Abstract
Findings of white matter pathology as indicated by diffusion tensor anisotropy values in schizophrenia are well established, but the differences in this measure between the onset of the disease and the chronic state are not well known. To investigate the differences between these states in the progression of the disease of schizophrenia we acquired 1.5 T diffusion tensor anisotropy images on 35 adult patients with schizophrenia and schizoaffective disorder, 23 adolescents having their first psychotic episode, and age and sex matched controls (33 adults and 15 adolescents). Regions of interest in major cortical white matter tracts chosen as salient to the prefrontal executive deficit in schizophrenia were assessed using stereotaxic coordinates from the Talairach and Tournoux atlas. Regions of each tract along anterior-posterior and/or inferior-superior directions in both hemispheres were evaluated in multiway ANOVA. Tracts between the frontal lobe and other brain regions, but not temporal, occipital and interhemispheric tracts, showed a differential aging pattern in normals and patients indicating that the white matter pathology in these regions is not stable between the onset and the chronic state in schizophrenia. This suggests that tracts involved in the connectivity of the temporal lobe white matter deficits were already well in place in adolescent patients, while frontal lobe pathology continues to develop from adolescence to adulthood.
1 Introduction
The white matter of the frontal lobe and temporal lobes goes through its final stages of development throughout adolescence and young adulthood, the most common period of onset of schizophrenia (Benes, 1989, Buchsbaum et al., 1992, Yakovlev and Lecours, 1967). Our recent study showed significant changes in the white matter connecting these regions of the cortex as indicated by diffusion tensor anisotropy between adolescence and adulthood (Schneiderman et al., 2007) and included both anisotropy increases with age in the anterior limb of the internal capsule and superior longitudinal fasciculus and decreases with age in the posterior limb of the internal capsule, cingulum bundle and temporal-occipital white matter.
Patients with schizophrenia have been reported to have lower anisotropy in the white matter of frontal and temporal lobes (for review, Buchsbaum et al., 2006) Connections between frontal lobes and posterior areas in adolescents with schizophrenia have been shown to have low anisotropy in the frontal inferior longitudinal fasciculus (Ashtari et al., 2007) and superior longitudinal fasciculus and corpus callosum (Kyriakopoulos et al., 2007) and cingulate (Kumra et al., 2005, White et al., 2007). Changes with age in anisotropy between normals and patients have also been reported within adult samples (Jones et al., 2006). However little has been done to directly compare the white matter changes in schizophrenia in these areas between the first break during adolescence and the chronic state of the disease during adulthood.
If the white matter pathology of schizophrenia is fully developed by the age of the first psychotic break in adolescence then we would expect the patients with schizophrenia to follow a parallel course of anisotropy changes with age in comparison to healthy subjects, as indicated by a significant diagnostic group effect in all regions, but no age group by diagnosis effects. However, if the white matter pathology of schizophrenia continues to progress after the first psychotic break, the patients might show an aberrant pattern of anisotropy changes with age. This would be indicated by a significant age group by diagnosis interaction in all regions.
It is also possible that the white matter pathology in schizophrenia may be in place in some but not all brain regions and that some regions might continue to pathologically progress between adolescence and adulthood. Because the temporal lobe contains many primary and secondary unimodal sensory areas that become myelinated earlier than association areas of the frontal cortex, we expect to see larger differences from the normal developmental pattern in tracts connecting the frontal lobe as indicated by significant age group by diagnosis interactions in the tracts involved in frontal connectivity. We would so expect that matter abnormalities in tracts connection the temporal lobe in schizophrenia would be in place at the onset of psychotic symptoms this would appear as a main effect of diagnosis but not a diagnosis by age interaction in regions involving temporal connectivity. Lastly because the female brain may mature earlier, possibly before the age in which subjects were enrolled in the study, we hypothesize that females may be less likely to show differences between age group we will obtain age group by sex interactions to test this.
2. Methods
2.1 Subjects
Thirty-five adult patients with the schizophrenia spectrum disorders (Table I) were recruited as described elsewhere (Mitelman et al., 2003). The patients were evaluated with the Comprehensive Assessment of Symptoms and History (CASH; (Andreasen et al., 1992)) and diagnosed as having schizophrenia (n=31) or schizoaffective disorder (n=4) according to the DSM-IV criteria. They were evaluated for handedness by the Edinburgh Handedness Inventory (Oldfield, 1971). The mean age of symptom onset was 20.67 years (SD=7.11). The mean age for beginning medication was 26 years and all patients were medicated. The adult patients has a mean score of 72.00 (range = 38 – 113, SD = 18.04, unavailable for n=4) on the Positive and Negative Syndrome Scale (PANSS) for Schizophrenia (Kay et al., 1987). No longitudinal data on these subjects was available.
Table I.
Subjects
| Age | Group | Total | Sex | Age | Handedness | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Mean | SD | Range | Right | Left | NA | |||
| Adult | Patient | 35 | 25 | 10 | 43.06 | 10.81 | 20–65 | 34 | 0 | 1 |
| Adult | Healthy | 33 | 20 | 13 | 42.18 | 11.48 | 22–64 | 31 | 2 | 0 |
| Adolescent | Patient | 23 | 15 | 8 | 16.08 | 2.06 | 13–20 | 20 | 2 | 1 |
| Adolescent | Healthy | 15 | 8 | 7 | 17.13 | 2.13 | 14–21 | 13 | 2 | 0 |
Twenty-three adolescent patients (Table I) experiencing their first psychotic episode were referred to the study by community physicians and emergency room clinicians. All patients met DSM-IV criteria for Psychotic Disorder NOS after a psychiatric examination with the Comprehensive Assessment of Symptoms and History (CASH; (Andreasen et al., 1992) by a board-certified psychiatrist. Based on a historical review of symptoms and/or longitudinal follow-up after at least 6 months, more specific DSM-IV diagnoses were made. No further follow-up data other then diagnosis was available. Diagnostic breakdown included 18 patients with schizophrenia, and 5 with bipolar disorder. The subjects with a final diagnosis of bipolar disorder were included because there is evidence of a possible shared etiology between bipolar disorder and schizophrenia (Thaker, 2008). Because of the shared initial diagnosis but differing final diagnosis results will be reported both including and excluding the patients with a final diagnosis of bipolar disorder. Adolescent patients had a mean score of 90.95 (range = 67–114, SD = 13.61, unavailable for n=4) on the PANSS. The higher score of the adolescent patients compared to the adults reflects the adolescent patients were not on active antipsychotic medication at the time of the evaluation. Participants were part of a study olanzapine versus haloperidol treatment in adolescent psychosis and were all drug naive at the time of the scan.
Thirty-three normal adult volunteers approximately matched in age and sex to the adult patients and 15 normal adolescent comparison subjects (Table I) were recruited by word of mouth or advertisement. All were evaluated with the CASH interview to exclude an Axis I psychiatric disorder in the subject or in a first-degree family member (Andreasen et al., 1992). Participants with significant neurological impairment, medical conditions interfering with cerebral functioning, history of head trauma, or meeting current or past criteria for substance abuse or dependence were excluded. For all subjects under age 18, consent for participation was given and written informed consent was obtained from a parent or legal guardian; written informed consent was obtained from subjects who were over 18-years-old. Measures of intelligence such as IQ were not consistently available across the whole study population and are not reported.
This is the first report to use all four subject groups as described above (Table I). White matter anisotropy in the normal adolescent and normal adults subjects were reported in an earlier report (Schneiderman et al., 2007). Volume of cortical and subcortical structures and cortical anisotropy in the adult patients and adult normal subjects have also been previously published by our group (Mitelman et al., 2005a, Mitelman et al., 2005b, Mitelman et al., 2005c, Mitelman et al., 2006, Mitelman et al., 2005d) but specific tracts were not evaluated in any of these patient studies. The neuropsychological functioning of the adolescent patients has been described by Brickman et al. (2004). The subjects do not overlap at with the sample in a previous report of anisotropy in schizophrenia by our group (Buchsbaum et al., 2006).
2.2 Image acquisition
The diffusion tensor sequence acquired fourteen 7.5-mm-thick axial slices with six diffusion weighted gradients and one baseline image (TR=4100 ms, TE=99 ms, TI=2.2 s, b=750 s/mm2, δ=31ms, Δ=73 ms, NEX=5, voxel=1.8×1.8×7.5 mm, FOV=230 mm) and the SPGR (Spoiled Gradient Recalled Acquisition) anatomical sequence acquired 124 1.2 mm-thick axial slices (TR=24 ms, echo time=5 ms, flip angle=40°, FOV=230 mm, voxel=0.898×0.898×1.20 mm), on a Signa LX/CV-1.5 T system (GE Medical Systems, Milwaukee). All scans were read by a radiologist and no clinically relevant anatomical abnormalities were reported.
2.3 Image processing
Anatomical MRI were resectioned to standard Talairach-Tournoux position using the algorithm of Woods et al. (1993) and a 6-parameter rigid-body transformation. The anisotropy images from each subject were then aligned to each participant’s own standard-position anatomical images using the same 6-parameter rigid-body transformation. A rigid-body transformation was chosen to be consistent with the method used in the previous report of frontal anisotropy and volume within the adult subset of the subjects (Mitelman et al., 2006). The cortical edge, as manually defined on the anatomical image, was displayed on the coregistered anisotropy image and visually checked for proper coregistration. To assess the degree of diffusion anisotropy in each voxel, we computed the relative anisotropy (RA) for each voxel (Buchsbaum et al., 1998). Relative anisotropy was chosen to allow for comparison to previously published reports from this sample of subjects that were published as RA values (Mitelman et al., 2006, Schneiderman et al., 2007).
2.4 Region of interest anisotropy assessment
On the basis of earlier studies of aging and schizophrenia, we selected specific white matter tracts of the brain that have been implicated in schizophrenia and/or development during adolescence that are related to the prefrontal deficit hypothesis (Table II and Figures). We defined the regions of interest as a serious of square 3 × 3 voxel boxes located at standard stereotaxic x, y, z locations for the chosen tracts as indicated in the print version of the Talairach and Tournoux (1988) atlas. The square region of interest (ROI) (3×3 voxels) was applied centered on that coordinate and at the proportion as the brain-bounding box in the Talairach-Tournoux atlas. An adjustment was made so that ROIs were moved closer to the centroid of the slice if the box fell partly outside the coregistered brain outline, as could happen in brains that were especially narrow in the x direction for boxes placed at 45° and 135°. The location of these regions of interest were then confirmed using the atlases of Mori (2005) and Ludwig (1956). Correct placement was confirmed by display of the regions of interest on the anisotropy image for each subject. We assessed stereotaxic error by using 35 normal adults who had had the anterior limb of the internal capsule manually traced (Brickman et al., 2006) and found that for this white matter structure, the observed centroid was within our 4.49×4.49 mm stereotaxically located region of interest. A more detailed examination of this methodology is available in our previous report on the non-patient portion of the subject population (Schneiderman et al., 2007).
Table II.
Areas of Interest - Talairach-Tournoux Coordinates
| z=−4 | 4 | 12 | 20 | 28 | 35 | |
|---|---|---|---|---|---|---|
| x,y | x,y | x,y | x,y | x,y | x,y | |
| Internal Capsule | 16,19 | 23,20 | 22,20 | 20,8 | 20,8 | |
| 13,12 | 15,13 | 17,10 | 20,3 | 20,3 | ||
| 9,7 | 10,4 | 15,0 | 20,0 | 20,0 | ||
| 5,2 | 9,0 | 5,−1 | 20,−1 | 20,−1 | ||
| 9,−4 | 19,−12 | 20,−10 | 21,−10 | 21,−10 | ||
| 22,−17 | 25,−23 | 25,−20 | 22,−24 | 22,−24 | ||
| Frontal Anterior Fasciculus (Includes Parts of Anterior Corona Radiata) | 22,45 | 22,45 | 22,52 | 22,35 | 22,32 | 25,30 |
| 22,32 | 22,32 | 26,25 | 22,25 | 22,20 | 25,20 | |
| Frontal Occipital Fasciculus (Includes Parts of Superior Longitudinal Fasciculus) | 19,30 | 20,28 | 20,18 | 18,−3 | 24,−7 | |
| 19,24 | 18,22 | 18,13 | 18,−9 | 24,−12 | ||
| Temporal Occipital White Matter | 50,−44 | 52,−44 | 57,−41 | |||
| 42,−54 | 42,−52 | 42,−51 | ||||
| 34,−64 | 34,−62 | 34,−61 | ||||
| 26,−74 | 25,−72 | 25,−71 | ||||
| 18,−84 | 16,−81 | 16,−81 | ||||
| Frontal Cingulate Bundle | 8,35 | 10,37 | 10,34 | 11,25 | 9,23 | |
| Frontal Interior Longitudinal Fasciculus | 38,−25 | 33,−42 | 35,−50 | |||
| 38,−35 | 34,−60 | 36,−60 | ||||
| Frontal Superior Longitudinal Fasciculus (Includes Frontal-Occipital Fibers) | 36,−38 | 34,−39 | 33,−20 | |||
| 36,−42 | 34,−42 | 33,−30 | ||||
| Optic Radiations | 23,−77 | 32,−63 | ||||
| Cingulum | 10,26 | 10,37 | 10,36 | 10,30 | 10,13 | 10,7 |
| 11,−48 | 10,−44 | 10,−17 | ||||
| Corpus Callosum | 5,26 | 5,24 | 5,12 | 11,−5 | ||
| 5,−36 | 5,−32 | 11,−12 | ||||
| Anterior Thalamic Radiations (Includes Parts of Anterior Corona Radiata) | 16,45 | 15,47 | 17,45 | 15,37 | ||
| 16,35 | 15,37 | 17,35 | 15,27 |
We evaluated the resolution and accuracy of key stereotaxic regions of interest by applying a strip of 17 square regions in y-direction steps of 1% of brain width centered on our corpus callosum stereotaxic location. We expected to see an anisotropy peak centered in the strip if it was positioned accurately. Strips for the center of the corpus callosum met these criteria well with peak anisotropy falling in the 8th to 10th box (90%). This is an error of 1–2%, equivalent to about 1.3–2.6mm and indicates that our ROI would fall entirely within the desired tract 90% of the time and that distortion of the anisotropy image was not appreciably greater than this range of error. Similar results were obtained for horizontal × steps and the anterior limb of the internal capsule. All four groups showed a similar pattern, indicating that diagnostic differences were not altering the best stereotaxic location.
2.5 Statistical analysis
Anisotropy values for each region of interest was entered into repeated measures analysis of variance with diagnostic group (patients/healthy volunteers), sex (male/female) and age (adolescent/adult) as independent group dimensions and region position (anterior/posterior, inferior/superior) and hemisphere (right, left) as repeated measures. All significant diagnosis effects and interactions are reported. Those interactions and effects that do not involve diagnosis and therefore are not part of our hypothesis are not reported. Higher order interactions with sex are reported as exploratory results. We also reported Huynh-Feldt and MANOVA F values for completeness. As probability values are obtained from multiway MANOVA interactions and multiple region plots have multiple line segments, standard error bars are not included.
A region of interest based strategy was used for several reasons. The first was that it allows specific hypothesis testing and demonstration of regional contrasts, which is not possible with statistical probability mapping. By not including white matter regions unrelated to the hypothesis, potential type I errors are reduced. This method also attempts to minimize type II errors resulting from assessing small individual potentially variable ROIs by sampling several points within each white matter region of interest. It was also chosen to ease replication by other readers using standard coordinates from well known atlases. We considered combining all anteroposterior dimensions in a single six or seven-way MANOVA for a single F, but thought that single tract interactions would be more interpretable under the general hypothesis of prefrontal connectivity.
3. Results
3.1 Internal Capsule
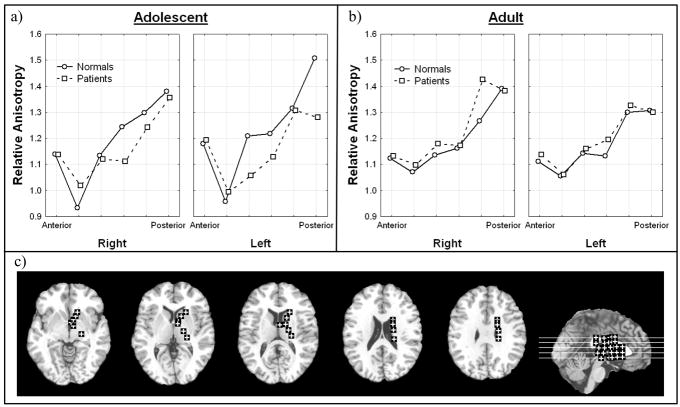
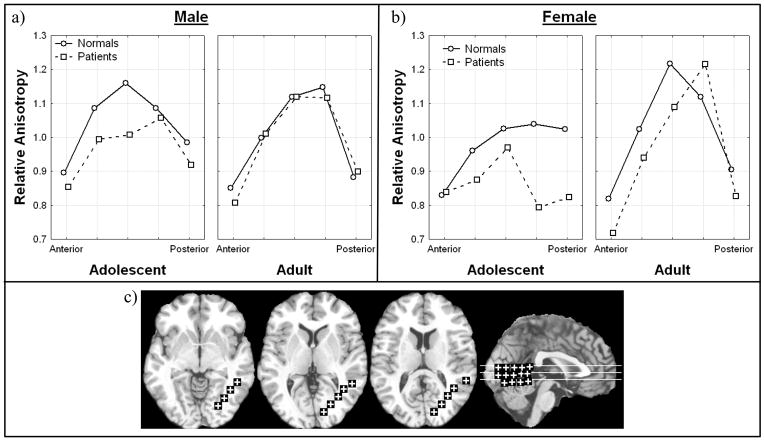
Adolescent patients had higher anisotropy in the two anterior most regions than adolescent normal volunteers but lower anisotropy in the more posterior regions, the genu (middle bend) and the posterior limb (Figure I; Diagnosis × anterior-posterior position × hemisphere × age, all subjects F = 2.30, df = 5, 490, p = 0.043, HF ε= .85, HF Adj. df = 4.26, 417.08, HF Adj. p = .054). This effect was more pronounced on the left side especially at the most posterior position. In adults, the normal subjects had slightly lower anisotropy than the patients in all but the most posterior positions in the internal capsule. When we excluded adolescent patients with a final diagnosis of bipolar disorder, this interaction was no longer significant. This tendency to have low anisotropy in patients in the posterior part of the internal capsule was most prominent in males in the left hemisphere, and suggests a greater effect on the motor system on the dominant side (Diagnosis × anterior-posterior position × hemisphere × sex, all subjects, F = 2.35, df = 5, 490, p = .040, HF = 0.85, HF Adj. df=4. 26, 417.08, HF Adj. p = .049). This pattern remained significant when excluding bipolar disorder (Diagnosis × anterior-posterior position × hemisphere × sex, excluding bipolar, F = 2.54, df = 5, 465, p = .028, HF ε = .85, HF Adj. df = 4.25, 395.20, HF Adj. p = .036).
Figure 1. Diagnosis, anterior-posterior position, hemisphere, age effects in relative anisotropy in the internal capsule.
a) Horizontal axis indicates anterior-posterior position within internal capsule in adolescents. Panels indicate right and left hemisphere. (see c, brain diagram below).
b) Horizontal axis indicates anterior-posterior position within internal capsule in adults. Panels indicate right and left hemisphere.
c) Representative slices from inferior to superior for Talairach z = −4, 4, 12, 20, 28 and a midsagittal slice are shown with regions of interest. For clarity in all figures regions of interest are enlarged (actual dimensions 5×5 pixels), were placed as mirror images in both hemispheres but are shown only in the right hemisphere, and are displayed on T1 images. The Talairach xyz coordinates are given in Table 1.
When looking at the anterior portion of the internal capsule only, normal adolescent males had a lower anisotropy then patients in the right hemisphere and slightly higher anisotropy in the left hemisphere (Diagnosis × age × sex × hemisphere, anterior portion only, all subjects, F = 8.56, df = 1, 98, p = 0.0043). Normal adult males had a higher anisotropy then patients in the right but slightly lower on the left. In female adolescents, higher anisotropy on the right and lower anisotropy on the left was seen in normal subjects in comparison to patients. Normal adult females had a lower anisotropy then patients in the right hemisphere, but in the left hemisphere there was little difference between anisotropy between normal subjects and patients. This trend persisted when excluding patients with a final diagnosis of bipolar disorder (Diagnosis × age × sex × hemisphere, anterior portion only, excluding bipolar, F = 7.83, df = 1, 93, p = .0062).
3.2 Anterior thalamic radiations
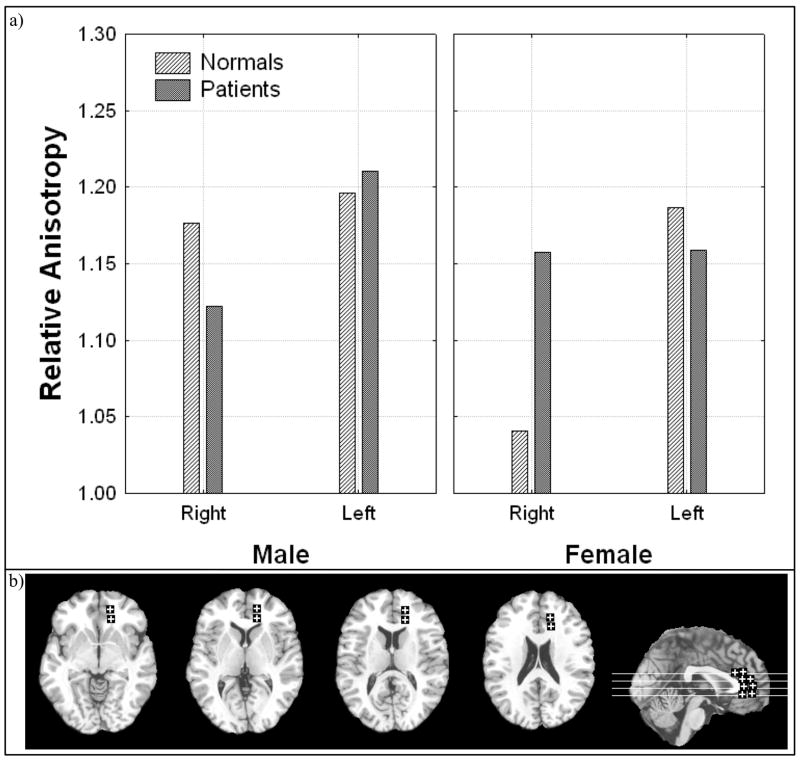
Males with schizophrenia had lower anisotropy in the right anterior thalamic radiations than normal males while little difference was seen on the left. In contrast, females with schizophrenia had higher anisotropy in the right anterior thalamic radiations than normal females (Figure II; Diagnosis × sex × hemisphere, all subjects, F = 5.82, df = 1, 93, p = 0.018). When bipolar patients were included this effect was no longer significant. When including all patients this effect was significant for the most anterior location and for males the effect was greater in the inferior portions; female patients had lower anisotropy in the left superior region (Diagnosis × sex × hemisphere × inferior-superior position × anterior-posterior region, all subjects, F = 2.1, df = 3, 294, p = .082). When excluding subjects with a final diagnosis of bipolar disorder this five-way interaction was no longer significant.
Figure 2. Diagnosis, sex, and hemisphere effects in relative anisotropy in the anterior thalamic radiations.
a) Horizontal axis indicates hemisphere. Panels represent male and female.
b) Representative slices from inferior to superior for z = −4, 4, 12, 20 and a midsagittal slice are shown with regions of interest.
Significant interactions:
Diagnosis × sex × hemisphere, all subjects, F = 5.82, df = 1, 93, p = 0.018;
Diagnosis × sex × hemisphere × inferior-superior position × anterior-posterior region, all subjects, F = 2.1, df = 3, 294, p = .082
3.3 Fronto-occipital fasciculus
Males with schizophrenia had lower anisotropy at the most inferior levels (Talairach z = 4 and 12) in the left hemisphere while females with schizophrenia had lower anisotropy at more superior levels (z = 20, 28; Figure III; Diagnosis × sex × hemisphere × inferior-superior position, excluding bipolar, F = 2.73, df = 4, 372, p = 0.029). This effect was no longer significant when bipolar patients were included.
Figure 3. Diagnosis, sex, hemisphere, inferior-superior position effects in relative anisotropy in the fronto-occipital fasciculus.
a) Horizontal axis indicates superior-inferior position within the fronto-occipital fasciculus as labeled by Talairach z in males. Panels indicate left and right hemisphere.
b) Horizontal axis indicates superior-inferior position within the fronto-occipital fasciculus as labeled by Talairach z in females. Panels indicate left and right hemisphere.
c) Representative slices from inferior to superior for z = 4, 12, 20, 28, 35 and a midsagittal slice are shown with regions of interest.
Significant interactions:
Diagnosis × sex × hemisphere × inferior-superior position, excluding bipolar, F = 2.73, df = 4, 372, p = 0.029
3.4 Frontal anterior fasciculus
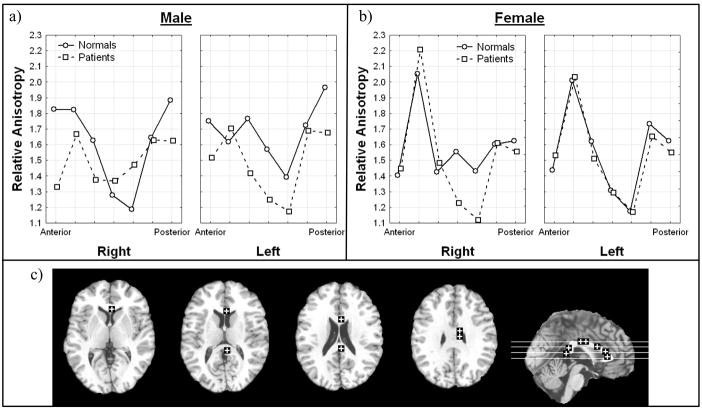
Consistent with our earlier report (Buchsbaum et al., 1998) patients with schizophrenia had lower anisotropy in the right frontal anterior fasciculus. This effect was found in both hemispheres for males but in female patients the anisotropy was lower than normal volunteers in the right hemisphere but higher in the left hemisphere (Figure IVa; Diagnosis × sex × hemisphere, all subjects, F = 8.05, df = 1, 98, p = 0.0055). This effect remained when excluding patients with a final diagnosis of bipolar disorder (Diagnosis × sex × hemisphere, excluding bipolar, F = 6.71, df = 1, 93, p = .011). The regions of interest were positioned so that the more posterior box was closer to the interhemispheric crossing of the corpus callosum, so we examined the anterior-posterior interactions. This revealed that the more posterior box showed the greatest normal-patient differences in adult patients (Figure IVb; Diagnosis × anterior-posterior position × age, all subjects, F = 6.93, df = 1, 98, p = 0.0098). When excluding bipolar patients this effect also remained significant (Diagnosis × anterior-posterior position × age, excluding bipolar, F = 5.84, df = 7, 93, p = .018).
Figure 4. Diagnosis, sex, and hemisphere effects and diagnosis, anterior-posterior position, and age effects in relative anisotropy in the frontal anterior fasciculus.
a) Horizontal axis indicates sex and panels indicate hemisphere.
b) Horizontal axis indicates anterior-posterior position in the frontal anterior fasciculus and panels indicate age.
c) Representative slices from inferior to superior for z = −4, 4, 12, 20, 28, 35 and a midsagittal slice are shown with regions of interest.
Significant interactions:
Diagnosis × sex × hemisphere, all subjects, F = 8.05, df = 1, 98, p = 0.0055
Diagnosis × sex × hemisphere, excluding bipolar, F = 6.71, df = 1, 93, p = .011
Diagnosis × anterior-posterior position × age, all subjects, F = 6.93, df = 1, 98, p = 0.0098
Diagnosis × anterior-posterior position × age, excluding bipolar, F = 5.84, df = 7, 93, p = .018
3.5 Frontal superior and inferior longitudinal fasciculi
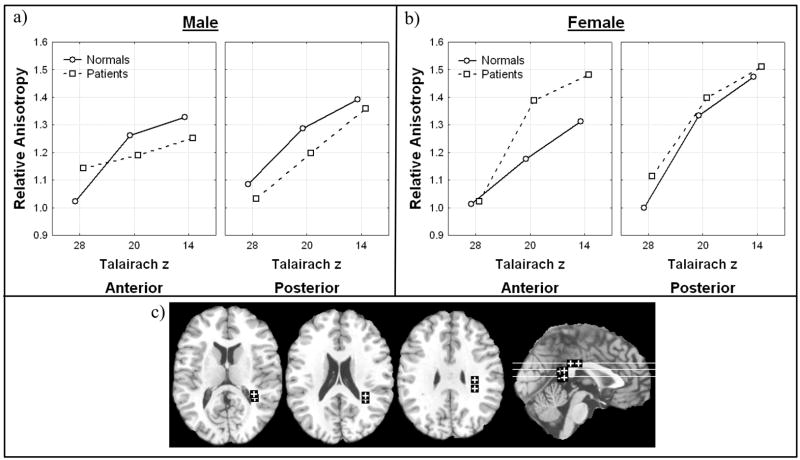
In the frontal superior longitudinal fasciculus, anisotropy was lower in male patients then controls in its more superior extent where fibers course to the temporal lobe while the reverse was found in females (Figure V; Diagnosis × inferior-superior level × anterior-posterior position × sex, all subjects, F=5.51, df = 2, 196, p = 0.0047, HF ε = .97, HF Adj. df = 1.94, 189.91, HF Adj. p = .0052). This effect was most prominent in adolescent males (Diagnosis × age × sex × hemisphere × inferior-superior level × anterior-posterior position, all subjects, F = 3.27, df = 2, 196, p = 0.040). Both of these interactions remained when excluding bipolar patients (Diagnosis × inferior-superior level × anterior-posterior position × sex, excluding bipolar, F = 7.10, df = 2, 186, p = 0.0011, HF ε = .98, HF Adj. df = 1.95, 181.75, HF Adj. p = .0012; Diagnosis × age × sex × hemisphere × inferior-superior level × anterior-posterior position, excluding bipolar, F = 3.63, df = 2, 186, p = 0.028). No effects involving diagnosis were seen in the frontal inferior longitudinal fasciculus.
Figure 5. Diagnosis, inferior-superior level, anterior-posterior position, and sex effects in relative anisotropy in the frontal superior longitudinal fasciculus.
a) Horizontal axis indicates superior-inferior position in the superior longitudinal fasciculus as labeled by Talairach z in males. Panels indicate anterior-posterior position in the superior longitudinal fasciculus.
b) Horizontal axis indicates superior-inferior position in the superior longitudinal fasciculus as labeled by Talairach z in females. Panels indicate anterior-posterior position in the superior longitudinal fasciculus.
c) Representative slices from inferior to superior for z = 12, 20, 28, and a midsagittal slice are shown with regions of interest.
Significant interactions:
Diagnosis × inferior-superior level × anterior-posterior position × sex, all subjects, F=5.51, df=2, 196, p=0.0047, HF ε = .97, HF Adj. df = 1.94, 189.91, HF Adj. p = .0052;
Diagnosis × age × sex × hemisphere × inferior-superior level × anterior-posterior position, all subjects, F = 3.27, df = 2, 196, p = 0.040
Diagnosis × inferior-superior level × anterior-posterior position × sex, excluding bipolar, F = 7.10, df = 2, 186, p = 0.0011, HF ε = .98, HF Adj. df = 1.95, 181.75, HF Adj. p = .0012
Diagnosis × age × sex × hemisphere × inferior-superior level × anterior-posterior position, excluding bipolar, F=3.63, df = 2, 186, p = 0.028
3.6 Cingulum bundle
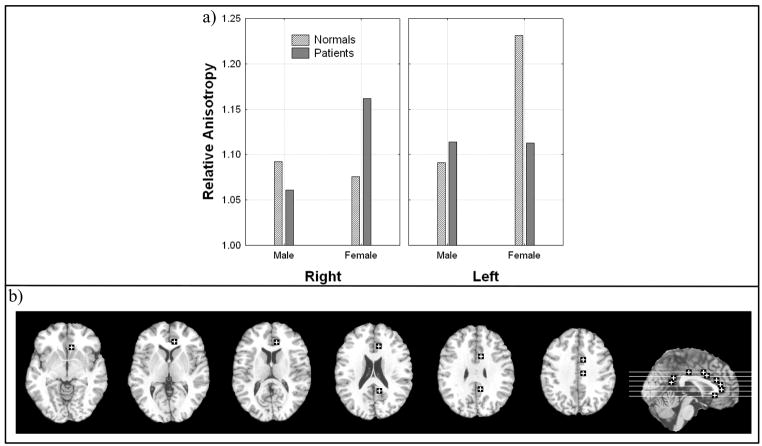
Male patients showed lower anisotropy than normal controls in the right hemisphere but female patients showed lower anisotropy in the left hemisphere (Figure VI; Diagnosis × sex × hemisphere, all subjects, F = 8.49, df = 1, 98, p = 0.0044). This effect remained when excluding patients with a final diagnosis of bipolar disorder (Diagnosis × sex × hemisphere, excluding bipolar, F = 10.19, df = 1, 93, p = 0.0019). In the right frontal portion of the cingulum bundle, the middle portion (z = 12 and 4) there was a large decrease in anisotropy in males patients but not in females (Diagnosis × sex × hemisphere × inferior-superior level, all subjects, F = 3.86, df = 4, 392, p = 0.0043, HF ε = .87, HFAdj. df = 3.46, 339.31, HF Adj. p = 0.0067).
Figure 6. Diagnosis, sex, and hemisphere effects in relative anisotropy in the cingulum bundle.
a) Horizontal axis sex and panels represent hemisphere.
b) Representative slices from inferior to superior for z = −4, 4, 12, 20, 28, 35 and a midsagittal slice are shown with regions of interest.
Significant interactions:
Diagnosis × sex × hemisphere, all subjects, F = 8.49, df = 1, 98, p = 0.0044
Diagnosis × sex × hemisphere, excluding bipolar, F = 10.19, df = 1, 93, p = 0.0019
Diagnosis × sex × hemisphere × inferior-superior level, all subjects, F = 3.86, df = 4, 392, p = 0.0043, HF ε = .87, HF Adj. df = 3.46, 339.31, HF Adj. p = 0.0067
3.7 Temporal-occipital white matter
In the temporal-occipital white matter patients had significantly lower anisotropy then healthy volunteers (Figure VII; Diagnosis, all subjects, F = 8.44, df = 1, 98, p = .0046). This effect remained significant when excluding subjects with a final diagnosis of bipolar disorder (Diagnosis, excluding bipolar, F = 9.55, df = 1, 93. p = .0026). This patient vs. normal difference was more prominent in the posterior temporal region and in adolescents, especially females (Figure VIIa, b; Age × diagnosis × sex × anterior-posterior position, all subjects, F = 2.50, df = 4, 392. p = .042, HF ε = 0.971, HF Adj. df = 3.88, 380.47, HF Adj. p = .043; Age × diagnosis × sex × hemisphere, all subjects, F = 7.46, df = 1, 98, p = .0075; Age × diagnosis × sex × hemisphere, excluding bipolar, F = 5.91, df = 1, 93, p = .0017).
Figure 7. Age, diagnosis, sex, and anterior-posterior position effects in relative anisotropy in the temporal occipital matter.
a) Horizontal axis indicates anterior-posterior position within the temporal occipital white matter in males, and panels indicate age.
b) Horizontal axis indicates anterior-posterior position within the temporal occipital white matter in females, and indicate age.
c) Representative slices from inferior to superior for z = −4, 4, 12, and a midsagittal slice are shown with regions of interest.
Significant interactions:
Diagnosis, all subjects, F = 8.44, df = 1, 98, p = .0046
Diagnosis, excluding bipolar, F = 9.55, df = 1, 93. p = .0026
Age × diagnosis × sex × anterior-posterior position, all subjects, F = 2.50, df = 4, 392. p = .042, HF ε = 0.971, HF Adj. df = 3.88, 380.47, HF Adj. p = .043
Age × diagnosis × sex × hemisphere, all subjects, F = 7.46, df = 1, 98, p = .0075
Age × diagnosis × sex × hemisphere, excluding bipolar, F = 5.91, df = 1, 93, p = .0017
3.8 Corpus callosum
In the right hemisphere male patients showed lower anisotropy than controls in the genu and splenium of the corpus callosum but higher anisotropy in the body. Female patients show lower anisotropy in the body and posterior portion of the splenium and slightly higher anisotropy in the genu and anterior portion of the splenium. In the left hemisphere male patients had lower anisotropy then controls with the exception of the anterior portion of the genu, females showed a similar trend in the body and splenium but with a lesser difference between groups and an increase in anisotropy in the genu of patients in comparison to normals (Figure VIII; Diagnosis × sex × hemisphere × anterior-posterior position, all subjects, F = 3.71, df = 6, 588, p = .0013, HF ε = .966, HF Adj. df = 5.79, 567.83, HF Adj. p = .0015). This effect remained significant when excluding subjects with bipolar disorder (Diagnosis × sex × hemisphere × anterior-posterior position, excluding bipolar, F = 4.10, df = 6, 558, p = .00049, HF ε= .956, HF Adj. df = 5.74, 533.67, HF Adj. p = .00061).
Figure 8. Diagnosis, sex, hemisphere, and anterior-posterior position effects in relative anisotropy in the corpus callosum.
a) Horizontal axis represents anterior-posterior position within the corpus callosum in males (2 most anterior points = genu, middle 3 points = body, 2 most posterior points = splenium), and panels represent hemisphere.
b) Horizontal axis represents anterior-posterior position within the corpus callosum in females, and panels represent hemisphere.
c) Representative slices from inferior to superior for z = 4, 12, 20, 28, and a midsagittal slice are shown with regions of interest.
Significant interactions:
Diagnosis × sex × hemisphere × anterior-posterior position, all subjects, F = 3.71, df = 6, 588, p = .0013, HF ε = .966, HF Adj. df = 5.79, 567.83, HF Adj. p = .0015;
Diagnosis × sex × hemisphere × anterior-posterior position, excluding bipolar, F = 4.10, df = 6, 558, p = .00049, HF ε = .956, HF Adj. df = 5.74, 533.67, HF Adj. p = .00061
3.9 Optic radiations
No significant interactions involving diagnosis were found in the optic radiations.
3.10 Age of onset and anisotropy
We entered the age of onset for adults and the current age for adolescents and examined correlations with anisotropy on an exploratory basis. Regions in the ventral internal capsule and ventral temporo-occipital white matter tended to show positive significant correlations with age of onset. Anterior frontal regions did not show consistent correlations. When current age was partialed out, these correlations remained significant.
4 Discussion
4.1 Differences between adolescents and adults
In agreement with studies of regional changes in adolescents with schizophrenia we also found changes in the frontal superior longitudinal fasciculus, corpus callosum and cingulate indicating that these areas are important in the development of schizophrenia (Kumra et al., 2005, Kyriakopoulos et al., 2007, White et al., 2007). Both of the tracts, the internal capsule and frontal anterior fasciculus, that showed a differential aging pattern in normals and patients were projections between the frontal lobe and other brain regions in agreement with the findings of Jones et al. (2006) that age is an important factor in white matter abnormalities in schizophrenia. The only main effect for diagnosis (across both age groups) was anisotropy decrease in the temporal lobe. These findings suggest that the temporal lobes already have a myelin-related pathology in place at the age of first psychotic break in adolescents while the pathology is still evolving in the frontal lobe, and supports the theory that the pathology of schizophrenia involves an abnormal course of myelin development.
4.2 Ventral dorsal dimension and age of onset
Older age of onset tended to be associated with higher anisotropy in the ventral internal capsule and ventral temporal-occipital white matter; these correlations were relatively unaffected as age was included as an independent variable in the analyses. This is consistent with later onset and possibly less severe illness being associated with higher anisotropy in these two regions. These are early developing regions, so this pattern is consistent with individuals with later onset schizophrenia having abnormalities more limited to the later developing tracts. The removal of subjects with the final diagnosis of bipolar disorder changed the significance of results in the internal capsule, anterior thalamic radiations, but did not affect the significance of results in other regions. This suggests that psychotic subjects with a final diagnosis of bipolar disorder may have different pathology then psychotic subjects with a final diagnosis of schizophrenia.
4.3. Specificity of regional findings
Because our overall control area, the optic radiations, which are myelinated early showed no diagnosis effects and that schizophrenia is a disease of higher cognitive functions such as decision making and language, our results are not consistent with a general defect in myelination in schizophrenia, but rather a specific regional and time-related deficit in myelin development. This pattern of specific developmental abnormalities in myelin suggests that symptoms associated with the temporal lobe including auditory hallucinations would present before frontal associated symptoms, including problems in executive functioning.
4.4 Exploratory Sex Effects
Significant interactions of sex and diagnosis were seen throughout connections to the frontal lobes and interhemispheric connections. These regions included the internal capsule, anterior thalamic radiations, frontal occipital fasciculus, frontal superior longitudinal fasciculus, cingulum bundle, and corpus callosum. In addition, effects involving sex, diagnosis and age group were seen in the internal capsule, frontal superior longitudinal fasciculus and temporal occipital white matter. These results suggest that sex is an influential factor in both white matter pathology in schizophrenia and the course that this pathology takes during the progression of the disease, and should be included in the design of future research experiments in this area.
4.5 Illness duration
One implication of our differential age effects in patients and normals is that the schizophrenia is a continuous process that alters white matter. Because the adolescents were imaged typically within a short interval after onset, the adult population had typically much longer period of illness before imaging. These group differences could be due to the effect of psychoactive medication, as the adolescents were all imaged before treatment and the adults after years of treatment. Konopaske et al.(2008) reported a decrease in the number of oligodendroglia in non-human primate cortex with exposure antipsychotics, though the trend was not statically significant. A second possibility is that the adolescent population has a different anatomical form of the disorder, characterized by earlier onset during adolescence. In our population, the adult patients had an average age of onset of 20.9 years, and the adolescents mean age was 16.1 years. Imprecision in recall and reporting of age of onset and the absence of sufficient numbers of adults with short durations of illness (less than 2 years) makes rigorous analysis of this issue impossible in our sample. A third possibility is that schizophrenia struck adolescents earlier during a potentially critical time window for prefrontal cortex development, altering frontothalamic and frontotemporal connections in a different manner than that in adults in whom schizophrenia began at a period after frontothalamic connectivity had be further established. Lastly possibilities of institutionalization, cohort effects, educational experience, and substance abuse, cannot be ruled out as contributors
4.6 Limitations
Limitations of the region of interest stereotaxic approach used include variation in the precision with which the positions of the regions fell within the tracts of interest in all subjects and the possibility that multiple tracts may be contributing to the region of interest. We used the Talairach atlas (Talairach and Tournoux, 1988) to place our regions of interest and to allow replication. The nomenclature varies somewhat between different atlases and this is reviewed elsewhere (Schneiderman et al., 2007). However, our anisotropy values might reflect regions with two or more pathways overlapping, so the anatomical name applied may represent an oversimplification.
Anisotropy itself is a limited measure. While linked to myelin integrity and development, it is also affected by the geometry of the tracts. A tract that fans out to reach multiple cortical targets might show low anisotropy whereas a mistargeted tract which extends only to a single cortical area might show higher anisotropy. Axon guidance and the development of tract orientation mainly occurs early in development before adolescence, so changes in anisotropy would appear to be primarily related to the maturation of tracts that have already been laid down. However the possibility of pruning and neuronal damage and death cannot entirely be ruled out as a possible cause of anisotropy changes in schizophrenia.
The sampling biases in adolescents and adults might be different, with adolescents being newly recruited through the emergency room to a greater extent than adults. Educational level is clearly different as adolescents were not old enough to have finished their education or reached their maximum years of education potential but the relation of education level and anisotropy is unknown and may be a confounding variable. Interpretation of our age effects may be limited by any differences in diagnostic features between adolescents and adults. Excluding bipolar adolescents enhanced diagnostic group effects for the cingulum bundle, frontal superior longitudinal fasciculus and fronto-occipital fasciculus, a finding that supports the DSM-IV division between bipolar disorder and schizophrenia.
4.7 Conclusion
In summary, diffusion tensor anisotropy measures differed between adolescence and adulthood. The differential developmental pattern was most marked in tracts involving the connectivity of the frontal lobe, a region noted to have reduced white matter volume and decreased functional activation in schizophrenia. In the tracts involved in the connectivity of the temporal temporal lobe it appears that the white matter deficit is already well in place by the first psychotic break, while in the frontal lobe the pathology continues to develop through adulthood.
Acknowledgments
This research was supported by grants to MSB: Normal metabolism and anatomy of the basal forebrain in man MH56489, Anatomy and function of the thalamus in schizophrenia MH60023 and PET studies of attention in schizophrenia MH 40071. The General Clinical Research Center of Mount Sinai (M01-RR-00071) provided clinical support. The Eli Lilly company partially supported the scanning of the adolescent sample as part of an investigator-initiated project. Rachel Bloom, Pauline Bokhoven, Karen Dahlman, and Desmond Heath assisted with the clinical aspects of the adolescent sample.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Chen S, Kumra S. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Archives of general psychiatry. 2007;64:1270–80. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–93. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Bloom R, Bokhoven P, Paul-Odouard R, Haznedar MM, Dahlman KL, Hazlett EA, Aronowitz J, Heath D, Shihabuddin L. Neuropsychological functioning in first-break, never-medicated adolescents with psychosis. J Nerv Ment Dis. 2004;192:615–22. doi: 10.1097/01.nmd.0000138229.29157.3e. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, Mitelman SA, Hazlett EA, Lincoln SJ, Newmark RE, Shihabuddin L. Internal capsule size in good-outcome and poor-outcome schizophrenia. The Journal of neuropsychiatry and clinical neurosciences. 2006;18:364–76. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Friedman J, Buchsbaum BR, Chu KW, Hazlett EA, Newmark R, Schneiderman JS, Torosjan Y, Tang C, Hof PR, Stewart D, Davis KL, Gorman J. Diffusion Tensor Imaging in Schizophrenia. Biological Psychiatry. 2006;60:1181–7. doi: 10.1016/j.biopsych.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Mansour CS, Teng DG, Zia AD, Siegel BV, Jr, Rice DM. Adolescent developmental change in topography of EEG amplitude. Schizophr Res. 1992;7:101–7. doi: 10.1016/0920-9964(92)90039-8. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, Downhill J, Haznedar M, Fallon JH, Atlas SW. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. NeuroReport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O’Sullivan M, Golesworthy P, McGuire P, Horsfield MA, Simmons A, Williams SC, Howard RJ. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Human brain mapping. 2006;27:230–8. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biological psychiatry. 2008;63:759–65. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, Wu J, Clarke T, Thaden E, Kane JM, Rhinewine J, Lencz T, Diamond A, Ardekani BA, Szeszko PR. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:934–41. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A Diffusion Tensor Imaging Study of White Matter in Early-Onset Schizophrenia. 2007 doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Ludwig E, Klingler J. Der innere Bau des Gehirns dargestellt auf Grund makroskopischer Prèaparate. The inner structure of the brain demonstrated on the basis of macroscopical preparations. Little, Brown; Boston: 1956. Atlas cerebri humani. [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark R, Chu KW, Buchsbaum MS. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann’s areas in schizophrenia. Schizophrenia research. 2005a;75:265–81. doi: 10.1016/j.schres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. NeuroImage. 2005b;27:753–70. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Metabolic disconnection between the mediodorsal nucleus of the thalamus and cortical Brodmann’s areas of the left hemisphere in schizophrenia. The American journal of psychiatry. 2005c;162:1733–5. doi: 10.1176/appi.ajp.162.9.1733. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. White matter fractional anisotropy and outcome in schizophrenia. Schizophr Res. 2006 doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–68. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophrenia research. 2005d;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. Elsevier; New York: 2005. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Schneiderman JS, Buchsbaum MS, Haznedar MM, Hazlett EA, Brickman AM, Shihabuddin L, Brand JG, Torosjan Y, Newmark RE, Tang C, Aronowitz J, Paul-Odouard R, Byne W, Hof PR. Diffusion tensor anisotropy in adolescents and adults. Neuropsychobiology. 2007;55:96–111. doi: 10.1159/000104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Thaker G. Psychosis Endophenotypes in Schizophrenia and Bipolar Disorder. Schizophrenia bulletin. 2008 doi: 10.1093/schbul/sbn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Kendi AT, Lehericy S, Kendi M, Karatekin C, Guimaraes A, Davenport N, Schulz SC, Lim KO. Disruption of hippocampal connectivity in children and adolescents with schizophrenia--a voxel-based diffusion tensor imaging study. Schizophrenia research. 2007;90:302–7. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Williamson PC, Kutcher SP, Cooper PW, Snow WG, Szalai JP, Kaye H, Morrison SL, Willinsky RA, Mamelak M. Psychological, topographic EEG, and CT scan correlates of frontal lobe function in schizophrenia. Psychiatry research. 1989;29:137–49. doi: 10.1016/0165-1781(89)90028-0. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Yakovlev P, Lecours A. The myelogenic cycles of regional maturation of the brain. In: Minowski A, editor. Regional development of the brain in early life. Blackwell Scientific; Oxford: 1967. pp. 3–70. [Google Scholar]