Abstract
Chronic oxidative stress has been associated with genomic instability following exposure to ionizing radiation (IR). However, data showing direct causal linkages between specific reactive oxygen species and the IR-induced mutator phenotype are lacking. The current study demonstrates that IR-induced genomically unstable cells (characterized by chromosomal instability and increased mutation and gene amplification frequencies) show a 3-fold increase in steady-state levels of hydrogen peroxide, but not superoxide. Furthermore, stable clones isolated from parallel studies showed significant increases in catalase and glutathione-peroxidase activity. Treatment of unstable cells with polyethylene glycol-conjugated catalase [PEG-CAT] reduced the mutation frequency and mutation rate in a dose dependent fashion. In addition, inhibiting catalase activity in the stable clones using 3-aminotriazole increased mutation frequency and rate. These results clearly demonstrate the causal relationship between chronic oxidative stress mediated by hydrogen peroxide and the mutator phenotype that persists many generations following exposure of mammalian cells to IR.
Keywords: Ionizing radiation, genomic instability, mutation rate, oxidative stress, Catalase
Introduction
For many years, it was thought that genetic changes caused by radiation were only due to DNA damage derived from the incident free radical species produced at the time of exposure. However, recently it has been discovered that the number of surviving cells exhibiting increased delayed mutation frequencies following IR is greater than would be predicted if only the cells exposed at the time of irradiation were involved [1,2]. In other words, the mutagenic effects of IR were found to be trans-generational. These persistent trans-generational mutagenic effects, apparently resulting from a mutator phenotype, have collectively been called non-targeted or bystander effects of radiation but the mechanisms governing these phenomena are not well understood [1,2].
Chronic oxidative stress mediated by continuous exposure to H2O2 or 95% O2 has been shown to induce genomic instability and gene amplification in hamster fibroblasts [3]. Additional studies investigating the cause of IR-induced genomic instability have suggested that persistent metabolic oxidative stress may be involved [4,5]. Hydroperoxides produced by metabolic processes have been suggested to contribute to persistent IR-induced genomic instability [6,7] based on data gathered using non-specific oxidation sensitive probes. However, direct causal linkages between increased steady-state levels of specific reactive oxygen species such as H2O2 and the mutator phenotype characterized by increased rates of delayed mutagenesis following exposure to IR are lacking.
In the current study, three subsets of Chinese hamster ovary fibroblasts (GM10115 cells) were used to determine the involvement of H2O2 in the IR-induced mutator phenotype and included: 1) the untreated parental GM10115 cell line, 2) unstable GM10115 clones designated CS-9 and LS-12, and 3) stable clones designated 114 and 118 [8]. We now show that the unstable clones not only exhibit increased mutation frequency and rates, but were found to produce greater steady-state levels of H2O2 relative to the parental cell line and stable clones. The stable clones were found to have increased catalase and glutathione peroxidase activities, relative to the unstable clones. Furthermore, both increased mutation frequency and increased mutation rates in the unstable clones were suppressed by a cell permeant H2O2 scavenging enzyme [PEG-CAT]. Mutation frequencies and rates could be increased in the stable clones treated with the catalase inhibitor, 3-aminotriazole. These results provide unambiguous evidence showing that H2O2 mediates the persistent mutator phenotype in these mammalian cells and suggest that scavengers of H2O2 could be used to inhibit chronic indications of genomic instability persisting many generations after exposure to a range of cytotoxic treatments.
Materials and methods
Cell culture
Chinese hamster ovary parental cell line GM10115 was obtained from ATCC. Cell stock flasks were grown in DMEM with high glucose and 1 mM sodium pyruvate (CellGrow, Herndon, VA), 10% FBS (Hyclone), 0.2 mM L-proline and 1% L-glutamine (Gibco). Previously described stable (114, 118) and unstable (CS-9, LS-12) clones were grown in the same media [8].
Mutation frequency assay
Three million cells from an exponentially growing culture were seeded onto 100 mm dishes. 6-thioguanine (Sigma, St Louis, MO) dissolved in DMSO was added at a final concentration of 40 µM. The dishes were left undisturbed for 2–3 three week until colonies with at least 50 cells appeared. The colonies were then fixed, stained and counted. Data were normalized to plating efficiency of identically treated cells in the absence of 6-thioguanine.
Carbamoyl-P synthetase/aspartate transcarbamylase/dihydroorotase (CAD) gene amplification assay
Amplification at the CAD gene locus was determined by plating 3.5 × 105 cells on a 100 mm dish. Media containing 100 µM PALA (N- phosphonoacetyl-L-aspartate) (Developmental Therapeutics Program, NCI/NIH) was added and dishes were placed in a 37°C incubator for 2 weeks. Colonies were then fixed stained and counted. Number of colonies was normalized to the plating efficiency.
Oxidation of 5-(and-6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate (c-DCFH2) as an indicator of steady-state levels of pro-oxidants
Cells were seeded (3.5 × 105) on a 60 mm dish in complete media (three replicates for each clone) and grown for 2 days. On day two, the cells were washed with phosphate buffered saline (PBS), following which 2 mL of PBS was added to each dish, then the dishes were incubated with 10 µg/mL c-DCFH (Invitrogen, Carlsbad, CA) (or the oxidation insensitive analog c-DCF) for 15 min at 37°C. The cells were then washed, trypsinized and centrifuged at 400 × g for 5 min. The pellet was re-suspended in 500 µL of PBS and filtered through a mesh to remove large aggregates. 10,000 live cells (gated on forward and side scatter) were analyzed for DCFH fluorescence (FL1 channel, λexcitation = 488 nm, λemission = 530 nm, 30 nm bandpass) using a Beckton Dickinson FACScan. DMSO (0.1% v/v) and antimycin A (10 µM) dissolved in DMSO were used as negative and positive controls, respectively. CellQuest Pro software was used for data analysis.
Oxidation of DHE as an indicator of steady-state levels of superoxide
Cells were seeded (3.5 × 105) on a 60 mm dishes (three replicates for each clone) and grown for two days in complete media. At the time of labeling the plates were washed with PBS and incubated at 37°C for 40 min with 10 µM DHE (Invitrogen) DMSO (0.1% v/v) in 2 mL PBS containing 5 mM pyruvate. The cells were then washed, trypsinized and centrifuged at 400 × g for 5 min. The pellet was re-suspended in 500 µL of PBS and filtered through a mesh to remove large aggregates. 10,000 live cells gated on forward and side scatter were analyzed for DHE fluorescence using a Beckton Dickinson FACScan (FL2 channel, λexcitation 488 nm, λemission 585 nm, 30 nm bandpass). DMSO (0.1% v/v) and antimycin A (10 µM) dissolved in DMSO were used as negative and positive controls, respectively. CellQuest Pro software was used for data analysis.
Extracellular hydrogen peroxide
Hydrogen peroxide assay was carried out as previously described [9]. Exponentially growing cells were washed with phenol red free HBSS three times. This was followed by the addition of glucose (final concentration 6.2 mM), HEPES (final concentration 1.0 mM), sodium bicarbonate (final concentration 6.0 mM), p-hydroxyphenylacetic acid (p-HPA, Sigma) (final concentration 1.6 mM) and HRP (final concentration 95 µg/mL). HBSS was used to bring the final volume to 1 mL. The cells were incubated at 37°C for 30 min during which time H2O2 accumulated in the extracellular medium. In case of catalase samples to verify the signal was coming from H2O2, the dishes were incubated with catalase (1000 units) for 30 min prior to the addition of reaction mixture. The media was collected in a cuvette and the release of H2O2 was followed fluorometrically (Perkin Elmer LS50B) by measuring the p-HPA dimer formed at excitation and emission wavelengths of 320 and 408 nm, respectively. Quantitation was accomplished using comparison to a standard curve obtained from genuine H2O2. The pH of the samples was checked following incubation and did not change significantly.
Isolation of mitochondria
Mitochondria were isolated from cells using density gradient centrifugation with a sucrose gradient in isolation buffer (0.25 M sucrose, 5 mM HEPES, 0.1 mM EDTA pH 7.25) [10]. Exponentially growing cells were harvested and centrifuged at 500 × g. The pellet was re-suspended in isolation buffer and homogenized using a Dounce homogenizer rinsed with dilute HCl followed by distilled water. Cell debris was separated by centrifugation at 1000 × g for 10 min. The supernatant was collected from this step and centrifuged at 10,000 × g for 10 min. The mitochondrial pellet was re-suspended in 500 µL isolation buffer and used for further analysis. Protein was determined using Bio-Rad protein assay protocol.
EPR
EPR analysis was carried out on isolated mitochondria using a Bruker EMX spectrometer. Mitochondrial protein samples (100 µg) were incubated with the DMPO spin trap (150 mM) for 2 min at room temperature. This was followed by 10 min incubation with 9 mM succinate (pH adjusted to 7.4) at 37°C. To ascertain that the DMPO-OH EPR signal observed was indeed due to superoxide, CuZnSOD (1000 U) was added 2 min prior to the addition of DMPO to suppress EPR signal. The part of the signal that was suppressed by the enzyme was considered a true measure of superoxide and thus this portion was plotted. Spectra were collected using TM cavity and a flat cell. Spectra were quantified by measuring the signal intensity of the two center lines of DMPO-OH spectra (aN = 14.9 G and aH = 14.9 G) and averaging these values [11]. The instrument parameters used were: sweep width 80 G, power 40 mW, ν = 9.79 GHz, scan rate 82 s, modulation amplitude 1 G, time constant 82 ms, number of scans = 10.
GPx activity
To make cell homogenates, 50 mM potassium phosphate buffer pH 7.8 containing 1.34 mM diethylenetriaminepentaacetic acid (DETAPAC) was added to cell pellets following 1 freeze thaw cycle. GPx-1 activity was measured using cumene hydroperoxide (Sigma) as the substrate by monitoring the disappearance of NADPH at 340 nm as described previously. Data was normalized per mg protein as determined by the Lowry protein assay [12,13].
Catalase assay
Catalase activity was determined by monitoring the disappearance of 10 mM H2O2 in reactions containing cell homogenate as previously described [14]. Activity was expressed in mκunits/milligram protein.
GSH measurements
Anderson and Griffith’s [15,16] recycling assay was used to determine glutathione content in cell homogenates and normalized to protein content. GSSG was determined by incubating the sample with 2-vinyl pyridine as previously described. GSH and GSSG concentrations were reported in GSH equivalents using a standard curve.
SOD activity assay
SOD activity assay was done as previously described [17]. Inhibition of nitro blue tetrazolium (NBT) reduction was used as a marker of SOD activity. Samples were run either in presence of 5 mM cyanide (for MnSOD activity) or in the absence of cyanide (for total SOD activity). MnSOD activity was subtracted from the total and the errors were propagated to obtain CuZnSOD activity.
PEG-CAT treatment
Exponentially growing cells (3 × 106) were seeded in 100 mm dishes and 100 U/mL PEG-CAT (Sigma) (reconstituted in PBS) was added to each dish 2h prior to the addition of 6-thioguanine. PEG was added at an equivalent concentration as the conjugated enzyme to control for any effects not specific to the enzyme activity.
Aminotriazole treatment
Cells were grown in complete media with 20 mM 3-aminotriazole (AT) for 4 days. On the fourth day, cells were trypsinized, counted and 3×106 cells were seeded in media containing 40 µM 6-thioguanine without AT. After 2 weeks colonies were stained and counted. The effect AT had on the plating efficiency of cells was corrected for in the mutation frequency assay.
Mutation Rate Assay
Cells were selected in media containing HAT (CellGrow) (hypoxanthine, aminopterin, thymidine) for 3 weeks. Following this, cells were grown in complete medium without HAT and mutation frequency was assayed immediately following removal from HAT selection (day 0) as well as after 10–15 days of passage in the absence of HAT. Mutation frequency was divided by the number of days out of selection to determine mutation rate/106 cells/day. The mutation rates for comparable groups were determined on the exact same day [18].
Statistics
Statistics were performed using one-way ANOVA for non-parametric measurements. Tukey post-hoc test was performed wherever appropriate. Results were considered significantly different if p ≤ 0.05 unless otherwise mentioned. All statistical tests were done using GraphPad Prism software (San Diego, CA)
Results
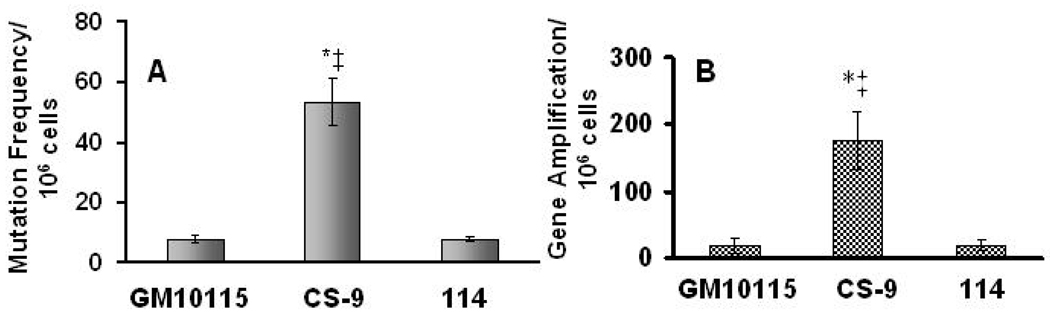
Clones used in this study were originally described by Limoli et al 1997. Briefly, colonies derived from single cells surviving exposure to 10 Gy, were expanded and analyzed for multiple endpoints of genomic instability. To confirm past findings, and to verify that an unstable vs stable clone possessed a mutator phenotype, cells were analyzed for mutation and gene amplification frequencies. Our data shows that the unstable clone CS9 exhibits higher (4- to 5- fold) mutation and gene amplification frequencies at the hprt and CAD gene loci respectively, relative to the parental GM10115 and stable 114 clone (Fig 1, Panel A and B).
Figure 1. CS-9 cells show increased genomic instability.
Panel A: To measure mutation frequency, 3 × 106 cells were plated in 100 mm dishes containing complete media and 40 µM 6-thioguanine. Dishes were left undisturbed in a 37°C incubator and colonies were counted after 2 weeks. Error bars represent ± 1 SD from 5 treatment dishes. p<0.01 * vs wild-type, ‡ vs 114. Panel B: To measure CAD gene amplification, 0.35 × 106 cells were plated on a 100 mm dish and media containing 100 µM PALA was added. Colonies were counted after 2 weeks. The error bars represent ± 1 SD from 6 dishes for GM10115 and CS-9, and 3 separate dishes for 114. p<0.01 * vs wild-type, ‡ vs 114.
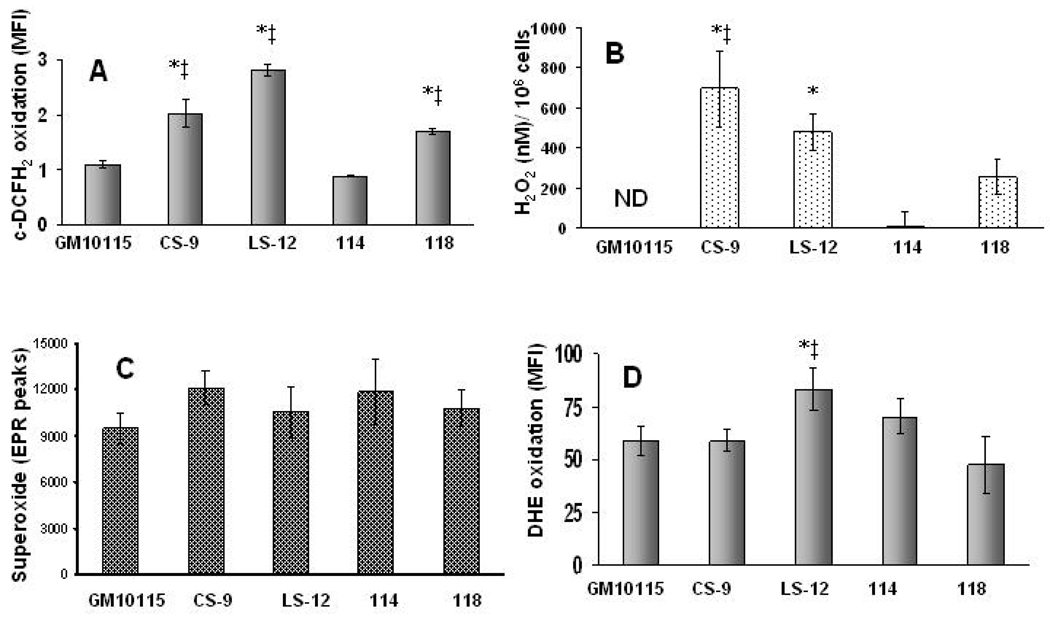
When steady-state levels of pro-oxidants were determined, the unstable clones (CS-9 and LS-12) demonstrated significantly increased c-DCFH2 oxidation relative to the parental and the stable clone 114 (Fig 2A). These changes in c-DCFH2 fluorescence were confirmed to be truly indicative of changes in steady-state pro-oxidant levels (and not changes in probe uptake, ester cleavage, or efflux) because no significant changes in fluorescence were noted in co-cultures labeled with the oxidation insensitive analog, c-DCF (data not shown). Consistent with the c-DCFH2 results, when a specific measure of extracelluar H2O2 production was determined (catalase inhibitable p-HPA oxidation) the unstable clones (CS-9 and LS-12) were found to produce significantly more H2O2, relative to the parental and stable 114 clone (Fig 2B). None of the unstable clones showed a consistent change in steady-state levels of superoxide in isolated mitochondria as determined by SOD inhibitable EPR spectroscopy (Fig 2C) and only LS-12 showed a modest change in superoxide sensitive DHE oxidation (Fig 2D). These results show that the unstable clones (CS-9 and LS-12) appear to demonstrate increases in steady-state levels of H2O2, relative to the parental and 114 stable clones. Interestingly the stable 118 clone showed what appeared to be some increase in H2O2 as well. However, since the intracellular redox status is also governed by antioxidant enzymes, the activities of primary antioxidant enzymes responsible for detoxification of superoxide (i.e. SOD) and hydrogen peroxide (i.e. GPx and catalase) were investigated.
Figure 2. Genomically unstable clones show increased steady-state levels of ROS.
Panel A: Intracellular pro-oxidant status of cells was assessed using c-DCFH2 staining. Cells were labeled with 10 µg/mL c-DCFH2 for 15 min at 37°C. Error bars represent ± 1 SD from 3 dishes. p<0.05 * vs wild-type, ‡ vs 114. Panel B: H2O2 produced by cells was measured in the medium using the catalase inhibitable p-HPA fluorescence. The error bars represent ± SEM of 6 dishes. ND stands for non-detectable. p<0.05 * vs wild-type, ‡ vs 114. Panel C: Superoxide was measured as the SOD inhibitable DMPO-OH EPR signal from isolated mitochondria from each cell line. The error bars represent ± SEM from 3 independently harvested samples. No statistical difference between cell lines was detected (p>0.05). Panel D: Estimation of intracellular superoxide using DHE oxidation. Cells were labeled with 10 µM DHE at 37°C for 40 min. Error bars represent ± 1 SD of 5 dishes from each cell line. p<0.05 * vs wild-type, ‡ vs 118.
Antioxidant enzyme analysis showed that the activities of H2O2 scavenging enzymes (Cat and GPx) were significantly elevated in the stable clones 114 and 118, relative to the wild type and unstable clones (Fig 3, panel A). On the other hand, Cat and GPx activities in the unstable clones were either reduced or comparable to the wild-type. Since GPx activity is affected by intracellular availability of reduced glutathione, glutathione levels were evaluated in these cell lines (Fig 3, panel B). Consistent with increases in GPx activity, the stable clones had increases in GSH levels, relative to the wild type. It had been proposed that the product of intracellular GSH and GPx activity gives a more accurate reflection of “effective GPx activity” [19], which is a better indicator of the H2O2 detoxifying capacity of the cell. On doing this calculation and propagating the errors in both measurements, we observed that the effective GPx activity was greatly elevated in both the stable clones (Fig 3, panel C). In addition, Fig 3, panel D, shows that there was no significant difference in either MnSOD or CuZnSOD activities in these clones. The results of the antioxidant enzyme analysis suggest that hydrogen peroxide removal may play a more critical role (as compared to superoxide removal) in governing radiation-induced genomic instability.
Figure 3. Hydroperoxide scavenging enzyme activity is increased in genomically stable clones.
Panel A: Glutathione peroxidase (GPx) and catalase activities were analyzed in cell homogenates and normalized to protein content. Data are represented as a fold change relative to the GM10115. The error bars for GPx activity represent ± 1 SD from 4 samples. p<0.05 * vs wild-type, ‡ vs CS-9. The error bars for catalase activity represent ± 1 SD from 3 independently harvested samples from each group. p<0.05 * vs wild-type, ‡ vs CS-9. Panel B: GSH was measured using the DTNB-recycling assay. Error bars represent ± 1 SD from 5 samples. p<0.05, * vs GM10115, ‡ vs CS-9 Panel C: Using data in panel A and C, effective GPx activity was calculated [19]. The errors in the GPx and GSH measurements were used to calculate the error for the effective GPx measurement using propagation of error theory. p<0.05, * vs GM10115, ‡ vs CS-9. Panel D: CuZnSOD and MnSOD activities were measured in whole cell lysates using inhibition of NBT reduction as an indicator. Error bars represent ± 1 SD from 4 samples. No statistical differences were noted among the groups (p>0.05).
Together, this data suggests that increases in steady-state levels of H2O2 may be causally related to radiation-induced genomic instability. To test this hypothesis the mutation frequency assay was repeated in the presence of 100 U/mL polyethylene glycol (PEG) conjugated catalase (CAT) using the unstable CS-9 cells. Fig 4 A demonstrates a dramatic suppression of mutation frequency in the PEG-CAT treated CS-9 cells, which also showed a 2-fold increase in catalase activity, relative to untreated cells. When the experiment was repeated using PEG alone, no suppression of mutation frequency was observed (data not shown) providing clear evidence for the role of catalase enzyme activity in the suppression of the mutator phenotype. Furthermore, PEG-CAT treatment demonstrated a dose response relationship to suppression of mutation frequency (Fig 4 B). To further confirm the causal role of H2O2 in radiation-induced genomic instability, catalase activity was inhibited in the 114 stable clone using 3-aminotriazole (AT) and the 6-thioguanine mutation frequency assay was repeated. When 114 cells were grown in the presence of 20 mM AT for four days (85–90% inhibition of catalase activity) and then plated into 6-thioguanine for 2 weeks mutation frequency was significantly increased 2–2.5-fold (Fig 4C).
Figure 4. H2O2 causes radiation-induced delayed mutagenesis.
Panel A: PEG-catalase (100 U/mL) was added to CS-9 cells 2 h prior to the addition of 6-thioguanine during the mutation frequency assay and left for 2-weeks. The results represent ± 1 SD from 4 samples. p<0.001 * vs CS-9. Panel B: The suppressive effect that PEG Cat had on mutation frequency in CS-9 was dose dependent. The results represent the average of 2 samples in the 25 U/mL as well as 50 U/mL group and 4 samples done in the 100 U/mL group. Panel C: The stable clone114 was grown in presence of 3-aminotriazole for 4 days. On the fourth day, treated cells were plated in presence of 6-thioguanine for the mutation frequency assay. The error bars represent ± 1 SD from 3 experiments. p<0.01 * vs 114. Panel D: The mutation rate in stable and unstable cells with or without manipulation to catalase activity was determined following selection in HAT media. The rates represent mutation frequency/106 cells/day. Each data set represents the average of 3 separate dishes. p<0.05, * vs GM10115, ‡ vs CS-9, £ vs 114, ε vs 118.
Since the mutator phenotype induced following exposure to radiation is believed to be a dynamic self perpetuating process, the next series of experiments were designed to determine mutation rates (as opposed to mutation frequency) in populations of genomically stable and unstable clones with or without manipulation of catalase activity. The cell populations were cleansed of pre-existing mutations at the hprt locus by passage in complete media supplemented with 1× HAT for three weeks. Following HAT selection, cells were grown with or without manipulation of catalase activity for 10–15 days and then plated for mutation frequency assay. Mutation frequency at a given time was divided by the number of days out of HAT selection to obtain the mutation rate. The results in Fig 4D show that the baseline mutation rate of the unstable clone (CS-9) was significantly higher than both wild-type (17-fold) and the stable clones (5- to 10-fold). Consistent with the hypothesis that H2O2 is causally related to the radiation-induced mutator phenotype, treatment with 100 U/mL PEG-CAT completely suppressed the increased mutation rate demonstrated by CS-9. In addition, treatment of the stable clones (114 and 118) with the catalase inhibitor AT, significantly increased mutation rates to a level greater than the wild-type. Infact, AT-treated 118 clone demonstrated mutation rates similar to the CS-9 unstable clone (Fig 4D). Interestingly, the 114 clone which had lower steady-state levels of H2O2 (Fig 2B) demonstrated a smaller increase in mutation rate with AT treatment (Fig 4D) when compared to the stable clones 118 which had a higher baseline steady-state levels of H2O2 (Fig 2B and Fig 4D) and showed a greater increase in peroxide levels following AT treatment (data not shown). This data clearly shows that radiation-induced genomic instability is a dynamic process that can be manipulated many generations following radiation exposure by altering the activity of the specific H2O2 scavenging enzyme catalase.
Discussion
Several theories have been proposed to explain the trans-generational nature of radiation-induced mutator phenotypes with the “DNA repair hypothesis” being one of the most prominent [20,1]. According to this theory, a mutator phenotype can arise if exposure to radiation damages a critical gene coding for a protein that plays a role in DNA repair. In such a case, it is thought that cells would continue to show increased mutagenesis that would persist as a trans-generational phenotype. While some evidence in favor of this theory has accumulated [21], the small “target size” presented by the genes encoding repair proteins coupled with uncertainty surrounding the underlying source of spontaneous DNA damage, make it unlikely that repair genes are the only targets for radiation-induced mutator phenotypes [Reviewed in [22]]. Further support of this idea comes from past work showing the frequency of IR-induced genomic instability in any surviving clone to be ~3% per Gy [23], an incidence far too high to be explained by single (or even multiple) hit theories. It has also been shown by a rigorous microarray analysis that even though the genes associated with various signaling pathways are differentially downregulated in the unstable clones, no single signaling pathway or repair gene correlated with a particular end-point of genomic instability [24].
Since genomic instability has also been associated with metabolic oxidative stress, a possible additional target for IR-induced genomic instability could be genes coding for proteins involved in metabolic processes capable of producing ROS [3,6,22,25,26]. This hypothesis is supported by the fact that the progeny of irradiated cells show some evidence dysfunctional mitochondria [6] and oxidative stress [4,6,7]. In addition, there have been recent reports that pretreatment with free radical scavengers is capable of inhibiting the occurrence of radiation-induced genomic instability [27]. However until now, studies identifying the specific ROS that cause persistent trans-generational radiation-induced mutator phenotypes were lacking.
The present study focused on identifying the role of O2•− and H2O2 in the mechanism of radiation-induced genomic instability. The results showed no consistent changes in steady-state levels of O2•− or the superoxide scavenging enzymes, CuZnSOD and MnSOD, in unstable vs stable clones. In contrast, radiation-induced genomically unstable cell lines demonstrated significantly increased steady-state levels of H2O2. Analysis of, genomically stable cell lines did however show a significant increase in the H2O2 scavenging enzymes, GPx and catalase. When the unstable cells were treated with a cell permeant specific scavenger of H2O2 (PEG-catalase) mutation frequency and mutation rates were dramatically suppressed. In addition, when catalase activity was inhibited in stable clones, mutation frequency and mutation rates were significantly increased. These results conclusively identify H2O2 as the primary cause of the radiation-induced mutator phenotype in this model system.
The identification of intracellular H2O2 as being causally involved with radiation-induced trans-generational genomic instability provides a mechanistic backdrop for the mutator phenotype hypothesis and suggests the critical importance of oxidative metabolic processes [7,20]. The central dogma in mammalian cell biology is that genetic material (DNA) undergoes transcription and translation to make functional proteins that execute essential cellular processes (i.e., production of energy and reducing equivalents necessary for metabolism and biosynthesis). In Fig 5 we show a theoretical model by which oxidative metabolism leading to the production of H2O2 can be incorporated into the mutator phenotype hypothesis. In this model, radiation can damage genes coding for proteins involved in either oxidative metabolism or DNA repair. When radiation damages genes coding for proteins capable of resulting in increased levels of H2O2, a heritable change in steady-state levels of pro-oxidants leading to metabolic oxidative stress could occur. The resulting pro-oxidant intracellular environment could then perpetuate the process of mutagenesis leading to genomic instability. Additional perturbation to the redox homeostasis in cells could increase stress induced damage to genes coding for DNA repair and further exacerbate genomic instability [20,21,28]. This model would predict a feed-forward loop where radiation-induced metabolic oxidative stress in concert with damage to DNA repair machinery, could progressively accelerate genomic destabilization leading to the plethora of deleterious biological effects associated with late radiation damage. This model also significantly increases the effective target size for heritable damage capable of leading to genomic instability which is consistent with the prevalence and variety of unstable phenotypes that have been observed following radiation.
Figure 5. Model by which oxidative metabolism can be incorporated into the mutator phenotype hypothesis to explain the progression of radiation-induced genomic instability.
This model (Fig 5) also predicts that cellular and dietary antioxidants specifically directed at mitigating the effects of hydroperoxides could rescue mammalian cells from this fate, effectively breaking the feed forward loop and thereby slowing the progression of the radiation-induced mutator phenotype. Antioxidants can slow the rate of oxidative damage and might also allow for high fidelity repair processes to work more effectively. The net result might be that in the presence of antioxidants, high fidelity repair capacity could offset the rate of damage, significantly reducing the progression of unstable phenotypes. Since genomic instability is thought to be an early step in tumorigenesis as well as degenerative diseases associated with aging, using specific scavengers of hydroperoxides to abrogate deleterious effects of radiation, provide an attractive biochemical rationale for the development of strategies to mitigate a broad range of radiation-induced effects.
Acknowledgements
This work is supported by the US department of energy DE-FG02-05ER64050, RO1CA100045, P30-CA086862. We would also like to thank Ling Li, Mitchell C. Coleman and Nükhet-Aykin Burns for helpful discussions regarding the antioxidant enzyme assays, Kjerstin M. Owens for helpful discussions related to the mutation rate assay, Melissa A. Fath for useful discussions regarding the EPR protocol, and Kellie L. Bodeker for editorial assistance. We would also like to thank the Flow Cytometry facility and the ESR facility at the University of Iowa.
Abbreviations
- AT
3-aminotriazole
- CAD
carbamoyl-P synthetase/aspartate transcarbamylase/dihydroorotase
- DHE
Dihydroethidium
- c-DCFH2
5-(and-6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- EPR
Electron paramagnetic resonance
- GPx
Glutathione peroxidase
- HAT
Hypoxanthine/Aminopterin/Thymidine
- HRP
Horseradish peroxidase
- IR
Ionizing radiation
- PALA
N-(phosphonoacetyl)-L-aspartate
- PEG
Polyethylene Glycol
- p-HPA
p-hydroxyphenylacetic acid
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
REFERENCES
- 1.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Rad. Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Azzam EI, de Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 3.Hunt CR, Sim JE, Sullivan SJ, Featherstone T, Golden W, Von Kapp-Herr C, Hock RA, Gomez RA, Parsian AJ, Spitz DR. Genomic instability and catalase gene amplification induced by chronic exposure to oxidative stress. Cancer Res. 1998;58:3986–3992. [PubMed] [Google Scholar]
- 4.Clutton SM, Townsend KM, Walker C, Ansell JD, Wright EG. Radiation-induced genomic instability and persisting oxidative stress in primary bone marrow cultures. Carcinogenesis. 1996;17:1633–1639. doi: 10.1093/carcin/17.8.1633. [DOI] [PubMed] [Google Scholar]
- 5.Limoli CL, Giedzinski E. Induction of chromosomal instability by chronic oxidative stress. Neoplasia. 2003;5:339–346. doi: 10.1016/S1476-5586(03)80027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limoli CL, Giedzinski E, Morgan WF, Swarts SG, Jones GD, Hyun W. Persistent oxidative stress in chromosomally unstable cells. Cancer Res. 2003;63:3107–3111. [PubMed] [Google Scholar]
- 7.Kim GJ, Fiskum GM, Morgan WF. A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res. 2006;66:10377–10383. doi: 10.1158/0008-5472.CAN-05-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limoli CL, Kaplan MI, Corcoran JJ, Meyers M, Boothman DA, Morgan WF. Chromosomal instability and its relationship to other end points of genomic instability. Cancer Res. 1997;57:5557–5563. [PubMed] [Google Scholar]
- 9.Panus PC, Radi R, Chumley PH, Lillard RH, Freeman BA. Detection of H2O2 release from vascular endothelial cells. Free Radic. Biol. Med. 1993;14:217–223. doi: 10.1016/0891-5849(93)90013-k. [DOI] [PubMed] [Google Scholar]
- 10.Kaschnitz RM, Hatefi Y, Pedersen PL, Morris HP. Isolation of mitochondria from morris hepatomas. Meth. Enzymol. 1979;55:79–88. doi: 10.1016/0076-6879(79)55011-3. [DOI] [PubMed] [Google Scholar]
- 11.Buettner GR. Spin trapping: ESR parameters of spin adducts. Free Radic.Biol.Med. 1987;3:259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Comm. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 15.Anderson ME. Handbook of methods for oxygen radical research. Florida: CRC Press Inc; 1985. [Google Scholar]
- 16.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 17.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 18.Glaab WE, Tindall KR. Mutation rate at the hprt locus in human cancer cell lines with specific mismatch repair-gene defects. Carcinogenesis. 1997;18:1–8. doi: 10.1093/carcin/18.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 20.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 21.Hinz JM, Tebbs RS, Wilson PF, Nham PB, Salazar EP, Nagasawa H, Urbin SS, Bedford JS, Thompson LH. Repression of mutagenesis by Rad51D-mediated homologous recombination. Nuc. Acid Res. 2006;34:1358–1368. doi: 10.1093/nar/gkl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metas. Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 23.Limoli CL, Corcoran JJ, Milligan JR, Ward JF, Morgan WF. Critical target and dose and dose-rate responses for the induction of chromosomal instability by ionizing radiation. Radiat. Res. 1999;151:677–685. [PubMed] [Google Scholar]
- 24.Snyder AR, Morgan WF. Lack of consensus gene expression changes associated with radiation-induced chromosomal instability. DNA Repair (Amst) 2005;4:958–970. doi: 10.1016/j.dnarep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Snyder AR, Morgan WF. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene. 2003;22:5848–5854. doi: 10.1038/sj.onc.1206697. [DOI] [PubMed] [Google Scholar]
- 26.Slane BG, Aykin-Burns N, Smith BJ, Kalen AL, Goswami PC, Domann FE, Spitz DR. Mutation of succinate dehydrogenase subunit C results in increased O2.−, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- 27.Limoli CL, Kaplan MI, Giedzinski E, Morgan WF. Attenuation of radiation-induced genomic instability by free radical scavengers and cellular proliferation. Free Radic. Biol. Med. 2001;31:10–19. doi: 10.1016/s0891-5849(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 28.Mondello C, Rebuzzini P, Dolzan M, Edmonson S, Taccioli GE, Giulotto E. Increased gene amplification in immortal rodent cells deficient for the DNA-dependent protein kinase catalytic subunit. Cancer Res. 2001;61:4520–4525. [PubMed] [Google Scholar]