Abstract
Arterial thrombosis is considered to arise from the interaction of tissue factor (TF) in the vascular wall with platelets and coagulation factors in circulating blood. According to this paradigm, coagulation is initiated after a vessel is damaged and blood is exposed to vessel-wall TF. We have examined thrombus formation on pig arterial media (which contains no stainable TF) and on collagen-coated glass slides (which are devoid of TF) exposed to flowing native human blood. In both systems the thrombi that formed during a 5-min perfusion stained intensely for TF, much of which was not associated with cells. Antibodies against TF caused ≈70% reduction in the amount of thrombus formed on the pig arterial media and also reduced thrombi on the collagen-coated glass slides. TF deposited on the slides was active, as there was abundant fibrin in the thrombi. Factor VIIai, a potent inhibitor of TF, essentially abolished fibrin production and markedly reduced the mass of the thrombi. Immunoelectron microscopy revealed TF-positive membrane vesicles that we frequently observed in large clusters near the surface of platelets. TF, measured by factor Xa formation, was extracted from whole blood and plasma of healthy subjects. By using immunostaining, TF-containing neutrophils and monocytes were identified in peripheral blood; our data raise the possibility that leukocytes are the main source of blood TF. We suggest that blood-borne TF is inherently thrombogenic and may be involved in thrombus propagation at the site of vascular injury.
Tissue factor (TF) present in the arterial wall has been considered to be responsible for the initiation of the coagulation cascade and thrombus formation (1). The role of vascular TF in acute thrombosis and atherosclerosis has been proposed based on our previous studies (2–5). To investigate the role of circulating TF in thrombogenesis, we have used a system in which pig aortic media or collagen-coated slides were mounted in a laminar flow chamber and perfused with native human blood. We noted that when stained either with derivatized factor VIIa (FVIIa) or with specific anti-TF antibodies, the thrombi contained large amounts of TF staining, whereas the media and collagen-coated slides were essentially negative. Thus, we surmised that the TF came from the blood; accordingly, we examined whole blood and plasma for TF activity that we have extracted and assayed. We conclude that there is circulating, potentially active TF in normal subjects. We present evidence that this pool is thrombogenic in model flow systems. We also present evidence suggesting the TF comes from leukocytes and hypothesize that the cell-surface TF is completely encrypted (6–8) but becomes available during thrombosis.
METHODS
Reagents.
Human recombinant FVIIa was a gift from Novo-Nordisk, Copenhagen. Factor X was purified from human plasma (9). Affigel-15 was purchased from Bio-Rad. The phospholipids used for relipidation of TF consisted of 30% 1,2-dioleoyl-sn-glycero-3-phosphatidylserine and 70% 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (Avanti Polar Lipids). Spectrozyme Xa was from American Diagnostica, Greenwich, CT. Immunopurified rabbit anti-human TF antibody (pAb-TF) and digoxygenin-labeled FVIIa (Dig-VIIa) was prepared as described (10). Monoclonal anti-TF antibodies (HTF1) have been described (11). Inhibited FVIIa (FVIIai) was prepared by addition of a 10-fold molar excess of d-phe l-phe arg chloromethyl ketone (Calbiochem) to FVIIa, incubation for 90 min at room temperature, and dialysis vs. Hepes buffer overnight. Monomeric bovine type III collagen (Vitrogen) was purchased from Collagen Corp.
Preparation of Collagen-Coated Glass Slides.
Microscope slides (Superfrost/Plus, Fisher Scientific) were incubated overnight with 10 μg ml−1 collagen followed by 1% BSA in PBS for 30 min. HTF1-coated slides were prepared similarly at 100 μg ml−1.
Perfusion systems for arterial segments have been described (12) and were modified to contain a three-port 1-ml mixing chamber containing a magnetic flea. Frozen pig aorta media (Pel-Freez Biologicals) were surgically altered to mimic severely injured arterial wall (13). Collagen-coated glass slides were mounted in a parallel plate flow chamber (14). Blood from the antecubital vein was obtained from healthy volunteers (who gave informed consent) and connected via Tygon tubing to the mixing chamber. The mixing chamber was mounted proximal to the flow chambers, and appropriate solutions were added and metered by a syringe pump at the rate of 1 ml min−1. The arterial chambers were maintained at 37°C whereas the slide perfusions were at room temperature. Flow rates, metered by a peristaltic pump, were 10 ml min−1, resulting in wall shear rates of 1,690 and 980 sec−1 for arterial segments and coated slides, respectively. All perfusions were for 5 min.
Histology.
Immediately after the perfusion, pig media were removed from the flow chamber, gently rinsed in PBS, placed in freshly prepared 4% phosphate buffered paraformaldehyde (pH 7.4), and fixed for at least 48 hr at room temperature. Every 1.5 mm, five cross sections from each specimen were obtained and paraffin-embedded. Tissue sections were serially cut at 5 μm and mounted on lysine-coated slides. For morphometry, slides were stained by a combined Masson elastin trichrome method.
Collagen-coated slides were removed from the chamber immediately after the perfusion, rinsed in PBS, and fixed in 4% paraformaldehyde for 1 hr.
Immunohistochemistry was performed by using 5-μm thick sections of pig media, deparaffinized, hydrated, and washed in PBS for 20 min and blocked with 1% peroxide in methanol and an appropriate normal serum. Sections were incubated with anti-TF antibodies at 1 μg/ml or with a murine monoclonal anti-fibrin II β chain (Bβ 15–42) antibody (NYB⋅T2G1, Accurate Scientific, Westbury, NY) at 1 μg ml−1 for 2 hr at 37°C. Anti-CD18 (Dako) was used identically at 4.1 μg ml−1. Bound antibodies were detected by using a biotin streptavidin-amplified detection system (BioGenex Laboratories, San Ramon, CA) developed with 3,3′-diaminobenzidine tetra-hydrochloride. After counterstaining with hematoxylin, slides were examined microscopically. TF also was identified by using Dig-VIIa (10). Flow chamber slides were stained for fibrin, TF, and CD18.
Controls for immunostaining consisted of substitution of primary antibodies with appropriate serum or nonspecific antibody. No Dig-VIIa staining was seen after preincubation with pAb-TF or dilution with unlabeled FVIIa.
Immunoelectron Microscopy.
Labeling of TF was performed before resin embedding at room temperature. The collagen-covered glass slides were fixed for 5 min with 4% paraformaldehyde in PBS, pH 7.4. Free aldehyde groups were inactivated with 50 mM glycine in PBS for 20 min. Nonspecific binding sites were blocked with 1.5% BSA and 0.5% ovalbumin in PBS, pH 7.4, three times for 5 min. Incubation with pAb-TF was done at 10 μg/ml in PBS with 0.1% BSA for 1 hr. After three 5-min washes in PBS with 0.1% BSA, samples were incubated with secondary goat anti-rabbit IgG coupled to 10 nm colloidal gold particles (Biocell Laboratories) diluted 1:40 in PBS containing 0.1% BSA and 0.05% Tween 20, pH 7.4, for 1 hr. After three 5-min washes in PBS, samples were fixed in 2% glutaraldehyde in 0.1 M Na Cacodylate buffer for 1 hr, briefly washed in 0.1 M Na Cacodylate buffer and postfixed in 1% osmium tetroxide in the same buffer, dehydrated in a series of aqueous ethanol solutions, exposed to propylene oxide, and embedded in Epon 812. Ultrathin sections were cut on a Reichert Ultracut S and stained with 5% aqueous uranyl acetate followed by lead citrate. Electron micrographs were taken with a JEOL 1210 at 100 kV. The controls treated with rabbit preimmune serum revealed no significant label with a negligible background of not more than 10 gold grains over 10 μm2.
Morphometry.
Thrombus on combined Masson elastin trichrome and fibrin-stained sections of pig media was quantified by computer-aided planimetry without knowledge of the sample treatment. Briefly, images of five cross sections per sample were digitized and the area of the thrombus was measured by using image-pro plus (Media Cybernetics, Silver Spring, MD). Areas were averaged to determine thrombus deposition per specimen.
Measurement of TF Activities in Plasma and Whole Blood.
Triton X-100 (4%) was added to plasma or whole blood. Plasma was prepared from blood less than 10 min after collection into 0.1 vol of 130 mM Na citrate by centrifugation at 2,000 g for 10 min at room temperature. pAb-TF coupled to Affigel (≈0.2 mg of pAb-TF per ml of Affigel) was added (100 μl ml−1) to the sample; after a 12-hr incubation period on a rocker the Affigel beads were removed by centrifugation. The beads were washed six times with Hepes buffer. The bound protein was eluted with a mixture of 100 mM β-octyl glucoside, 25 μM phospholipid (1,2-dioleoyl-sn-glycero-3-phosphatidylserine/1,2-dioleoyl-sn-glycero-3-phosphatidylcholine, 30:70 wt/wt), and 6 M guanidine-HCL. The Affigel was removed by centrifugation and the supernatant was dialyzed for 3 days against Hepes-buffered saline (10 mM Hepes, pH 7.5/140 mM NaCl).
TF activity was measured by adding the sample to a solution containing 1 nM FVIIa, 150 nM factor X, and 5 mM CaCl2. At intervals, samples were transferred to a microtiter plate in which each well contained 100 μl of EDTA buffer (50 mM Bicine, pH 8.5/20 mM EDTA/1 mg/ml BSA), which terminated production of Xa. A chromogenic substrate, Spectrozyme Xa (25 μl, 0.5 mM final concentration), was added to each well, and the increase in OD was measured at 405 nm for 10 min by using a kinetic ELISA plate reader at 35°C (Tmax, Molecular Devices).
RESULTS
TF in Experimental Thrombi.
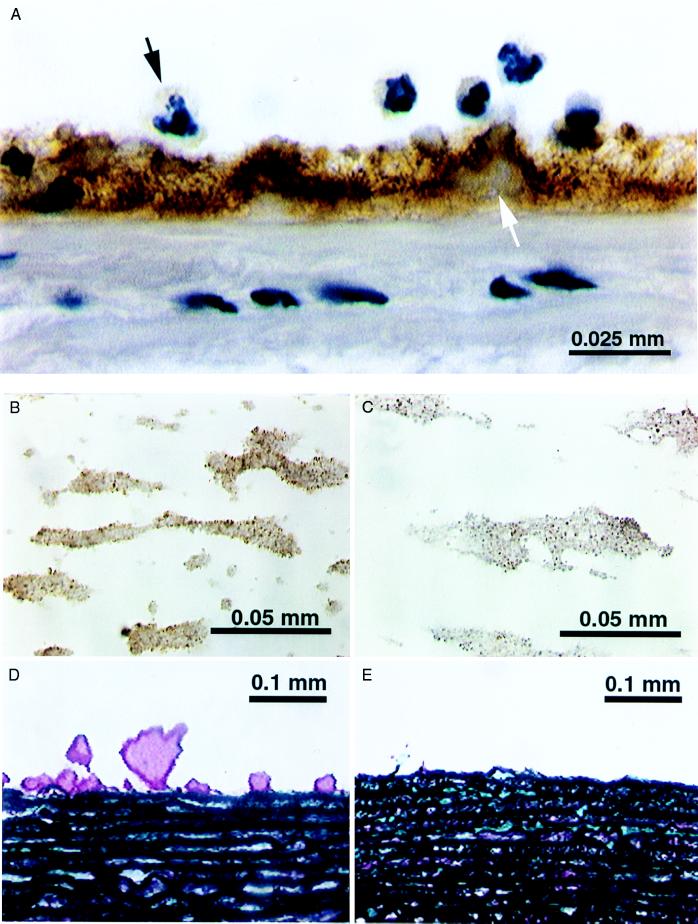
When native human blood was perfused over pig arterial media the thrombus was intensely stained for TF by using pAb-TF whereas the media itself was essentially negative (Fig. 1A). Identical results were obtained with monoclonal anti-TF antibody and Dig-VIIa (not shown) (10). Note that the thrombi are intensely stained for TF whereas none was seen in the media. This finding suggests that the TF originated in the blood (although leeching from the arterial segment could not be excluded). Interestingly, some of the neutrophils stained for TF. To determine whether the formation of these thrombi was TF dependent, we added pAb-TF (20 μg ml−1 of blood) to the mixing chamber to which the blood was added directly from the donor vein. The formation of thrombus on pig media was diminished by ≈70% (Fig. 1 D and E). Because of the potential presence of TF within the arterial media, we could not conclude unambiguously that this decrease was a result of inhibition of blood TF.
Figure 1.
(A) TF staining (brown) of a thrombus formed on pig aortic media. Note the absence of staining of the media and the positive staining of the thrombus and some of the neutrophils; much of the immunostained (pAb-TF) TF appears to be extracellular and associated with fibrin. (B) Microthrombi on collagen-coated glass slide stained by using pAb-TF (brown). Note the 1- to 2-μm granules, which stain intensely. (C) Microthrombi on collagen-coated glass slide stained for CD-18 (brown). Note the staining of the granules. (D) Thrombus (purple) formed on pig media perfused with native human blood. (E) Pig media perfused with native human blood containing pAb-TF; note the markedly diminished thrombi.
To establish that TF in thrombi could arise from the blood, studies were performed by using a system that contains no exogenous TF, namely collagen-coated glass slides that were placed in a parallel plate flow chamber (14) and perfused with native human blood. Microthrombi formed on the collagen again stained strongly for TF by using pAb-TF (Fig. 1B) or Dig-VIIa (not shown), and the material included TF-positive particles of ≈1–2 μm diameter. These particles stained positively for CD-18, suggesting a leukocyte origin (Fig. 1C).
TF-Dependent Properties of the Experimental Thrombi.
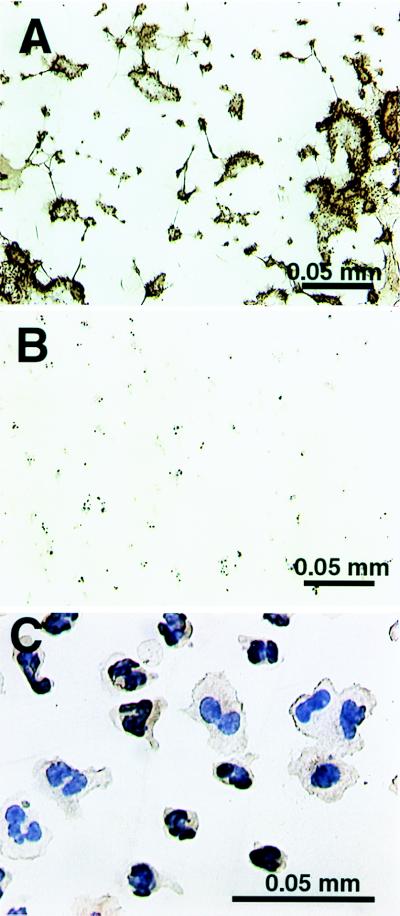
Deposited TF is active. The ultimate product of coagulation, fibrin formation, was chosen as an indicator of TF activity. We investigated fibrin generation by perfusing native blood over the collagen-coated slides. As shown in Fig. 2 A and B, fibrin formation was markedly reduced when FVIIai (10 nM), a potent inhibitor of TF (15), was included in the perfusion; further, the mass of deposited thrombus was clearly decreased.
Figure 2.
(A) Collagen-coated glass slide perfused with native human blood and stained for fibrin (brown). A fibrin network as well as microthrombi are evident. (B) Same as A but the TF inhibitor FVIIai was included in the flow. Compared with A, the fibrin staining is minimal, and the thrombus mass is considerably smaller. (C) Glass slides coated with pAb-TF and perfused with anticoagulated whole blood shows deposition of leukocytes. Both neutrophils and macrophages are positive (pAb-TF stain).
Source of blood TF.
To capture leukocytes with TF on their surface, we perfused monoclonal anti-TF antibody-coated slides with anticoagulated blood. A thrombin inhibitor (RO 46–6240) (16) and a platelet GP IIb-IIIa inhibitor (RO 44–9883) (17) were added to ensure adhesion of TF-positive cells without thrombin or platelet aggregate formation. We found mostly neutrophils adhering (≈75%) with a lesser number of mononuclear cells (≈25%), whereas no adherent cells were seen when an irrelevant antibody was used (not shown). Of note, when these slides were stained by using the pAb-TF, virtually all adherent cells were positive for TF (Fig. 2C).
Immunoelecron microscopy.
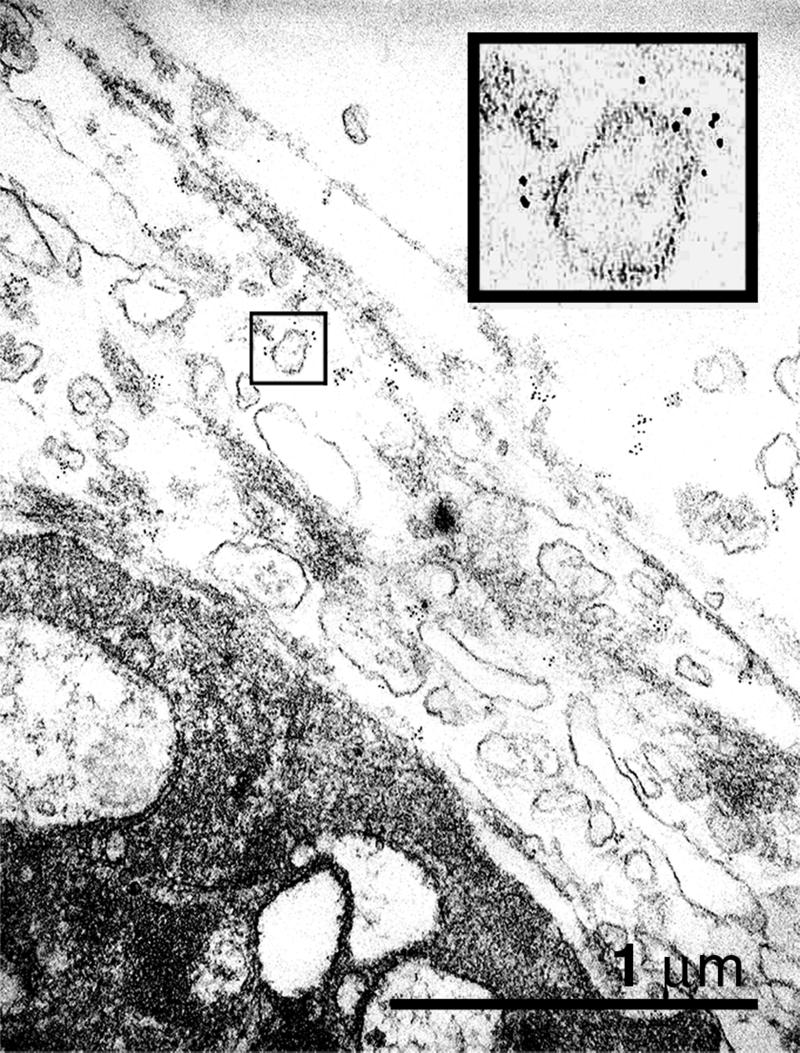
ImmunoGold label for TF was found predominantly colocalized to the surface of membrane vesicles. Frequently these vesicles were clustered adjacent to platelet surfaces in thrombi (Fig. 3), suggesting that adsorbed TF-positive vesicles explain the positive platelets we repeatedly observed close to thrombi. Staining of TF was not detectable on all sections through vesicles. Because the thickness of an ultrathin section is 60–80 nm this finding indicates that either TF is present only at a subpopulation of vesicles or the distribution of TF is heterogeneous on the vesicle surface.
Figure 3.
Electron micrograph of thrombi that were deposited on collagen-coated glass slide. ImmunoGold staining for TF shows that vesicular structures adjacent to the platelet membrane are positive. (Inset) A magnification (×4) of a stained vesicle.
TF Activity in Blood and Plasma.
To confirm the activity of circulating TF, we extracted it from blood and plasma. The extracted protein was inserted in lipid vesicles, which is required for activity. The resultant mixture was assayed: the average TF content of blood was 0.26 pM (SE = 0.16) and 0.35 pM (SE = 0.05) for plasma. This material was 60% inhibited by the pAb-TF, which indicates a true concentration of ≈0.2 pM TF.
DISCUSSION
Our evidence for the presence of thrombogenic TF in circulating blood is as follows: (i) thrombi formed on perfused pig media display intense staining for TF as did thrombi deposited on collagen-coated slides whereas neither substrate did; (ii) when circulating TF activity was inhibited the amount of thrombus in both systems was reduced, and fibrin, which was abundantly stained in control experiments, was essentially absent from the collagen-coated slides; (iii) we isolated active TF from freshly collected whole blood; and (iv) TF-positive neutrophils and monocytes from whole blood were isolated. We emphasize that these 5-min perfusions were performed on-line by using blood directly from the donors’ veins, thereby obviating any consideration of in vitro protein synthesis. Moreover, others have found by using a collagen-coated capillary that no changes were observed in the coagulation of whole blood for 20 min, owing to the activation of factor XII (18).Thus, contrary to existing consensus, the blood contains all of the elements required for coagulation to occur via the “TF pathway.” Others have demonstrated the presence of TF antigen and activity in blood (19–21), but its source and significance have not been studied.
The current view of coronary artery thrombosis is that the process is initiated when atherosclerotic plaques, which contain large amounts of TF, rupture, thereby exposing vascular TF to flowing blood and that propagation of the thrombus involves coagulation reactions that occur on the surface of platelets. Our findings suggest a view of thrombosis that does not necessarily require the exposure of TF at the site of vascular injury. However, because of the large amount of TF within atheromatous plaques, we surmise that thrombosis may be initiated by vascular TF, but our findings suggest that propagation results from the deposition of blood-borne TF on platelets in the nascent thrombus thereby forming “TF-platelet hybrids.” This phenomenon would clearly favor thrombus propagation because factors IXa and Xa, both initial products of the TF pathway, would be generated on TF-containing microvesicles at the platelet surface, thereby reducing the distance they must diffuse from the source of TF to the platelet surface. Because the time to capture of any diffusing species rises as the square of the distance, the shortened distance can have very significant effects on subsequent reaction velocities (22). Moreover, our findings may explain previous studies in which it was shown that platelets increase the TF activity of stimulated leukocytes (23, 24).
The thrombogenicity of blood-borne TF may explain why experimental therapies, such as anti-TF antibodies and FVIIai, are extremely antithrombotic while causing no bleeding in the experimental animals (25). In this case, the therapies simply may inhibit circulating TF at levels far below those required to inhibit the hemostatic vascular TF.
To summarize, we have quantified TF activity in native human blood and plasma and have identified TF-bearing cells in experimental thrombi and circulating in blood. Thus, we have demonstrated the thrombogenic potential of this blood-borne pool by showing that blood-generated thrombi have the essential ingredients to promote thrombosis, namely TF, which initiates coagulation, and procoagulant platelets, which support the reactions leading to thrombin generation. If this same process occurs in vivo on vascular surfaces, then circulating TF must be considered when formulating a scenario of thrombus formation. We note that cell-surface TF is capable of being encrypted, which, in effect, means that it binds to FVIIa and to anti-TF, but is not competent to initiate coagulation. In cell culture systems ≈50% of the TF is encrypted, but in vivo, it might well be totally encrypted, which would allow circulating and endothelial cell TF to be present without generalized coagulation ensuing.
Although we have found TF in plasma, we do not know whether this finding reflects the in vivo situation or whether TF is released in vitro from TF-positive cells. In addition, although we repeatedly have observed TF-positive neutrophils, we do not as yet know whether these cells synthesize TF or engulf and transport it to the site of thrombus growth.
Acknowledgments
This work was supported, in part, by Grants HL 54469 and HL 29019 from the National Heart Lung and Blood Institute.
ABBREVIATIONS
- TF
tissue factor
- FVII
factor VII
- pAb-TF
immunopurified rabbit anti-human TF antibody
- Dig
digoxygenin
References
- 1.Fuster, V., Fallon, J. T. & Nemerson, Y. (1996) Lancet348, Suppl. 1, s7–s10. [DOI] [PubMed]
- 2.Zeldis S M, Nemerson Y, Pitlick F A, Lentz T L. Science. 1972;175:766–768. doi: 10.1126/science.175.4023.766. [DOI] [PubMed] [Google Scholar]
- 3.Badimon J J, Weng D, Chesebro J H, Fuster V, Badimon L. Thromb Haemostasis. 1994;71:511–516. [PubMed] [Google Scholar]
- 4.Kirchhofer D, Riederer M A, Baumgartner H R. Blood. 1997;89:1270–1278. [PubMed] [Google Scholar]
- 5.Fuster V, Fallon J T, Badimon J J, Nemerson Y. Thromb Haemostasis. 1997;78:247–255. [PubMed] [Google Scholar]
- 6.Bach R, Rifkin D B. Proc Natl Acad Sci USA. 1990;87:6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le D T, Rapaport S I, Rao L V. J Biol Chem. 1992;267:15447–15454. [PubMed] [Google Scholar]
- 8.Maynard J R, Heckman C A, Pitlick F A, Nemerson Y. J Clin Invest. 1975;55:814–824. doi: 10.1172/JCI107992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miletich J P, Broze G J, Jr, Majerus P W. Methods Enzymol. 1981;80:221–228. [Google Scholar]
- 10.Thiruvikraman S V, Guha A, Roboz J, Taubman M B, Nemerson Y, Fallon J T. Lab Invest. 1996;75:451–461. [PubMed] [Google Scholar]
- 11.Carson S D, Ross S E, Bach R, Guha A. Blood. 1987;70:490–493. [PubMed] [Google Scholar]
- 12.Badimon L, Badimon J J, Galvez A, Chesebro J H, Fuster V. Arteriosclerosis. 1986;6:312–320. doi: 10.1161/01.atv.6.3.312. [DOI] [PubMed] [Google Scholar]
- 13.Meyer B J, Badimon J J, Mailhac A, Fernandez-Ortiz A, Chesebro J H, Fuster V, Badimon L. Circulation. 1994;90:2432–2438. doi: 10.1161/01.cir.90.5.2432. [DOI] [PubMed] [Google Scholar]
- 14.Grabowski E F. Am J Cardiol. 1989;64:10E–15E. doi: 10.1016/0002-9149(89)90728-5. [DOI] [PubMed] [Google Scholar]
- 15.Nemerson Y, Gentry R. Biochemistry. 1986;25:4020–4033. doi: 10.1021/bi00362a006. [DOI] [PubMed] [Google Scholar]
- 16.Hilpert K, Ackermann J, Banner D W, Gast A, Gubernator K, Hadvary P, Labler L, Muller K, Schmid G, Tschopp T B, et al. J Med Chem. 1994;37:3889–3901. doi: 10.1021/jm00049a008. [DOI] [PubMed] [Google Scholar]
- 17.Alig L, Edenhofer A, Hadvary P, Hurzeler M, Knopp D, Muller M, Steiner B, Trzeciak A, Weller T. J Med Chem. 1992;35:4393–4407. doi: 10.1021/jm00101a017. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto Y, Kaibara M. Biochim Biophys Acta. 1990;1035:361–368. doi: 10.1016/0304-4165(90)90101-2. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda C, Iijima K, Nakamura K. Clin Chem. 1989;35:1897–1900. [PubMed] [Google Scholar]
- 20.Suefuji H, Ogawa H, Yasue H, Kaikita K, Soejima H, Motoyama T, Mizuno Y, Oshima S, Saito T, Tsuji I, et al. Am Heart J. 1997;134:253–259. doi: 10.1016/s0002-8703(97)70132-7. [DOI] [PubMed] [Google Scholar]
- 21.Key N S, Slungaard A, Dandelet L, Nelson S C, Moertel C, Styles L A, Kuypers F A, Bach R R. Blood. 1998;91:4216–4223. [PubMed] [Google Scholar]
- 22.Berg H. Random Walks in Biology. Princeton, NJ: Princeton Univ. Press; 1993. [Google Scholar]
- 23.Niemetz J, Marcus A J. J Clin Invest. 1974;54:1437–1443. doi: 10.1172/JCI107891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halvorsen H, Olsen J O, Osterud B. J Leukocyte Biol. 1993;54:275–282. doi: 10.1002/jlb.54.4.275. [DOI] [PubMed] [Google Scholar]
- 25.Harker L A, Hanson S R, Kelly A B. Thromb Haemostasis. 1997;78:736–741. [PubMed] [Google Scholar]