal | Empirical | Nominal | Empirical |
| p-value | p-value | p-value | p-value | p-value | p-value | p-value | p-value | ||
| (corresponding LOD) | |||||||||
| ** BMI(5) = multiple terms of BMI | |||||||||
| 6 | 80.4 | 0.04352 | - | 0.00003 | 0.00005 | 0.03317 | - | 0.03317 | - |
| (0.6) | (3.5) | (3.3) | (0.7) | ||||||
| 6 | 162 | 0.000002 | 0.00002 | 0.09494 | - | 0.17314 | - | 0.73447 | - |
| (4.7) | (3.7) | (0.4) | (0.2) | ||||||
| 10 | 118.3 | 0.00020 | 0.00050 | 0.03945 | - | 0.13155 | - | 0.36026 | - |
| (2.7) | (2.4) | (0.7) | (.3) | ||||||
| 17 | 74.6 | 0.48657 | - | 0.00159 | - | 0.03231 | - | 0.00019 | 0.00071 |
| (0) | (1.9) | (.7) | (2.7) | (2.2) | |||||
Figure 1.
Haseman-Elston Regression of AHI and BMI in European Americans. Black solid line: AHI adjusted for age, age2, sex, and age*sex, black dotted: AHI adjusted for age, age2, sex, age*sex, and multiple terms of BMI; gray solid line: BMI adjusted for age, age2, sex, and age*sex; gray dotted: BMI adjusted for age, age2, sex, age*sex, and AHI
With BMI adjustment, including consideration of multiple BMI terms, additional evidence of linkage was observed on chromosomes 6 and 11. For example, on chromosome 6 (80.4 cM) the significance of the Haseman-Elston test statistic with supplementary BMI covariates included increased to empirical p= 0.00005 (LOD = 3.3), compared to AHI unadjusted for BMI (pn = .04) or adjusted for only a single term of BMI (pn=0.00112; LOD=2.0). Similarly, on chromosome 11 at 38 cM a marked increase in linkage evidence was observed after fully adjusting for BMI (pn=0.00104 LOD=2.1) when adjusting for multiple terms of BMI instead of just a single BMI term (pn=0.006; LOD=1.4) or unadjusted (pn=0.2). LOD scores for BMI adjusting for age and sex did not exceed 2.0. However, adjusting BMI for AHI resulted in a higher linkage peak on chromosome 17 at 74.6 cM (pn= .00159; LOD=1.9 increasing to pn=0.00019; LOD=2.7).
Linkage Results, African Americans
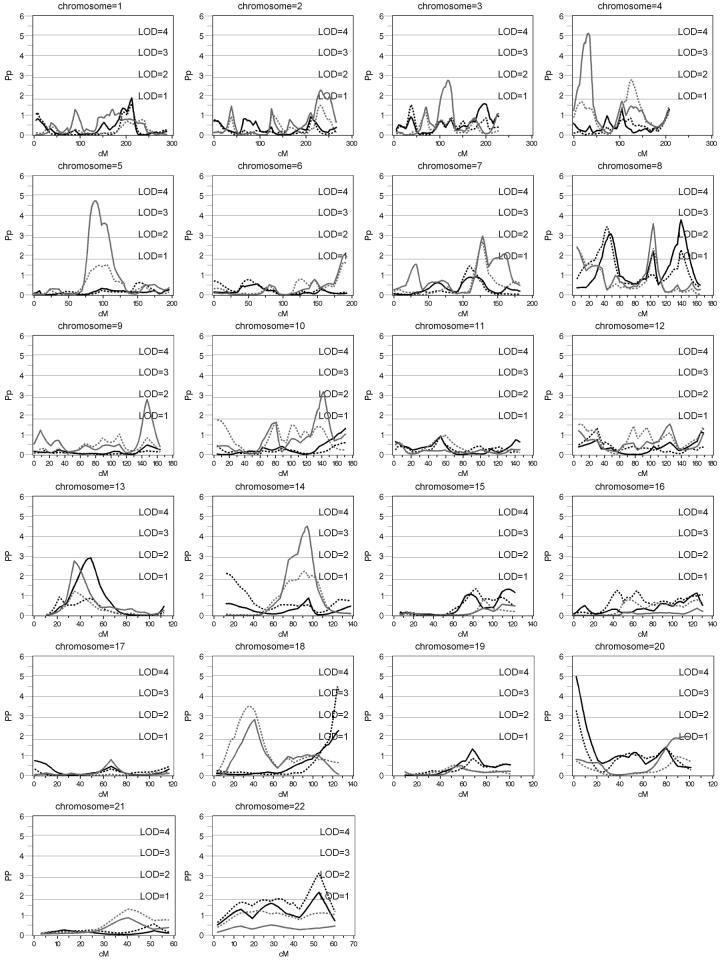
Linkage peaks for AHI unadjusted for BMI were observed on chromosome 8 at 45 cM (pn=0.00082; LOD=2.2), 100 cM (pn=0.00688; LOD=1.31) and 140 cM (pn=0.00016; LOD=2.8), chromosome 13 at 49 cM (pn = 0.00124; LOD=2.0) and the telomeric end of the p-arm of chromosome 20 (pn=0.00001; LOD=3.9). Linear adjustment of AHI for BMI decreased the evidence for linkage on chromosome 8 at 100 (pn=0.09547; LOD=0.4) and 140 cM (pn=0.0054; LOD=1.4), but not at 43 cM. At this site the linkage peak slightly increased (pn=0.00036; LOD=2.5). Linkage evidence decreased slightly to pn=0.00054; LOD=2.3. Increased significance with BMI adjustment was demonstrated at chromosome 18 at 126 cM (pn=0.00003, LOD=3.5), and at chromosome 22 at 52 cM (pn=0.00072, LOD=2.2). Linkage evidence on chromosome 13 decreased (pn=0.14) with BMI adjustment. Evidence for linkage for AHI did not substantively change with inclusion of additional covariate terms for BMI (squared and interaction terms) from that observed with a single linear term for BMI.
Age and sex adjusted BMI demonstrated the highest linkage peaks on chromosome 4 at 30 cM (pn=0.00001; LOD=4.1), chromosome 5 at 88 cm (pn=0.00002; LOD=3.7), chromosome 8 at 100 cM (pn=0.00026; LOD=2.6), chromosome 10 at 139 cM (pn=0.00068; LOD=2.2), and chromosome 14 at 94 cM (pn=0.00003; LOD=3.5) (Figure 2). A peak for BMI was shifted 14 cM away from the AHI peak on chromosome 13 (pn=0.0018; LOD=1.8) at 35 cM. Adjusting BMI for AHI resulted in increased evidence for linkage on chromosome 18 at ∼ 40 cM from pn= 0.00154 (LOD=1.9) to pn= 0.00033 (LOD=2.5).
Figure 2.
Haseman-Elston Regression of AHI and BMI in African Americans. Black solid line: AHI adjusted for age, age2, sex, and age*sex; black dotted: AHI adjusted for age, age2, sex, age*sex, and BMI; gray solid line: BMI adjusted for age, age2, sex, and age*sex; gray dotted: BMI adjusted for age, age2, sex, age*sex, and AHI
Empirical p-values obtained by permutation testing of full sibling pairs alone (because it is not possible to perform an appropriate permutation test for both types of sibs together) reduced the evidence for linkage because of the loss of the information contained in the half-sibling pairs, which comprise approximately 50% of the sample. Linkage to AHI, adjusted for age and sex, nevertheless remained significant at the 0.001 level on chromosomes 8 (138 cM) and 20 (0 cM). Adjusting AHI for BMI increased evidence for linkage (p<.001) on chromosomes 8 at 44 cM, 18 at 132 cM, and 22 at 52 cM.
Discussion
This analysis uses linkage techniques to tease apart genes for AHI that are independent from BMI from genes that operate jointly for BMI by examining the relative magnitude of AHI peaks before and after BMI adjustment and in the presence or absence of BMI linkage. In each racial group, these analyses identified several linkage peaks that were unique to each trait, as well as others that suggested underlying QTL likely to harbor variants common to both traits. Table 4 summarizes the linkage peaks from this analysis (p< 0.001) and points to other genome-wide association and linkage studies that offer similar results primarily for obesity traits, but also website only results for AHI adjusted for BMI, found in publicly available Framingham SNP Health Association Resource data (SHARe) on dbGAP (see http://www.ncbi.nlm.nih.gov/projects/gap/cgibin/study.cgi?study_id=phs000007.v1.p1 (Cupples et al. 2007)). Genes were identified if they fell within 10 cM each way of the linkage peak, with the interval length based on a simulation study that found most linkage peaks to be within a total 20 cM region of a true simulated disease locus (Cordell 2001).
Table 4.
Summary of replicated linkage peaks and candidate genes within 10 cM of observed linkage peaks
| Region | cM* | Nearest Marker | Published Linkage Studies for BMI | Published Association Studies** | Other Hypothesized Candidate Genes |
|---|---|---|---|---|---|
| Bold indicates candidate genes / SNPs hypothesized for sleep apnea phenotype; otherwise candidate genes refer to obesity related traits, per se. | |||||
| Centimorgans were interpolated using an integrated map (Duffy 2006) | |||||
| ACE = angiotensin I converting enzyme; ADRA2A= adrenergic, α-2A receptor; ADRB1 = adrenergic, β1 receptor; AHI = apnea hypopnea index; BMI = body mass index; CART= CART prepropeptide; CCKAR= cholecystokinin A receptor; CRHR1 = corticotropin releasing hormone receptor 1; ESR1 = estrogen receptor 1; FBAT = family based association test; FGFR1= fibroblast growth factor receptor 1; FGFR2= fibroblast growth factor receptor 2; FTO= fat mass and obesity associated; HCRTR2 = hypocretin receptor 2; HLOD = Heterogeneity logarithm of the odds of linkage; HTR1B = 5-hydroxytryptamine (serotonin) receptor 1B; IDE = insulin-degrading enzyme; LOD = logarithm of the odds of linkage; LPL = lipoprotein lipase; MCHR1 = melanin-concentrating hormone receptor 1; MLS = maximum logarithm of the odds of linkage; NPL = nonparametric linkage; PPARA = peroxisome proliferator-activated receptor α; PPARGC1A = peroxisome proliferator-activated receptor γ coactivator 1α; SGK = serum/glucocorticoid regulated kinase 1; SORBS1 = sorbin and SH3 domain containing 1; TSHR = thyroid stimulating hormone receptor isoform 1 | |||||
| European | |||||
| Americans | |||||
| 6q11-q13 | 84 | GATA64D02 | - | COL21A1 (Fox et al. 2007: BMI FBAT p = 5.1*10-6) | HCRTR2 |
| HTR1B (Levitan et al. 2001: minimum BMI p=.001) | |||||
| 6q26 | 162 | AFM242ZG5 | Feitosa et al. 2002, (MLS=1.6) | ESR1 (Mansur et al. 2005: BMI p=.024; Fox et al. 2007: BMI, p<.05) | |
| Atwood et al. 2002, 2002, (LOD=3.1) | VIP (Fox et al. 2007: BMI p=.001) rs4869783** (Cupples et al. 2007: AHI p= 9.4*10-6) | ||||
| 10q24 | 117 | GATA64A09 | IDE (Gu et al. 2004: BMI p=.0067) | ||
| SORBS1 (San Millan et al. 2004: BMI p=0.008) | |||||
| 17q21 | 75 | GATA49C09N | Norris et al. 2005, | CRHR1 (Challis et al. 2004: BMI p=0.0083) | |
| (MLS=2.8) | ACE (Kramer et al. 2005: 2005 obesity p<0.05) | ||||
| African Americans | |||||
| 4p15.3 | 32 | GATA70E01 | Arya et al. 2004, (LOD=4.5) | CCKAR (Funakoshi et al. 2000: BMI p=.08, Leptin: p=0.003) | |
| PPARGC1A (Pihlajamaki et al. 2005: | |||||
| Stone et al. 2002, (HLOD=2.2) | BMI p=.03; (Esterbauer et al. 2002: BMI p=0.006) | ||||
| 5q14 | 88 | GATA52A12 | CART (Yamada et al. 2002: BMI: p=.034) | ||
| 8p21.3 | 43 | AFMA127YE5 | Stone et al. 2002, (HLOD=2.0) | LPL (Corella et al. 2001: BMI: p=.02) | |
| 8q23.1 | 104 | GATA8B01 | Dong et al. 2005, (NPL=1.9) | rs10504675** (Cupples et al. 2007: AHI p= 3.79*10-5) | FGFR1 |
| 8q24.1 | 140 | GATA21C12 | |||
| 10q24-q25 | 143 | ATA29C03 | Chen et al. 2005, (LOD=1.0) | SGK (Dieter et al. 2004: BMI p<0.008) | |
| Bell et al. 2004, (LOD=2.5) | ADRB1 (Linne et al. 2005: BMI p=.05; Fox et al. 2007: BMI FBAT p=0.004; Lima et al. 2007: BMI p=.02) | FGFR2 | |||
| Turner et al. 2004, (LOD=2.0) | ADRA2A (Garenc et al. 2002: fat deposition p < 0.05; Lima et al. 2007: BMI p=0.05) | ||||
| 14q31 | 94 | GATA193A07 | Wu et al. 2002, (LOD=2.2) | TSHR (Hinney et al. 2007: obesity p=1.38*10-4) | |
| 18q23 | 125 | Z18QTEL11 | - | - | |
| 20p11.2 | 4 | AFM077XD3 | - | - | |
| 22q13 | 52 | UT7136 | - | MCHR1 (Bell et al. 2005: obesity p=.006) | |
| PPARA (Bosse et al. 2003: BMI p=.023) | |||||
data available on dbGAP website only
In each race, several loci appeared to influence AHI but not BMI; i.e., a concomitant linkage peak for BMI was not observed and/or evidence for linkage to AHI did not change, or increased, after BMI adjustment. In this regard, an area of interest in EAs is on chromosome 6 at 72 cM where a maximum lod score of 3.5 (for the fully BMI adjusted AHI trait) was observed. Although the region on chromosome 6p11-6q11 likely harbors many genes, it includes the gene for the hypocretin/orexin-2 receptor (HCRTR2/OXR2), which is part of a biologic system involved in appetite regulation, sleep/wake control, and the hypothalamic control of circulation (Ehmke & Just 2003;Taylor & Samson 2003). HCRTR2/OXR2 knockout mice show abnormal regulation of sleep/wake transition similar to human narcolepsy (Willie et al. 2003). Sleep and ventilatory phenotypes may interact, as evidenced by the findings from prepro-orexin knockout mice, which demonstrate a sleep/wake dependent influence of orexin on the control of breathing (Nakamura et al. 2007). Since orexin neurons also project to the dorsal raphe nuclei and pre-Botzinger complex, areas in the brainstem that control ventilation(Peyron et al. 1998;Young et al. 2005) as well as to hypoglossal neurons (which innervate the major upper airway dilator muscle)(Fung et al. 2001;Peever et al. 2003), it is plausible that orexins influence propensity for sleep apnea through effects on ventilatory phenotypes and upper airway neuromuscular function. Although the role of orexins in sleep apnea has not yet been defined, OSA patients have been reported to have low levels of orexin A that improved upon initiation of CPAP therapy (Sakurai et al. 2004;Sakurai et al. 2005).
The area in AAs that showed the strongest evidence for linkage for AHI independent of BMI is on chromosome 8 at 44 cM. The LOD score increased from 2.0 to 2.4 after adjusting for BMI. This genetic region represents a novel linkage peak, without known candidate genes nearby.
A number of linkage peaks were also observed that appear to influence both AHI and BMI. In AAs, linkage peaks for both AHI and BMI were observed on chromosome 13 near the serotonin 2a receptor (HTR2A). Although the linkage peaks did not reach the threshold of p < 0.001 for inclusion in table 4, this gene is of particular interest given its putative roles in control of both respiration and obesity. Furthermore, a meta-analysis of 37 BMI linkage studies identified this region as one of few areas suggestive for linkage (Saunders et al. 2007). Because OSA resolves completely during wakefulness, neurotransmitters that stimulate upper airway dilator muscles during sleep are implicated (Kubin et al. 1998). Evidence exists of the importance of serotonin in the role of sleep/wake control of airway patency (Veasey 2003). The pharmacology of serotonin involves multiple receptor subtypes, including HTR2A, which can cause direct excitatory effects on the control of respiration. Reductions in serotonin activation of the dilator motor neurons of the airway are believed to occur during sleep, contributing to the loss of upper airway patency and obstructive episodes. Abnormalities in brainstem serotonin activity also have been demonstrated in immunopathological studies of victims of sudden infant death syndrome (Kinney 2005), which may occur as a result of failed ventilatory protective responses in young and stressed infants and which may co-aggregate with OSA (Tishler et al. 1996). Moreover, in animal models microperfusion of serotonin into the hypoglossal area enhances Non-REM airway dilator muscle activity, which is usually reduced in sleep (Jelev et al. 2001). Two association analyses have examined the functional variants of the HTR2A receptor and OSA with conflicting results (Bayazit et al. 2006;Sakai et al. 2005). In Ay mice (dominant for ectopic expression of agouti protein), obesity is associated with increased HTR2A mRNA gene expression over that in wild-type mice, while pharmacologic inactivation of HTR2A decreases overeating and weight gain (Nonogaki et al. 2006).
In several chromosomal regions, it is unclear whether the linkage signals represent QTLs that are unique to or pleiotropic for AHI and BMI. In EAs, the telomeric end of the q arm of chromosome 6 shows strong evidence for linkage to AHI that is reduced after adjusting for BMI (LOD=4.7 decreasing to 0.4). This reduction in the linkage peak may suggest that a putative QTL is not acting independently of BMI, despite the lack of linkage evidence for BMI. The Framingham 100K SHARe reported a significant association between rs4869783 and AHI adjusted for BMI in this chromosome 6q region as well p=9.4*10−6 (Cupples et al. 2007) Region 17q also suggests actions due to pleiotropic QTL in EA. While ACE was listed as a candidate gene in table 4, there was no significant association between the ACE polymorphism and AHI or BMI in the Cleveland Family Study (Patel et al. 2007).
Particularly in AAs, there appear to be several strong linkage peaks for BMI on 4p15.3-15.2, 5q13-15, 10q24-25 and 14q23.The peaks decreased upon adjustment for AHI, suggesting that candidate genes in these areas may also influence AHI or that the BMI peaks represent false positive findings. Candidate genes identified from the 2005 obesity map (Rankinen et al. 2006) and other association studies are listed in table 4, although not all of the associations have been replicated in other studies. . Linkage peaks observed on the tail ends of chromosome 18 and 20 are noteworthy; however, the absence of flanking markers limits the ability to accurately estimate multipoint IBD allele sharing.
Because genotyping data of individuals from overlapping families were combined into one analysis, the results of the study could not be analyzed as a separate replication study of the initial published scans (Palmer et al. 2003;Palmer et al. 2004). Although the published results used different AHI measurements and different analytic techniques, there are some important consistencies. In AAs, the peak at 104 cM on chromosome 8p12-8p11.2 for BMI retained the same relative magnitude, approximately LOD=2.5, but in the current linkage analysis there is now an additional modest peak for AHI. This is consistent with a pleiotropic QTL for both BMI and AHI, the first genome scan being underpowered to detect the genetic effect on AHI. Adjusting each trait for the other reduced the magnitude of the linkage peak, further supporting the hypothesis of pleiotropy. Evidence for linkage to BMI alone, in the absence of linkage to AHI, remained consistent between the first genome and the pooled genome scan for the region 2q35-2q37.1. The linkage signal at 14p12 (12 cM) in the first genome scan for AHI adjusted for BMI was also strengthened with the larger sample, from LOD=.05 to LOD=2.1.
Other areas identified on the initial scan were not confirmed using a larger pooled sample. The relatively modest size of the linkage peaks in the first genome scan may not have represented true linkage. Alternatively, if the added families were heterogeneous with respect to disease, it is possible that this could cause the loss of evidence for linkage as the sample size was increased. In particular, as compared with the first genome scan, the second genome scan included a larger proportion of EA families recruited through individuals without known sleep apnea rather than through probands with laboratory diagnosed sleep apnea. The addition of these families to the pooled dataset may have diluted the QTL effects observed in several areas, including the initial area on chromosome 2 near the pro-opio-melanocortin gene, and the area on chromosome 19 at 70 cM near the Apolipoprotein E (APOE) gene. We suggest this based on an additional fine-mapping study of 9 microsatellites (average spacing: 2 cM) localized to the APOE region in which increased evidence was found for linkage to AHI adjusted for BMI, suggesting that the region is not a false positive, although APOE was excluded as a causative locus (Larkin et al. 2006). This seemingly contradictory finding highlights the fact that genetic heterogeneity can reduce power to detect genes in linkage and candidate gene studies.
In the original genome scan on EAs, a very modest peak for BMI on chromosome 10q23-q24 at 118 cM was observed (LOD=1.87) that dropped to 0.60 after adjusting for AHI, in the absence of a linkage peak for AHI (Palmer et al. 2003), while the current analyses show stronger evidence in this region to AHI. Two candidate genes there are in tight linkage disequilibrium, ADRB1 and ADRA2A (Hoehe et al. 1995), for which there is evidence of co-regulation and crosstalk in tissues where they are expressed together (Xu et al. 2003). There have been conflicting studies on the relationship between the Arg-389-Gly allele of the ADRB1 gene and measures of adiposity, (Dionne et al. 2002;Ellsworth et al. 2005;Linne et al. 2005;Rasmussen et al. 2005;Ryden et al. 2001;Terra et al. 2005).
A limitation of this study and many genetic studies is the lack of agreement between results for EAs and AAs. A measure of average marker information content for siblings, defined by Kruglyak and Lander in 1995 (54% EA vs. 55% AA) (Kruglyak & Lander 1995), does not explain the lack of concordance observed in the results. Likely explanations include: genetic heterogeneity of disease, differences in QTL allele frequency, population specific gene by environment interactions, or different phenotypic presentation of disease, particularly sleep apnea. Also, several genome-wide association studies of obesity have identified genes that were not observed in this study, including fat mass and obesity associated (FTO), insulin induced gene 2 (INSIG2), melanocortin 4 receptor (MC4R), catenin β-like 1 CTNNBL1 and phosphofructokinase, platelet (PFKP) (Herbert et al. 2006;Hinney et al. 2007;Liu et al. 2008;Loos et al. 2008;Scuteri et al. 2007).
In summary, our analyses highlight the potential utility of jointly analyzing an outcome (e.g., AHI) and a major risk factor (e.g., BMI) as initial steps in dissecting genetic pathways important for each trait. For most linkage peaks, adjusting AHI for BMI, and vice versa, resulted in decreased linkage peaks, pointing to scenarios where it was not possible to tease apart AHI from BMI. However, other analyses identified areas where linkage evidence increased following adjusting AHI for BMI, pointing to areas where putative genes may influence one trait independently of the other. The current linkage results, which represent the largest study to date of linkage for sleep apnea, indicate several promising areas to pursue for further understanding the genetics of OSA, including the region on chromosome 6, which harbors the orexin-2 receptor, a gene well recognized for its role in animal models of narcolepsy in EAs. In AAs, evidence suggests the presence of a QTL for AHI on chromosome 13, where there is a candidate gene for serotonin receptor 2a that may influence both obesity and OSA. However, these observations will require additional corroborating or replicating evidence, including fine mapping and candidate gene association studies, as well as use of appropriate animal models to further identify genetic control of these complex phenotypes. All linkage areas harbor many other genes as well, and it is possible that genetic association studies will implicate additional genes in these as well as other areas. Use of linkage findings, such as these, may be used is such future genetic association studies to help weight the significance of association tests (Chen & Witte 2007).
Acknowledgements
Genotyping was conducted by the NHLBI's Marshfield Mammalian Genotyping Services. We are indebted to Joan Aylor, Kathryn Clark, Jennifer Frame, Rawan Salem and Heather Rogers for their dedication to this study. We also gratefully acknowledge the participants of the Cleveland Family Study, who have been very generous with their time. We thank Dr. Jill Kilanowski for editorial comments and Robert Goodloe for help with the tables. Some of the results of this paper were obtained by using the software package S.A.G.E., which is supported by a U.S. Public Health Service Resource Grant from the National Center for Research. This work was supported by grants: T32-HL07567, RO1HL46380, RO1GM28356, RR03655, GCRC R-949-02-0130, and KL2-RR024990.
This work was supported by grants: T32-HL07567, RO1HL46380, RO1GM28356, RR03655, GCRC R-949-02-0130, and KL2-RR024990
Abbreviations
- AHI
apnea hypopnea index
- BMI
body mass index
- AA
African Americans
- EA
European Americans
- OSA
obstructive sleep apnea
- LOD
logarithm of the odds of linkage
- pP
-log(p-value)
- QTL(s)
quantitative trait locus (loci)
Footnotes
Disclosure: The authors report no conflicts of interest.
Supplementary Material
References
- Arya R, Duggirala R, Jenkinson CP, Almasy L, Blangero J, O'Connell P, Stern MP. Evidence of a novel quantitative-trait locus for obesity on chromosome 4p in Mexican Americans. Am.J.Hum.Genet. 2004;74(2):272–282. doi: 10.1086/381717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood LD, Heard-Costa NL, Cupples LA, Jaquish CE, Wilson PW, D'Agostino RB. Genomewide linkage analysis of body mass index across 28 years of the Framingham Heart Study. Am.J.Hum.Genet. 2002;71(5):1044–1050. doi: 10.1086/343822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayazit YA, Yilmaz M, Ciftci T, Erdal E, Kokturk O, Gokdogan T, Kemaloglu YK, Inal E. Association of the -1438G/A polymorphism of the 5-HT2A receptor gene with obstructive sleep apnea syndrome. ORL J.Otorhinolaryngol. Relat Spec. 2006;68(3):123–128. doi: 10.1159/000091216. [DOI] [PubMed] [Google Scholar]
- Bell CG, Benzinou M, Siddiq A, Lecoeur C, Dina C, Lemainque A, Clement K, Basdevant A, Guy-Grand B, Mein CA, Meyre D, Froguel P. Genome-wide linkage analysis for severe obesity in french caucasians finds significant susceptibility locus on chromosome 19q. Diabetes. 2004;53(7):1857–1865. doi: 10.2337/diabetes.53.7.1857. [DOI] [PubMed] [Google Scholar]
- Bell CG, Meyre D, Samson C, Boyle C, Lecoeur C, Tauber M, Jouret B, Jaquet D, Levy-Marchal C, Charles MA, Weill J, Gibson F, Mein CA, Froguel P, Walley AJ. Association of melanin-concentrating hormone receptor 1 5′ polymorphism with early-onset extreme obesity. Diabetes. 2005;54(10):3049–3055. doi: 10.2337/diabetes.54.10.3049. [DOI] [PubMed] [Google Scholar]
- Bosse Y, Despres JP, Bouchard C, Perusse L, Vohl MC. The peroxisome proliferator-activated receptor alpha L162V mutation is associated with reduced adiposity. Obes.Res. 2003;11(7):809–816. doi: 10.1038/oby.2003.112. [DOI] [PubMed] [Google Scholar]
- Challis BG, Luan J, Keogh J, Wareham NJ, Farooqi IS, O'Rahilly S. Genetic variation in the corticotrophin-releasing factor receptors: identification of single-nucleotide polymorphisms and association studies with obesity in UK Caucasians. Int.J.Obes.Relat Metab Disord. 2004;28(3):442–446. doi: 10.1038/sj.ijo.0802564. [DOI] [PubMed] [Google Scholar]
- Chen G, Adeyemo AA, Johnson T, Zhou J, Amoah A, Owusu S, Acheampong J, Agyenim-Boateng K, Eghan BA, Oli J, Okafor G, Abbiyesuku F, Dunston GM, Chen Y, Collins F, Rotimi C. A genome-wide scan for quantitative trait loci linked to obesity phenotypes among West Africans. Int.J.Obes.(Lond) 2005;29(3):255–259. doi: 10.1038/sj.ijo.0802873. [DOI] [PubMed] [Google Scholar]
- Chen GK, Witte JS. Enriching the analysis of genomewide association studies with hierarchical modeling. Am.J.Hum.Genet. 2007;81(2):397–404. doi: 10.1086/519794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell HJ. Sample size requirements to control for stochastic variation in magnitude and location of allele-sharing linkage statistics in affected sibling pairs. Ann.Hum.Genet. 2001;65(Pt 5):491–502. doi: 10.1017/S0003480001008831. [DOI] [PubMed] [Google Scholar]
- Corella D, Guillen M, Portoles O, Sorli JV, Alonso V, Folch J, Saiz C. Gender specific associations of the Trp64Arg mutation in the beta3-adrenergic receptor gene with obesity-related phenotypes in a Mediterranean population: interaction with a common lipoprotein lipase gene variation. J.Intern.Med. 2001;250(4):348–360. doi: 10.1046/j.1365-2796.2001.00894.x. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Arruda HT, Benjamin EJ, D'Agostino RB, Sr., Demissie S, DeStefano AL, Dupuis J, Falls KM, Fox CS, Gottlieb DJ, Govindaraju DR, Guo CY, Heard-Costa NL, Hwang SJ, Kathiresan S, Kiel DP, Laramie JM, Larson MG, Levy D, Liu CY, Lunetta KL, Mailman MD, Manning AK, Meigs JB, Murabito JM, Newton-Cheh C, O'Connor GT, O'Donnell CJ, Pandey M, Seshadri S, Vasan RS, Wang ZY, Wilk JB, Wolf PA, Yang Q, Atwood LD. The Framingham Heart Study 100K SNP genome-wide association study resource: overview of 17 phenotype working group reports. BMC Med.Genet. 2007;8(Suppl):1S1. doi: 10.1186/1471-2350-8-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes.Res. 2004;12(5):862–870. doi: 10.1038/oby.2004.104. [DOI] [PubMed] [Google Scholar]
- Dionne IJ, Garant MJ, Nolan AA, Pollin TI, Lewis DG, Shuldiner AR, Poehlman ET. Association between obesity and a polymorphism in the beta(1)-adrenoceptor gene (Gly389Arg ADRB1) in Caucasian women. Int.J.Obes.Relat Metab Disord. 2002;26(5):633–639. doi: 10.1038/sj.ijo.0801971. [DOI] [PubMed] [Google Scholar]
- Dong C, Li WD, Li D, Price RA. Interaction between obesitysusceptibility loci in chromosome regions 2p25-p24 and 13q13-q21. Eur.J.Hum.Genet. 2005;13(1):102–108. doi: 10.1038/sj.ejhg.5201292. [DOI] [PubMed] [Google Scholar]
- Duffy DL. An integrated genetic map for linkage analysis. Behav.Genet. 2006;36(1):4–6. doi: 10.1007/s10519-005-9015-x. [DOI] [PubMed] [Google Scholar]
- Ehmke H, Just A. The orexins: linking circulatory control with behavior. Am.J.Physiol Regul.Integr.Comp Physiol. 2003;285(3):R519–R521. doi: 10.1152/ajpregu.00311.2003. [DOI] [PubMed] [Google Scholar]
- Ellsworth DL, Coady SA, Chen W, Srinivasan SR, Boerwinkle E, Berenson GS. Interactive effects between polymorphisms in the beta-adrenergic receptors and longitudinal changes in obesity. Obes.Res. 2005;13(3):519–526. doi: 10.1038/oby.2005.55. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Oberkofler H, Linnemayr V, Iglseder B, Hedegger M, Wolfsgruber P, Paulweber B, Fastner G, Krempler F, Patsch W. Peroxisome proliferator-activated receptor-gamma coactivator-1 gene locus: associations with obesity indices in middle-aged women. Diabetes. 2002;51(4):1281–1286. doi: 10.2337/diabetes.51.4.1281. [DOI] [PubMed] [Google Scholar]
- Feitosa MF, Borecki IB, Rich SS, Arnett DK, Sholinsky P, Myers RH, Leppert M, Province MA. Quantitative-trait loci influencing bodymass index reside on chromosomes 7 and 13: the National Heart, Lung, and Blood Institute Family Heart Study. Am.J.Hum.Genet. 2002;70(1):72–82. doi: 10.1086/338144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CS, Heard-Costa N, Cupples LA, Dupuis J, Vasan RS, Atwood LD. Genome-wide association to body mass index and waist circumference: the Framingham Heart Study 100K project. BMC Med.Genet. 2007;8(Suppl):1S18. doi: 10.1186/1471-2350-8-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi A, Miyasaka K, Matsumoto H, Yamamori S, Takiguchi S, Kataoka K, Takata Y, Matsusue K, Kono A, Shimokata H. Gene structure of human cholecystokinin (CCK) type-A receptor: body fat content is related to CCK type-A receptor gene promoter polymorphism. FEBS Lett. 2000;466(23):264–266. doi: 10.1016/s0014-5793(00)01080-2. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res. 2001;903(12):257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- Garenc C, Perusse L, Chagnon YC, Rankinen T, Gagnon J, Borecki IB, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. The alpha 2-adrenergic receptor gene and body fat content and distribution: the HERITAGE Family Study. Mol.Med. 2002;8(2):88–94. [PMC free article] [PubMed] [Google Scholar]
- Gu HF, Efendic S, Nordman S, Ostenson CG, Brismar K, Brookes AJ, Prince JA. Quantitative trait loci near the insulin-degrading enzyme (IDE) gene contribute to variation in plasma insulin levels. Diabetes. 2004;53(8):2137–2142. doi: 10.2337/diabetes.53.8.2137. [DOI] [PubMed] [Google Scholar]
- Herbert A, Gerry NP, McQueen MB, Heid IM, Pfeufer A, Illig T, Wichmann HE, Meitinger T, Hunter D, Hu FB, Colditz G, Hinney A, Hebebrand J, Koberwitz K, Zhu X, Cooper R, Ardlie K, Lyon H, Hirschhorn JN, Laird NM, Lenburg ME, Lange C, Christman MF. A common genetic variant is associated with adult and childhood obesity. Science. 2006;312(5771):279–283. doi: 10.1126/science.1124779. [DOI] [PubMed] [Google Scholar]
- Hinney A, Nguyen TT, Scherag A, Friedel S, Bronner G, Muller TD, Grallert H, Illig T, Wichmann HE, Rief W, Schafer H, Hebebrand J. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS.ONE. 2007;2(12):e1361. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehe MR, Otterud B, Hsieh WT, Martinez MM, Stauffer D, Holik J, Berrettini WH, Byerley WF, Gershon ES, Lalouel JM. Genetic mapping of adrenergic receptor genes in humans. J.Mol.Med. 1995;73(6):299–306. doi: 10.1007/BF00231616. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J.Physiol. 2001;532(Pt 2):467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr.Dev.Pathol. 2005;8(5):507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Kramer H, Wu X, Kan D, Luke A, Zhu X, Adeyemo A, McKenzie C, Cooper R. Angiotensin-converting enzyme gene polymorphisms and obesity: an examination of three black populations. Obes.Res. 2005;13(5):823–828. doi: 10.1038/oby.2005.94. [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Lander ES. Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am.J.Hum.Genet. 1995;57(2):439–454. [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack AI. Control of Upper Airway Motoneurons During REM Sleep. News Physiol Sci. 1998:1391–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Larkin EK, Patel SR, Redline S, Mignot E, Elston RC, Hallmayer J. Apolipoprotein E and obstructive sleep apnea: evaluating whether a candidate gene explains a linkage peak. Genet.Epidemiol. 2006;30(2):101–110. doi: 10.1002/gepi.20127. [DOI] [PubMed] [Google Scholar]
- Levitan RD, Kaplan AS, Masellis M, Basile VS, Walker ML, Lipson N, Siegel GI, Woodside DB, Macciardi FM, Kennedy SH, Kennedy JL. Polymorphism of the serotonin 5-HT1B receptor gene (HTR1B) associated with minimum lifetime body mass index in women with bulimia nervosa. Biol.Psychiatry. 2001;50(8):640–643. doi: 10.1016/s0006-3223(01)01201-x. [DOI] [PubMed] [Google Scholar]
- Lima JJ, Feng H, Duckworth L, Wang J, Sylvester JE, Kissoon N, Garg H. Association analyses of adrenergic receptor polymorphisms with obesity and metabolic alterations. Metabolism. 2007;56(6):757–765. doi: 10.1016/j.metabol.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linne Y, Dahlman I, Hoffstedt J. beta1-Adrenoceptor gene polymorphism predicts long-term changes in body weight. Int.J.Obes.(Lond) 2005;29(5):458–462. doi: 10.1038/sj.ijo.0802892. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Liu XG, Wang L, Dina C, Yan H, Liu JF, Levy S, Papasian CJ, Drees BM, Hamilton JJ, Meyre D, Delplanque J, Pei YF, Zhang L, Recker RR, Froguel P, Deng HW. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum.Mol.Genet. 2008;17(12):1803–1813. doi: 10.1093/hmg/ddn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf F, I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O'Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M, Vogel CI, Wallace C, Waterworth DM, Weedon MN, Willer CJ, Wraight, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Jacobs KB, Chanock SJ, Hayes RB, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat.Genet. 2008;40(6):768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur AP, Nogueira CC, Strunz CM, Aldrighi JM, Ramires JA. Genetic polymorphisms of estrogen receptors in patients with premature coronary artery disease. Arch.Med.Res. 2005;36(5):511–517. doi: 10.1016/j.arcmed.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J.Appl.Physiol. 2007;102(1):241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Oka Y. Increased hypothalamic 5-HT2A receptor gene expression and effects of pharmacologic 5-HT2A receptor inactivation in obese Ay mice. Biochem.Biophys.Res.Commun. 2006;351(4):1078–1082. doi: 10.1016/j.bbrc.2006.10.173. [DOI] [PubMed] [Google Scholar]
- Norris JM, Langefeld CD, Scherzinger AL, Rich SS, Bookman E, Beck SR, Saad MF, Haffner SM, Bergman RN, Bowden DW, Wagenknecht LE. Quantitative trait loci for abdominal fat and BMI in Hispanic-Americans and African-Americans: the IRAS Family study. Int.J.Obes.(Lond) 2005;29(1):67–77. doi: 10.1038/sj.ijo.0802793. [DOI] [PubMed] [Google Scholar]
- Palmer LJ, Buxbaum SG, Larkin E, Patel SR, Elston RC, Tishler PV, Redline S. A whole-genome scan for obstructive sleep apnea and obesity. Am.J.Hum.Genet. 2003;72(2):340–350. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LJ, Buxbaum SG, Larkin EK, Patel SR, Elston RC, Tishler PV, Redline S. Whole genome scan for obstructive sleep apnea and obesity in african-american families. Am.J.Respir.Crit Care Med. 2004;169(12):1314–1321. doi: 10.1164/rccm.200304-493OC. [DOI] [PubMed] [Google Scholar]
- Patel SR, Larkin EK, Mignot E, Lin L, Redline S. The association of angiotensin converting enzyme (ACE) polymorphisms with sleep apnea and hypertension. Sleep. 2007;30(4):531–533. doi: 10.1093/sleep/30.4.531. [DOI] [PubMed] [Google Scholar]
- Patel SR, Tishler P. Familial and genetic factors. In: Kushida CA, editor. Obstructive Sleep Apnea: Pathophysiology, Comorbidities, and Consequences. Informa Healthcare; New York: 2007. [Google Scholar]
- Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. Journal of Neurophysiology. 2003;89(5):2591–2600. doi: 10.1152/jn.00968.2002. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J.Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki J, Kinnunen M, Ruotsalainen E, Salmenniemi U, Vauhkonen I, Kuulasmaa T, Kainulainen S, Laakso M. Haplotypes of PPARGC1A are associated with glucose tolerance, body mass index and insulin sensitivity in offspring of patients with type 2 diabetes. Diabetologia. 2005;48(7):1331–1334. doi: 10.1007/s00125-005-1800-9. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Perusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity.(Silver.Spring) 2006;14(4):529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Rasmussen M, Belza A, Almdal T, Toubro S, Bratholm P, Astrup A, Christensen NJ. Change in beta1-adrenergic receptor protein concentration in adipose tissue correlates with diet-induced weight loss. Clin.Sci.(Lond) 2005;108(4):323–329. doi: 10.1042/CS20040238. [DOI] [PubMed] [Google Scholar]
- Redline S, Patel SR. Genetics of Obstructive Sleep Apnea: Evidence for a Role of Variation in Ventilatory Control. In: Gaultier C, editor. Genetics of Respiratory Control Disorders. 2007. [Google Scholar]
- Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26(6):703–709. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
- Redline S, Tishler PV, Tosteson TD, Williamson J, Kump K, Browner I, Ferrette V, Krejci P. The familial aggregation of obstructive sleep apnea. Am.J.Respir.Crit Care Med. 1995;151(3 Pt 1):682–687. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- Ryden M, Hoffstedt J, Eriksson P, Bringman S, Arner P. The Arg 389 Gly beta1-adrenergic receptor gene polymorphism and human fat cell lipolysis. Int.J.Obes.Relat Metab Disord. 2001;25(11):1599–1603. doi: 10.1038/sj.ijo.0801815. [DOI] [PubMed] [Google Scholar]
- Sakai K, Takada T, Nakayama H, Kubota Y, Nakamata M, Satoh M, Suzuki E, Akazawa K, Gejyo F. Serotonin-2A and 2C receptor gene polymorphisms in Japanese patients with obstructive sleep apnea. Intern.Med. 2005;44(9):928–933. doi: 10.2169/internalmedicine.44.928. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Nishijima T, Arihara Z, Takahashi K. Plasma orexin-A levels in obstructive sleep apnea-hypopnea syndrome. Chest. 2004;125(5):1963–1964. doi: 10.1378/chest.125.5.1963. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Nishijima T, Takahashi S, Yamauchi K, Arihara Z, Takahashi K. Low plasma orexin-A levels were improved by continuous positive airway pressure treatment in patients with severe obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127(3):731–737. doi: 10.1378/chest.127.3.731. [DOI] [PubMed] [Google Scholar]
- San Millan JL, Corton M, Villuendas G, Sancho J, Peral B, Escobar-Morreale HF. Association of the polycystic ovary syndrome with genomic variants related to insulin resistance, type 2 diabetes mellitus, and obesity. J.Clin.Endocrinol.Metab. 2004;89(6):2640–2646. doi: 10.1210/jc.2003-031252. [DOI] [PubMed] [Google Scholar]
- Saunders CL, Chiodini BD, Sham P, Lewis CM, Abkevich V, Adeyemo AA, de Andrade M, Arya R, Berenson GS, Blangero J, Boehnke M, Borecki IB, Chagnon YC, Chen W, Comuzzie AG, Deng HW, Duggirala R, Feitosa MF, Froguel P, Hanson RL, Hebebrand J, Huezo-Dias P, Kissebah AH, Li W, Luke A, Martin LJ, Nash M, Ohman M, Palmer LJ, Peltonen L, Perola M, Price RA, Redline S, Srinivasan SR, Stern MP, Stone S, Stringham H, Turner S, Wijmenga C, Collier A. Meta-analysis of genome-wide linkage studies in BMI and obesity. Obesity.(Silver.Spring) 2007;15(9):2263–2275. doi: 10.1038/oby.2007.269. [DOI] [PubMed] [Google Scholar]
- Schaid DJ, Olson JM, Gauderman WJ, Elston RC. Regression models for linkage: issues of traits, covariates, heterogeneity, and interaction. Hum.Hered. 2003;55(23):86–96. doi: 10.1159/000072313. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S, Abkevich V, Hunt SC, Gutin A, Russell DL, Neff CD, Riley R, Frech GC, Hensel CH, Jammulapati S, Potter J, Sexton D, Tran T, Gibbs D, Iliev D, Gress R, Bloomquist B, Amatruda J, Rae PM, Adams TD, Skolnick MH, Shattuck D. A major predisposition locus for severe obesity, at 4p15-p14. Am.J.Hum.Genet. 2002;70(6):1459–1468. doi: 10.1086/340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MM, Samson WK. The other side of the orexins: endocrine and metabolic actions. Am.J.Physiol Endocrinol.Metab. 2003;284(1):E13–E17. doi: 10.1152/ajpendo.00359.2002. [DOI] [PubMed] [Google Scholar]
- Terra SG, McGorray SP, Wu R, McNamara DM, Cavallari LH, Walker JR, Wallace MR, Johnson BD, Bairey Merz CN, Sopko G, Pepine CJ, Johnson JA. Association between beta-adrenergic receptor polymorphisms and their G-protein-coupled receptors with body mass index and obesity in women: a report from the NHLBI-sponsored WISE study. Int.J.Obes.(Lond) 2005;29(7):746–754. doi: 10.1038/sj.ijo.0802978. [DOI] [PubMed] [Google Scholar]
- Tishler PV, Redline S, Ferrette V, Hans MG, Altose MD. The association of sudden unexpected infant death with obstructive sleep apnea. Am.J.Respir.Crit Care Med. 1996;153(6 Pt 1):1857–1863. doi: 10.1164/ajrccm.153.6.8665046. [DOI] [PubMed] [Google Scholar]
- Turner ST, Kardia SL, Boerwinkle E, Andrade MM. Multivariate linkage analysis of blood pressure and body mass index. Genet.Epidemiol. 2004;27(1):64–73. doi: 10.1002/gepi.20002. [DOI] [PubMed] [Google Scholar]
- Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential. Am.J.Respir.Med. 2003;2(1):21–29. doi: 10.1007/BF03256636. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38(5):715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Wu X, Cooper RS, Borecki I, Hanis C, Bray M, Lewis CE, Zhu X, Kan D, Luke A, Curb D. A combined analysis of genomewide linkage scans for body mass index from the National Heart, Lung, and Blood Institute Family Blood Pressure Program. Am.J.Hum.Genet. 2002;70(5):1247–1256. doi: 10.1086/340362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J.Biol.Chem. 2003;278(12):10770–10777. doi: 10.1074/jbc.M207968200. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yuan X, Otabe S, Koyanagi A, Koyama W, Makita Z. Sequencing of the putative promoter region of the cocaine- and amphetamine-regulated-transcript gene and identification of polymorphic sites associated with obesity. Int.J.Obes.Relat Metab Disord. 2002;26(1):132–136. doi: 10.1038/sj.ijo.0801848. [DOI] [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am.J.Respir.Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J.Appl.Physiol. 2005;99(4):1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- Zeegers M, Rijsdijk F, Sham P. Adjusting for covariates in variance components QTL linkage analysis. Behav.Genet. 2004;34(2):127–133. doi: 10.1023/B:BEGE.0000013726.65708.c2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.