Abstract
Para-aminosalicylic acid (PAS), an FDA-approved anti-tuberculosis drug, has been used successfully in the treatment of severe manganese (Mn)-induced Parkinsonism in humans (Jiang et al., JOEM 48:644, 2006). This study was conducted to explore the capability of PAS in reducing Mn concentrations in body fluids and tissues of Mn-exposed animals. Sprague-Dawley rats received daily intraperitoneally (i.p.) injections of 6 mg Mn/kg, 5 d/wk for 4 wks, followed by a daily subcutaneously (sc.) dose of PAS (100 and 200 mg/kg as the PAS-L and PAS-H group, respectively) for another 2, 3 or 6 wks. Mn exposure significantly increased the concentrations of Mn in plasma, red blood cells (RBC), cerebrospinal fluid (CSF), brain and soft tissues. Following PAS-H treatment for 3 wks, Mn levels in liver, heart, spleen and pancreas were significantly reduced by 25 to 33%, while 3 wks of PAS-L treatment did not show any effect. Further therapy with PAS-H for 6 wk reduced Mn levels in striatum, thalamus, choroid plexus, hippocampus and frontal cortex by 16 to 29% (p<0.05). Mn exposure greatly increased iron (Fe) and copper (Cu) concentrations in CSF, brain and liver. Treatment with PAS-H restored Fe and Cu levels comparable with control. These data suggest that PAS likely acts as a chelating agent to mobilize and remove tissue Mn. A high-dose and prolonged PAS treatment appears necessary for its therapeutic effectiveness.
Keywords: para-aminosalicylic acid or PAS, manganism, manganese, iron, copper, chelation cerebrospinal fluid, choroid plexus, striatum
Introduction
Occupational exposure to manganese (Mn) has been linked to the majority of the reported cases of Mn intoxication. Neurotoxicity due to inhalation exposure to airborne Mn has been reported in miners, smelters, welders, as well as workers in dry-cell battery factories (Bowler et al., 2006; Chandra et al., 1981; Couper, 1837; Huang et al., 1989; Myers et al., 2003; Ono et al., 2002). Patients who suffered from Mn intoxication, namely manganism, display an extrapyramidal syndrome in a pattern similar to, but not identical to idiopathic Parkinson’s disease, including tremor, bradykinesia and gait difficulties. Patients can also display neuropsychological difficulties that include memory loss, apathy, and even psychosis (Aschner et al., 2007; Crossgrove and Zheng, 2004). Patients with severe manganism have difficulties in coping with daily life. While an increasing and immediate demand exists for an effective therapy for Mn-induced neurological impairment, a viable treatment has yet to be discovered.
Clinically, levodopa has been used to treat extrapyramidal syndromes, but with limited benefits (Huang et al., 1993; Lee, 2000; Mena et al., 1970; Rosenstock et al., 1971). In a more rigorous designed clinical trial, Koller et al. (2004) found that treatment with levodopa among parkinsonian welders did not lead to a significant beneficial effect. The strategy to remove the body burden of Mn to normal levels has also been tested in manganism patients. Chelation therapy with ethylene-diamine-tetraacetic acid (EDTA) has shown in some cases to produce promising clinical results (Hernandez et al., 2006), while in other cases it increases Mn elimination in urine but does not improve clinical syndromes (Calne et al., 1994; Cook et al., 1974; Crossgrove and Zheng, 2004; Ono et al., 2002). Thus, a search for other chelating agents for Mn intoxication has become necessary.
Para-aminosalicylic acid (PAS, 4-amino-2-hydroxybenzoic acid, 4-aminosalicylic acid, CAS#89-57-6, MW 153.14), also nicknamed PASER, Paramycin, or Parasal, has been used as an anti-tuberculosis drug since the early 1950s. The therapeutic benefit is believed to be due to its inhibitory effect on folic acid synthesis and therefore the synthesis of the cell wall of the tuberculosis mycobacterium, the primary bacterium causing tuberculosis (Physician’s Desk Reference, 2000; Rengarajan et al., 2004). The chemical structure of PAS is comprised of carboxyl, hydroxyl and amine groups, which provide promising chelating moieties for metals. Ky et al. (1992) first reported two successful clinical cases using PAS for treatment of chronic severe Mn poisoning. This group subsequently conducted a 17-year follow-up study on one of the patients and found that the PAS therapy led to a promising long-term prognosis (Jiang et al., 2006). Combined with 86 other cases effectively treated with PAS in the literature, this evidence suggests that PAS may be a promising therapy for manganism. However, the exact mechanism of drug action (i.e., by chelation, anti-inflammation, or both) remains unknown. Thus, one of the major purposes of this study was to investigate the effectiveness of PAS in reducing the body burden of Mn.
Upon exposure, Mn accumulates in brain regions, including the basal ganglia structures, and to a lesser extent, the caudate nucleus and putamen (Calne et al., 1994; Reaney et al., 2006; Roels et al., 1997; Yamada et al., 1986). T1-weighted magnetic resonance images (MRI) of patients with Parkinson-like symptoms exhibits high signal densities in the basal ganglia attributed to Mn, especially the globus pallidus (Jiang et al., 2007; Kim, 2006; Nagatomo et al., 1999). Mn exposure is known to alter iron (Fe) homeostasis in the cerebrospinal fluid (CSF) and brain tissues (Li et al., 2005, 2006; Zheng et al., 1999). An increased Fe concentration in the CSF is believed to be the result of Mn interference with Fe transport by the blood-CSF barrier at the choroid plexus (Wang et al., 2008a,b). Early studies by Lai et al. (1999) also suggest an increased Cu level in the striatum of rats exposed to Mn in drinking water. In light of these studies, we were interested in investigating whether PAS treatment would restore the altered Fe and Cu status to normal physiological levels.
The aims of this study were (1) to investigate whether PAS treatment reduced Mn levels in selected brain regions and organs of rats subchronically exposed to Mn, (2) to study the time-dose response of PAS treatment in reducing Mn tissue levels, (3) to verify the alteration of Fe and Cu in body fluids and brain tissues following Mn exposure, and (4) to investigate whether PAS restored tissue Fe and Cu to the normal levels.
Materials and Methods
Materials
Chemicals were obtained from the following sources: Manganese chloride (MnCl2) from Fisher scientific (Pittsburgh, PA); para-aminosalicylic acid (PAS) from Sigma (St Louis, MO); nitric acid form Mallinckrodt (Hazelwood, MO); atomic spectrophotometry standard solutions for Mn (as Mn nitrate in 3% nitric acid), iron (Fe, in 3% nitric acid) and copper (Cu, in 3% nitric acid) from Ricca Chemical Company(Fenton MO). All reagents were of analytical grade, HPLC grade or the best available pharmaceutical grade.
Animals
Male Sprague–Dawley rats were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN). At the onset of the study, the rats were 7–8 weeks old, weighing 220±10g (Mean± SD). Upon arrival, the rats were housed in a temperature-controlled, 12/12 light/dark room, and acclimated for one week prior to experimentation. They were allowed to have free access to pelleted Purina semi-purified rat chow (Purina Mills Test Diet 5755C) purchased from Purina Mills (Richmond, IN) and the distilled, deionized water. Purina Mills Diet 5755C contains 0.60% calcium, 0.57% phosphorus, 0.40% potassium, 0.07% magnesium, 0.21% sodium, 60 ppm Fe, 20 ppm Zn, 65 ppm Mn, 15 ppm Cu, 3.2 ppm cobalt, 0.6 ppm iodine, 3.0 ppm chromium, and 0.2 ppm selenium. The diet is highly consistent in its composition and has been used in our past studies on chronic lead exposure (Zhao et al., 1998; Zheng et al., 1996). The study was conducted in compliance with standard animal use practices and was approved by Institutional Committee on Animal Uses at Purdue University.
Mn and PAS administrations and sample collection
There were 8 rats in each study group. Both MnCl2 and PAS sodium salt were dissolved in sterile saline each day prior to administration. The study was designed as follows: Rats in the Mn-only Group received i.p. injections of 6 mg Mn/kg (6 mg Mn/mL) once daily, between 9:00 am and 10:00 am, 5 days per week for 4 weeks; they were then injected with saline subcutaneously once daily for 2, 3 or 6 weeks until tissue dissection (designated as the Mn-only Group).
Rats in PAS treatment groups received the same daily i.p. injections of 6 mg Mn/kg as those in the Mn-only group. Following four weeks of subchronic Mn exposure, exposure ceased and the animals were treated with PAS subcutaneously at dose of 100 mg/kg (designated as PAS-Low-Dose Group or PAS-L) or 200 mg/kg (PAS-High-Dose Group or PAS-H) once daily, between 9:00 am and 10:00 am, 5 days per week for additional 2, 3, or 6 weeks. The animals were then subjected to tissue dissection and subsequent metal analyses. Two control groups were designated as follows: Rats in the saline control group received the daily i.p. injections of saline at a volume equivalent to the Mn-only group throughout the experiment (designated as the Control Group). Another group of rats received the same saline injections as those in the control group for 4 weeks, followed by subcutaneous injection of 200 mg/kg of PAS for 2, 3, or 6 weeks (designated as the PAS-only group).
The dose regimen for Mn exposure was chosen because it was known to be associated with a significant increase of Mn concentration in brain tissues and altered biochemical parameters in rats (Seth et al., 1977, 1981; Wang et al., 2008b; Zheng et al., 1998, 1999). Subcutaneous injection of PAS was chosen in order to avoid the in situ chemical interaction between PAS and Mn at the same dosing site.
Twenty-four hour after the last injection, rats were anesthetized with ketamine/xylazine (75:10 mg/kg, l mg/kg i.p.). CSF samples were obtained through a 26-gauge needle inserted between the protuberance and the spine of the atlas, and were free of the blood. Blood samples were collected from the inferior vena cava into a 2-mL heparinized syringes. Following standing in room temperature for at least half an hour, the blood was centrifuged at 3400 g for 10 min at which point the plasma was transferred to an Eppendorf tube. The pellets were then washed with saline for 3 times. The red blood cells (RBC) were obtained; an aliquot of the RBC was used for determining the hemoglobin concentration; and the rest was for metal analyses. All CSF, plasma and RBC samples were stored at −20°C prior to analysis.
Rat brains were dissected from the skull and the choroid plexus was collected from lateral and third ventricles. Various brain regions, i.e., striatum, hippocampus, motor cortex, and cerebellum and thalamus, were dissected as we previously described (Zheng et al., 1998; Li et al., 2006). The major organs, i.e., heart, kidney, liver, testis, pancreas and spleen, were also dissected. All collected tissues were stored at −80°C prior to analysis.
Samples digestion and Atomic Absorption Spectrophotometry (AAS) analysis
All brain tissues and organ samples were thawed at room temperature and a 200-mg tissue sample was weighed in a 10 mL-MARSXpress microwave digestion vessel (CEM Corp., Matthews, NC). An aliquot of 2 mL ultrapure nitric acid was added into the vessel. The tightly capped vessels were placed in a model MARSXpress Microwave Reaction System (CEM Corp.) and digested at 600W power output with 15-min stepwise rise to 200 °C, which was maintained for 10 min. After cooling to room temperature, the resulting solution was mixed with another 2 mL of distilled, deionized (DDI-) water. The solution was then transferred to a 10-mL metal-free polypropylene conical tube (Falcon) and stored at 4 °C until analysis. To determine the metal concentrations in the CSF, plasma and RBC samples, aliquots of 0.5 mL plasma and 0.4 mL RBC were mixed with 1.5 mL and 1.6 mL of ultrapure nitric acid, respectively, in microwave vessels and digested under the same condition as described above. The CSF samples were digested with the equal volume of nitric acid at 56 °C overnight.
A Varian SpectroAA-20 Plus GTA-96 flameless graphite furnace AAS was used to quantify Mn, Fe and Cu concentrations in body fluids, brain tissues, and selected organs. Digested samples were diluted 50, 500, and 1000 folds with 1.0% (v/v) HNO3 for Mn, Fe, and Cu, respectively, in order to maintain the reading within the concentration ranges of the standard curves. The ranges of calibration standards were 0–5 µg/L for Mn, 0–10 µg/L for Fe, and 0–20 µg/L for Cu. The detection limit for Mn, Cu and iron were 0.09 ng/mL, 0.9 ng/mL, 0.18 ng/mL of the assay solution. Intra-day precision of the method for Mn, Cu and Fe were 2.9 %, 1.6% and 3.1%, respectively, and the inter-day precision were 3.3 %, 3.7 % and 4.6 %, respectively (Zheng et al., 1998, 1999).
Statistical analysis
All data are presented as mean ± SD and analyzed by SPSS 15.0 statisticas software (SPSS Inc., Chicago, IL). Prior to the analysis of variance (ANOVA), a homogeneity of variance test was conducted to determine the homogeneity of tested values. Comparison of two means was then performed using a one-way ANOVA, followed by Dunnett’s multiple comparison tests. In all cases, the differences between two means were considered significant if p values were equal or less than 0.05.
Results
Reduction of Mn concentrations in body fluids and tissues by PAS
Following subchronic Mn exposure, Mn concentrations in rat plasma, CSF and RBC were significantly increased as compared to the control group. Treatment with the low-dose PAS (PAS-L) did not affect plasma or RBC Mn concentrations but significantly reduced CSF Mn concentration (by 28%) after 3-week treatment (Table 1). Rats treated with the high-dose PAS (PAS-H) for 3 and 6 weeks showed a significant 22–26% reduction of Mn concentrations in RBC. While a 2-week PAS-H treatment significantly reduced CSF Mn by 33%, the treatment with 3 and 6 weeks of PAS-H did not significantly affect Mn concentration in the CSF (Table 1). Administration of PAS-H to the animals without Mn exposure did not alter Mn levels to any significant extent.
Table 1.
Effect of PAS treatment on Mn concentrations in plasma, RBC and CSF of rats subchronically exposed to Mn
| Group | Control Groups |
Mn alone |
Mn + PAS Treatment |
||
|---|---|---|---|---|---|
| Saline | PAS-H | PAS-L | PAS-H | ||
| Plasma (ng/mL) | |||||
| 2-week | 5.14±2.11 | 4.32±0.74 | 10.9±1.74* | 12.4±1.93 | 10.4±1.71 |
| 3-week | 5.60±0.64 | 5.08±1.20 | 8.12±1.06* | 10.8±3.41 | 10.1±1.86 |
| 6-week | 4.60±1.00 | 4.63±0.87 | 8.46±1.82** | 8.75±2.60 | |
| RBC (ng/mg Hb) | |||||
| 2-week | 0.10±0.03 | 0.09±0.02 | 2.26±0.40** | 2.50±0.23 | 2.16±0.39 |
| 3-week | 0.09±0.02 | 0.07±0.02 | 2.32±0.40** | 2.32±0.48 | 1.81±0.36# |
| 6-week | 0.08±0.02 | 0.07±0.02 | 0.77±0.19** | 0.57±0.11## | |
| CSF (ng/mL) | |||||
| 2-week | 4.88±1.63 | 4.32±0.74 | 11.5±2.41** | 9.96±2.90 | 7.75±2.28# |
| 3-week | 4.09±1.30 | 4.20±0.44 | 10.4±2.05** | 7.42±0.56# | 8.88±2.03 |
| 6-week | 5.09±0.44 | 4.79±1.34 | 10.2±1.78** | 11.2±1.69 | |
Rats in the control group received daily ip. saline injection throughout the entire experiment. Rats in the PAS control group received ip. saline for 4 weeks, followed by sc. PAS-H for 2, 3, or 6 weeks prior to tissue dissection. Animals in the Mn-alone group received ip. 6 mg Mn/kg, 5 days/week for 4 weeks, followed by sc. saline for 2, 3, or 6 weeks. Rats in the Mn-PAS groups received ip. 6 mg Mn/kg, 5 days/week for 4 weeks, followed by either low-dose PAS (100 mg/kg) or high-dose PAS (200 mg/kg) sc. injection for 2, 3, or 6 weeks. Data represent mean ± SD, n=6–8.
p<0.05
p<0.01 as compared to the saline group
p<0.05
p<0.01 as compared to the Mn-alone group.
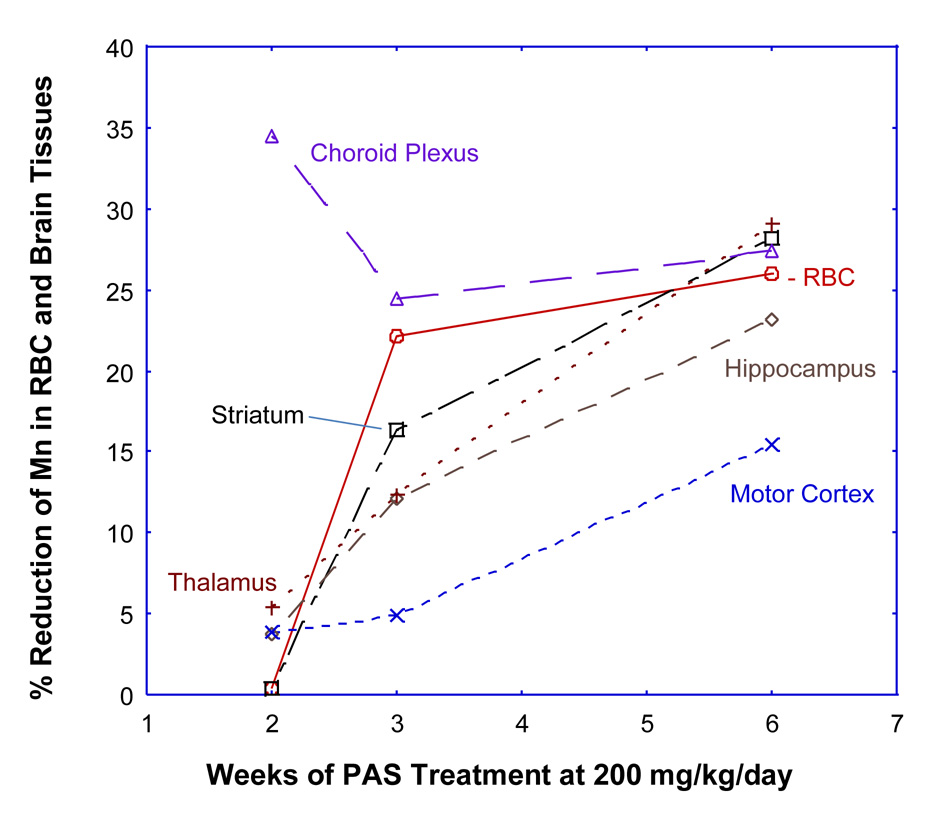
Mn tended to accumulate in brain tissues for a prolonged period. Concentrations of Mn in all brain regions examined remained to be significantly higher than those in controls even at 6 weeks of post-Mn exposure (p<0.01) except for the choroid plexus at 6 weeks (p>0.05) (Table 2). The highest Mn concentrations were found in striatum and thalamus. Treatment with PAS-L did not significantly affect Mn levels in all brain regions examined. However, a treatment of PAS-H for 2 weeks significantly reduced Mn concentrations in striatum, thalamus and choroid plexus. Following PAS-H treatment for 6 weeks, Mn levels in the striatum, thalamus, choroid plexus, hippocampus and motor cortex were reduced by 28.2%, 29.1%, 27.4%, 23.2%, and 15.5%, respectively (p<0.05) (Table 2). Noticeably also, the % of reduction of tissue Mn by PAS-H was increased with the PAS-H treatment time (Fig. 1), suggesting that a prolonged PAS therapy may be necessary to bring down Mn levels in brain tissues.
Table 2.
Effect of PAS treatment on Mn concentrations in selected brain regions (µg/g) of rats subchronically exposed to Mn
| Group | Control Groups |
Mn alone |
Mn + PAS Treatment |
||
|---|---|---|---|---|---|
| Saline | PAS-H | PAS-L | PAS-H | ||
| Striatum | |||||
| 2-week | 0.42±0.05 | 0.38±0.03 | 0.94±0.20** | 0.93±0.09 | 0.93±0.19 |
| 3-week | 0.40±0.02 | 0.37±0.03 | 0.73±0.09** | 0.71±0.09 | 0.61±0.06# |
| 6-week | 0.45±0.11 | 0.40±0.05 | 0.72±0.10** | 0.52±0.04## | |
| Hippocampus | |||||
| 2-week | 0.38±0.04 | 0.35±0.05 | 0.72±0.10** | 0.70±0.04 | 0.69±0.16 |
| 3-week | 0.36±0.02 | 0.37±0.02 | 0.60±0.08** | 0.54±0.09 | 0.53±0.08 |
| 6-week | 0.36±0.01 | 0.35±0.09 | 0.61±0.14** | 0.47±0.03## | |
| Motor Cortex | |||||
| 2-week | 0.43±0.03 | 0.38±0.04 | 0.79±0.04** | 0.79±0.05 | 0.76±0.07 |
| 3-week | 0.41±0.05 | 0.32±0.08 | 0.66±0.05** | 0.66±0.08 | 0.62±0.04 |
| 6-week | 0.40±0.03 | 0.37±0.10 | 0.58±0.04** | 0.49±0.08# | |
| Thalamus | |||||
| 2-week | 0.47±0.03 | 0.50±0.04 | 1.08±0.07** | 1.13±0.13 | 1.03±0.02 |
| 3-week | 0.49±0.04 | 0.48±0.08 | 0.83±0.09** | 0.79±0.08 | 0.73±0.04# |
| 6-week | 0.46±0.03 | 0.46±0.06 | 0.66±0.06** | 0.47±0.03## | |
| Cerebellum | |||||
| 2-week | 0.06±0.01 | 0.05±0.01 | 0.10±0.04** | 0.09±0.00 | 0.08±0.01 |
| 3-week | 0.06±0.01 | 0.06±0.00 | 0.06±0.01 | 0.06±0.01 | 0.06±0.01 |
| 6-week | 0.06±0.01 | 0.06±0.00 | 0.07±0.00* | 0.06±0.01 | |
| Choroid Plexus | |||||
| 2-week | 0.27±0.07 | 0.27±0.06 | 0.85±0.03** | 0.72±0.14 | 0.56±0.09## |
| 3-week | 0.29±0.10 | 0.32±0.09 | 0.62±0.13** | 0.43±0.12 | 0.46±0.11## |
| 6-week | 0.37±0.08 | 0.30±0.11 | 0.42±0.08 | 0.30±0.06## | |
Please see the notes to Table 1 for detailed dose regimen. Data represent mean ± SD, n=6–8.
p<0.05
p<0.01 as compared to the saline group
p<0.05
p<0.01 as compared to the Mn-alone group.
Figure 1.
% Reduction of Mn concentrations in selected brain regions and RBC following PAS chelation therapy. Sprague-Dawley rats in the Mn-alone group received ip. injections of 6 mg Mn/kg, 5 days/week for 4 weeks, followed by sc. saline for 2, 3, or 6 weeks. Rats in the Mn-PAS groups received ip. injections of 6 mg Mn/kg, 5 days/week for 4 weeks, followed by sc. injections of 200 mg PAS/kg for 2, 3, or 6 weeks. Mn concentrations were determined by AAS (n=5–8 in each group). The values in the Mn-PAS group were compared with those in the Mn-alone group and expressed as % of reduction.
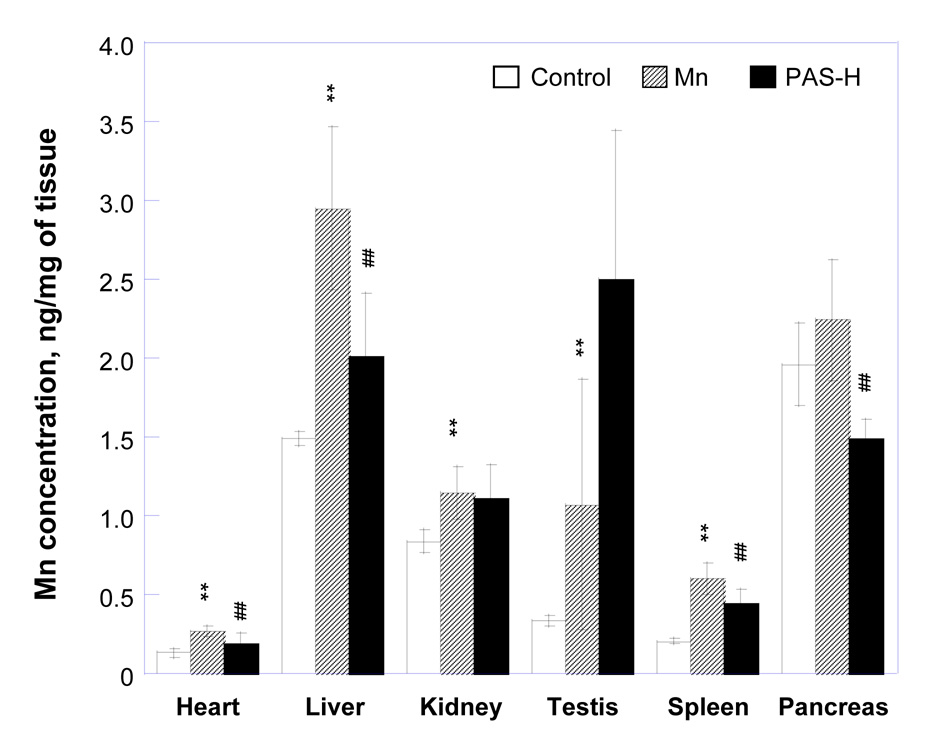
Similar to brain tissues, subchronic Mn exposure markedly increased Mn concentrations in major organs such as heart, liver, kidney, testes, spleen and pancreas even after 6 weeks of cessation of Mn exposure (p<0.01) (data not shown). Dosing with PAS-L did not greatly affect Mn concentrations in these organs in Mn-exposed animals. Treatment with PAS-H for 3 weeks significantly reduced tissue Mn levels by 25% in spleen, 31% in heart, 32% in liver and 33% in pancreas, with no effect on the testes and kidney (Fig. 2). Prolonged treatment with PAS-H for 6 weeks, however, did not further reduce Mn levels in these organs. PAS-H treatment in control rats did not significantly alter Mn concentrations in all organs examined (data not shown).
Figure 2.
Changes of Mn concentrations in major organs of animals exposed to Mn and treated with PAS. Rats in the control group received daily ip. saline injections throughout the entire experiment. Animals in the Mn-alone group received ip. injections of 6 mg Mn/kg, 5 days/week for 4 weeks, followed by sc. saline for 2, 3, or 6 weeks. Rats in the Mn-PAS groups received ip. injections of 6 mg Mn/kg, 5 days/week for 4 weeks, followed by sc. injection of 200 mg PAS/kg for another 2, 3, or 6 weeks. Data represent mean ± SD, n=6–8.*: p<0.05,**: p<0.01 as compared to the saline group; #: p<0.05, ##: p<0.01 as compared to the Mn-alone group.
Fe concentrations in body fluids and tissues of Mn-exposed rats and the effect of PAS treatment
Following Mn exposure, Fe concentration in RBC and CSF were markedly increased as compared to saline controls (p<0.01) (Table 3). While PAS-L treatment for 3 weeks did not affect Fe levels in RBC and CSF, treatment with PAS-H for 2 and 3 weeks significantly reduced Fe levels by 47% and 28%, respectively. In control rats without Mn exposure, PAS-H did not cause any significant effect on Fe concentrations in plasma, RBC or CSF.
Table 3.
Effect of PAS treatment on Fe concentrations in plasma, RBC and CSF of rats subchronically exposed to Mn
| Group | Control Groups |
Mn alone |
Mn + PAS Treatment |
||
|---|---|---|---|---|---|
| Saline | PAS-H | PAS-L | PAS-H | ||
| Plasma (µg/mL) | |||||
| 2-week | 2.43±1.20 | 2.02±0.60 | 2.34±0.11 | 2.39±0.59 | 2.09±0.97 |
| 3-week | 2.39±0.58 | 2.47±0.93 | 2.11±0.69 | 2.15±0.52 | 2.30±0.93 |
| 6-week | 2.78±0.76 | 2.41±0.99 | 2.67±0.88 | 2.21±0.72 | |
| RBC (µg/mg Hb) | |||||
| 2-week | 1.87±0.29 | 1.76±0.25 | 3.75±0.64** | 3.78±0.42 | 3.87±0.27 |
| 3-week | 1.76±0.24 | 1.76±0.48 | 2.99±0.31** | 3.25±0.80 | 3.02±0.38 |
| 6-week | 1.60±0.27 | 1.71±0.18 | 2.34±0.75** | 2.65±0.69 | |
| CSF (ng/mL) | |||||
| 2-week | 69.5±15.4 | 40.6±31.99 | 113.4±33.8** | 86.4±25.3## | 60.0±32.8## |
| 3-week | 68.4±1.58 | 71.6±10.25 | 89.6±16.0** | 78.5±22.6 | 64.7±15.6## |
| 6-week | 74.0±2.99 | 69.1±2.19 | 90.4±36.8** | 79.9±24.3 | |
Please see the notes to Table 1 for detailed dose regimen. Data represent mean ± SD, n=6–8.
p<0.05
p<0.01 as compared to the saline group
p<0.05
p<0.01 as compared to the Mn-alone group.
Two to six weeks following cessation of Mn exposure, Fe concentrations were statistically significantly increased in the hippocampus (23–110% as compared to saline control) and choroid plexus (26–59%), moderately increased in striatum (6–10%), motor cortex (4–29%) and thalamus (13% at post 3 week), and not changed in cerebellum (Table 4). Treatment with PAS-L and PAS-H caused significant reductions in Mn levels by 29.4% and 41.5%, respectively, in the choroid plexus, and 30.9% and 23.0%, respectively, in hippocampus with 2-week treatment, and 27.8% and 19.9%, respectively, in choroid plexus and 51.7% and 53.1%, respectively, in hippocampus with 3-week treatment. Prolonged PAS therapy for 6 weeks did not affect Fe status as compared to Mn-exposed animals (Table 4). Fe levels in all the brain tissues of control rats having received only PAS-H treatment did not show any significant changes, suggesting that PAS treatment by itself did not affect Fe status and the reduction may be a consequence of Mn removal.
Table 4.
Effect of PAS treatment on Fe concentrations in selected brain regions (µg/g) of rats subchronically exposed to Mn
| Group | Control Groups |
Mn alone |
Mn + PAS Treatment |
||
|---|---|---|---|---|---|
| Saline | PAS-H | PAS-L | PAS-H | ||
| Striatum | |||||
| 2-week | 17.4±2.99 | 15.5±0.86 | 17.6±3.44* | 13.7±0.87 | 14.5±1.38 |
| 3-week | 17.5±0.95 | 15.8±2.11 | 18.5±1.91** | 13.7±1.83 | 14.8±2.77# |
| 6-week | 18.5±1.51 | 16.9±3.16 | 20.3±2.82** | 19.2±3.46 | |
| Hippocampus | |||||
| 2-week | 12.4±3.44 | 15.3±2.73 | 19.2±0.88** | 13.2±1.24## | 14.7±1.16## |
| 3-week | 13.2±1.71 | 14.9±1.35 | 27.8±2.41** | 13.4±1.89## | 14.8±1.57## |
| 6-week | 13.9±2.03 | 13.9±2.62 | 17.1±2.25** | 17.8±0.51 | |
| Motor Cortex | |||||
| 2-week | 13.2±1.19 | 14.7±1.88 | 16.4±1.13** | 15.6±1.92 | 17.2±2.51 |
| 3-week | 12.8±1.65 | 16.9±0.98 | 16.5±2.99** | 15.7±2.82 | 16.4±1.35 |
| 6-week | 14.9±2.31 | 14.7±1.26 | 15.5±3.21** | 13.4±5.86 | |
| Thalamus | |||||
| 2-week | 15.9±2.70 | 18.6±1.31 | 16.6±2.06 | 16.5±1.81 | 17.2±2.64 |
| 3-week | 16.6±1.95 | 18.4±3.60 | 18.8±2.98** | 18.2±1.22 | 18.8±2.27 |
| 6-week | 17.1±2.00 | 16.9±1.73 | 16.3±1.53 | 16.8±1.28 | |
| Cerebellum | |||||
| 2-week | 16.2±3.66 | 16.4±1.65 | 16.5±1.07 | 15.7±2.93 | 15.3±2.93 |
| 3-week | 17.6±1.98 | 18.1±1.00 | 16.2±2.65 | 15.5±1.57 | 16.9±2.96 |
| 6-week | 18.6±0.31 | 18.3±2.97 | 17.8±2.91 | 15.6±1.80 | |
| Choroid Plexus | |||||
| 2-week | 87.9±8.50 | 82.4±20.6 | 140.0±9.18** | 98.9±21.7## | 81.9±26.1## |
| 3-week | 80.9±12.2 | 81.5±15.9 | 102.2±20.9* | 73.8±21.2## | 81.7±23.9## |
| 6-week | 79.7±24.2 | 90.1±13.2 | 77.0±19.9* | 68.7±16.9 | |
Please see the notes to Table 1 for detailed dose regimen. Data represent mean ± SD, n=6–8.
p<0.05
p<0.01 as compared to the saline group
p<0.05
p<0.01 as compared to the Mn-alone group.
For all the organs tested, Mn exposure did not significantly affect Fe concentrations except for a 99% increase in the liver (control: 160±62 ng/mg; Mn-treated: 297±65 ng/mg) after 2 weeks and 21% increase in spleen after 6 weeks (control: 729±59 ng/mg; Mn-treated: 885±110 ng/mg). PAS-H therapy significantly reduced these increased Fe levels (liver: 196±44 ng/mg); spleen: 436±127).
Cu concentrations in body fluids and tissues of Mn-exposed rats and the effect of PAS treatment
The concentrations of Cu in plasma were significantly reduced by 14–33% following Mn exposure, whereas they were increased by 31–88% in RBC and 14–17% in the CSF in the same animals at 2, 3, and 6 weeks post Mn dose administration (Table 5). PAS-L treatment had no effect on Cu levels, but PAS-H restored Cu levels to normal in plasma and CSF.
Table 5.
Effect of PAS treatment on Cu concentrations in plasma, RBC and CSF of rats subchronically exposed to Mn
| Group | Control Groups |
Mn alone |
Mn + PAS Treatment |
||
|---|---|---|---|---|---|
| Saline | PAS-H | PAS-L | PAS-H | ||
| Plasma(µg/mL) | |||||
| 2-week | 1.11±0.14 | 1.02±0.18 | 0.95±0.09** | 0.94±0.05 | 1.00±0.06 |
| 3-week | 1.20±0.19 | 1.10±0.11 | 0.92±0.11** | 0.93±0.10 | 1.10±0.09 |
| 6-week | 0.97±0.14 | 1.31±0.04 | 0.65±0.10** | 0.47±0.07## | |
| RBC(ng/mg Hb) | |||||
| 2-week | 1.49±0.36 | 1.49±0.26 | 2.51±0.32** | 2.45±0.14 | 2.72±0.25 |
| 3-week | 1.51±0.22 | 1.44±0.48 | 2.84±0.25** | 2.86±0.25 | 2.62±0.19 |
| 6-week | 1.84±0.23 | 1.79±0.21 | 2.41±0.66** | 1.98±0.11 | |
| CSF(ng/mL) | |||||
| 2-week | 32.8±1.95 | 32.6±2.68 | 31.3±1.12 | 31.3±2.76 | 30.1±2.06 |
| 3-week | 31.6±4.04 | 34.9±4.47 | 37.1±1.32** | 34.8±2.60 | 29.3±3.92## |
| 6-week | 34.5±3.16 | 35.5±2.61 | 39.5±3.52** | 36.9±1.72 | |
Please see the notes to Table 1 for detailed dose regimen. Data represent mean ± SD, n=6–8.
p<0.05
p<0.01 as compared to the saline group
p<0.05
p<0.01 as compared to the Mn-alone group.
Subchronic Mn exposure significantly increased Cu concentrations in striatum by 18–32% compared to saline controls, hippocampus by 18–52%, motor cortex by 25–41%, thalamus by 7–27%, cerebellum by 32% (at 2 weeks post-Mn) and choroid plexus by 16–18% (at 2–3 weeks post-Mn) compared to saline controls (Table 6). Treatment with PAS-L and PAS-H for 2 weeks decreased the Cu levels in striatum (15%–22% of those Mn-alone animal tissues, same below) and choroid plexus (65–69%). Following 3-week treatment of PAS-L and PAS-H, Cu levels were reduced in hippocampus (12–17%), motor cortex (20–21%), and choroid plexus (20% only with PAS-L). PAS-H for 6 weeks resulted in a significant reduction of tissue Cu levels in hippocampus (17%), motor cortex (16%), cerebellum (25%) and choroid plexus (15%).
Table 6.
Effect of PAS treatment on Cu concentrations in selected brain regions (µg/g) of rats subchronically exposed to Mn
| Group | Control Groups |
Mn alone |
Mn + PAS Treatment |
||
|---|---|---|---|---|---|
| Saline | PAS-H | PAS-L | PAS-H | ||
| Striatum | |||||
| 2-week | 2.68±0.28 | 2.71±0.14 | 3.54±0.78** | 3.01±0.18# | 2.76±0.16## |
| 3-week | 2.61±0.12 | 2.21±0.12 | 3.09±0.40* | 2.76±0.80 | 2.75±0.26 |
| 6-week | 2.64±0.19 | 2.69±0.11 | 3.18±0.17* | 2.78±0.46 | |
| Hippocampus | |||||
| 2-week | 1.91±0.049 | 1.82±0.08 | 2.26±0.23* | 2.14±0.15 | 2.22±0.16 |
| 3-week | 1.99±0.06 | 1.76±0.06 | 2.42±0.29** | 2.01±0.22## | 2.14±0.15# |
| 6-week | 1.63±0.064 | 1.82±0.21 | 2.48±0.16* | 2.06±0.32# | |
| Motor Cortex | |||||
| 2-week | 2.41±0.32 | 2.33±0.33 | 2.51±0.26* | 2.56±0.41 | 2.61±0.16 |
| 3-week | 2.20±0.27 | 1.98±0.22 | 2.96±0.47** | 2.36±0.12## | 2.33±0.10## |
| 6-week | 1.93±0.31 | 2.05±0.18 | 2.42±0.16* | 2.04±0.23## | |
| Thalamus | |||||
| 2-week | 1.25±0.09 | 1.44±0.15 | 1.52±0.29** | 1.51±0.13 | 1.50±0.198 |
| 3-week | 1.13±0.10 | 1.29±0.15 | 1.44±0.15** | 1.38±0.16 | 1.30±0.16 |
| 6-week | 1.35±0.04 | 1.27±0.22 | 1.45±0.27* | 1.51±0.08 | |
| Cerebellum | |||||
| 2-week | 2.68±0.32 | 2.64±0.12 | 3.55±0.76** | 2.61±0.35## | 2.31±0.19## |
| 3-week | 2.54±0.09 | 2.34±0.21 | 2.52±0.25 | 2.23±0.16 | 2.35±0.18 |
| 6-week | 2.50±0.12 | 2.54±0.18 | 2.52±0.35 | 1.88±0.27## | |
| Choroid Plexus | |||||
| 2-week | 1.41±0.16 | 1.35±0.31 | 4.02±2.09** | 1.25±0.31## | 1.42±0.28## |
| 3-week | 1.35±0.47 | 1.34±0.16 | 1.56±0.17* | 1.25±0.32## | 1.41±0.30 |
| 6-week | 1.36±0.19 | 1.50±0.35 | 1.32±0.26 | 1.12±0.35## | |
Please see the notes to Table 1 for detailed dose regimen. Data represent mean ± SD, n=6–8.
p<0.05
p<0.01 as compared to the saline group
p<0.05
p<0.01 as compared to the Mn-alone group.
Among 6 organs tested, Cu levels were increased to various degrees in liver, spleen pancreas and kidney. Treatment with PAS-H for 6 weeks did not significantly affect Cu levels in these organs except for pancreas, where a 24% reduction was observed (data not shown).
Discussion
The results of the current study clearly demonstrated that subchronic Mn exposure not only resulted in a marked increase of Mn concentrations in body fluids, brain tissues and major organs, but also caused significant increases of Fe and Cu concentrations in RBC, CSF and selected brain regions. Treatment with PAS evidently reduced Mn levels in selected brain regions as well as in major organs, suggesting a chelating function of PAS, in addition to its known anti-tuberculosis effect. Moreover, PAS therapy appeared to be capable of restoring the concentrations of Fe and Cu in body fluids and tissues close to the normal levels. These observations seem likely in a good agreement with the previously observed effectiveness of PAS in treating manganism patients in clinics (Jiang et al., 2006).
The chelation and ensuing reduction of Mn by PAS possesses several characteristics. First, only the higher dose of PAS was effective in decreasing tissue Mn concentrations. In all body fluids, brain tissues and major organs examined, the treatment with high dose of PAS achieved a far more effective outcome in bringing down Mn levels than did the low dose PAS treatment. This observation is consistent with the results from human studies. As an anti-tuberculosis drug, the recommended oral dosage of PAS is 8–12 gram/day. While PAS is slowly and relatively completed absorbed (Wan et al., 1974), the short blood half-life (t1/2: 2–3 hr) indicates that PAS is quickly removed from the systemic circulation, leaving a relatively low blood level (Peloquin et al., 2001). This blood level may be sufficient for anti-tuberculosis purpose. However, to achieve the therapeutic effect in manganism, the drug is usually given by intravenous infusion at 4–8 gram per day (Jiang et al., 2006; Ky et al., 1992). A high sustainable blood level following a high dose of PAS may allow sufficient PAS molecules to pass across the brain barriers, thus being able to mobilize and remove Mn from its intracellular depots. It is known that Mn is mainly intracellular distributed and accumulated in nuclei (Crossgrove and Zheng, 2004; Kalia et al., 2008); some studies also suggest a sequestration of Mn by mitochondria (Gavin et al. 1999; Liccione and Maines 1988).
Second, the time course of this PAS study suggested that a prolonged PAS treatment was necessary to maintain the reduced Mn concentrations in RBC and brain tissues. Again, this finding is in a good agreement with our previous clinical outcomes where the patients underwent 3-month iv. infusion therapy and showed a visible improvement (Ky et al., 1992; Jiang et al., 2006). The exact reason as to why such a prolonged dose regimen is required remains unknown. Conceivably, however, a continued presence of PAS molecules in the blood stream likely facilitate the movement of Mn from cell compartments such that Mn ions can be made available to be transported to the extracellular fluid and subsequently eliminated from the body. This hypothesis deserves further exploration.
Finally, PAS treatment effectively removed Mn from the CSF, a major component of brain extracellular fluids, as well as from the choroid plexus. The CSF is primarily produced by the choroid plexus in brain ventricles; Mn is known to be transported across this tissue and into the CSF (Michalke et al., 2007; Murphy et al., 1991; Rabin et al., 1993; Zheng et al., 2003). An earlier study by Spector and coworker’s (Spector 1973) showed that PAS enters into the CSF from blood and that the drug molecules can return to the blood from the CSF by an active efflux transport mechanism. The unique ability of PAS in passing across the blood-CSF barrier makes it possible for drug molecules to gain access to Mn ions that are deeply bound to brain targeted cells. In clinics, EDTA has been evaluated for use in the treatment of manganism with unsuccessful results. The lack of symptomatic improvement following EDTA chelation is thought to be due to its four highly water soluble carboxyl groups, which prevent the molecule from effectively crossing the blood-brain barrier. In fact, radiolabeled EDTA is used as an extracellular tracer to monitor the leakage of blood brain barrier (Fenstermacher et al., 1988; Schlageter et al., 1987). Our current data clearly support a beneficial effect of PAS over EDTA for PAS’s ability to reduce Mn in the CSF, choroid plexus as well as other brain regions and systemic organs.
The targeted regions of brain Mn accumulation from this study were the striatum and hippocampus; this observation is consistent with the reports by this and other groups (Lai 1999; Li et al., 2006; Reaney et al., 2006; Roels et al., 1997; Zheng et al., 1999). Similar to early literature data (Ponnapakkam et al., 2003; Thomsen et al., 2004), we found a significant accumulation of Mn in major organs following subchronic Mn exposure. Moreover, we observed a clear chelating effect of PAS in removing Mn from tissues. In a pioneering study by Tandon et al. (1975) in India, PAS was shown to remove 52% of Mn from liver and testis in Mn-exposed rats. In the similar animal model, PAS reduced brain Mn concentration by 26% (Tandon and Singh, 1975). The same investigators also showed that PAS treatment increased Mn excretion in feces by 140% in Mn-exposed rabbits during a 24 hour sample collection (Tandon et al., 1978). However, these early studies did not investigate Mn levels in CSF, RBC, specific brain regions and major organs before and after PAS therapy. Domingo’s group in Spain, however, found no reduction of tissue Mn concentrations by PAS treatment in a mouse model (Sanchez et al., 1995). Noticeably, they treated mice with PAS for only 5 days; such a short period of PAS treatment is unlikely to produce any therapeutic outcomes as is shown by our current results.
Mn exposure is known to be associated with a distorted Fe metabolism at both systemic and cellular levels (Aschner and Aschner, 1990; Li et al., 2004, 2005; Zheng et al., 1999; Zheng and Zhao, 2001). The present study provides the further evidence for increased Fe levels in the CSF and in nearly all brain areas examined except for cerebellum. While Fe concentrations were not altered as a consequence of Mn exposure in most organs tested, the liver indeed displayed a nearly 2-fold increase in Fe levels. Treatment with PAS restored brain Fe to control levels. Interestingly, PAS at the high dose did not seem to affect the normal Mn concentrations in body fluids as well as in most tissues. This characteristic may prove to be a significant clinical advantage, since PAS treatment apparently did not induce Fe deficiency, a phenomenon which is often encountered during metal chelation therapy.
One of the major findings from the current study pertains to a remarkable elevation of Cu concentrations in RBC, CSF, and nearly all brain regions examined, particularly in the choroid plexus where a nearly 3-fold increase was observed. The increased Cu levels in body fluids have been observed among smelters (Jiang et al., 2007) and welders (Wang et al., 2008) as well as in brains of animals exposed to Mn (Guilarte et al., 2006; Lai et al, 1999.) Among smelting workers, the whole blood Cu is increased by 14% (Jiang et al., 2007). Career welders show 18% and 45% increases of Cu in serum and saliva, respectively (Wang et al., 2008). Chronic Mn exposure in non-human primates results in increased Cu concentrations in the basal ganglia (Guilarte et al., 2006). In a rat model, Mn exposure also results in an increase in Cu levels in brain tissues (Lai, 1999). The questions as to how Mn exposure causes elevated Cu status in the CSF and brain tissues and how the increased Cu may lead to neurodegeneration remain unanswered and are exciting research subjects for further investigation. PAS treatment reduced Cu levels in hippocampus and choroid plexus. Similar to the Fe results from this study, PAS treatment in control animals without Mn exposure did not cause any significant changes in Cu concentrations in body fluids, brain tissues and major organs. With this limited information, it is difficult to predict whether PAS is more selective toward Mn than to Fe or Cu. Nonetheless, lack of chelation by PAS of essential elements such as Fe and Cu appear to be an encouraging feature for clinical application of PAS.
The exact mechanism by which PAS chelates Mn is unclear. From the chemistry point of view, PAS structure possesses a carboxyl group, along with hydroxyl and amine groups, providing an ideal chelating moiety for metals. Although our data imply a chelating action of PAS, the conclusive evidence from urinary and fecal elimination of Mn following PAS treatment should be obtained to substantiate PAS chelation function. It is also interesting to investigate the chelating chemistry between PAS, Mn and other metals. Such a study may pave the way for future development of PAS analog for Mn chelation therapy.
In summary, our data show that subchronic exposure to Mn leads to significant accumulation of Mn in targeted brain area. More importantly, an altered Cu homeostasis is observed in the CSF, choroid plexus, striatum and hippocampus following Mn exposure. PAS appears to be effective in reducing Mn concentrations and restoring Fe and Cu concentrations in body fluids and brain tissues to the normal physiological level. A high-dose and prolonged PAS treatment is necessary for its therapeutic effectiveness. These findings support the clinical effectiveness of PAS in treatment of manganism.
Acknowledgements
The authors wish to thank Andrew Monnot, Mamta Behl and Changhe Xiao for their technical assistance during the experiment. This study was supported by NIH/National Institute of Environmental Health Sciences grants RO1 ES08146 and DoD USAMRMC W81XWH-05-1-0239.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Aschner JL. Manganese transport across the blood–brain barrier: relationship to iron homeostasis. Brain Res Bull. 1990;24:857–860. doi: 10.1016/0361-9230(90)90152-p. [DOI] [PubMed] [Google Scholar]
- Aschner M, Nass R, Guilarte TR, Schneider JS, Zheng W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS, Srivastawa RS, Singh H, Gupta VP. An exploratory study of manganese exposure to welders. Clin Toxicol. 1981;18:407–416. doi: 10.3109/15563658108990264. [DOI] [PubMed] [Google Scholar]
- Cook DG, Fahn S, Brait KA. Chronic manganese intoxication. Arch Neurol. 1974;30:59–64. doi: 10.1001/archneur.1974.00490310061010. [DOI] [PubMed] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Brit Ann Med Pharm Vital Stat Gen Sci. 1837;1:41–42. [Google Scholar]
- Crossgrove JS, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004:17544–17553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenstermacher J, Kaye T. Drug "diffusion" within the brain. Ann NY Acad Sci. 1988;531:29–39. doi: 10.1111/j.1749-6632.1988.tb31809.x. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20:445–454. [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hernandez HE, Discalzi G, Valentini C, Venturi F, Chio A, Carmellino C, Rossi L, Sacchetti A, Pira E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neuro Toxicology. 2006;27:333–339. doi: 10.1016/j.neuro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne DB. Chronic manganese intoxication. Arch Neurol. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W, Calne DB. Progression after chronic manganese exposure. Neurology. 1993;43:1479–1483. doi: 10.1212/wnl.43.8.1479. [DOI] [PubMed] [Google Scholar]
- Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med. 2006;48:644–649. doi: 10.1097/01.jom.0000204114.01893.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YM, Zheng W, Long LL, Zhao WJ, Li XG, Mo XA, Lu JP, Fu X, Li WM, Liu SF, Long QY, Huang JL, Pira E. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. NeuroToxicology. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia K, Jiang W, Zheng W. Manganese accumulates primarily in nuclei of cultured brain cells. NeuroToxicology. 2008;29:466–470. doi: 10.1016/j.neuro.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. Neuroimaging in manganism. NeuroToxicology. 2006;27:369–372. doi: 10.1016/j.neuro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W. Effect of levodopa treatment of parkinsonism in welders: A double-blind study. Neurology. 2004;62:730–733. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- Ky SQ, Deng HS, Xie PY, Hu W. A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Br J Ind Med. 1992;49:66–69. doi: 10.1136/oem.49.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. NeuroToxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- Lee JW. Manganese Intoxication. Arch Neurol. 2000;57:597–599. doi: 10.1001/archneur.57.4.597. [DOI] [PubMed] [Google Scholar]
- Li GJ, Zhang LL, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004;46:241–248. doi: 10.1097/01.jom.0000116900.49159.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GJ, Zhao Q, Zheng W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood-CSF barrier in vitro. Toxicol Appl Pharmacol. 2005;205:188–200. doi: 10.1016/j.taap.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GJ, Choi BS, Wang X, Liu J, Waalkes MP, Zheng W. Molecular mechanism of distorted iron regulation in the choroids plexus and selected brain regional capillaries following in vivo manganese exposure. NeuroToxicology. 2006;27:737–744. doi: 10.1016/j.neuro.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liccione J, Maines M. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther. 1988;247:156–161. [PubMed] [Google Scholar]
- Mena I, Court J, Fuenzalida S, Papavasiliou PS, Cotzias GC. Modification of chronic manganese poisoning. Treatment with L-dopa or 5-OH tryptophane. N Engl J Med. 1970;282:5–10. doi: 10.1056/NEJM197001012820102. [DOI] [PubMed] [Google Scholar]
- Michalke B, Berthele A, Mistriotis P, Ochsenkuhn-Petropoulou M, Halbach S. Manganese speciation in human cerebrospinal fluid using CZE coupled to inductively coupled plasma MS. Electrophoresis. 2007;28:1380–1386. doi: 10.1002/elps.200600686. [DOI] [PubMed] [Google Scholar]
- Murphy VA, Wadhwani KC, Smith QR, Rapoport SI. Saturable transport of manganese(II) across the rat blood-brain barrier. J Neurochem. 1991;57:948–954. doi: 10.1111/j.1471-4159.1991.tb08242.x. [DOI] [PubMed] [Google Scholar]
- Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, London L, Esswein E, Renton K, Spies A, Boulle A, Naik I, Iregren A, Rees DJ. The nervous system effects of occupational exposure on workers in a South African manganese smelter. NeuroToxicology. 2003;24:885–894. doi: 10.1016/S0161-813X(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci. 1999;162:102–105. doi: 10.1016/s0022-510x(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Ono K, Komai K, Yamada M. Myoclonic involuntary movement associated with chronic manganese poisoning. J Neurol Sci. 2002;199:93–96. doi: 10.1016/s0022-510x(02)00111-9. [DOI] [PubMed] [Google Scholar]
- Peloquin CA, Zhu M, Adam RD, Singleton MD, Nix DE. Pharmacokinetics of para-aminosalicylic acid granules under four dosing conditions. Ann Pharmacother. 2001;35:1332–1338. doi: 10.1345/aph.1A088. [DOI] [PubMed] [Google Scholar]
- Physicians’ Desk Reference. Medical Economics Company; Montvale: 2000. pp. 1443–1444. [Google Scholar]
- Ponnapakkam T, Iszard M, Henry-Sam G. Effects of oral administration of manganese on the kidneys and urinary bladder of Sprague-Dawley rats. Int J Toxicol. 2003;22:227–232. doi: 10.1080/10915810305103. [DOI] [PubMed] [Google Scholar]
- Rabin O, Hegedus L, Bourre JM, Smith QR. Rapid brain uptake of manganese(II) across the blood-brain barrier. J Neurochem. 1993;61:509–517. doi: 10.1111/j.1471-4159.1993.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol Sci. 2006;93:114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- Rengarajan J, Sassetti CM, Naroditskaya V, Sloutsky A, Bloom BR, Rubin EJ. The folate pathway is a target for resistance to the drug para-aminosalicylic acid (PAS) in mycobacteria. Mol Microbiol. 2004;53:275–282. doi: 10.1111/j.1365-2958.2004.04120.x. [DOI] [PubMed] [Google Scholar]
- Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet JP, Lison D. Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol. 1997;71:223–230. doi: 10.1007/s002040050380. [DOI] [PubMed] [Google Scholar]
- Rosenstock HA, Simons DG, Meyer JS. Chronic manganism. Neurologic and laboratory studies during treatment with levodopa. JAMA. 1971;217:1354–1358. doi: 10.1001/jama.217.10.1354. [DOI] [PubMed] [Google Scholar]
- Sanchez DJ, Gomez M, Domingo JL, Llobet JM, Corbella J. Relative efficacy of chelating agents on excretion and tissue distribution of manganese in mice. J Appl Toxicol. 1995;15:285–288. doi: 10.1002/jat.2550150409. [DOI] [PubMed] [Google Scholar]
- Schlageter NL, Carson RE, Rapoport SI. Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:1–8. doi: 10.1038/jcbfm.1987.1. [DOI] [PubMed] [Google Scholar]
- Seth PK, Husain R, Mushtaq M, Chandra SV. Effect of manganese on neonatal rat: Manganese concentration and enzymatic alterations in brain. Acta Pharmacol Toxicol. 1977;40:553–560. [PubMed] [Google Scholar]
- Seth PK, Hong JS, Kilts CD, Bondy SC. Alteration of cerebral neurotransmitter receptor function by exposure of rats to manganese. Toxicol Lett. 1981;9:247–254. doi: 10.1016/0378-4274(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Spector R, Loremzo AV. The active transport of para-aminosalicylic acid from the cerebrospinal fluid. J Pharm Exp Ther. 1973;85:642–648. [PubMed] [Google Scholar]
- Tandon SK, Chandra SV, Singh J, Husain R, Seth PK. Chelation in metal intoxication. I. In vivo effect of chelating agents on liver and testes of manganese administered rats. Environ. Res. 1975;9:18–25. doi: 10.1016/0013-9351(75)90045-6. [DOI] [PubMed] [Google Scholar]
- Tandon SK, Singh J. Removal of manganese by chelating agents from brain and liver of manganese treated rats: An in vitro and an in vivo study. Toxicology. 1975;5:237–241. doi: 10.1016/0300-483x(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Tandon SK. Chelation in metal intoxication. VI. Influence of PAS and CDTA on the excretion of manganese in rabbits given MnO2. Toxicology. 1978;9:379–385. doi: 10.1016/0300-483x(78)90021-5. [DOI] [PubMed] [Google Scholar]
- Thomsen HS, Svendsen O, Klastrup S. Increased manganese concentration in the liver after oral intake. Acad Radiol. 2004;11:38–44. doi: 10.1016/s1076-6332(03)00571-3. [DOI] [PubMed] [Google Scholar]
- Wan SH, Pentikainen P, Azarnoff DL. Bioavailability studies on para-aminosalicylic acid and its various salts in man. I. Absorption from solution and suspension. J Pharmacokinet Pharmacodyn. 1974;2:1–12. [Google Scholar]
- Wang XQ, Miller DS, Zheng W. Intracellular trafficking of metal transporters in intact rat choroid plexus following in vitro treatment of manganese or iron. Toxicol Appl Pharmacol. 2008a;230:167–174. doi: 10.1016/j.taap.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li GJ, Zheng W. Efflux of iron from the cerebrospinal fluid to the blood at the blood-CSF barrier: Effect of manganese exposure. 2008b. Submitted. [DOI] [PMC free article] [PubMed]
- Yamada M, Ohno S, Okayasu I, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol (Berl) 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Slavkovich V, Zheng W. Lead exposure promotes translocation of protein kinase C activity in rat choroid plexus in vitro, but not in vivo. Toxicol Appl Pharmacol. 1998;149:99–106. doi: 10.1006/taap.1997.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Shen H, Blaner SB, Zhao Q, Ren X, Graziano JH. Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: The role of the choroid plexus. Toxicol Appl Pharmacol. 1996;139:445–50. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: A mechanism of manganese neurotoxicity. Brain Res. 1998;799:334–342. doi: 10.1016/s0006-8993(98)00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q. Iron overload following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res. 2001;897:175–179. doi: 10.1016/s0006-8993(01)02049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]