Abstract
The ability of vaccines to induce immunity both in mucosal and systemic compartments may be required for prevention of HIV infection and AIDS. We compared DNA-MVA vaccination regimens adjuvanted by IL-12 DNA, administered intramuscularly and nasally or only nasally. Most of the vaccinated Rhesus macaques developed mucosal and systemic humoral and cell-mediated SHIV-specific immune responses. Stimulation of mucosal anti-Env IgA responses was limited. After rectal challenge with SHIV 89.6P, all vaccinated and naive animals became infected. However, most of the vaccinated animals showed significant control of viremia and protection from CD4+ T cell loss and AIDS progression compared to the control animals. The levels of CD4+ and CD8+ T cell virus-specific responses measured on the day of challenge correlated with the level of viremia control observed later during the chronic infection. Postchallenge viremia levels inversely correlated with the preservation of SHIV-specific CD4+/IL-2+ and CD8+/TNF-α+ T cells but not with CD4+/IFN-γ+ T cells measured over time after challenge. We also found that during the early chronic infection SHIV vaccination permitted a more significant preservation of both naive and memory CD4+ T cells compared to controls. In addition, we observed a more significant and prolonged preservation of memory CD4+ T cells after SHIV vaccination and challenge than that observed after SIV vaccination and challenge. As the antiviral immunity stimulated by vaccination is present in the memory CD4+ T cell subpopulations, its more limited targeting by SHIV compared to SIV may explain the better control of X4 tropic SHIV than R5 tropic SIVs by vaccination.
INTRODUCTION
THE SEVERITY OF THE AIDS EPIDEMIC in the developing world and the limited resources that some of these countries have available for health care render the need of an AIDS vaccine more urgent every day. When tested in Rhesus macaques, the candidate vaccines developed thus far do not protect from chronic infection but provide a reduction in the viremia set point and a delay of progression to AIDS. The control of viremia and the length of disease-free infection depend on the model of infection used for the vaccine evaluation in nonhuman primates. When the simian-human immunodeficiency virus (SHIV) virus was used as the challenge agent after recombinant DNA/MVA or recombinant DNA/adenovirus prime boost vaccinations, excellent viremia control has been achieved after challenge and a prolonged chronic infection without disease was observed.1-3 However, the same vaccination provided more limited protection when the challenge was carried out with SIV.4,5 In this case the set point of chronic viremia achieved in vaccinated animals was higher than after SHIV challenge and the median of the disease-free interval was approximately 24 months compared to 12 months in the naive controls.4 The more acute course of the SHIV89.6P infection compared to SIV infection and the general ease of achieving protection against acute viruses compared to chronic viruses were linked to these results.6
SHIV89.6P and most SIVs used in vaccine research differ in coreceptor usage, the former using the CXCR4 coreceptor and the latter using CCR5.7,8 It is possible that the different tropism of SHIV89.6P may have an impact on the outcome of the challenge after vaccination. The CCR5 coreceptor is more abundant in the mononuclear cells (MCs) of the gastrointestinal (GI) tract than in the peripheral blood mononuclear cells (PBMCs), while the CXCR4 coreceptor is similarly abundant in PBMCs and in GI MCs and substantially more abundant than the CCR5 coreceptor in PBMCs.9-13 The tropism for different target cells and their abundance and relative tissue distribution may ultimately impact the magnitude of depletion in a subpopulation, viral reservoirs, and the efficacy of a particular immunization regime.
The route of vaccination has a profound effect on the homing receptors induced in virus-specific cells and on where these cells ultimately reside.14-20 Achieving virus-specific responses at sites of viral entry and major sites of virus replication may be important for the efficacy of the immunization. A number of studies investigated mucosal approaches in AIDS vaccine candidates.21-35 Mucosal routes of immunizations are better suited than systemic routes for stimulation of immunity in both the systemic and mucosal compartments, and the nasal route may be best suited for this goal.36,37 It is important to evaluate different routes of immunization in addition to different candidate vaccines. Challenge viruses that have different tropism and possibly different quantitative tissue distributions may respond differently to immunization via different routes.
In this study, we compared a recombinant SHIV + interleukin (IL)-12 DNA/MVA immunization administered nasally with a combined nasal-intramuscular immunization and evaluated whether the different immunization routes stimulate forms of anti-SHIV immunity that produce different levels of protection. We also investigated whether the vaccine regimens had an impact on how SHIV89.6P affects the decline of CD4+ T cell subpopulations during the early stages of infection. We found that the combined intramuscular-nasal DNA-MVA regimen provided the most significant protection in these experiments and that it favored more significant levels of preservation of memory CD4 T cells early during chronic infection than that observed during early SIV infection after SIV vaccination.
MATERIALS AND METHODS
Animal vaccines
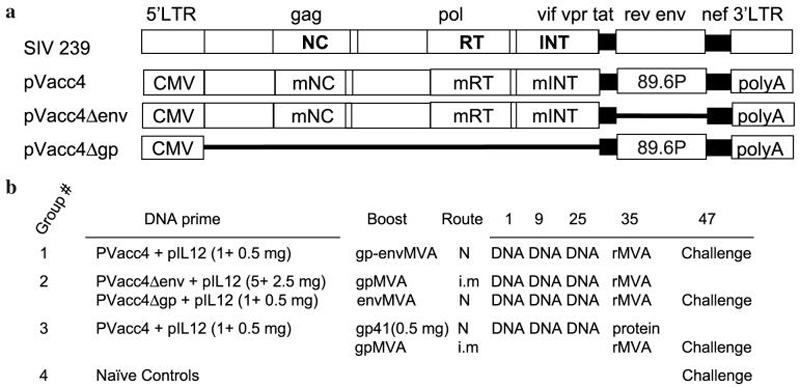
The DNA plasmid pVacc4 used in the vaccination is a derivative of pVacc1.33 pVacc1 includes a full SIVmac239 genome with multiple mutations in the NC basic domain and the functional domains of RT and INT, under the control of the cytomegalovirus (CMV) promoter. A 3.1-kb SphI-NcoI fragment that includes the env gene from pSHIV-KB9 3′ (NIH AIDS Research and Reference Reagent Program, catalog #4129) replaced the corresponding SphI-SnaBI fragment of pVacc1 that includes the SIV env of SIVmac239. In addition, a stop codon replaced the initiation codon of the vpr gene. The DNA sequence was confirmed by sequencing and the profile of viral particles produced by pVacc4 was evaluated by Western blot using a macaque SIV positive and a human HIV-positive sera followed by 125I-labeled protein A. Recombinant modified vaccinia virus Ankara (rMVA) expressing SIV Gag-Pol and HIV Env proteins was prepared as previously described.38,39 The rhesus macaque IL-12 expression plasmid was previously described.34 IL-12 production by rmIL-12.p40.DLp35 was tested in 293T transfection supernatant by enzyme-linked immunosorbent assay (ELISA) (IL-12 p70 assay, R&D Systems, Minneapolis, MN) and its biological activity was measured according to an assay described.40 Recombinant gp41 was expressed in Escherichia coli as a fusion of residues 29-167 of HIV-1 HXB2 gp41 with residues 43-88 of influenza virus HA2 and purified from inclusion bodies. This protein was mixed with the E. coli heat-labile enterotoxin (LT), which has been proven to be an effective mucosal adjuvant.
Immunization and challenge
Male Rhesus macaques were cared for at the New England Regional Primate Research Center using approved protocols under the guidelines established by the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Twenty animals were divided into four groups and treated as follows (Fig. 1). Group 1 received 1 mg pVacc4 DNA + 0.5 mg pIL-12 on day 1 of week 1, 9, and 25 and 109 plaque-forming units (pfu) of rMVA, expressing SIV gag, pol, and HIV env genes, were delivered to the nasal mucosa in volumes of 100 μl per nostril on week 34. Group 2 received 5 mg of pVacc4Δenv combined with 2.5 mg IL-12 DNA intramuscularly and 1 mg pVacc4Δgp DNA combined with 0.5 mg IL-12 DNA nasally on week 1, 9, and 25. SIV Gag-Pol rMVA (109 pfu in 200 μl) was administered intramuscularly and HIV89.6 Env rMVA (109 pfu in 200 μl) was administered nasally on week 34. Group 3 received 1 mg pVacc4 DNA mixed with 0.5 mg pIL-12 on week 1, 9, and 25. SIV gag-pol rMVA (109 pfu in 200 μl) was administered intramuscularly and purified gp41HA41 (0.5 mg mixed with 10 μl of LT in 80 μl) nasally on week 34. Group 4 provided naive controls for the challenge. DNA was administered nasally as drops of a DNA solution in saline. For this administration, the vaccine DNA was formulated at a concentration of 10 mg/ml and each DNA immunization consisted of 1.5 mg of DNA administered as a 150 μl dose, 75 μl in each nostril of the animal. Concentration and volumes were chosen to maximize the amount of DNA delivered in the smallest volume possible to facilitate retention of vaccine in the nasal cavity. At week 47, each animal was inoculated with a lethal dose (10 AID50, titrated via the rectal route) of pathogenic SHIV89.6P virus, administered nontraumatically with a needleless tuberculin syringe as cell-free virus in the rectum.
FIG. 1.
Vaccination protocol. Schematic representation of the proviral DNAs used in the vaccine regimens (a) and regimens administered to the four groups (b). N, nasal immunization; i.m., intramuscular immunization.
ELISA for anti-SIV and anti-HIV gp120 antibodies
Plasma obtained by standard venipuncture before and at intervals after vaccination was analyzed by ELISA for antiviral IgG antibodies. Both antiviral and total IgA in rectal secretions collected with Weck-Cel Surgical Spears (“sponges”; Medtronic Solan, Jacksonville, FL) were measured by ELISA. Blood contamination in secretions was assessed through measurement of hemoglobin with ChemStrips 4 (Boehringer-Mannheim) as described.42 Hemoglobin in secretions was, on average, 0.06% of that in blood. The collection method and procedure for secretion extraction have been described.42 Total IgA was measured as described33 with the exception that unlabeled and biotinylated purified polyclonal goat antimonkey IgA antibodies (Rockland Immunochemicals, Gilbertsville, PA) were used as the coating and secondary reagents, respectively.
ELISAs for antiviral antibodies were performed as described.33 Briefly, plates were coated with 250 ng SIVmac251 viral lysate (Advanced Biotechnologies Inc., Columbia, MD), 100 ng HIV-1 rgp120MN, or 100 ng HIV-1 rgp41MN (both ImmunoDiagnostics, Woburn, MA). In ELISAs for antigen-specific IgA, 12 wells of each plate were coated with serial dilutions (6-200 ng/ml) of macaque IgA that had been purified in the laboratory from breast milk (Yerkes Primate Center, Atlanta, GA). After blocking, plates were loaded with plasma samples and positive control or with secretions and buffer (macaque IgA wells). Positive controls for antiviral IgG were purified human polyclonal anti-HIV p24 (cross-reactive with SIV p27), -gp120, or -gp41 IgG antibodies (all ImmunoDiagnostics). In assays for antiviral IgA, wells coated with macaque IgA were used to generate a standard curve. Plates were developed with biotinylated, purified goat antihuman IgG antibody (Southern Biotechnology Associates, Birmingham, AL) or antimonkey IgA antibody (Rockland Immunochemicals, Gilbertsville, PA).33 Final development was with avidin-peroxidase and ABTS (Sigma), followed by measurement of absorbance at 414 nm.
The endpoint titer of anti-SIV or gp120MN IgG antibody was determined as the last serial 2-fold dilution of plasma that produced an absorbance value that was greater than the mean absorbance plus 3 standard deviations of eight blank (buffer diluent) wells that were reacted with the same reagents. Concentrations of SIV-, gp120-, or gp41-specific IgA were interpolated from standard curves constructed with the SoftMax Pro computer software program (Molecular Devices, Sunnyvale, CA). Antiviral IgA concentrations were divided by the total IgA concentration in each rectal secretion to obtain specific activity (ng antibody/μg immunoglobulin), which correlates directly with the proportion of virus-specific antibody-secreting cells in the rectal mucosa. Using the above assays, we have previously established that antibody titers or specific activity in postvaccination plasma or secretions are significant if they are 3.4-fold greater than those in corresponding preimmune specimens.33
Measurement of neutralizing antibodies
Neutralization titers were evaluated by an inhibition of infectivity assay.43 The virus used in the neutralization assay was SHIV89.6P.44 Antibody-mediated neutralization of SHIV was measured in an MT-2 cell-killing assay as described previously.45 Neutralization was measured at a time when virus-induced cell killing in virus control wells is greater than 70% but less than 100%. Neutralizing antibody titers were given as the reciprocal dilution required to protect 50% of cells from virus-induced killing.
Interferon (IFN)-γ ELISPOT
This assay was based on previously described methods46 and followed the protocol provided by the manufacturer (U-CyTech monkey ELISPOT kit, Utrecht, Netherlands). Briefly, 96-well microtiter plates were coated with 100 μl of anti-IFN-γ antibody in phosphate-buffered saline (PBS) as instructed by the manufacturer and stored overnight at 4°C. After the plates were washed and blocked, PBMCs were plated in triplicate at two concentrations (105 and 5 × 105 cells) with either medium alone [RPMI 1640 containing 25 mM HEPES, 10% fetal bovine serum (FBS), L-glutamine, penicillin, and streptomycin], or medium containing 10 μg/ml concanavalin A (Sigma), 5 μg/ml pooled SIV gag or SHIV89.6P Env peptides (AIDS Reference and Reagent Program, catalog #6204 and #4827), or an unrelated peptide pool. The plates were covered with a low evaporation lid and incubated at 37°C in 5% CO2 for 20 h. Following incubation, cells were flicked-off and residual cells were lysed with 200 ml of ice-cold water for 10 min. After washing the plates, spots were developed in accordance with the manufacturer's instructions by consecutive treatments with biotinylated anti-IFN-γ antibody, GABA-conjugated anti-biotin antibody, and activator in substrate buffer. The spots in each well were counted by an independent automated immunospot image analyzer (Zellnet Inc., New York, NY). The average number of spots present in the triplicate irrelevant cultures was subtracted from the average number in virus-specific peptide-stimulated cultures.
Intracellular staining for IL-2, IFN-γ, and tumor necrosis factor (TNF)-α
Intracellular staining was carried out as in a previously described protocol.47 Briefly, 106 PBMCs were stimulated with 1 μg/ml CD28 and CD49d antibodies (Becton Dickinson) in the above described RPMI medium and cultured for 1 h at 37°C in 5% CO2 with medium alone or medium containing 1 μg/ml staphylococcal enterotoxin B (SEB; Sigma) or 5 μg/ml pooled SIV Gag and HIV Env peptides. After 1 h, brefeldin A (final 10 μg/ml; Sigma) was added to each tube and incubation was continued for 5 h. Cells were treated with 100 μl of ice-cold 20 mM EDTA for 15 min, washed with PBS containing 1% bovine serum albumin (BSA), then stained for 30 min on ice with anti-CD3-FITC (Pharmingen) and anti-CD4-PerCP or anti-CD8-PE (Becton Dickinson). Cells were washed, permeabilized with FACS Permeabilizing Buffer (Becton Dickinson), washed, and then stained with APC-conjugated anti-IL-2, anti-IFN-γ, or anti-TNF-α antibody (Pharmingen). Cells were fixed with 1% paraformaldehyde and analyzed for fluorescence by flow cytometry using a Beckman Cytomics FC500 (Beckman Coulter). The CD3+ cells were used as the gate for CD8+ cells. Data for peptide-stimulated PBMCs are reported as the percentage of TNF-α+, CD3+, CD8+ cells or IL-2+, CD3+, CD8+ cells, determined after subtracting the percentage of these cells observed after stimulation with an unrelated peptide pool.
Immunostaining of CD4+ T cell memory subsets
Frozen stocks pf PBMCs were thawed and immunofluorescent stained for flow cytometric analysis with the following combination of fluorochrome-conjugated monoclonal antibodies (mAbs) specific for cell surface molecules: CD3 [peridinin chlorophyll protein-Cy5.5 (PerCP-Cy5.5)], CD4 [fluorescein isothiocyanate48 (FITC)], CD95 [phycoerythrin (PE)], and CD28 [allophycocyanin (APC)]. All antibodies were obtained from BD Bioscience and samples were analyzed by four-color flow cytometry (MoFlo, Dako Cytomation). Data analysis was performed using Summit v.4 software (Dako Cytomation). In this study naive CD4+ CD3+ T cells were identified by their CD95low CD28high phenotype, whereas memory CD4+ T cells were CD95high CD28high (central memory, CM) and CD95high CD28low (effector memory, EM) in the CD4+ CD3+ lymphocyte gate.
Detection of Gag p11c-tetramer-staining CD3+ CD8+ T lymphocytes
Rhesus macaques were screened for Mamu-A*01 positivity by a polymerase chain reaction (PCR)-based technique49 and in the two animals in each of the vaccine groups that were MamuA*01 positive p11c-tetramer staining was carried out on PBMCs and rectal biopsy-derived MCs. When the analysis was carried out on pooled biopsies, MCs were isolated by collagenase digestion (400 U/ml, SIGMA) followed by Ficoll-Hypaque density gradient centrifugation. PBMCs and MCs were stained with antihuman CD3 antibodies (FITC-labeled, Pharmingen), antihuman CD8αβ antibody (PerCP-labeled, Beckman Coulter), antihuman β7 integrin (APC-labeled, Pharmingen), and a PE-labeled Mamu-A*01-Gag p11c (Gag181-189) conjugate (a gift from J. Altman, Emory University).50,51 The samples were analyzed by four-color flow cytometry on a Beckman Cytomics FC500 and the data are presented as percentage of tetramerpositive cells of all CD3+ CD8+ cells. PBMCs or mucosal MNCs from two Mamu-A*01-negative animals were stained at every time point that was analyzed and the average of these results was subtracted from the values observed in Mamu-A*01 positive animals. The mean for the Gag p11c tetrameric complex staining background was 0.04%. Values higher than 0.1%, which is 2-fold higher than the mean background, were used as the threshold for positive responses in all the PBMC tetramer data analysis.
Viral load quantization
Plasma SHIV RNA levels were measured by a real time RTPCR assay, as described.52,53 The assay has a threshold sensitivity of 60 copy equivalent per ml. Interassay variation is <25% (coefficient of variation).
Flow cytometric analysis of blood-derived immune cells
Quantitative evaluation of CD4+ and CD8+ T cell subpopulations in PBMC samples was carried out during the postchallenge follow-up according to previously published procedures.54
Statistical analysis
Calculations and statistical analyses were performed using the Statview 4 computer program (Abacus Concepts, Berkeley, CA). Endpoint antibody titers and RNA viral loads were logarithmically transformed and the geometric means were calculated for each vaccination group. Between group comparisons were performed by analysis of variance (ANOVA) using Fisher's protected least significant difference set to the 95% confidence level. Within group comparisons were by two-tailed, paired t-test or Wilcoxon rank-sum test. Correlation analyses were done by Spearman rank followed by Fisher's r-to-z conversion of correlation coefficients to p values. Survival distributions were estimated using the Kaplan-Meyer method and the log-rank test statistics was used to compare survival curves between two or more groups. Results of statistical analyses were considered significant if they produced p values ≤0.05.
RESULTS
HIV and SIV are predominantly transmitted via mucosal surfaces and establish systemic chronic infections with a significant acute and chronic component localized at the GI mucosa.12,55-57 Many studies have evaluated the impact of intramuscular prime-boosting immunization regimens in the SHIV model. Our working hypothesis is that immunity in both the mucosal and systemic compartments may provide a better control of infection than immunity localized predominantly in the systemic compartment. In particular, humoral mucosal immunity against the viral Env protein may be important to prevent transmission, as this protein is exposed on the outer surface of the virion and could be easily targeted by mucosal IgA. Toward the goal of stimulating these diverse responses and evaluating their role in infection and disease progression, we have compared three vaccination regimens (Fig. 1). We evaluated a prime-boosting DNA-MVA regimen that included all the SHIV structural proteins and most accessory proteins and was administered mucosally (Group 1) and two combined mucosal and systemic DNA-MVA regimens that differ from each other in the following: in the Group 2 regimen the Gag-Pol antigen produced after DNA or MVA immunizations was administered only systemically and the Env antigen produced after DNA or MVA immunizations was administered only nasally; in the Group 3 regimen DNA priming for all antigens occurred nasally as for Group 1, while boosting with Gag-Pol MVA was administered systemically and Env as purified HIV gp41 in LT adjuvant was given nasally. IL-12 DNA was used as the source of the adjuvant cytokine in association with viral DNA in all DNA vaccinations. Humoral and cell-mediated systemic and mucosal responses were assessed during the immunization regimen and their role in infection and protection was assessed after rectal challenge with virus.
Humoral and cell-mediated responses during the immunization
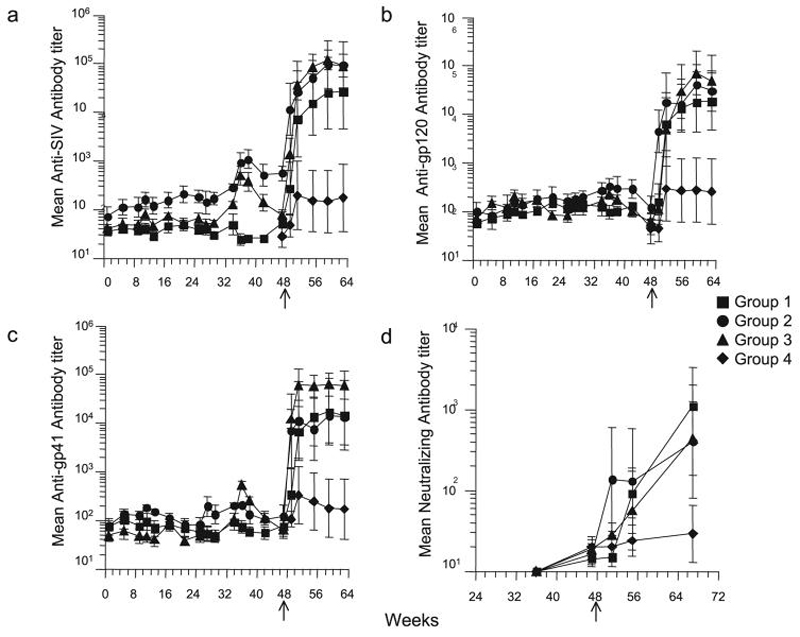
Humoral and cell-mediated mucosal and systemic responses were measured during the vaccination and after challenge (week 47). Virus-specific IgA production was evaluated in rectal secretions and some but not all animals were found to have developed SHIV responses during the vaccination. There was no significant difference between the different regimens in their ability to stimulate antiviral IgA responses in the rectal secretions (Table 1). These data indicate that an IL-12-augmented SHIV DNA/MVA vaccine administered nasally or intramuscularly can stimulate rectal antiviral IgA, but this stimulation varies among the animals. Moderate increases in anti-SHIV IgG compared to the preimmune sera were found in the serum of some animals during the vaccination protocol (Fig. 2). We could not detect boosting of serum humoral responses after the nasal rMVA vaccination. Interestingly, a consistent increase in serum anti-gp41 IgG titers could be achieved after a single nasal boost with gp41 protein with LT adjuvant. Antiviral IgG and IgA had declined to baseline in most of the animals by the day of challenge.
Table 1.
ANTI-SHIV MUCOSAL HUMORAL RESPONSES DURING THE VACCINE TRIALa
|
SIV |
gp120 |
gp41 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wk 33 | Wk 37 | Wk 41 | Wk 33 | Wk 37 | Wk 41 | Wk 33 | Wk 37 | Wk 41 | |
| Group 1 | |||||||||
| 177 | — | — | — | — | — | — | 2.3 | 1.7 | — |
| 180 | 2.4 | — | — | 4.8 | — | — | 2.9 | 1.3 | — |
| 182 | — | — | 5.0 | — | — | 4.4 | — | — | — |
| 184 | — | — | — | — | 8.6 | 9.7 | — | — | — |
| 187 | 1.1 | 1.5 | — | — | 2.5 | — | |||
| Group 2 | |||||||||
| 185 | — | 2.6 | — | — | — | — | — | — | — |
| 186 | — | — | — | — | — | — | 2.9 | 6.6 | — |
| 188 | 1.1 | — | — | — | — | — | — | — | — |
| 316 | — | — | — | — | — | 3.4 | — | 1.1 | — |
| 319 | — | — | 3.5 | — | — | — | 1.1 | — | 1.8 |
| Group 3 | |||||||||
| 189 | 2.0 | — | — | — | — | — | — | — | — |
| 190 | — | — | — | — | 2.6 | — | — | — | — |
| 192 | — | 1.3 | 4.0 | 1.0 | 1.0 | 4.2 | 1.6 | — | 2.0 |
| 320 | 12.3 | — | 2.7 | — | — | 2.0 | 2.7 | 8.8 | — |
| 321 | 2.5 | — | — | — | — | — | 14.5 | 12.6 | 1.1 |
SHIV-specific IgA fold increase in rectal secretions during the immunization. The specific activity (ng SHIV IgA/μg total IgA) was obtained by dividing the antiviral IgA concentration by the total IgA concentration in each sample. Numbers in bold indicate values that were significantly higher (mean + 3 standard deviations) than the preimmune samples. The fold increase is expressed as a ratio between postimmune sample specific activity and background specific activity measured before immunization. (—) indicates absence of increase.
FIG. 2.
Plasma anti-SHIV IgG titers. Geometric mean of reciprocal endpoint titers of (a) anti-SIV Gag/Pol, (b) anti-HIV gp120, (c) anti-HIV gp41-specific IgG, and (d) neutralizing antibodies measured in plasma collected during vaccination, on the day of challenge (week 47) and postchallenge at 4 week intervals up to week 64. Error bars represent SEM. Arrows indicate day of challenge.
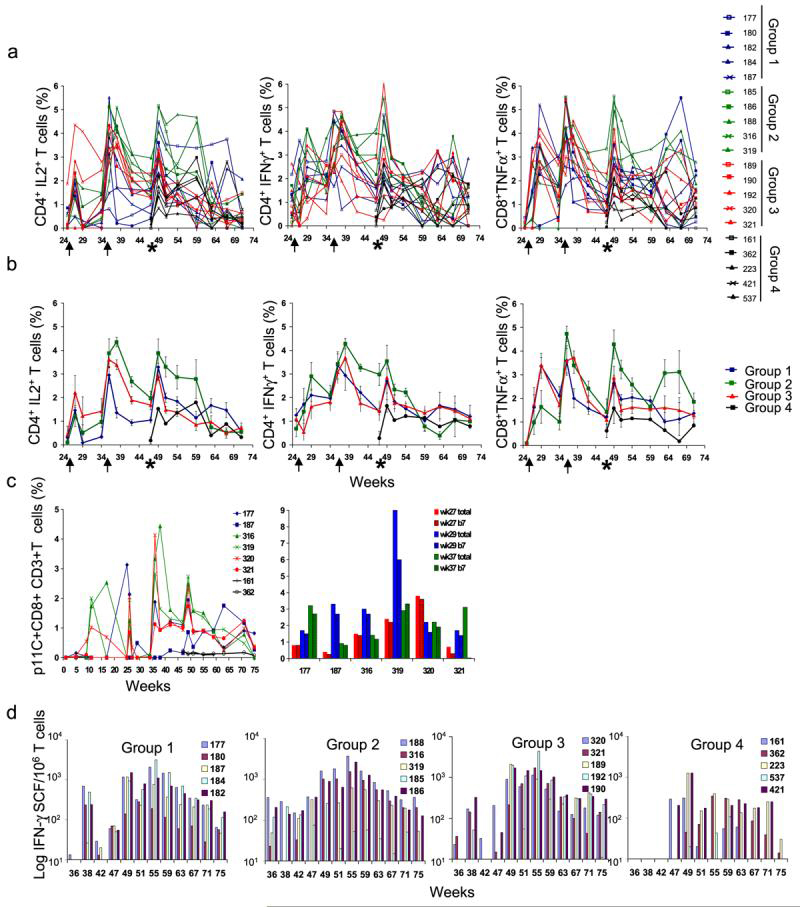
The differences in cellular responses observed in the three vaccinated groups before challenge were not statistically significant in most cases (p > 0.05), with the following exceptions: the levels of Gag-specific CD4+/IL-2+ T cells measured after Gag stimulation in Group 2 compared to Group 1; the levels of Gag-specific CD8+/TNF-α+ T cells measured after Gag stimulation in Group 3 compared to Group 1; and the levels of Gag-specific CD4+/IFN-γ+ T cells measured after Gag stimulation in Group 3 compared to Group 2 (Fig. 3a and b). In addition, IFN-γ ELISPOT, measured after Gag and Env peptide stimulation in PBMCs of Group 2 animals from week 36 and 47, was significant when compared to that observed in Group 3 animals (p < 0.008) (Fig. 3d). On the day of challenge, the difference between Group 2 systemic CD4+ T cell-mediated response levels and those of other groups was significant. These responses may have been critical to the better control of peak and chronic viremia observed in these animals.
FIG. 3.
SHIV-specific systemic cell-mediated immune responses. Percentage of SHIV-specific T cells detected in PBMCs by intracellular staining after stimulation with an SIV Gag or an HIV-1 89.6 Env peptide pool. (a) Values for the individual animals are reported as the sum of percentages of Gag-specific and Env-specific cells. (b) Group means of the values reported in (a). (c) Percentage of CD3+/CD8+/p11c tetramer-positive T cells in PBMCs of Mamu-A*01-positive animals in each group during the entire experiment (left) and in rectal MC 2 and 4 weeks after the third DNA vaccination and 2 weeks after rMVA boosting in Mamu-A*01+ animals (right). The two graph bars for each Mamu-A*01 animal represent the percentage of rectal mononuclear cells. CD3+/CD8+ (open bar) and CD3+/CD8+/β7+ (striped bar) T cells colabeled with Gag p11C tetramer. (d) Number of IFN-γ-secreting PBMCs per million cells detected by ELISPOT after stimulation with SIV Gag or Env peptide pools. 50 SFU corresponds to the level of 2-fold the average background and is the threshold for significant responses. Graph bars represent the mean number of spots per million cells of Gag-specific plus Env-specific cells, detected in triplicate cultures of PBMCs cultured with peptides, after subtracting of the mean number of spots found in triplicate control cultures of PBMCs in medium alone. The arrows indicate the days of the second and third vaccinations. The asterisks indicate the day of challenge.
DNA vaccination stimulated the induction of CD3+/CD8+/p11c+ T cells in the PBMCs of Mamu-A*01+ animals (Fig. 3c, left). At week 29, higher percentages of p11c+ T cells were found in rectal MCs compared to those found in PBMCs (Fig. 3c, right). The majority of p11c+ T cells detected in MCs were also β7 positive, suggesting the mucosal priming of these cells (Fig. 3c, right, striped bars). The percentages of p11c+ T cells measured in PBMCs on week 26 in most of the animals were higher than those measured at weeks 27 and 29, probably reflecting the redistribution of anti-Gag CD8+ T cells from the blood to other tissues after the third DNA vaccination. Two weeks after MVA boosting (week 37), an increase in the PBMC percentage of CD3+/CD8+/p11c+ T cells was observed for five of the six Mamu-A*01+ animals. Elevated levels of PBMC CD3+/CD8+/p11c+ T cells were still present on the day of challenge in those five animals, although these levels had contracted from the peak reached after the rMVA vaccination. The small number of Mamu-A*01+ animals in each group prevents statistical evaluation of these results.
Rectal challenge and postchallenge follow-up
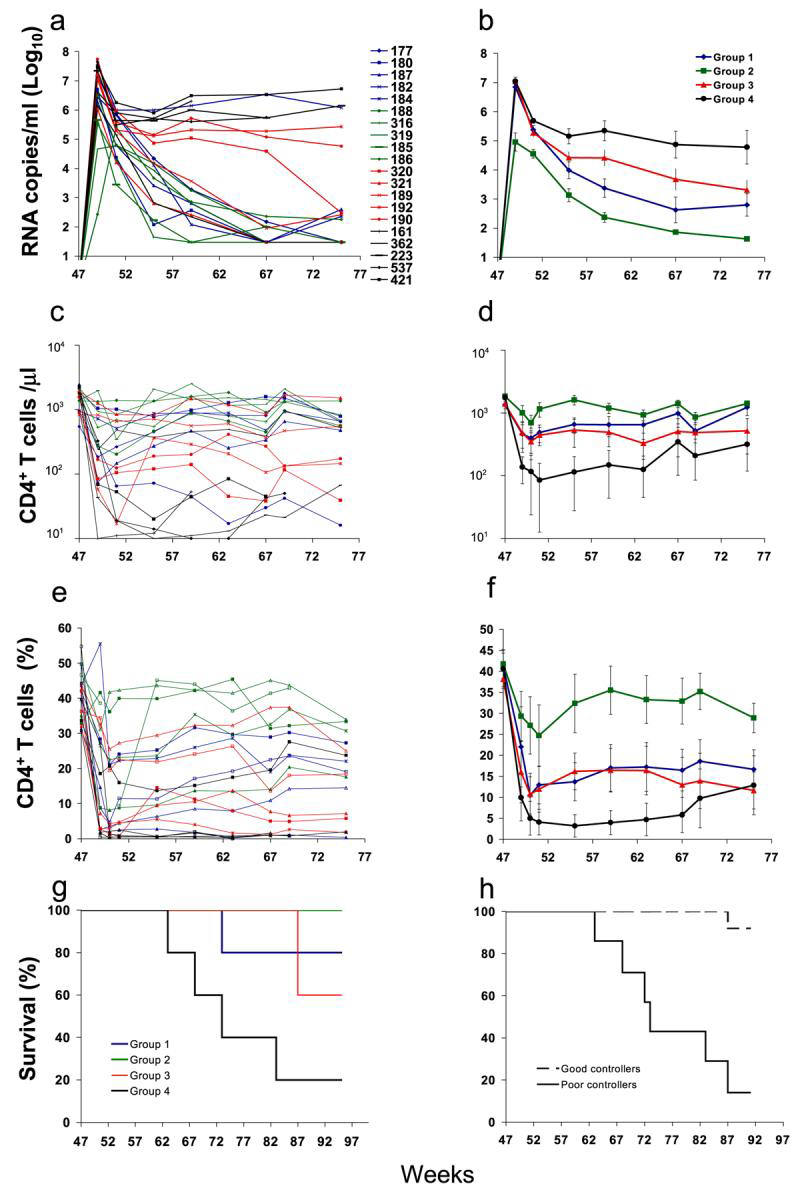
When vaccinated and naive animals were challenged rectally with SHIV 89.6P on week 47, they all became infected. However, most of the vaccinated animals, including all Group 2 animals, showed better viremia control and were better protected from CD4+ T cell loss and AIDS progression than the control Group 4 animals (Fig. 4). Differences in viremia control between groups were evaluated at peak (week 2 postchallenge) and from week 8 to week 28 postchallenge (Fig. 4a and b). Differences in CD4+ T cell counts were evaluated from week 4 postchallenge to week 28 (Fig. 4c and d). Group 2 viremia levels and CD4+ T cell counts observed throughout the postchallenge time course were statistically significant when compared to the values observed for the Group 4 control animals. Peak viremia (week 2) was significantly lower in Group 2 compared to all the other groups (Group 2 compared to Group 1: p = 0.05; Group 2 compared to Group 3 or Group 4: p < 0.04). Peak viremia differences between Group 1 or 3 and 4 were not significant. The differences in viremia levels and CD4+ T cell counts observed between Group 1 and Group 4 or Group 3 and Group 4 animals were statistically significant once the viremia set point was reached (week 8). The significance of the differences of chronic viremia between groups during the postchallenge time course (week 8 to week 28 postchallenge) was the following: Group 1 vs. Group 4: p < 0.003; Group 2 vs. Group 4:, p < 5.6E-6; Group 3 vs. Group 4: p < 0.034. The differences of chronic viral loads (week 8-28) among vaccinated groups were significant when Group 2 was compared to Group 1 (p < 0.04) and to Group 3 (p < 0.0003). The statistical significance in the differences of absolute CD4+ T cell counts and percentages during the postchallenge time course between groups is the following: (1) when Group 2 was compared to Group 4, both at peak viremia (week 2, p < 0.05) and after week 8 (p < 1.9E-13); when Group 2 was compared to Group 1 or 3, from week 8 onward (p < 0.0002); (2) when Group 1 or 3 was compared to Group 4, from week 8 onward (p < 0.0002; p < 0.0031); (3) comparison between Group 1 and 3 did not show statistical significance. When the survival in the control group became lower than 50% (week 35 postchallenge), all vaccinated animals with one exception were still alive. There was statistical significance when survival in each vaccine group was compared to the survival in the control group (Fig. 4g).
FIG. 4.
RNA viral loads in macaques challenged rectally with SHIV89.6P (expressed as log10). (a) Values of viral RNA copies detected in the plasma of each animal are reported for individual time points. (b) The geometric mean of viral RNA copy numbers detected in the animals of each group at each time point is reported. Postchallenge absolute CD4+ T cell counts per μl (c) and percentage (e) in PBMCs of animals in the four groups. CD4+ T cells were evaluated in PBMCs on the day of challenge (week 47) and up to 28 weeks postchallenge (week 75). (d) The mean of the absolute CD4 T cell counts (d) and percentage (f) detected in the PBMC of the animals of each regimen group is reported. Error bars represent SEM. (g,h) Kaplan-Meier survival curves following challenge for the different groups (g) or after dividing vaccinated and control animals into two groups based on their viral load at set point (week 8 postchallenge) (h). Significance, evaluated by the log rank test, was observed when Group 2 was compared to Group 4 (p < 0.01), when Group 3 was compared to Group 4 (p < 0.03), and when Group A (good controllers) was compared to Group B (poor controllers) (p < 0.01). Animals included in Group B are 182 (Group 1), 190, 192, 320 (Group 3), and 161, 223, 212, 537 (control Group 4). All the remaining animals were included in Group A.
A significant increase in antibody titers could be documented for all the animals by detection of SHIV-specific serum IgG in infected animals, beginning 2-4 weeks after challenge (Fig. 2). The kinetics and magnitude of the antiviral IgG antibody response during the first 4 weeks after challenge were significantly different in vaccinated animals compared to naive controls, suggesting that the virus-specific antibodies detected in the vaccinated animals after challenge were a secondary response against viral antigens or, alternatively, that the protection of CD4+ T cells afforded by the vaccine permitted the establishment of these responses to an extent that did not occur in the control animals. All vaccinated animals maintained significantly higher antibody titers than the naive controls during the postchallenge follow-up (all p < 0.05 by ANOVA). The magnitude and the persistence of serum IgG responses detected up to 17 weeks after challenge in the chronically infected animals paralleled the preservation of CD4+ T cell counts and the control of viremia. Neutralizing antibody titers were also significantly higher in vaccinated animals than in controls (p < 0.05 by ANOVA). This result suggests that containment of virus replication due to the vaccine-induced cell-mediated responses and consequent preservation of the immune system is critical to the ability to establish significant antibody responses, and more specifically neutralizing antibodies, and these in turn contribute to a further reduction of viremia and protection of the immune system.
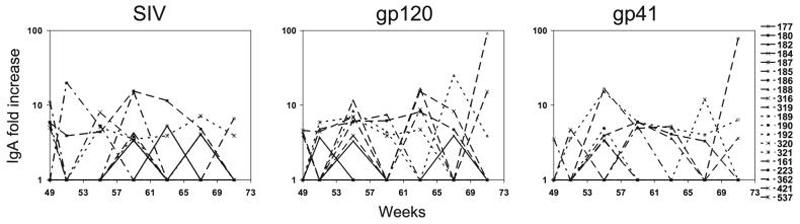
The analysis of virus-specific IgA responses in the rectal secretions after challenge revealed puzzling differences among the groups (Fig. 5). Virus-specific IgA responses were present only sporadically (once or twice per animal) in four of five Group 1 animals, which had received nasal vaccination. They were more consistently present and of higher magnitude against the whole virus lysate; gp120 and gp41 in Group 2 animals and Group 3 animals showed a pattern intermediate between the two. Three of the four controls tested also showed antiviral IgA responses. These responses are hard to interpret as animals with good outcome and CD4+ T cell preservation showed no, low, and persistent IgA responses, and the same was true for animals with poor outcome and low CD4+ T cell count. It is possible that limited viral replication combined with preserved CD4+ T cell counts in the GI tract may be important for the development of IgA responses and that these do not occur if the viral load in the GI tract is very low or when the CD4+ T cells are depleted. As viral loads and CD4+ T cells were not measured in the GI tract, this may be the reason why the observed results cannot be correlated with the outcome of the animals.
FIG. 5.
SHIV-specific IgA fold increase in rectal secretions after challenge. The fold increase is expressed as a ratio between postimmune sample-specific activity and background-specific activity measured before immunization. The first panel shows the IgA fold increase detected using an SIV viral lysate, the second panel shows the anti-gp120 IgA fold increase, and the third panel shows the anti-gp41 IgA fold increase.
Correlates of protection
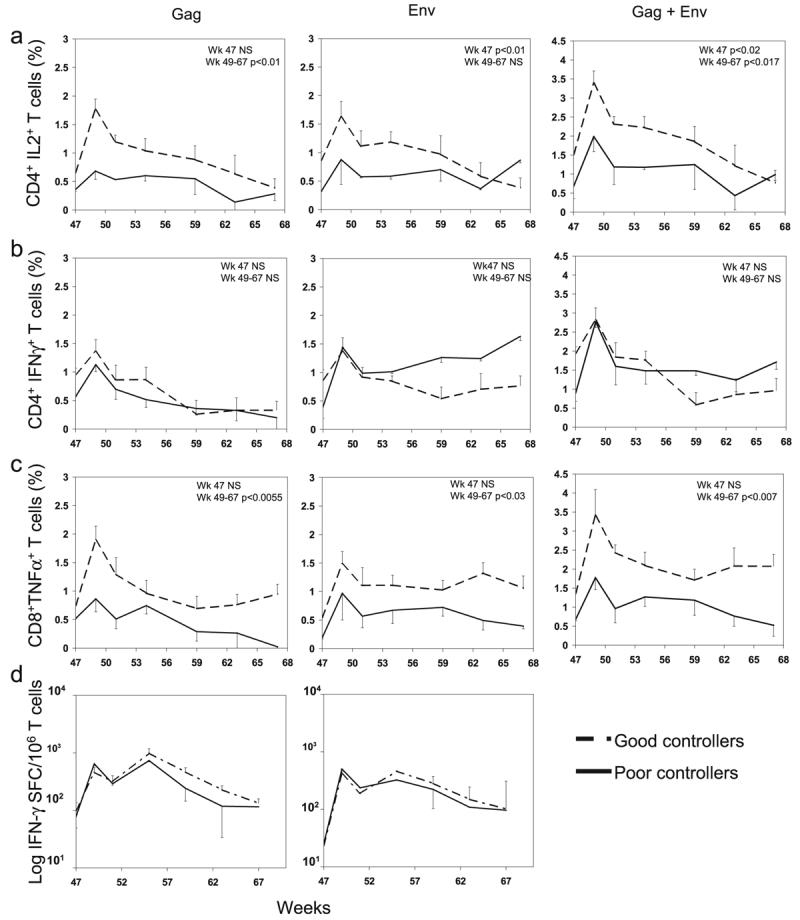
Because the vaccinated animals did significantly better than the controls in terms of CD4+ T cell preservation and AIDS progression, the differences in some of the virus-specific responses stimulated by the vaccination may have been critical to the better control of viremia observed in these animals, but we could not establish a correlation between the responses measured during the vaccination phase of the trial and the outcome in the individual vaccinated groups. This suggests that parameters in addition to those monitored in this experiments need to be evaluated or that the group size was limited. However, when we divided the animals included in this trial into two groups, animals that controlled viremia below 1000 copies/ml (Group A, or good controllers) and animals whose viremia persisted above 1000 copies/ml (Group B, or poor controllers) 28 weeks after challenge (week 75 of trial), we found that survival was significantly higher in Group A (Fig. 4h), confirming the already established correlation between viremia control and disease protection. In addition, on the day of challenge (week 47) there were statistical significances (by Wilcoxon rank-sum test) in the following comparisons: (1) the numbers of Env-specific IL-2+/CD4+ T cells (p < 0.01), detected after Env peptide stimulation in Group A PBMC samples compared to Group B PBMC samples, and (2) the cumulative numbers of Gag-specific plus Env-specific IL-2+/CD4+ T cells (p < 0.02), detected after peptide stimulation in Group A PBMC samples compared to Group B PBMC samples (Fig. 6a). The comparative analysis of the virus-specific immune responses measured from week 26 to 47 in these two groups did not show statistical significance (Fig. 6a-d). These results indicate that the level of memory virus-specific response present at the time of challenge, regardless of the vaccine regimen used to induce it, correlates with the level of protection observed later during the chronic infection. These results also suggest that poor vaccine responders, present in all groups, combined with the small number of animals in the groups, affected the interpretation of the results observed with the individual vaccine regimens.
FIG. 6.
SHIV-specific cell-mediated responses in good (dashed line) and poor (solid line) virus controllers (a-d). Group averages and SEM are represented at each time point. p values for the comparison of the values measured on the day of challenge (week 47) and for the areas under the curve of the two groups from week 24 to 27 (immunization) and from 49 to 67 (postchallenge) are reported. NS, nonsignificant.
Postchallenge, significant differences were observed in some of the responses detected in the vaccinated animals compared to those observed in the nonvaccinated animals (Fig. 3). Significance was observed for the comparison of Gag-stimulated CD8+/TNF-α+ T cells observed in Group 1 and 2 compared to Group 4, and Group 2 compared to Group 1 and 3, and for Gag-stimulated CD4+/IFN-γ+ cells observed in Group 2 compared to Group 3 and 4. The postchallenge antiviral cell-mediated reponses in vaccinated animals paralleled the control of viremia and preservation of the CD4+ T cell counts observed in these animals, suggesting the importance of these responses in chronic SHIV containment and in delayed progression to AIDS. Indeed, when responses measured in Group A, good controllers, and Group B, poor controllers, were compared, the differences of CD4+/IL-2+ T cells and CD8+/TNF-α+ T cells measured over the course of the infection (week 49 to 68, corresponding to week 2 to 21 postchallenge) in PBMCs of the two groups after SIV stimulation were statistically significant (Fig. 6a and c). This was not true for CD4+/IFN-γ+ T cells, where the differences between the two groups were not significant. Similar results were observed with the IFN-γ ELISPOT analysis carried out after challenge in PBMCs, where no differences were observed in the numbers of spots detected after Gag or Env stimulation between the two groups (Fig. 6d).
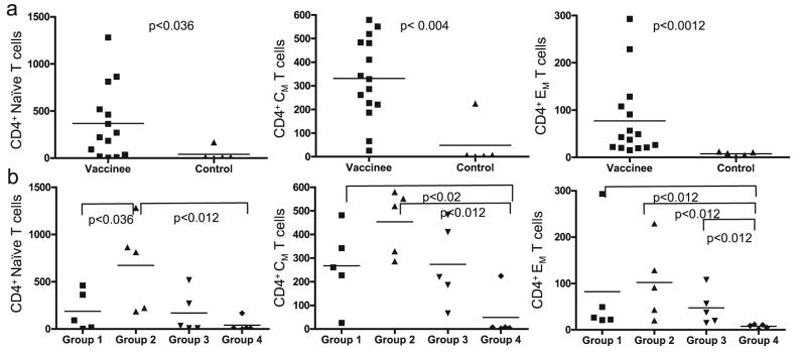
The SHIV89.6 virus used for the challenge is an X4-tropic virus and it can infect naive as well as memory CD4+ T cells. We evaluated the frequency of CD4+ T cell subsets in the PBMCs at early time points of the chronic infection phase (week 8 and 12 postchallenge) and compared the levels observed in vaccinated and control animals (Fig. 7a). As expected, considering the virus tropism, we found that all three CD4+ subpopulations were significantly affected by the infection in the controls, and the difference of the levels of these subpopulations between the two groups was statistically significant when analyzed by the Wilcoxon rank-sum test. This was also true when the comparison was carried out between all CD4+ T cell subpopulations of Group 2 animals and the naive controls, between CD4+ CM and EM subpopulations in Group 1 animals and the naive controls, between the CD4+ EM subpopulation in Group 3 animals and Group 4 naive controls, and between CD4+ naive T cells of Group 2 and Group 1 (Fig. 7b). The significance of comparison of the levels of all CD4 subpopulations between Group 2 and Group 4 indicates that this vaccination regime provided the best protection from long-term CD4+ T cell depletion. This analysis also indicates that unlike during SIV infection, the naive CD4+ T cell population is more substantially targeted by SHIV89.6P. This targeting limits the depletion of the memory population, which includes the anti-SHIV memory T cells established by the vaccination.
FIG. 7.
Naive, CM, and EM CD4+ T cell populations after challenge. Percentage of naive (CD95-/CD28+), CM (CD95+/CD28+), and EM (CD95+/CD28-) CD4+ T cells in vaccinated and control animals (a) and in individual vaccine and control groups (b). The average of values measured 8 and 12 weeks after challenge is represented for each animal. p values for each pair comparison were calculated using the Wilcoxon rank-sum test.
In summary, the data indicate that the IL-12-augmented, combined intramuscular and nasal administration of SIV recombinant DNA/MVA can provide more significant long-term protection from CD4+ T cell loss and AIDS development than the IL-12-augmented SHIV DNA/MVA nasal vaccination alone. However, the nasal vaccination regimen provided nearly the benefit seen with IL-12-augmented intramuscular/nasal vaccination, as the outcome of CD4+ T cell preservation in Group 1 and Group 3 animals is also significantly different than that observed in Group 4 controls.
DISCUSSION
HIV infection, occurring in most cases via mucosal surfaces, leads to a chronic disease with systemic dissemination and a significant component localized in the GI tract. Prevention from infection may benefit from strong mucosal immunity, as containment of the viral inoculum needs to occur at the site of exposure. Both humoral and cell-mediated responses, especially against Env determinants that are most exposed on the virus surface and could be easily targeted by antibodies, could play a significant role at mucosal sites. Interestingly, clearance of chronic infection is extremely hard to achieve, even when the infectious agent is a very immunogenic, highly attenuated virus.58-61 It is therefore unlikely that this may happen with wild-type viruses, if chronic infection occurs after vaccination. Once the infection becomes disseminated, both mucosal and systemic immunity may be necessary for significant and prolonged viral containment. As the route of immunization strongly influences where the immune responses reside, it is important to investigate where vaccine regimens stimulating systemic, mucosal, or combined systemic and mucosal antiviral immunity may be better suited to prevent disease occurrence long term.
The studies presented here indicate that a regimen that involved administering Gag/Pol and other nonstructural SHIV antigens systemically, and the Env antigen mucosally, provided the best level of protection from disease development when compared to a regimen where all the SHIV antigens were administered entirely nasally. However, protection provided by the exclusive nasal immunization regimen closely approached that provided by the combined nasal/intramuscular regimen administered to Group 2 animals. Furthermore, nasal Env immunization, whether based on DNA + MVA or DNA + protein, did not stimulate consistent rectal Env humoral responses and the antiviral immunity induced was not sufficient to prevent the establishment of chronic infection after rectal challenge.
The addition of IL-12 DNA did not reduce the efficacy of vaccination compared to SHIV DNA alone. Although a group receiving nasally viral DNA + rMVA only was not included in this trial, we reported such a group in a previous trial34 and in that trial the outcome of the group that received SHIV DNA with IL-12 DNA, boosted by rMVA, was worse than that of the SHIV DNA + rMVA group. We hypothesized that the small size of the groups may have been a factor in this result. Indeed in this trial the addition of IL-12 DNA, administered to all the animals in the three vaccine groups, did not adversely affect the outcome of the vaccination. This cytokine was included in the vaccination because of its positive effect on mucosal responses and our interest in achieving consistent mucosal humoral responses. The Env antigen was administered nasally to all animals as DNA expressing HIV Env together with IL-12 DNA. The anti-gp120 IgA response was limited to a few animals, suggesting limited efficacy of this mode of antigen administration and of the capability of IL-12 DNA to provide adjuvancy to IgA stimulation via the nasal route.
As observed with a number of other vaccines, the protection from SHIV-induced disease induced by any of the vaccine regimens was more significant than the protection achieved by vaccination against SIV.1-5 SHIV infection in naive macaques induces a drastic decline of CD4+ T cells that does not faithfully recapitulate the HIV or SIV course of infection and disease progression observed in humans.6 It has been suggested that the more acute nature of X4 SHIVs may result in an infection that can be more easily controlled by vaccination. However, X4 SHIVs, like SIVs, establish a chronic stage that persists in the host even when the infection occurs after vaccination, supporting the chronic nature of this virus. It is possible that the broader tropism of X4 SHIV compared to R5 SIV and HIV, which unlike the R5 viruses extends also to naive CD4+ T cells, may play a role in the different outcome of the vaccination in this infection model.62,63 The availability of viral targets in the naive CD4+ T cell population may result in reduced damage to the memory CD4+ T cell compartment that contains the virus-specific cells induced by the vaccination. Limited targeting of these cells, which become activated by the replicating virus, may in turn result in more efficient elimination of virus-infected cells and in more significant reduction of the viremia. This could be harder to achieve if the activated virus-specific cells are directly and almost exclusively targeted by the R5 viruses. Indeed with the SHIV-89.6P we observed higher losses in the naive compartment but a more significant preservation of memory cells during the early stages of viremia, despite levels of virus replication that were similar or higher than during the same stages of SIV infection. The differential preservation of the memory compartment may be the key to a better outcome of SHIV vaccination compared to SIV.
The relative abundance and distribution of CXCR4+ and CCR5+ cells between the systemic and the mucosal compartment may also have an impact on whether mucosal or systemic immunity is more critical to the elimination of the infected cells. A larger proportion of CCR5+ cells resides in the GI mucosa than in systemic lymphoid organs while CXCR4+ CD4+ T cells are overall more abundant and more similarly distributed between the mucosal and systemic compartment.9,13 In our experiments a regimen with immunization administered at both compartments provided better protection than a nasal immunization alone or a regimen in which only part of the MVA boosting, but not the DNA priming, occurred systemically. A group immunized only systemically was not included and therefore a direct comparison cannot be done. Results by others indicate that intramuscular vaccination provides excellent protection against X4 SHIV, in agreement with the concept that this vaccination would stimulate immunity at systemic sites where a substantial fraction of viral targets is present. Our recent experiment with R5 SIV vaccination and challenge indicates that in this model a mucosal vaccination provides better protection than a systemic one (M. Marique et al., unpublished observations). There is limited information on how the route of vaccination affects the vaccination outcome, in particular as a direct comparison with the same vaccine in the same challenge model. The experiments presented here suggest that assessment of a vaccine via different routes and consideration of the tropism of the strain used for challenge may be significant issues in the development of an AIDS vaccine.
ACKNOWLEDGMENTS
We thank Dr. Jeff Lifson and Mike Piatak for assistance with the measurements of viral loads in macaques. This project was funded by NIH Grants AI41365 (to A.A.) and AI48133 (to P.A.K.) and CFAR Grant 5P30AI060354 (to M.G.) and was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-124000.
REFERENCES
- 1.Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- 2.Robinson HL. New hope for an AIDS vaccine. Nat Rev Immunol. 2002;2:239–250. doi: 10.1038/nri776. [DOI] [PubMed] [Google Scholar]
- 3.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective antiimmunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 4.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O'Connor DH, Fridman A, Bagchi A, Tussey LG, Bett AJ, Finnefrock AC, Fu TM, Tang A, Wilson KA, Chen M, Perry HC, Heidecker GJ, Freed DC, Carella A, Punt KS, Sykes KJ, Huang L, Ausensi VI, Bachinsky M, Sadasivan-Nair U, Watkins DI, Emini EA, Shiver JW. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79:15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinberg MB, Moore JP. AIDS vaccine models: challenging challenge viruses. Nat Med. 2002;8:207–210. doi: 10.1038/nm0302-207. [DOI] [PubMed] [Google Scholar]
- 7.Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- 8.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 9.Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, Grovit-Ferbas K, Mackay CR, Chen ISY, Giorgi JV. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. Aids. 2000;14:1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 10.Oppermann M. Chemokine receptor CCR5: Insights into structure, function, and regulation. Cell Signal. 2004;16:1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Kinter A, Arthos J, Cicala C, Fauci AS. Chemokines, cytokines and HIV: A complex network of interactions that influence HIV pathogenesis. Immunol Rev. 2000;177:88–98. doi: 10.1034/j.1600-065x.2000.17708.x. [DOI] [PubMed] [Google Scholar]
- 12.Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantele A, Kantele JM, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher EC, Makela PH. Homing potentials of circulating lymphocytes in humans depend on the site of activation: Oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol. 1997;158:574–579. [PubMed] [Google Scholar]
- 15.Kantele A, Hakkinen M, Moldoveanu Z, Lu A, Savilahti E, Alvarez RD, Michalek S, Mestecky J. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: Evidence for compartmentalization within the common mucosal immune system in humans. Infect Immun. 1998;66:5630–5635. doi: 10.1128/iai.66.12.5630-5635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantele A, Zivny J, Hakkinen M, Elson CO, Mestecky J. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J Immunol. 1999;162:5173–5177. [PubMed] [Google Scholar]
- 17.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 19.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: New concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: Role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J Immunol. 2004;172:857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 21.Belyakov IM, Ahlers JD, Brandwein BY, Earl P, Kelsall BL, Moss B, Strober W, Berzofsky JA. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J Clin Invest. 1998;102:2072–2081. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cromwell MA, Veazey RS, Altman JD, Mansfield KG, Glickman R, Allen TM, Watkins DI, Lackner AA, Johnson RP. Induction of mucosal homing virus-specific CD8(+) T lymphocytes by attenuated simian immunodeficiency virus. J Virol. 2000;74:8762–8766. doi: 10.1128/jvi.74.18.8762-8766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klavinskis LS, Bergmeier LA, Gao L, Mitchell E, Ward RG, Layton G, Brookes R, Meyers NJ, Lehner T. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J Immunol. 1996;157:2521–2527. [PubMed] [Google Scholar]
- 24.Lehner T, Tao L, Panagiotidi C, Klavinskis LS, Brookes R, Hussain L, Meyers N, Adams SE, Gearing AJ, Bergmeier LA. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J Virol. 1994;68:1624–1632. doi: 10.1128/jvi.68.3.1624-1632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehner T, Wang Y, Ping L, Bergmeier L, Mitchell E, Cranage M, Hall G, Dennis M, Cook N, Doyle C, Jones I. The effect of route of immunization on mucosal immunity and protection. J Infect Dis. 1999;179(Suppl 3):S489–492. doi: 10.1086/314809. [DOI] [PubMed] [Google Scholar]
- 26.Lehner T, Wang Y, Cranage M, Bergmeier LA, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Pauza CD, Lu X, Montefiori DC, Miller CJ. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 28.Marx PA, Compans RW, Gettie A, Staas JK, Gilley RM, Mulligan MJ, Yamshchikov GV, Chen D, Eldridge JH. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 29.McChesney MB, Collins JR, Miller CJ. Mucosal phenotype of antiviral cytotoxic T lymphocytes in the vaginal mucosa of SIV-infected rhesus macaques. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S63–66. [PubMed] [Google Scholar]
- 30.Polacino PS, Stallard V, Klaniecki JE, Pennathur S, Montefiori DC, Langlois AJ, Richardson BA, Morton WR, Benveniste RE, Hu SL. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in macaques. J Virol. 1999;73:8201–8215. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevceva L, Alvarez X, Lackner AA, Tryniszewska E, Kelsall B, Nacsa J, Tartaglia J, Strober W, Franchini G. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques. J Virol. 2002;76:11659–11676. doi: 10.1128/JVI.76.22.11659-11676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veazey RS, Gauduin MC, Mansfield KG, Tham IC, Altman JD, Lifson JD, Lackner AA, Johnson RP. Emergence and kinetics of simian immunodeficiency virus-specific CD8(+) T cells in the intestines of macaques during primary infection. J Virol. 2001;75:10515–10519. doi: 10.1128/JVI.75.21.10515-10519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SW, Kozlowski PA, Schmelz G, Manson K, Wyand MS, Glickman R, Montefiori D, Lifson JD, Johnson RP, Neutra MR, Aldovini A. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J Virol. 2000;74:10514–10522. doi: 10.1128/jvi.74.22.10514-10522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertley FM, Kozlowski PA, Wang SW, Chappelle J, Patel J, Sonuyi O, Mazzara G, Montefiori D, Carville A, Mansfield KG, Aldovini A. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol. 2004;172:3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- 35.Wang SW, Bertley FM, Kozlowski PA, Herrmann L, Manson K, Mazzara G, Piatak M, Johnson RP, Carville A, Mansfield K, Aldovini A. An SHIV DNA/MVA rectal vaccination in macaques provides systemic and mucosal virus-specific responses and protection against AIDS. AIDS Res Hum Retroviruses. 2004;20:846–859. doi: 10.1089/0889222041725253. [DOI] [PubMed] [Google Scholar]
- 36.Bergquist C, Johansson EL, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: Influence of the menstrual cycle. J Immunol. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 38.Moss B, Carroll MW, Wyatt LS, Bennink JR, Hirsch VM, Goldstein S, Elkins WR, Fuerst TR, Lifson JD, Piatak M, Restifo NP, Overwijk W, Chamberlain R, Rosenberg SA, Sutter G. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieschke GJ, Rao PK, Gately MK, Mulligan RC. Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo. Nat Biotechnol. 1997;15:35–40. doi: 10.1038/nbt0197-35. [DOI] [PubMed] [Google Scholar]
- 41.Mantis NJ, Kozlowski PA, Mielcarz DW, Weissenhorn W, Neutra MR. Immunization of mice with recombinant gp41 in a systemic prime/mucosal boost protocol induces HIV-1-specific serum IgG and secretory IgA antibodies. Vaccine. 2001;19:3990–4001. doi: 10.1016/s0264-410x(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 42.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24:297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 43.Montefiori DC, Baba TW, Li A, Bilska M, Ruprecht RM. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239delta3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 44.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford JM, Earl PL, Moss B, Reimann KA, Wyand MS, Manson KH, Bilska M, Zhou JT, Pauza CD, Parren PW, Burton DR, Sodroski JG, Letvin NL, Montefiori DC. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73:10199–10207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Meide PH, Groenestein RJ, de Labie MC, Heeney J, Pala P, Slaoui M. Enumeration of lymphokine-secreting cells as a quantitative measure for cellular immune responses in rhesus macaques. J Med Primatol. 1995;24:271–281. doi: 10.1111/j.1600-0684.1995.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 47.Weynants V, Walravens K, Didembourg C, Flanagan P, Godfroid J, Letesson JJ. Quantitative assessment by flow cytometry of T-lymphocytes producing antigen-specific gamma-interferon in Brucella immune cattle. Vet Immunol Immunopathol. 1998;66:309–320. doi: 10.1016/s0165-2427(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 48.Ito T, Inaba M, Inaba K, Toki J, Sogo S, Iguchi T, Adachi Y, Yamaguchi K, Amakawa R, Valladeau J, Saeland S, Fukuhara S, Ikehara S. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999;163:1409–1419. [PubMed] [Google Scholar]
- 49.Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 50.Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–617. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- 51.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 52.Lifson JD, Rossio JL, Piatak M, Jr., Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, Hirsch VM, Reimann KA, Schmitz JE, Ghrayeb J, Bischofberger N, Nowak MA, Desrosiers RC, Wodarz D. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;75:10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suryanarayana K, Wiltrout TA, Vasquez GM, Hirsch VM, Lifson JD. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 54.Mansfield KG, Veazey RS, Hancock A, Carville A, Elliott M, Lin KC, Lackner AA. Induction of disseminated Mycobacterium avium in simian AIDS is dependent upon simian immunodeficiency virus strain and defective granuloma formation. Am J Pathol. 2001;159:693–702. doi: 10.1016/S0002-9440(10)61740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 56.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dandekar S. Pathogenesis of HIV in the gastrointestinal tract. Curr HIV/AIDS Rep. 2007;4:10–15. doi: 10.1007/s11904-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 58.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 59.Johnson RP, Glickman RL, Yang JQ, Kaur A, Dion JT, Mulligan MJ, Desrosiers RC. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyand MS, Manson KH, Garcia-Moll M, Montefiori D, Desrosiers RC. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar A, Mukherjee S, Shen J, Buch S, Li Z, Adany I, Liu Z, Zhuge W, Piatak M, Jr., Lifson J, McClure H, Narayan O. Immunization of macaques with live simian human immunodeficiency virus (SHIV) vaccines conferred protection against AIDS induced by homologous and heterologous SHIVs and simian immunodeficiency virus. Virology. 2002;301:189–205. doi: 10.1006/viro.2002.1544. [DOI] [PubMed] [Google Scholar]
- 62.Brown CR, Czapiga M, Kabat J, Dang Q, Ourmanov I, Nishimura Y, Martin MA, Hirsch VM. Unique pathology in simian immunodeficiency virus-infected rapid progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J Virol. 2007;81:5594–5606. doi: 10.1128/JVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishimura Y, Igarashi T, Donau OK, Buckler-White A, Buckler C, Lafont BA, Goeken RM, Goldstein S, Hirsch VM, Martin MA. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc Natl Acad Sci USA. 2004;101:12324–12329. doi: 10.1073/pnas.0404620101. [DOI] [PMC free article] [PubMed] [Google Scholar]