Abstract
The mechanism of copper (Cu) transport into the brain is unclear. This study evaluated the main species and route of Cu transport into the brain using in situ brain perfusion technique, and assessed the levels of mRNA encoding Cu transporters using real time RT-PCR. Free 64Cu uptake in rat choroid plexus (CP), where the blood-cerebrospinal fluid barrier (BCB) is primarily located, is about 50 and 1,000 times higher than 64Cu-albumin and 64Cu-ceruloplasmin uptake, respectively. The unidirectional transport rate constants (Kin) for Cu in the CP and brain capillaries of the blood-brain barrier (BBB) were 1,034 and 319 μl/s/g, respectively, while Kin in CSF and capillary-depleted parenchyma were much reduced, 0.8 and 112 μl/s/g, respectively. The Kin in cerebellum was significantly lower than that in hippocampus. The mRNAs encoding Cu transporter-1 (Ctr1) and ATP7A were higher in the CP than those in brain capillaries and parenchyma, whereas ATP7B mRNA was higher in brain capillaries than those in the CP and brain parenchyma. Taken together, these data suggest that the expression of Cu transporters is higher in brain barriers than in brain parenchyma; the Cu transport into the brain is mainly achieved through the BBB as a free Cu ion and the BCB may serve as a main regulatory site of Cu in the CSF.
Keywords: copper, blood-brain barrier, blood-CSF barrier, choroid plexus, cerebrospinal fluid, brain capillaries, ceruloplasmin, albumin, copper transporter-1 (Ctr1), ATP7A
1. Introduction
Copper (Cu), as an essential metal, plays a crucial role in biochemical reactions and in physiological regulations (Linder and Hazegh-Azam 1996). Cu is required as a cofactor in various Cu-containing enzymes. As a free metal ion, Cu also participates in angiogenesis, nerve myelination and endorphin action (Turnlund 1998; Gaggelli et al. 2006). From the toxicological point of view, free Cu ions can interact readily with oxygen to initiate a cascade of biochemical events leading to the production of the highly damaging hydroxyl radical. It is because of its essentiality to the cellular function and its cytotoxic nature in oxidative stress that Cu is strictly regulated in the body (Linder and Hazegh-Azam 1996; Turnlund 1998; Li and Zheng 2005).
Genetic defects in expression of Cu transport-related proteins have been linked to several diseases associated with neurologic disorders. For example, a mutation in Cu-transporter ATP7B gene, which is located on the long arm (q) of chromosome 13 (13q14.3), causes the overload of Cu in the body, leading to Wilson’s disease with neurologic symptoms including tremors, abnormal postures, dystonia and bradykinesia (Kodama 1996; Gaggelli et al. 2006). A genetic disorder in the expression of the Cu-transporter ATP7A in Menkes disease, on the other hand, results in Cu deficiency particularly in liver and brain. Patients display syndromes of seizures, psychomotor deterioration, failure to thrive, hypothermia, accompanied by extensive neurodegeneration in brain gray matter (Dexter et al. 1991). It has been suggested that a defect in these Cu transporters at blood-brain barrier (BBB) may partly contribute to the altered brain homeostasis of Cu (Li and Zheng 2005).
Cumulative evidence has implied that an imbalance of Cu homeostasis in brain may play a role in the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, spongiform encephalopathies, and familial amyotrophic lateral sclerosis (Hartmann and Evenson 1992; Deibel et al. 1996; Waggoner et al. 1999; Strausak et al. 2001; Rossi et al. 2004; Gaggelli et al., 2006). More recently, human studies have shown a significant increase in Cu concentrations in blood and saliva among manganese-exposed smelters and welders (Jiang et al., 2007; Wang et al., 2008). Studies on manganese-exposed non-human primates also reveal a significant increase in brain Cu concentrations (Guilarte et al., 2008). Noticeably, manganese-caused neurotoxicity exhibits the clinical syndromes highly resembling those of Parkinson’s disease (Aschner et al. 2007; Zheng 2005).
Most of Cu ions absorbed from the small intestine are distributed to liver and kidneys; they are transported in blood by binding to ceruloplasmin. In serum, about 65–90% of Cu tightly binds with ceruloplasmin, and the rest of Cu loosely binds with albumin, transcuprein and amino acids (Linder 1991). At the cellular level, the uptake of Cu into the cells is thought to be mediated by two transporter proteins, i.e., Cu transporter 1 (Ctr1) and divalent metal transporter 1 (DMT1). The former has a high affinity selective to Cu and the latter is relatively non-specific and available also to other divalent metals (Tandy et al. 2000). Inside of cells, ATP7A and ATP7B are Cu transport proteins that participate in Cu efflux (Lutsenko et al. 2002). While the proteins pertaining to Cu transport within and between body compartments have been identified, the mechanism by which Cu was transported into the brain and the relative role of the BBB and blood-cerebrospinal fluid (CSF) barrier (BCB) in regulating brain homeostasis of Cu remained largely unclear. The primary species of Cu either as bound or free that contributes to brain transport was also unknown. Moreover, little has been learned about Cu transport and distribution in different brain regions.
This study was designed to test the hypothesis that transport of Cu into various brain regions was determined by the Cu species present in blood and the BBB and BCB may play different roles in transporting and regulating Cu homeostasis in the brain. We used a well-described (Preston et al. 1995) and well-established brain perfusion technique in this laboratory (Deane et al. 2004) to determine the unidirectional uptake rates (Kin) of three Cu species (i.e., free 64Cu, 64Cu-albumin and 64Cu-ceruloplasmin) into brain capillaries, parenchyma, choroid plexus (CP) and CSF. In addition, we assessed the levels of mRNA expression of several key Cu transporters (i.e., Ctr1, DMT1, ATP7A, and ATP7B) in brain capillaries, parenchyma and CP. The research should help better understand Cu transport by brain barriers under physiological conditions.
2. Results
2.1. Free Cu as the main species responsible for Cu transport into brain
To understand the primary species responsible for Cu transport into the brain, three species of Cu, i.e., unbound 64Cu, 64Cu-albumin and 64Cu-ceruloplasmin, were individually perfused into brain via the internal carotid artery. The Cu uptake by brain capillaries, parenchyma (capillary-depleted brain tissue), CP and CSF, expressed as the distributing volume, was then examined. Data in Fig. 1A showed that Cu uptakes by the CP in the form of free 64Cu, 64Cu-albumin, and 64Cu-ceruloplasmin were 78.4 ± 8.61, 1.70 ± 0.45, and 0.08 ± 0.02 mL/g, respectively. Regardless of the Cu species investigated, the uptake of Cu was highest in the CP, followed by, in a descending order, brain capillaries, brain parenchyma, and CSF.
Fig. 1.
64Cu uptake (as a volume of distribution, mL/g) into choroid plexus (CP), cerebrospinal fluid (CSF), brain capillaries (BC) and brain parenchyma (BP), after rat brain was perfused with free 64Cu, 64Cu-albumin (Alb) and 64Cu-ceruloplasmin (CPN) for 2 min. Data represent mean ± SEM, n=4.
Data in Fig. 1B further revealed that the volume of distribution of free Cu in the CP was about 50 times greater than that of albumin-bound Cu, and about 1000 times greater than that of ceruloplasmin-bound Cu. Interestingly, the similar pattern of Cu uptake among all three tested Cu species was observed in brain capillaries, parenchyma, and CSF (Fig. 1B). These data suggest that free Cu was the major species entering brain barriers and being transported into brain parenchyma.
2.2. Accumulation of 64Cu by brain barrier cells and transport into CSF and capillary-depleted brain parenchyma
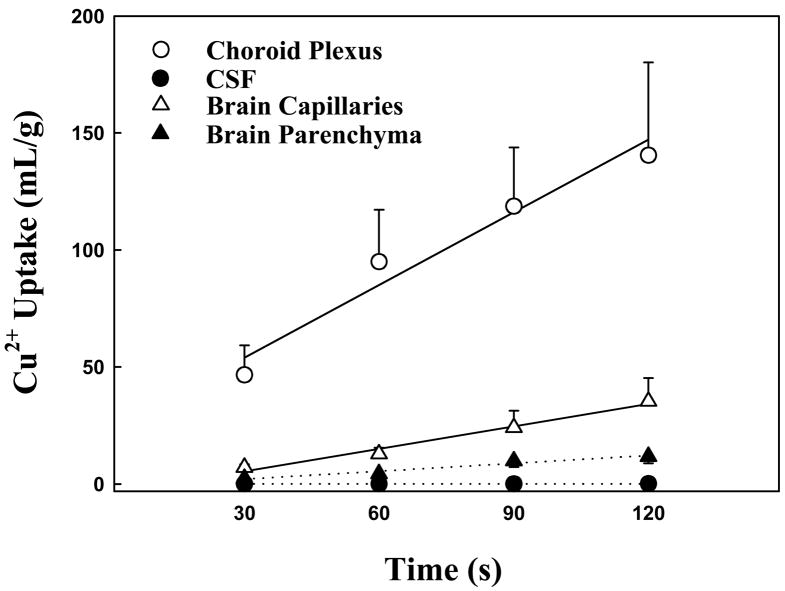
Upon knowing the free Cu as a major species in brain transport from the above studies, we used free 64Cu to study the route by which Cu entered the brain. 64Cu uptake by the CP and transport into the CSF were linear up to 120 sec (Fig. 2). The uptake rate constants, Kin, estimated from the slopes of the uptake curves indicated the rate of Cu entering the CP was nearly 1300 fold greater than the rate of Cu entering the CSF (Table 2). This observation suggested that the CP was capable of sequestering Cu ions from the blood circulation; yet such a remarkable accumulation of Cu in the BCB did not result in a substantial transport of Cu into the CSF. Thus, the CP seemed unlikely to be a primary site for Cu to enter brain extracellular fluid.
Fig. 2.
Time courses of 64Cu uptake into choroid plexus, CSF, brain capillaries and brain parenchyma after rat brain was perfused with only free 64Cu. Data represent mean ± SEM, n=3–5.
Table 2.
Unidirectional uptake rate constants (Kin) of free 64Cu in the brain capillaries, brain parenchyma, choroid plexus and CSF
| Brain regions | Kin (μL/sec/g) |
|---|---|
| Choroid plexus | 1034.5 ± 369.1 |
| CSF | 0.8 ± 0.4 |
| Brain capillaries | 319.6 ± 89.5 |
| Brain parenchyma | 111.5 ± 28.7 |
Data represent mean ± SEM, n=3–5.
64Cu uptake by cerebral capillaries and transport into capillary-depleted brain parenchyma were also linear within the duration of the perfusing time (Fig. 2). The Kin in brain capillaries was about 3 fold greater than that in capillary-depleted brain tissues (Table 2). The rapid uptake of Cu by brain capillaries (320 ul/s/g) and a moderate transport of Cu into brain parenchyma (112 ul/s/g) suggested that free Cu ions were transported by the blood-brain barrier in a rate-limited fashion.
2.3. Regional brain 64Cu uptake
Differences in brain regional Cu uptake may contribute to the toxicity of Cu on particular brain areas or pathways. When free 64Cu was perfused via internal carotid artery, the uptake of Cu by all brain areas studied was linear within the perfusion time (Fig. 3). The homogeneity of variance test of the Table 3 revealed that there were homogeneities of Kin values within the regions of frontal cortex, striatum, hippocampus, midbrain and cerebellum (F = 1.216, P = 0.310). Thus, the one-way ANOVA was performed to examine the differences among brain regions. The ANOVA did not reveal any significant differences in Kin values between frontal cortex, striatum, hippocampus, and midbrain (Table 3). However, the Kin value in cerebellum was statistically differently lower than that in hippocampus (p<0.05).
Fig. 3.
Time courses of 64Cu uptake into various brain regions (i.e., frontal cortex, striatum, hippocampus, midbrain and cerebellum) after rat brain was perfused with only free 64Cu. Data represent mean ± SEM, n=5.
Table 3.
The Kin of free 64Cu in different brain regions
| Brain regions | Kin (μL/sec/g) |
|---|---|
| Frontal cortex | 78.8 ± 29.6 |
| Striatum | 54.0 ± 18.4 |
| Hippocampus | 95.0 ± 23.4 |
| Midbrain | 89.5 ± 22.8 |
| Cerebellum | 43.4 ± 8.1* |
Data represent mean ± SEM, n=5.
: p<0.05 as compared to hippocampus.
2.4. Expression of mRNAs encoding Cu transporters
Since free Cu ions were apparently the major species in crossing brain barriers, there was a need to understand the relative abundance of potential metal ion transporters in barrier system, which may be responsible for Cu transport. Ctr1, DMT1, ATP7A, and ATP7B are known to be associated with Cu transport. Quantitative real-time R-PCR was used to qualify the mRNA expression levels of these putative Cu transporters in cerebral capillaries, brain parenchyma, and the CP. The expressions of Cu transporter genes were significantly higher in cerebral capillary and CP than those in brain parenchyma (p<0.05) (Fig. 4), except for ATP7B whose level in parenchyma was close to that of the CP. The data suggest that brain barriers express high levels of Cu transporters. Between two brain barriers, Ctr1 and ATP7A expression levels in the CP were about 1.2 and 3.4 fold higher than those in cerebral capillaries, respectively, (p<0.05); yet ATP7B mRNA level in cerebral capillaries was about 4.0 fold higher than that in the CP (p<0.05) (Fig. 4). The mRNA levels of DMT1 in the CP and cerebral capillaries were similar, but about 2.6-fold higher than that in brain parenchyma (p<0.05).
Fig. 4.
Relative abundance of mRNAs encoding Cu transporters (Ctr1, DMT1, ATP7A, ATP7B) in brain parenchyma and capillaries and choroid plexus by real time RT-PCR. Data represent mean ± SEM, n=7. Different letters (a, b, and c) above each bar indicate a significant difference between each other by ANAVA
3. Discussion
The results of this study clearly indicate that Cu is transported into the brain as a free Cu ion. Among three tested Cu species, i.e., free Cu, ceruloplasmin-bound Cu or albumin-bound Cu, the highest accumulation of Cu in brain barrier tissues (CP and cerebral capillaries) and brain parenchyma is associated with free Cu perfusion. The values of distributing volume in all four tested brain fractions are several magnitudes higher following free Cu perfusion than following perfusion of protein-bound Cu species. This observation supports the view that Cu in the blood circulation is carried by its binding proteins; upon reaching brain barriers, it is the free Cu ions but not protein-bound Cu species that enter brain barriers and subsequently distribute to brain parenchyma and the CSF.
Under physiological conditions, most serum Cu ions are bound to ceruloplasmin with a small portion of Cu carried by albumin, transcuprein and other amino acids (Weiss and Linder 1985; Linder 1991). Based on the observations such as abundant ceruloplasmin in serum, a high capacity of ceruloplasmin in binding Cu, and the presence of unique membrane receptors for ceruloplasmin, some earlier studies have suggested that ceruloplasmin may play a critical role in distributing, transporting and metabolizing Cu (Hsieh and Frieden 1975; Campbell et al. 1981; Stevens et al. 1984). However, in a study using aceruloplasminemic mice with genetically altered, low expression of ceruloplasmine, Meyer et al. (2001) report that systemic Cu kinetics with regard to gastrointestinal absorption, hepatic uptake, or biliary excretion does not differ significantly between aceruloplasminemic mice and wild type mice. These results lead authors to conclude that ceruloplasmin is not essential for Cu absorption and metabolism (Meyer et al. 2001). Our data, by using three major Cu species in the blood circulation, demonstrated that the uptake rate of unbound, free Cu ion far exceeded that of ceruloplasmin-bound Cu, a direct experimental evidence that supports a lesser role of ceruloplasmin in Cu transport into brain tissues.
Serum albumin possesses a binding site for Cu. On target tissues such as liver the presence of albumin inhibits Cu uptake by hepatocytes (Darwish et al. 1984; Ettinger et al. 1986). These data indicate that albumin, in addition to ceruloplasmin, may contribute to Cu transport and metabolism (Marceau and Aspin 1973; Wirth and Linder 1985; Gordon et al. 1987). However, the result from analbuminemic rats does not provide the evidence to support an important role of albumin in Cu distribution and metabolism, as there is essentially no difference in Cu disposition between albumin-null and wild animals (Vargas et al. 1994). Our data with a much less brain Cu uptake in albumin-bound Cu than free Cu are in a good agreement with the report by Vargas et al. (1994). Since Cu is loosely bound to albumin (Pietrangelo et al. 1992), it is not entirely surprised that Cu ions may be released from albumin-bound moiety at brain barriers and these free Cu ions are then transported into the brain.
Considering the route for Cu to enter the brain, the CP among all tested brain fractions exhibited the highest capacity in acquiring Cu from the blood circulation irrespective to what Cu species was used. It is therefore curious that the unidirectional uptake rate of Cu by the CSF, most of which is produced by the CP, is nearly 1200 fold slower than that in the CP, particularly in the face of a high accumulation of this metal in the CP. This may indicate that the CP sequesters Cu and thus tightly regulates the movement of Cu into the CSF. In cerebral capillaries, however, the Cu uptake is much slower (about 3.2 fold less) than in the CP; yet the acquired Cu ions in cerebral capillaries may be more readily transported to the parenchyma than Cu in the CP to the CSF. Since there is no apparent barrier between the CSF and interstitial fluid, the Cu in the CSF may be derived from the Cu in the bulk flow of the interstitial fluid spilled by neurons and glial cells. Taken together, we hypothesize that the greater transport of Cu into the brain parenchyma compared with its slow transport across the CP to the CSF reflects the presence of a concentration gradient for brain Cu under physiological conditions. The BBB appears to be a more important route than the BCB in the transport of Cu into brain parenchyma, where Cu is utilized and subsequently released into the CSF via the interstitial fluid. The BCB may function as a regulatory site to maintain the homeostasis of Cu in brain extracellular fluid.
The differences in the density of perfused capillaries and local blood flow between brain regions are known to affect brain regional differences in chemical distribution (Klein et al. 1986; Zheng 2001). Cu distribution in the hippocampus is nearly 1.7 fold higher than that in striatum, although the regional Kin values are not statistically significantly different. On the other hand, Cu uptake is significantly lower in cerebellum than in hippocampus. Whether these regional differences in Cu uptake reflect the diversities in capillary density, blood flow, and/or the abundance of Cu transporter expression in these brain regions deserves further exploration.
Several transporters have been suggested to transport Cu across the cell membrane, including Ctr1, DMT1, ATP1A and ATP7B (Li and Zheng 2005). In Ctr1-knockout mice, brain Cu content is decreased by approximately 50%, substantiating the need of Ctr1 for copper transport at cellular level (Iwase et al. 1996; Nishihara et al. 1998; Kuo et al. 2001; Lee et al. 2001). ATP7A has been linked to Cu efflux from most of tissues (Mercer and Llanos 2003). By using APT7A inhibitor, Qian and colleagues (Qian et al. 1998) demonstrate that Cu efflux can be blocked. The ATP7B gene is mainly expressed in the liver, where it is responsible for biliary excretion of Cu. Lack of ATP7B expression in Wilson’s disease results in overload of hepatic Cu and a consequential increase of brain Cu concentration (Buiakova et al. 1999; Faa et al. 2001). DMT1 is known to present in neurons, cerebral capillary endothelial cells and the CP epithelial cells and is thought to transport nonselectively divalent metal ions including Mn2+, Fe2+, Co2+, Ni2+, and Cu2+ (Burdo et al. 2001; Wang et al. 2006). The current data show that all these putative Cu transporters are expressed more profoundly in brain barrier fractions (cerebral capillaries and CP) than brain parenchyma, suggesting the possible involvement of these transporters in brain Cu uptake. The observation of high expression of ATP7A in BCB as opposed to a high expression of ATP7B in BBB is interesting; yet their functional relevance to Cu homeostasis in the brain is unknown. In addition, the question as to what are the relative roles of these transporters in regulating Cu transport by two brain barriers remains unanswered. An in-depth study on these questions is well warranted.
In summary, the current study demonstrates that free Cu ions are the main species for Cu transport into the brain. The BBB appears to serve as the main entrance for Cu to get access to brain parenchyma whereas the BCB is likely involved in the regulation of Cu homeostasis in the CSF. Both BBB and BCB express metal transporters associated with Cu transport and the levels of their gene expression are higher in brain barrier cells than in brain parenchyma.
4. Experimental procedures
4.1. Materials
Chemicals were obtained from the following sources: copper chloride, sodium pyruvate, calcium chloride, HEPES, rat albumin from Sigma Chemical Co. (St. Louis, MO); Chelex® 100 resin from Bio-Rad Laboratories (Hercules, CA); Solvable® and Hionic Fluor® from Packard BioScience B.V. (Groningen, Netherlands); [14C]sucrose (specific activity: 495 mCi/mmol) from Moravek Biochemicals, Inc. (Brea, CA). All reagents were analytical grade, HPLC grade, or the best available pharmaceutical grade.
64CuCl2 (specific activity 15–30 mCi/μg) was produced at Washington University by cyclotron irradiation of an enriched 64Ni target by using methods reported (McCarthy et al. 1997).
4.2. Animals
All rats (Sprague-Dawley, male) were purchased from Harlan Inc. (Indianapolis, IN). They were housed in a temperature-controlled, 12:12 light/dark room and were allowed free access to tap water and food. All rats used in this study were 8 – 9 weeks old (240 – 280 g) at the time of experimentation. The experimental protocol was approved by the Purdue Animal Care and Use Committee (PACUC).
4.3. Experimental design
Study 1 (n=4 for each group) was performed to evaluate the main species of Cu that was transported into the brain. In situ brain perfusion was performed at single time point (120 sec) with 3 Cu species (free 64Cu, 64Cu-albumin or 64Cu-ceruloplasmin). Our preliminary study showed that perfusion longer than 5 min may cause damage to capillary cells. To ensure a reliable result, we limited the perfusion time to 120 sec.
Study 2 (n=5 in each time point) was performed to determine the unidirectional uptake rates (Kin) of Cu in brain capillaries and parenchyma, CP, CSF and regional brain (frontal cortex, striatum, hippocampus, midbrain and cerebellum) with free, unbound 64Cu (0.2 μM) perfused at various time points (30, 60, 90, and 120 sec).
Beta radioactivities of 64Cu and 14C were measured with a Packard Tri-Carb 2200A liquid scintillation counter. Brain samples were weighed and digested with Solvable® (Packard BioScience B.V., Groningen, Netherlands) for 2–3 h at 55 °C. After digestion, Hionic Fluor® (Packard BioScience B.V., Groningen, Netherlands) was added to the sample.
4.4. Preparation of 64Cu-albumin and 64Cu-ceruloplasmin complexes
64Cu-albumin complex was prepared by incubating 64CuCl2 with 1 mg/mL of rat albumin for 1 h at room temperature (Mas and Sarkar 1992). Excess free Cu was removed by ultrafiltration using a membrane with molecular weight cut off of 50,000-Da (Micropore, Bedford, MA).
64Cu-ceruloplasmin complex was made as described by Lee et al. (1993). Briefly, a donor rat was injected intravenously via tail vein with 4 mCi of 64Cu as the 1:1 Cu-nitriloacetate complex (Cu-NTA). The rat was sacrificed 24 h later and plasma collected. Chelex® 100 resin (1 mg/mL) was added into the plasma sample; the mixture was placed on a horizontal shaker with continuous shaking for 1 h, followed by centrifugation to remove any loosely bound 64Cu (Campbell et al. 1981).
4.5. In situ brain perfusion procedure
The method described by Takasato et al. (1984) and Smith (1996) with modification was used in this study. Briefly, the rat was anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and the right common carotid artery was exposed. Following ligation of the external carotid, pterygopalatine and common carotid arteries, the internal carotid was cannulated with a polyethylene catheter (PE-10). The brain was perfused with a Ringer solution in a syringe containing (g/L): NaCl, 7.31; KCl, 0.356; NaHCO3, 2.1; KH2PO4, 0.166; MgSO4·3H2O, 0.213; glucose, 1.50; and sodium pyruvate, 1 mmol/L and CaCl2, 2.5 mmol/L, at a flow rate of 9 mL/min (Variable Flow, Mini Pump, VWR), 37°C, pH 7.4 and gassed continuously with 5% CO2–95% O2. Prior to the start of the experiment, the solution was filtered and pre-gassed with 5% CO2–95% O2. The “hot” solution in a separate syringe, which contained the radioisotopes of [14C] sucrose (as a space marker) and 64Cu alone, 64Cu-albumin, or 64Cu-ceruloplasmin in pre-gassed Ringer solution, was delivered to cannulated internal carotid artery via a second syringe pump (Harvard Compact Infusion Pump, Model 975) at a flow rate of 1.0 mL/min. Thus, the total flow rate of perfusion was 10 mL/min. To prevent re-circulation of the rat blood, the left ventricle of the heart was cut before the start of perfusion. This technique was validated for CNS transport studies (Takasoto et al. 1984; Smith 1996; Zlokovic et al. 1986; Deane and Bradbury 1990), and well established and used in this laboratory (Dean et al. 2004).
At the end of each perfusion (30, 60, 90, and 120 sec), the Harvard syringe pump was switched off and the brain vascular system was washed for 30 sec with the Ringer solution to remove Cu adsorbed to the luminal surface and the luminal content (Deane and Bradbury 1990).
Immediately after perfusion, a sample of CSF was collected from the cisterna magna, using a 25-gauge butterfly needle (Becton Dickinson, Franklin Lakes, NJ). The brain was harvested and washed with ice-cold saline, and then placed on an ice-cold filter paper, saturated with cold saline on the surface of a beaker filled with ice. Following removal of meninges and surface blood vessels, the ipsilateral CP was collected from the lateral ventricle. In Study 1, the ipsilaterally perfused cerebrum was used for capillary separation and subsequent assays. In Study 2, the perfused brain tissue was further dissected to collect the frontal cortex (FC), striatum (ST), hippocampus (HP), midbrain (MB) and cerebellum (CE).
4.6. Capillary separation
The capillary separation was carried out as previously described (Preston et al. 1995; Triguero et al. 1990; Zlokovic 1995; Dean et al. 2004). Briefly, the cold brain was weighed and homogenized in 3 volumes of cold solution of buffer with 7–8 strokes in a 7 mL tissue grind pestle (Kontes, Vineland, NJ). The buffer solution contains (mmol/L) HEPES, 10; NaCl, 141; KCl, 4; MgSO4, 1; NaH2PO4, 1; CaCl2, 2.5; and glucose, 10 at pH 7.4. Dextran 70 was added to a final concentration of 15% (w/v) and the solution homogenized with 3 additional strokes. The homogenate was then spun at 5,400 × g for 15 min at 4°C. The supernatant (capillary-depleted fraction) and pellet (capillary-enriched fraction) were separated carefully and counted for radioactivity. Light microscopic examination confirmed that the pellet consisted mainly of networks of brain vessels, while the supernatant was essentially free of vasculature.
4.7. Calculations
64Cu uptake was expressed as a volume of distribution, Vd, and calculated as (CTissue or CCSF/CPerfusate), where CTissue or CCSF are d.p.m./g of brain tissue (e.g. cerebral capillaries, capillary free brain homogenate, whole brain tissue, choroid plexus, brain regions) or CSF, and CPerfusate are d.p.m./mL of the perfusion fluid. Vd for the capillaries was calculated from d.p.m./g. The unidirectional uptake rates, Kin (μL/s/g), corresponding to the slope of the uptake curve was determined using linear regression analysis of Vd against the perfusion time (T, s) as Vd = Kin T + Vi, where Vi is the ordinate intercept of the regression line. 64Cu uptake was corrected for residual radioactivity by deducting Vd for [14C] sucrose from the total 64Cu distributing volume.
4.8. Quantitative Real-time RT-PCR
Total RNAs were isolated from brain capillaries, parenchyma and CP by acid guanidium-phenol-chloroform method using TRIzol® Reagent (Gibco-BRL, Gaithersburg, MD) and purified with RNeasy® Mini kit (Qiagen GmbH, Hilden, Germany). cDNA was synthesized in a reaction volume of 100 μL containing 1 μg of total RNA, 2.5 μM of oligo dT primer, 1 mM dNTP mixtures, 0.4 U of RNase inhibitor, 1.25 U of MuLV reverse transcriptase, 5 mM MgCl2 and 1×PCR buffer according to the manufacturer’s instruction (Applied Biosystems, Foster City, CA).
Real-time RT-PCR using the Mx3000p (Stratagene, Cedar Creek, TX) was used to quantify mRNA levels. The primers for selected genes listed in Table 1 were designed using Primer Express software (Applied Biosystems, Foster City, CA). Each PCR reaction contained 5 μL of cDNA, 12.5 μL of 2× Absolute™ QPCR SYBR Green® mix (ABgene, Epsom, UK), and 200–400 nM of the forward and reverse primers. Each reaction was run in duplicate to obtain an average value. A total of 7 rats were used in this study. After an initial denaturation at 95°C for 15 min, the amplification program consist of 40 cycles of denaturation at 95 °C for 30 sec and annealing for at 60 °C for 1 min and extension at 72 °C for 30 sec. The relative differences in mRNA expression among brain capillaries and parenchyma, and CP were expressed using cycle time (Ct) values as follows: the relative differences between the brain parenchyma and the other two tissues were calculated and expressed as a relative increase, setting the brain parenchyma at 100%.
Table 1.
Primers used for real-time RT-PCR analysis
| mRNA | Sequence | Position |
|---|---|---|
| Ctr1 : GenBank accession number NM_133600 | ||
| Forward primer | 5′-CTAAACGCAGGCCGAGTTTC-3′ | 1275–1294 |
| Reverse primer | 5′-TTGGGATGGGCAGGTTCA-3′ | 1342–1359 |
| DMT1 : GenBank accession number NM_013173 | ||
| Forward primer | 5′-CAGTGCTCTGTACGTAACCTGTAAGC-3′ | 4055–4080 |
| Reverse primer | 5′-CGCAGAAGAACGAGGACCAA-3′ | 4146–4165 |
| ATP7A : GenBank accession number NM_052803 | ||
| Forward primer | 5′-CCCTCAACAGCGTCGTCACT-3′ | 4420–4439 |
| Reverse primer | 5′-GACTAGCAGCATCCCCAAAGG-3′ | 4511–4530 |
| ATP7B : GenBank accession number NM_012511 | ||
| Forward primer | 5′-GCCTACAAATCGCTGAGACAC-3′ | 2148–2168 |
| Reverse primer | 5′-CTCCGCCTTTTCAGCTATGG-3′ | 2248–2267 |
4.9. Statistics
All data were expressed as a mean ± SEM. Prior to the analysis of variance (ANOVA), a homogeneity of variance test was conducted to determine the homogeneity of tested values. The differences between groups were then compared by one-way ANOVA, followed by a post hoc multiple comparison with Dunnett test using the SPSS 12.0 statistics package for Windows. The differences between two means were considered significant if the p value was equal or less than 0.05.
Acknowledgments
We are grateful to Dr. Janelle Crossgrove and Ms. Shirley Wang for their technical assistance during experimentation. This study was supported in part by NIH/National Institute of Environmental Health Sciences grant ES08146 and ES013118 and Johnson & Johnson Focus Program. The production of 64Cu at Washington University School of Medicine is supported by the NCI grant R24 CA86307.
Abbreviation
- CSF
cerebrospinal fluid
- BBB
blood-brain barrier
- BCB
blood-CSF barrier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Nass R, Guilarte TR, Schneider JS, Zheng W. Manganese neurotoxicity: From worms to men. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet. 1999;8:1665–1671. doi: 10.1093/hmg/8.9.1665. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- Campbell CH, Brown R, Linder MC. Circulating ceruloplasmin is an important source of copper for normal and malignant animal cells. Biochim Biophys Acta. 1981;678:27–38. doi: 10.1016/0304-4165(81)90044-1. [DOI] [PubMed] [Google Scholar]
- Darwish HM, Cheney JC, Schmitt RC, Ettinger MJ. Mobilization of copper(II) from plasma components and mechanisms of hepatic copper transport. Am J Physiol. 1984;246:G72–G79. doi: 10.1152/ajpgi.1984.246.1.G72. [DOI] [PubMed] [Google Scholar]
- Deane R, Bradbury MW. Transport of lead-203 at the blood-brain barrier during short cerebrovascular perfusion with saline in the rat. J Neurochem. 1990;54:905–914. doi: 10.1111/j.1471-4159.1990.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Deane R, Zheng W, Zlokovic BV. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J Neurochem. 2004;88:813–820. doi: 10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J Neurol Sci. 1996;143:137–142. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Ettinger MJ, Darwish HM, Schmitt RC. Mechanism of copper transport from plasma to hepatocytes. Fed Proc. 1986;45:2800–2804. [PubMed] [Google Scholar]
- Faa G, Lisci M, Caria MP, Ambu R, Sciot R, Nurchi VM, Silvagni R, Diaz A, Crisponi G. Brain copper, iron, magnesium, zinc, calcium, sulfur and phosphorus storage in Wilson’s disease. J Trace Elem Med Biol. 2001;15:155–160. doi: 10.1016/S0946-672X(01)80060-2. [DOI] [PubMed] [Google Scholar]
- Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, Prion, and Parkinson’s diseases and Amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- Gordon DT, Leinart AS, Cousins RJ. Portal copper transport in rats by albumin. Am J Physiol. 1987;252:E327–E333. doi: 10.1152/ajpendo.1987.252.3.E327. [DOI] [PubMed] [Google Scholar]
- Guilarte RT, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008 doi: 10.1111/j.1471–4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann HA, Evenson MA. Deficiency of copper can cause neuronal degeneration. Med Hypotheses. 1992;38:75–85. doi: 10.1016/0306-9877(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Hsieh HS, Frieden E. Evidence for ceruloplasmin as a copper transport protein. Biochem Biophys Res Commun. 1975;67:1326–1331. doi: 10.1016/0006-291x(75)90172-2. [DOI] [PubMed] [Google Scholar]
- Iwase T, Nishimura M, Sugimura H, Igarashi H, Ozawa F, Shinmura K, Suzuki M, Tanaka M, Kino I. Localization of Menkes gene expression in the mouse brain; its association with neurological manifestations in Menkes model mice. Acta Neuropathol. 1996;91:482–488. doi: 10.1007/s004010050455. [DOI] [PubMed] [Google Scholar]
- Jiang YM, Zheng W, Long LL, Zhao WJ, Li XG, Mo XA, Lu JP, Fu X, Li WM, Liu SF, Long QY, Huang JL, Pira E. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. NeuroToxicology. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B, Kuschinsky W, Schrock H, Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol. 1986;251:H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- Kodama H. Essential trace elements and immunity. Niphon Rinsho. 1996;54:46–51. [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci U S A. 2001;98:6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci U S A. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lancey R, Montaser A, Madani N, Linder MC. Ceruloplasmin and copper transport during the latter part of gestation in the rat. Proc Soc Exp Biol Med. 1993;203:428–439. doi: 10.3181/00379727-203-43619. [DOI] [PubMed] [Google Scholar]
- Li GJ, Zheng W. Regulation of neuroactive metals by the choroid plexus. In: Zheng W, Chodobski A, editors. The Blood-Cerebrospinal Barrier. CRC Press; New York: 2005. pp. 211–239. [Google Scholar]
- Linder MC. Biochemistry of copper. Plenum Press; New York: 1991. [Google Scholar]
- Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Efremov RG, Tsivkovskii R, Walker JM. Human copper-transporting ATPase ATP7B (the Wilson’s disease protein): biochemical properties and regulation. J Bioenerg Biomembr. 2002;34:351–362. doi: 10.1023/a:1021297919034. [DOI] [PubMed] [Google Scholar]
- Marceau N, Aspin N. The intracellular distribution of the radiocopper derived from ceruloplasmin and from albumin. Biochim Biophys Acta. 1973;328:338–350. doi: 10.1016/0005-2795(73)90267-5. [DOI] [PubMed] [Google Scholar]
- Mas A, Sarkar B. Uptake of 67Cu by isolated human trophoblast cells. Biochim Biophys Acta. 1992;1135:123–128. doi: 10.1016/0167-4889(92)90127-w. [DOI] [PubMed] [Google Scholar]
- McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- Mercer JF, Llanos RM. Molecular and cellular aspects of copper transport in developing mammals. J Nutr. 2003;133:1481S–1484S. doi: 10.1093/jn/133.5.1481S. [DOI] [PubMed] [Google Scholar]
- Meyer LA, Durley AP, Prohaska JR, Harris ZL. Copper transport and metabolism are normal in aceruloplasminemic mice. J Biol Chem. 2001;276:36857–36861. doi: 10.1074/jbc.M105361200. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Furuyama T, Yamashita S, Mori N. Expression of copper trafficking genes in the mouse brain. Neuroreport. 1998;9:3259–3263. doi: 10.1097/00001756-199810050-00023. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A, Panduro A, Chowdhury JR, Shafritz DA. Albumin gene expression is down-regulated by albumin or macromolecule infusion in the rat. J Clin Invest. 1992;89:1755–1760. doi: 10.1172/JCI115778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JE, al Sarraf H, Segal MB. Permeability of the developing blood-brain barrier to 14C-mannitol using the rat in situ brain perfusion technique. Brain Res Dev Brain Res. 1995;87:69–76. doi: 10.1016/0165-3806(95)00060-q. [DOI] [PubMed] [Google Scholar]
- Qian Y, Tiffany-Castiglioni E, Welsh J, Harris ED. Copper efflux from murine microvascular cells requires expression of the menkes disease Cu-ATPase. J Nutr. 1998;128:1276–1282. doi: 10.1093/jn/128.8.1276. [DOI] [PubMed] [Google Scholar]
- Rossi L, Lombardo MF, Ciriolo MR. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem Res. 2004;29:493–504. doi: 10.1023/b:nere.0000014820.99232.8a. [DOI] [PubMed] [Google Scholar]
- Smith QR. Brain perfusion systems for studies of drug uptake and metabolism in the central nervous system. Pharm Biotechnol. 1996;8:285–307. doi: 10.1007/978-1-4899-1863-5_15. [DOI] [PubMed] [Google Scholar]
- Stevens MD, DiSilvestro RA, Harris ED. Specific receptor for ceruloplasmin in membrane fragments from aortic and heart tissues. Biochemistry. 1984;23:261–266. doi: 10.1021/bi00297a014. [DOI] [PubMed] [Google Scholar]
- Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull. 2001;55:175–185. doi: 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]
- Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol. 1984;247:H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, Srai SK, Sharp P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J Biol Chem. 2000;275:1023–1029. doi: 10.1074/jbc.275.2.1023. [DOI] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Turnlund JR. Human whole-body copper metabolism. Am J Clin Nutr. 1998;67:960S–964S. doi: 10.1093/ajcn/67.5.960S. [DOI] [PubMed] [Google Scholar]
- Vargas EJ, Shoho AR, Linder MC. Copper transport in the Nagase analbuminemic rat. Am J Physiol. 1994;267:G259–G269. doi: 10.1152/ajpgi.1994.267.2.G259. [DOI] [PubMed] [Google Scholar]
- Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- Wang DX, Du XQ, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Letters. 2008;176:40–47. doi: 10.1016/j.toxlet.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li GJ, Zheng W. Up-regulation of DMT1 expression in choroidal epithelial cells following manganese exposure. Brain Res. 2006;1097:1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KC, Linder MC. Copper transport in rats involving a new plasma protein. Am J Physiol. 1985;249:E77–E88. doi: 10.1152/ajpendo.1985.249.1.E77. [DOI] [PubMed] [Google Scholar]
- Wirth PL, Linder MC. Distribution of copper among components of human serum. J Natl Cancer Inst. 1985;75:277–284. [PubMed] [Google Scholar]
- Zheng W. Neurotoxicology of the brain barrier system: New implications. J Toxicol Clin Toxicol. 2001;39:711–719. doi: 10.1081/clt-100108512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W. Blood-CSF barrier in iron regulation and manganese-induced Parkinsonism. In: Zheng W, Chodobski A, editors. The Blood-Cerebrospinal Barrier. CRC Press; New York: 2005. pp. 413–436. [Google Scholar]
- Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12:1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Begley DJ, Djuricic BM, Mitrovic DM. Measurement of solute transport across the blood-brain barrier in the perfused guinea pig brain: method and application to N-methyl-alpha-aminoisobutyric acid. J Neurochem. 1986;46:1444–1451. doi: 10.1111/j.1471-4159.1986.tb01760.x. [DOI] [PubMed] [Google Scholar]