Abstract
Repeated amphetamine treatment results in behavioral sensitization in a high percentage of rats. Alterations to plasma corticosterone, neural monoamines and stress behavior can accompany amphetamine sensitization. Whether these changes occur following repeated amphetamine treatment in the absence of behavioral sensitization is not known. Male Sprague-Dawley rats were treated with amphetamine (2.5 mg/kg, i.p.) or saline once daily for 6 days. Amphetamine-induced locomotion and stereotypy, open-field anxiety behavior, plasma corticosterone and limbic monoamines were measured during withdrawal. Sixty-two percent of amphetamine-treated rats showed behavioral sensitization over the test periods. Only amphetamine-sensitized rats showed increased latency to enter the center of the open-field, as well as increased plasma corticosterone when compared to saline-treated controls. Amphetamine-sensitized rats showed increased dopamine concentrations in the shell of the nucleus accumbens and increased serotonin concentrations in the dorsal hippocampus, which were not observed in amphetamine-treated non-sensitized rats. These findings suggest that anxiety behavior, plasma corticosterone and limbic monoamines concentrations are altered by repeated amphetamine (2.5 mg/kg) treatment, and that these neuroendocrine and behavioral changes are often associated with sensitization to the psychostimulant effects of amphetamine.
Keywords: Anxiety, Corticosterone, Dopamine, Sensitization, Serotonin
1. Introduction
Increased behavioral sensitivity to psychostimulants for extended periods of time suggests that the drugs induce long-term or permanent changes to motor and reward systems within the central nervous system [1]. Behavioral sensitization to amphetamine in rats is characterized by increased motor activity and alterations to neural systems involved in reward [1–5]. Amphetamine-sensitized rats also show increased acquisition to amphetamine self-administration when compared to drug naïve controls [6]. This sensitized state can last for months to years following withdrawal of the drug [7,8].
Individual responses to stress are believed to be a major underlying cause of relapse during psychostimulant withdrawal and detoxification [9–12]. Some studies indicate that amphetamine-sensitized rats show increased plasma levels of stress-induced corticosterone release (for example; [13 – 14] although see [15]). Similarly, amphetamine-treated rats show increased behavioral sensitivity to mild stressors during the withdrawal period when compared to saline-treated controls [12;16, 17]. Altered stress responses following sensitization could explain, in part, why stress increases the probability of relapse and drug seeking behavior during a withdrawal period.
Enhanced stress-induced corticosterone levels and behavioral responses in amphetamine-treated rats may also result simply from the pharmacological actions of repeated amphetamine on neural and endocrine stress systems, regardless of whether sensitization occurs. Acute and chronic administration of psychostimulants increases plasma corticosterone concentrations [18; 19]. Furthermore, administration of amphetamine causes widespread increases in monoamine release in the central nervous system, including brain regions linked to stress responsiveness such as the hippocampus, medial prefrontal cortex, ventral tegmental area and nucleus accumbens [8]. Following amphetamine administration, exposure to acute and chronic stressors alters limbic serotoninergic activity [20]. This neurotransmitter is strongly linked with fear, anxiety and depression [21 – 23]. Chronic exposure to psychostimulant treatment is believed to induce intense and repeated activation of stress-related neural and endocrine systems [12, 24]. In turn, repeated drug-induced activation of stress pathways may result in long-term neural and endocrine dysfunction, as reflected by increased sensitivity to stress and the induction of anxiety states during drug abstinence.
Currently, it is not known whether alterations in neural, endocrine and anxiety measures are a consequence of behavioral sensitization to amphetamine, or alternatively, result from the pharmacological actions of chronic amphetamine regardless of sensitization. To partially address this question, the goal of this study was to determine whether basal corticosterone levels, anxiety behavior and limbic monoaminergic concentrations of rats that sensitize to the behavioral effects of amphetamine differ from rats that receive amphetamine treatment but do not exhibit behavioral sensitization. The use of male Sprague-Dawley rats as an experimental model offers the opportunity to examine physiological and behavioral differences between amphetamine-sensitized and amphetamine-treated non-sensitized rats, since unselected populations of this strain shows individual variability in the ability to sensitize to amphetamine [25].
2. Materials and Methods
2.1 Animals
Adult male outbred Sprague-Dawley rats (n = 32, mass 300–320 g; Charles River, Raleigh, NC, USA) were housed in pairs in a light controlled room (12-h reverse light/dark cycle: lights off at 8:00 AM). Food and water were available ad libitum. All experiments were conducted during the dark phase between the hours of 9:00 AM and 5:00 PM. The following procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
2.2 Amphetamine Treatment and Activity Measures
Animals were handled and placed for 40 min in a locomotion chamber (52 × 33.5 × 30 cm; divided into a 2 × 3 grid) once daily for five days to habituate to the testing enclosure. Following habituation, rats received either d-amphetamine sulfate (2.5 mg/kg salt weight, i.p.; Sigma Chemical Co., St. Louis, MO, USA) or the equivalent volumes of sterile saline (0.9%) (n = 16 per treatment group) administered once daily for six days. With the exception of the first day of administration, rats were placed back into their home cages following injection.
Locomotion testing was conducted in response to the injection on the first day of treatment (first exposure), and 7 and 14 days following the last repeated injection (i.e., 7 and 14 days of withdrawal period). On day 7 of withdrawal, both amphetamine and saline pre-treated rats received 2.5 mg/kg amphetamine. On day 14 of withdrawal, amphetamine and saline pre-treated rats were injected with amphetamine or saline, respectively. Immediately following injection, rats were placed into the locomotion chamber for 70 min under red light illumination, since behavioral sensitization to a variety of amphetamine doses is apparent within 10–60 min of injection (for example; [14, 15, 26 – 29]). Two scorers blind to treatment reviewed the behavioral recordings, counting the number of consecutive line-crossings completed by each animal and the total number of rears during the final 60 min of the 70 min testing period [30].
Measures of focused stereotypy intensity were scored during the first exposure test and the day 14 withdrawal testing session. Stereotypy ratings were based on a 0–6 scale, scored over two min for every 10 min interval. Scores were adapted from [30], and intensity ratings and behaviors are summarized in Table 1. The higher ratings (3–6) represent intense focused stereotypy that excludes locomotion [30, 31].
Table 1.
Stereotypy intensity ratings and associated behaviors.
| Rating | Behaviors expressed over 2-min observation periods |
|---|---|
| 0 | Inactive/Locomotion only |
| 1 | Intermittent, non-oral-based stereotypy such as repetitive head bobbing, grooming with limbs and rearing |
| 2 | Continuous, non-oral based stereotypy expressed over wide area of enclosure |
| 3 | Continuous, non-oral-based stereotypy expressed in restricted area of enclosure |
| 4 | Pronounced, oral-based stereotypy directed at self in a restricted area such as repetitive licking, biting and grooming with mouth |
| 5 | Intermittent, oral-based stereotypy directed externally such as repetitive licking or biting of walls and floor of enclosure |
| 6 | Continuous, oral-based stereotypy directed externally |
2.3 Open Field Testing
On day 11 of withdrawal, rats were placed in an open field chamber (54.5 × 80 cm) with a center area (21 × 46 cm) outlined in white, under white room lighting. Behavior was recorded for 30 min using a video camera and VCR. Two scorers blind to treatment reviewed the recordings and measured latency to enter the center of the chamber, and total activity over the 30 min period, which comprised rearing and line crossing. Scoring was performed using a computer-based event recorder (Etholog V. 2.2.5, Sao Paulo, Brazil; [32])
2.4 Collection of Brains and Plasma
Three days following final locomotion test (at day 17 of withdrawal), animals were decapitated and brains collected and frozen at −70°C until sectioned. This method of storage has been shown to preserve monoamine content at levels comparable to that of fresh tissue [33, 34]. Trunk blood was collected, and centrifuged at 5,000 rpm. Plasma was drawn off and frozen at −70°C until assayed. Samples were collected three days following amphetamine challenge to ensure complete recovery from the pharmacological effects of amphetamine treatment and the effects of testing procedures.
2.5 Plasma Corticosterone Assay
Measurement of plasma corticosterone was performed using a corticosterone enzyme-linked immunoassay kit, following the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Ten µl of plasma and 0.5 µl steroid displacement reagent were diluted with 990 µl of assay buffer for a 100-fold dilution. Duplicates of each sample, controls and standards were assayed. Plasma corticosterone levels were detected by absorbance of samples at 405 nm (wavelength correction set at 595 nm) and compared to known corticosterone standards using automated plate reader reader and KinetiCalc Jr. software (Bio-Tek Instruments, Winooski, VT, USA). Absorbance values were used to calculate maximum binding percent, which ranged between 19.9 – 20.9 %, and percent of non-specific binding, which was 2.4%; both values were within the manufacturer’s range. The detection limit sensitivity of this assay was 27.0 pg/ml.
2.6 Brain Monoamine Analysis
Frozen brains were serially sliced at 300 µm in the coronal plane, at −10°C, and stored at −70°C until microdissection. Limbic and striatal terminal fields (medial prefrontal cortex, central nucleus of the amygdala, dorsal hippocampus, ventral hippocampus, dorsal striatum, shell and core of the nucleus accumbens), and the monoaminergic cell body regions (ventral tegmental area, substantia nigra pars compata, dorsal raphe nucleus, median raphe nucleus) were identified using published guides [35, 36], and bilaterally microdissected with a 500 µm id cannula using a dissecting microscope and freezing stage. The microdissected tissue was expelled into 60 µl of sodium acetate buffer (pH 5.0) containing 9.42 pg/µl of internal standard (dihydroxybenzylamine, DHBA), and the cells were lyzed by freeze-thawing.
Brain regions were analyzed for serotonin, its metabolite 5-hydroxyindoleacetic acid (5-HIAA), dopamine, and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), using high-pressure liquid chromatography. A detailed description of this assay has been published elsewhere [37–38]. Levels of central monoamines were obtained via comparison to known standards, and corrected for recovery using CSW32 v1.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). Neurotransmitter and metabolite levels were expressed as pg amine/µg protein.
2.7 Data and Statistical Analysis
The percentage change in amphetamine-induced locomotion from first exposure locomotion test to the day 14 of withdrawal locomotion test was determined for each individual amphetamine pre-treated rat. Rats were allocated into sensitized and non-sensitized groups based on the following criteria that were formed from preliminary data indicating a bimodal distribution of responses over time: 25% or greater increase in locomotion indicated sensitization and a 25 % or greater decrease in locomotion over the testing sessions indicated non-sensitization. In this particular study, 10 of the 16 amphetamine-treated rats were sensitized and 6 of the 16 rats were allocated to the non-sensitized group based on these criteria.
The effects of repeated amphetamine administration on either total locomotion or total rearing was compared across the testing sessions and within each testing session using separate 2-way ANOVAs with one repeated measure (time). Significant effects of time, treatment, or time × treatment interactions were followed by one-way repeated measure ANOVAs. Locomotion and rearing data obtained from day 7 of withdrawal were analyzed independently from the other test days using separate one-way ANOVAs, since all rats received amphetamine injection on this test day. Significant main effects were further analyzed using Student Newman-Keuls (SNK) post-hoc test for multiple comparisons.
To ensure that decreased locomotion exhibited by the non-sensitized group was not due to augmented focused stereotyped behavior that excludes locomotion (thus indicating greater sensitivity to amphetamine), amphetamine-induced stereotypy scores for the first exposure and day 14 withdrawal tests were compared among experimental groups across the testing session. These comparisons were made using separate Kruskal-Wallis ANOVAs on ranks, since the data did not meet assumptions of normality and equal variance.
Open-field behavior (latency and total activity), plasma corticosterone, and monoamine concentrations were all compared between groups using separate one-way ANOVAs. Significant effects were further analyzed using SNK post-hoc tests. All analyses were performed using SigmaStat (version 2.03; © 1992–1997, SPSS Inc., Point Richmond, CA), with the alpha level set at 0.050.
3. Results
3.1 Amphetamine-Induced Locomotion, Rearing and Stereotypy Intensity
During the first exposure and on day 14 of withdrawal (Fig. 1), sensitized and non-sensitized groups received a single injection of amphetamine, while control rats received a saline injection. For locomotion exhibited during the first exposure test (Fig. 1A), there was a significant effect of treatment F(2,29) = 76.459, p < 0.001, time F(5,145) = 10.368, p < 0.001 and a treatment × time interaction F(10, 145) = 6.002, p < 0.001. One-way repeated measure ANOVA showed a significant effect for locomotion across time for all three groups (sensitized p < 0.010; non-sensitized p = 0.016; saline p = 0.007). Sensitized rats showed greater locomotion at 30, 40 and 50 min compared to 10 min post-injection (p < 0.010). In contrast, post-injection locomotion counts for the non-sensitized rats did not statistically differ at any time point when compared to 10 min post-injection (p > 0.05). Saline-treated rats showed a significant decrease in locomotion over the testing session (p = 0.007). When locomotion scores were compared between groups, sensitized and non-sensitized groups showed higher locomotion compared to the saline-treated group at each post-injection time point (p < 0.001). Furthermore, non-sensitized rats exhibited higher amphetamine-induced locomotion when compared to sensitized rats at 10 min (p = 0.006) and 20 min (p = 0.023) post-injection.
Fig. 1.
Locomotion counts over time (A) following first injection and (B) at 14 days of withdrawal, in response to a challenge of 2.5 mg/kg, i.p. amphetamine (sensitized and non-sensitized groups), or saline. (C) Total locomotion during first exposure and at 14 days of withdrawal, during the 60 min observation period. * represents significant differences between sensitized and non-sensitized rats, # represents significant differences from pre-test. Both amphetamine-treated groups were significantly higher than saline-treated rats at all time points and testing sessions (see Results for statistics). (Amphetamine-sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
For locomotion scores at day 14 of withdrawal (Fig. 1B), there was a significant effect of treatment F(2,29) = 76.459, p < 0.001, a treatment×time interaction F(10, 145) = 6.002, p < 0.001 and a trend for a significant effect of time F(5,145) = 10.368, p = 0.051). One-way repeated measure ANOVA showed a significant effect of time on locomotion for saline-treated (p < 0.001) and sensitized (p < 0.001) rats but not for non-sensitized rats (p = 0.418). Sensitized rats had greater locomotion at all post-injection time points compared to 10 min post-injection (p < 0.001). Levels of locomotion exhibited by saline-treated rats were lower at all post-injection time points compared to 10 min post-injection (p < 0.01). Sensitized and non-sensitized groups showed higher locomotion compared to the saline-treated group at each time point (p < 0.010). However, non-sensitized rats exhibited lower post-treatment locomotion when compared to sensitized rats at all time periods after amphetamine injection (p < 0.010), with the exception of 10 min post-injection (p = 0.170).
Total locomotion within the testing sessions was compared between first exposure and day 14 of withdrawal (Fig. 1C). There was a significant effect of treatment F(2,29) = 87.568, p < 0.001 and a significant interaction between treatment and time F(2,29) = 26.348, p < 0.001. Total locomotion scores within the first exposure test were not significantly different between sensitized and non-sensitized groups (p = 0.156). However, both amphetamine-treated groups exhibited higher total locomotion compared to saline-treated rats (p < 0.001). Sensitized rats showed an increase (p < 0.001) in total amphetamine-induced locomotion between first exposure and day 14 of withdrawal. In contrast, non-sensitized rats showed a decrease in total amphetamine-induced locomotion during this period (p < 0.001). Thus, total amphetamine-induced locomotion was higher in the sensitized group compared to non-sensitized rats (p < 0.001) at day 14 of withdrawal. Saline-treated rats showed a small but significant increase in total locomotion over the testing sessions (p = 0.044), but both amphetamine-treated groups exhibited higher total locomotion at day 14 of withdrawal when compared to saline-treated controls (p < 0.001).
For rearing during the first exposure test (Fig. 2A), there was a significant effect of treatment F(2,29) = 17.151, p < 0.001. but neither time F(5,145) = 2.166, p = 0.065 nor treatment × time interaction F(10, 145) = 1.174, p = 0.320 were significant. Analysis of the treatment effect revealed that non-sensitized rats exhibited greater rearing counts compared to both sensitized and saline-treated rats (p = 0.001). At day 14 of withdrawal (Fig. 2B), there was also a significant effect of treatment F(2,29) = 17.485, p < 0.001. Neither time F(5,145) = 0.391, p 0.855 nor interaction between treatment × time F(10, 145) = 1.742, p = 0.077 were significant. In contrast to their first exposure, non-sensitized rats exhibited lower amphetamine-induced rearing counts compared to sensitized rats (p = 0.041). Both sensitized and non-sensitized rats showed greater rearing counts than saline-treated controls (p < 0.001 and p = 0.014, respectively).
Fig. 2.
Rearing counts over time (A) following first injection and (B) at 14 days of withdrawal, in response to a challenge of 2.5 mg/kg, i.p. amphetamine (sensitized and non-sensitized groups), or saline. (C) Total rearing during first exposure and at 14 days of withdrawal, during the 60 min observation period. * represents significant differences between sensitized and non-sensitized rats, # represents significant differences from pre-test. Both amphetamine-treated groups were significantly higher in total rearing than saline-treated rats at 14 days of withdrawal, but only non-sensitized rats were significantly higher compared to saline-treated rats in the first exposure test (see Results for statistics). (Amphetamine-sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
Total rearing across the testing sessions is summarized in Fig. 2C. There was a significant effect of treatment F(2,29) = 70.705, p < 0.001, and a significant interaction between treatment and time F(2,29) = 12.401, p < 0.001. In the first exposure test, non-sensitized rats showed greater total rearing counts than sensitized and saline-treated groups (p < 0.001). Rearing counts between sensitized and saline-treated groups were similar (p = 0.597). However, sensitized rats showed a significant increase in total amphetamine-induced rearing counts between first exposure and day 14 of withdrawal tests post-treatment sessions (p < 0.001), while non-sensitized rats showed a significant decrease in total amphetamine-induced rearing counts across the two testing sessions (p < 0.001). This resulted in sensitized rats exhibiting more total amphetamine-induced rearing counts compared to non-sensitized rats (p = 0.032). Total rearing counts from saline-treated rats did not significantly differ between the two testing sessions (p = 0.310). Both amphetamine-treated groups showed greater total rearing counts at day 14 of withdrawal compared with saline rats (p < 0.010).
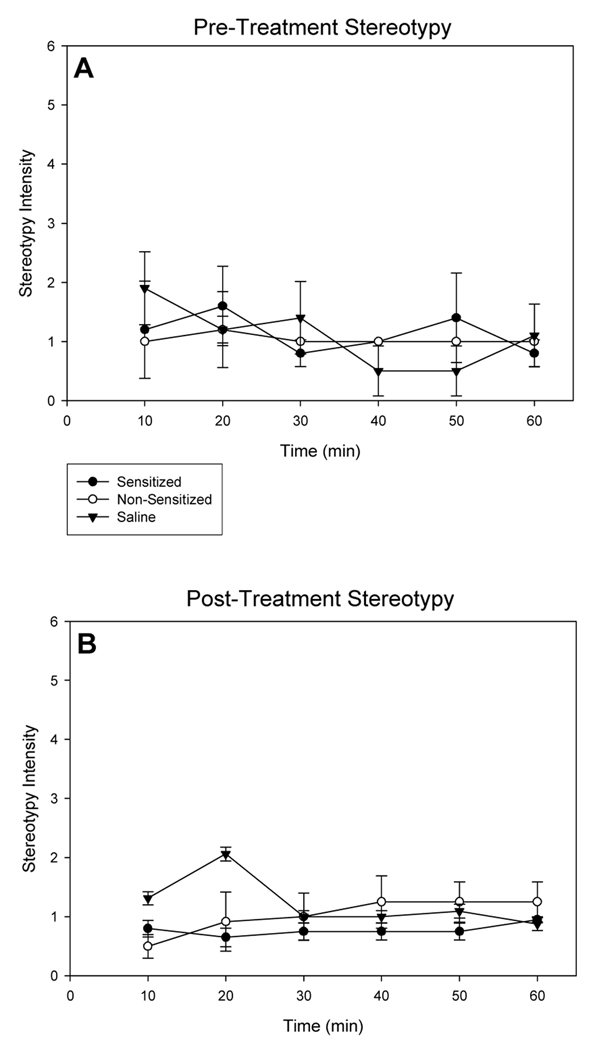
Stereotypy intensity scores were recorded for the two amphetamine-treated groups and saline controls to ensure that reductions in amphetamine-induced locomotion and rearing observed for non-sensitized rats did not result from heightened focused stereotyped behavior that normally excludes locomotion [5, 30, 31]. Rats did not show pronounced focused stereotyped behavior regardless of treatment, indicating that locomotion was the predominant behavior exhibited over the testing session (Fig. 3). No significant effects of treatment at any time point were observed for stereotypy ratings during the first exposure or during the day 14 of withdrawal tests ( p < 0.05 for all comparisons).
Fig. 3.
Stereotypy scores as a measure of intensity over time (A) following first injection and (B) at 14 days of withdrawal, in response to a challenge of 2.5 mg/kg, i.p. amphetamine (sensitized and non-sensitized groups), or saline. (Amphetamine-sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
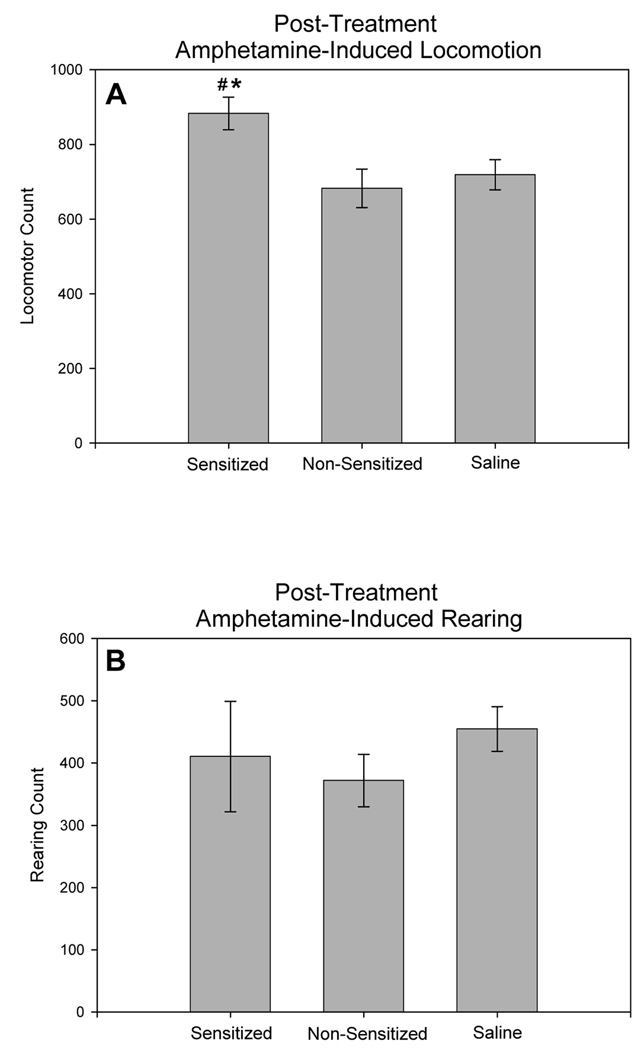
All rats were given a single injection of amphetamine at 7 days of withdrawal. In this test, amphetamine-induced locomotion was significantly different among the groups (Fig. 4A; F2,29, = 4.776, p = 0.017). Sensitized rats exhibited greater amphetamine-induced locomotion when compared to both non-sensitized rats (p = 0.035) and saline pre-treated rats (p = 0.012). Non-sensitized and saline pre-treated rats showed similar levels of amphetamine-induced locomotion (p = 0.604). Amphetamine-induced rearing counts did not differ among the groups in this testing session (Fig. 4B; F(2,29) = 0.486, p = 0.621)
Fig. 4.
Total amphetamine-induced locomotion (A) and total amphetamine-induced rearing (B) during 60 mins of observation in response to amphetamine-challenge (2.5 mg/kg, i.p.) at 7 days of withdrawal, for amphetamine pre-treated (sensitized and non-sensitized) and saline pre-treated rats. * represents significant differences from saline controls, # represents significant differences between sensitized and non-sensitized rats. (Amphetamine-sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
3.2 Open Field Behavior
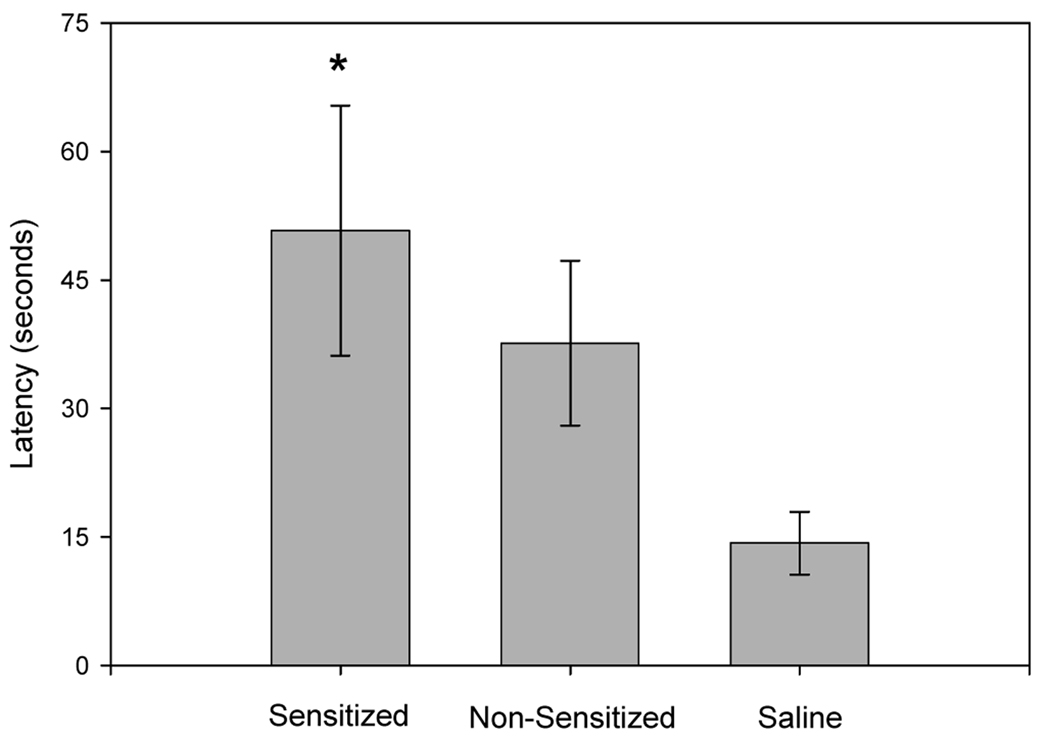
Latency to enter the center of the open field on day 11 of withdrawal varied among sensitized, non-sensitized and controls rats (Fig. 5; F(2,27) = 4.584, p = 0.020). Sensitized rats took a longer time to enter the center region compared to the saline-treated group (p = 0.018). Latency to enter the center of the open field for amphetamine-treated non-sensitized rats was not significantly different from either saline-treated (p = 0.109) or sensitized rats (p = 0.389). Total activity, determined from rearing counts and line-crossing, did not significantly differ between any of the groups during the 30 min observation period F(2,27) = 0.270, p = 0.765, suggesting that increased latency to enter the center was not a result of decreased activity within the open field.
Fig. 5.
Latency to enter center of open field, at 11 days of withdrawal. * represents significant difference from saline-treated controls. (Amphetamine-sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
3.3 Plasma Corticosterone
Plasma corticosterone levels, measured on day 17 of withdrawal, differed among sensitized, non-sensitized and control rats (Fig. 6; F(2,28) = 4.429, p = 0.022). Sensitized rats had higher plasma corticosterone concentrations than saline-treated rats (p = 0.021). In contrast, non-sensitized and saline-treated rats had similar plasma corticosterone concentrations (p = 0.808). There was a trend for higher plasma corticosterone levels in sensitized rats compared to non-sensitized rats (p = 0.061).
Fig. 6.
Plasma corticosterone concentrations at 17 days of withdrawal. * represents significant differences from the saline-treated group. (Amphetamine-sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
3.4 Neural Concentrations of Dopamine and DOPAC
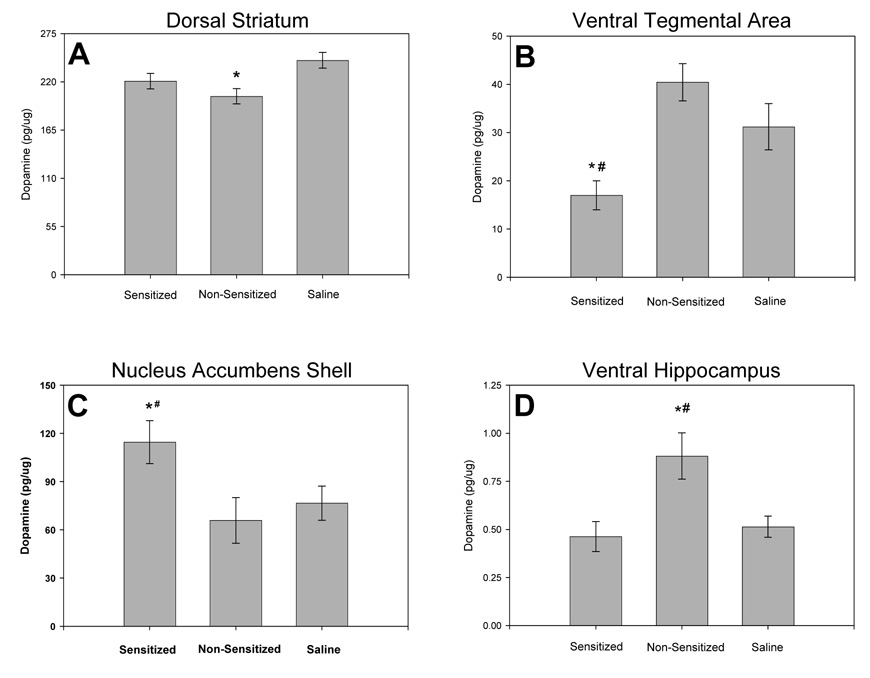
Figure 7 illustrates significant effects of amphetamine sensitization on concentrations of dopamine in selected brain regions, and the non-significant results are summarized in Table 2. Concentrations of the dopamine metabolite DOPAC were not significantly different in any of the brain regions studied (Table 3).
Fig. 7.
Dopamine concentrations in the limbic and nigrostriatal systems at 17 days of withdrawal. Dopamine concentrations in (A) the dorsal striatum, (B) the ventral tegmental area, (C) the shell of the nucleus accumbens, and (D) in the ventral hippocampus, for sensitized rats, non-sensitized rats and saline-treated controls. * represents significant differences from the saline-treated group, # represents significant differences between sensitized and non-sensitized groups. (Amphetamine-sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
Table 2.
Basal concentrations of dopamine within the limbic and nigrostriatal systems 17 days following repeated amphetamine (2.5 mg/kg, ip.) or saline pre-treatment.
| DA Concentrations (pg/µg tissue) | ||||
|---|---|---|---|---|
| Region | Treatment | Mean±SEM | F | p |
| Central Nucleus of the Amygdala |
Sensitized | 10.7±4.7 | 3.667 | 0.055 |
| Non-Sensitized | 29.8±2.8 | |||
| Control | 24.3±5.9 | |||
| Medial Prefrontal Cortex |
Sensitized | 1.3±0.1 | 2.198 | 0.133 |
| Non-Sensitized | 1.2±0.1 | |||
| Control | 1.6±0.1 | |||
| Dorsal Raphe Nucleus |
Sensitized | 7.0±0.7 | 2.156 | 0.138 |
| Non-Sensitized | 10.6±0.8 | |||
| Control | 9.4±1.0 | |||
| Median Raphe Nucleus |
Sensitized | 3.3±0.5 | 2.982 | 0.071 |
| Non-Sensitized | 5.0±0.3 | |||
| Control | 4.6±0.4 | |||
| Nucleus Accumbens Core |
Sensitized | 142.3±7.5 | 0.363 | 0.700 |
| Non-Sensitized | 150.9±4.5 | |||
| Control | 146.9±5.8 | |||
| Substantia Nigra |
Sensitized | 8.4±2.1 | 0.524 | 0.599 |
| Non-Sensitized | 5.0±1.1 | |||
| Control | 8.2±2.0 | |||
Table 3.
Basal concentrations of DOPAC within the limbic and nigrostriatal systems 17 days following repeated amphetamine (2.5 mg/kg, ip.) or saline pre-treatment.
| DOPAC Concentrations (pg/µg tissue) | ||||
|---|---|---|---|---|
| Region | Treatment | Mean±SEM | F | P |
| Central Nucleus of the Amygdala |
Sensitized | 3.1±1.4 | 2.003 | 0.181 |
| Non-Sensitized | 5.5±0.4 | |||
| Control | 5.3±0.4 | |||
| Medial Prefrontal Cortex |
Sensitized | 0.6±0.0 | 1.596 | 0.227 |
| Non-Sensitized | 0.6±0.1 | |||
| Control | 0.7±0.1 | |||
| Dorsal Raphe Nucleus |
Sensitized | 2.2±0.3 | 2.161 | 0.146 |
| Non-Sensitized | 3.3±0.4 | |||
| Control | 2.6±0.3 | |||
| Median Raphe Nucleus |
Sensitized | 2.8±0.7 | 0.219 | 0.805 |
| Non-Sensitized | 3.2±0.3 | |||
| Control | 2.7±0.4 | |||
| Ventral Tegmental Area |
Sensitized | 5.2±0.6 | 2.035 | 0.149 |
| Non-Sensitized | 7.8±1.1 | |||
| Control | 7.0±0.8 | |||
| Nucleus Accumbens Shell |
Sensitized | 27.9±3.2 | 2.006 | 0.163 |
| Non-Sensitized | 19.1±2.3 | |||
| Control | 23.3±1.7 | |||
| Nucleus Accumbens Core |
Sensitized | 41.8±3.9 | 0.712 | 0.500 |
| Non-Sensitized | 35.8±1.2 | |||
| Control | 38.6±2.4 | |||
| Substantia Nigra |
Sensitized | 3.1±0.6 | 1.238 | 0.310 |
| Non-Sensitized | 2.1±0.3 | |||
| Control | 3.2±0.4 | |||
| Dorsal Striatum |
Sensitized | 23.1±2.4 | 1.891 | 0.173 |
| Non-Sensitized | 19.5±0.5 | |||
| Control | 23.7±1.1 | |||
Dorsal striatal dopamine concentrations were significantly different among groups (Fig. 7A; F(2,27) = 4.564, p = 0.020). Non-sensitized rats had lower dopamine concentrations in the dorsal striatum when compared to saline-treated controls (p = 0.022), but were not significantly different from sensitized rats (p = 0.079). Concentrations of dopamine in the dorsal striatum of sensitized rats were similar to values in saline-treated rats (p = 0.285).
In the ventral tegmental area, dopamine concentrations also differed among sensitized, non-sensitized and control rats (Fig. 7B; F(2,29) = 4.856, p <0.016). Sensitized rats had lower dopamine concentrations in the ventral tegmental area when compared to non-sensitized (p = 0.020) and saline-treated rats (p = 0.027). However, concentrations of dopamine in the ventral tegmental area of non-sensitized and saline-treated groups were not significantly different (p = 0.239).
In the shell of the nucleus accumbens, dopamine concentrations differed among groups (Fig. 7C; F(2,27) = 3.496, p = 0.047). Dopamine concentrations in the nucleus accumbens shell were higher in sensitized rats when compared to saline-treated controls (p = 0.030) or to non-sensitized rats (p = 0.043). Dopamine concentrations in the nucleus accumbens of non-sensitized and saline-treated groups were not significantly different (p = 0.698).
Sensitized, non-sensitized and saline-treated rats differed in dopamine concentrations within the ventral hippocampus (Fig. 7D; F(2,28) = 6.585, p = 0.005). Dopamine concentrations in the ventral hippocampus of non-sensitized rats were higher values measured in sensitized (p = 0.008) and saline-treated rats (p = 0.003). In contrast, ventral hippocampal dopamine concentrations in the sensitized and saline-treated groups were not significantly different (p = 0.626).
3.5 Neural Concentrations of Serotonin and 5-HIAA
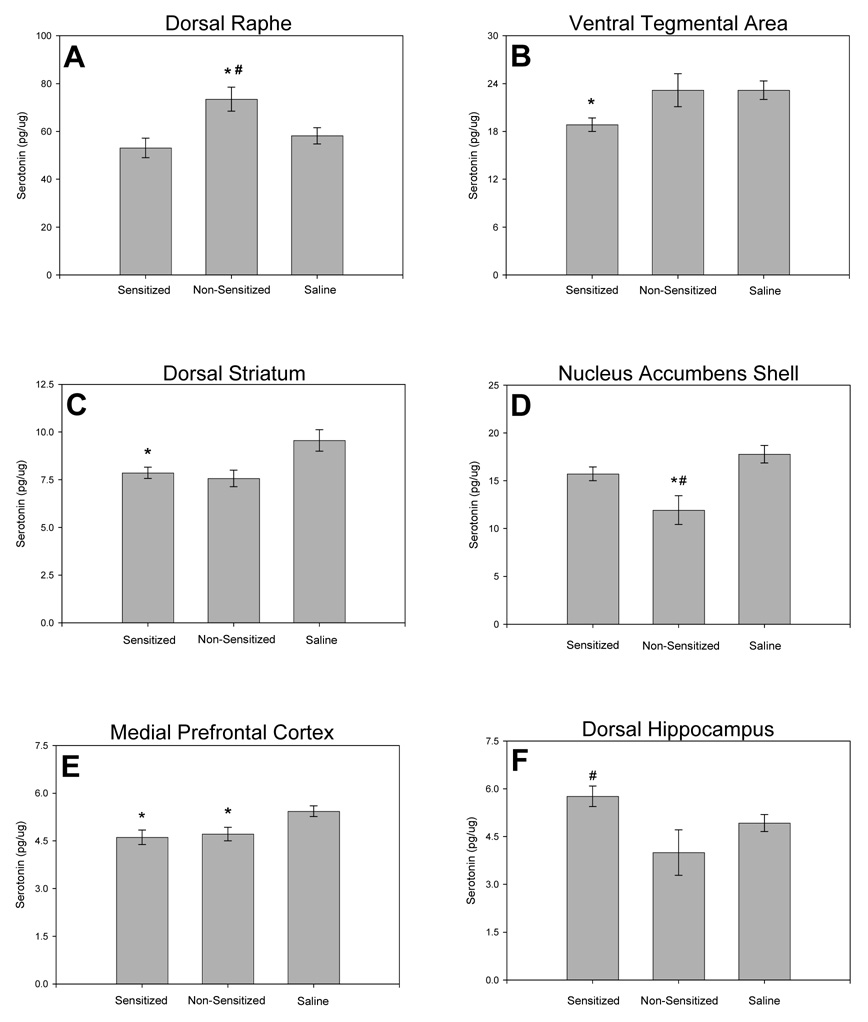
The significant effects of amphetamine sensitization on serotonin concentrations in selected brain regions are illustrated in Fig. 8, and the non-significant results are summarized in Table 4. Concentrations of the serotonin metabolite 5-HIAA were not significantly different in any of the brain regions studied (Table 5).
Fig. 8.
Serotonin concentrations in the limbic and nigrostriatal systems at 17 days of withdrawal. Serotonin concentrations in (A) the dorsal raphe, (B) the ventral tegmental area, (C) the dorsal striatum, (D) the shell of the nucleus accumbens, (E) the medial prefrontal cortex, and (F) in the dorsal hippocampus, for sensitized rats, non-sensitized rats and saline-treated controls. * represents significant differences from the saline-treated group, # represents significant differences between sensitized and non-sensitized groups. (Amphetamine sensitized: N=10, non-sensitized: N=6, saline: N=16; means ± S.E.M. are shown for all groups).
Table 4.
Basal concentrations of serotonin within the limbic and nigrostriatal systems 17 days following repeated amphetamine (2.5 mg/kg, ip.) or saline pre-treatment
| 5-HT Concentrations (pg/µg tissue) | ||||
|---|---|---|---|---|
| Region | Treatment | Mean±SEM | F | p |
| Central Nucleus of the Amygdala |
Sensitized | 21.4±2.0 | 0.965 | 0.397 |
| Non-Sensitized | 23.3±2.3 | |||
| Control | 26.3±2.6 | |||
| Median Raphe Nucleus |
Sensitized | 52.1±4.3 | 1.590 | 0.226 |
| Non-Sensitized | 68.2±6.3 | |||
| Control | 58.1±3.4 | |||
| Nucleus Accumbens Core |
Sensitized | 9.6±0.2 | 0.777 | 0.472 |
| Non-Sensitized | 9.3±0.4 | |||
| Control | 10.1±0.4 | |||
| Substantia Nigra |
Sensitized | 15.6±1.2 | 1.017 | 0.378 |
| Non-Sensitized | 14.0±1.3 | |||
| Control | 16.5±1.0 | |||
| Ventral Hippocampus |
Sensitized | 9.9±0.6 | 2.130 | 0.140 |
| Non-Sensitized | 8.0±0.7 | |||
| Control | 8.7±0.5 | |||
Table 5.
Basal concentrations of 5-HIAA within the limbic and nigrostriatal systems 17 days following repeated amphetamine (2.5 mg/kg, ip.) or saline pre-treatment.
| 5-HIAA Concentrations (pg/µg tissue) | ||||
|---|---|---|---|---|
| Region | Treatment | Mean±SEM | F | p |
| Central Nucleus of the Amygdala |
Sensitized | 16.1±01.3 | 1.712 | 0.201 |
| Non-Sensitized | 20.3±02.0 | |||
| Control | 18.7±01.2 | |||
| Medial Prefrontal Cortex |
Sensitized | 4.4±00.2 | 1.079 | 0.353 |
| Non-Sensitized | 4.8±00.1 | |||
| Control | 4.9±00.3 | |||
| Dorsal Raphe Nucleus |
Sensitized | 61.7±04.2 | 1.423 | 0.261 |
| Non-Sensitized | 80.9±06.5 | |||
| Control | 70.7±05.4 | |||
| Median Raphe Nucleus |
Sensitized | 82.0±11.2 | 0.972 | 0.393 |
| Non-Sensitized | 106.9±04.9 | |||
| Control | 89.1±07.7 | |||
| Ventral Tegmental Area |
Sensitized | 17.0±00.6 | 1.559 | 0.227 |
| Non-Sensitized | 18.4±00.8 | |||
| Control | 18.7±00.7 | |||
| Nucleus Accumbens Shell |
Sensitized | 8.4±00.5 | 2.679 | 0.086 |
| Non-Sensitized | 7.5±00.7 | |||
| Control | 9.2±00.4 | |||
| Nucleus Accumbens Core |
Sensitized | 8.1±00.4 | 0.359 | 0.701 |
| Non-Sensitized | 8.4±00.6 | |||
| Control | 8.6±00.4 | |||
| Substantia Nigra |
Sensitized | 12.3±00.6 | 0.288 | 0.752 |
| Non-Sensitized | 13.5±01.3 | |||
| Control | 14.1±01.5 | |||
| Dorsal Striatum |
Sensitized | 6.4±00.5 | 2.720 | 0.085 |
| Non-Sensitized | 6.2±00.2 | |||
| Control | 7.5±00.4 | |||
| Dorsal Hippocampus |
Sensitized | 7.8±00.5 | 0.0815 | 0.922 |
| Non-Sensitized | 7.6±00.6 | |||
| Control | 7.6±00.4 | |||
| Ventral Hippocampus |
Sensitized | 8.3±00.9 | 0.951 | 0.400 |
| Non-Sensitized | 7.0±00.5 | |||
| Control | 7.7±00.3 | |||
Serotonin concentrations in the dorsal raphe nucleus differed among sensitized, non-sensitized and control rats (Fig. 8A; F(2,27) = 3.681, p = 0.040). Non-sensitized rats had higher serotonin concentrations in the dorsal raphe nucleus than sensitized (p = 0.032), and saline-treated rats (p = 0.040). However, dorsal raphe serotonin concentrations in sensitized and saline-treated groups were not significantly different (p = 0.348).
Serotonin concentrations in the ventral tegmental area also differed among groups (Fig. 8B; F(2,29) = 3.606, p = 0.040). Sensitized rats had lower serotonin concentrations in the ventral tegmental area than saline-treated rats (p = 0.043), and there was a trend for lower serotonin concentrations when sensitized rats were compared to non-sensitized rats (p = 0.057). Ventral tegmental area serotonin concentrations did not differ significantly between non-sensitized and saline-treated groups (p = 0.997).
In the dorsal striatum, there were differences in serotonin concentrations among sensitized, non-sensitized and control rats (Fig. 8C; F(2,27) = 4.363, p = 0.024). Sensitized rats had lower dorsal striatal serotonin concentrations than saline-treated rats (p = 0.028), but were not significantly different from non-sensitized rats (p = 0.747). A trend towards lower serotonin concentrations of dorsal striatal serotonin was evident when non-sensitized rats were compared to saline-treated controls (p = 0.051).
Serotonin concentrations the shell of the nucleus accumbens also differed among sensitized, non-sensitized and control rats (Fig. 8D; F(2,27) = 6.076, p = 0.007). Non-sensitized rats had lower nucleus accumbens shell serotonin concentrations than saline-treated (p = 0.006) and sensitized rats (p = 0.043). Nucleus accumbens shell serotonin concentrations were not significantly different in sensitized and saline-treated groups (p = 0.112).
In the medial prefrontal cortex, serotonin concentrations in sensitized, non-sensitized and saline-treated differed (Fig. 8E; F(2,29) = 5.490, p = 0.010). Decreases in medial prefrontal cortex serotonin concentrations were detected in both sensitized and non-sensitized rats when compared to the saline-treated group (sensitized; p = 0.014, non-sensitized; p = 0.033), but no significant differences were found between sensitized and non-sensitized rats (p = 0.766).
In the dorsal hippocampus, serotonin concentrations differed between groups (Fig. 8F; F(2,27) = 3.916, p = 0.033). Sensitized rats had higher dorsal hippocampal serotonin than non-sensitized rats (p = 0.026), but neither sensitized (p = 0.117) nor non-sensitized (p = 0.119) groups were significantly different from saline-treated rats.
4. Discussion
4.1 Behavioral Sensitization
In rodents, sensitization to repeated amphetamine treatments is often characterized by enhanced locomotor responses to low drug doses and enhanced stereotypy to higher doses of amphetamine [5,7]. In our study, repeated treatment with 2.5 mg/kg amphetamine produced hyperactivity which predominated over focused stereotyped behavior when tested on day 14 of withdrawal. The majority (62%) of amphetamine-treated rats showed sensitization to the locomotor effects of amphetamine as evidenced by increased total amphetamine-induced locomotion and rearing over the testing sessions. These rats also showed greater amphetamine-induced locomotion when compared to saline-treated rats challenged with amphetamine 7 days after the last repeated injection, indicating sensitization to the locomotion effects of amphetamine (Fig. 4A).
In contrast, 38% of amphetamine-treated rats did not show locomotor sensitization. These rats, classified as non-sensitized, exhibited decreased locomotion and amphetamine-induced rearing over the testing sessions. Non-sensitized rats also showed similar amphetamine-induced locomotor responses at 7 days of withdrawal when compared to rats undergoing their first exposure to amphetamine (Fig. 4A), further suggesting that they did not sensitize to the locomotor effects of repeated amphetamine treatment. The absence of locomotor sensitization following repeated amphetamine treatment could not be explained by an increase in amphetamine-induced focused stereotyped behavior that would normally exclude locomotion [30]. These results suggest that non-sensitized rats are desensitized [39] and may have habituated to the behavioral effects of repeated amphetamine. The proportion of sensitized to non-sensitized rats in our study was similar to that demonstrated for cocaine sensitization studies in rats [40]. Interestingly, the distribution of sensitization measures in the current study was bimodal, such that all the rats treated with amphetamine clearly divided into the two groups (sensitized and non-sensitized). Further studies are required to determine whether this distinct bimodal distribution holds for this strain and other strains of rats.
Many studies have focused on pre-existing differences that may predict amphetamine sensitization in rats and addiction susceptibility in humans. For example, individuals that have high sensation-seeking scores also express higher self-report measures associated with the reinforcing effects of amphetamine [41]. Likewise, rats that have high locomotor counts in response to a novel environment (high responders) or have high novelty preference show greater locomotor responses to an initial administration of amphetamine, and greater amphetamine self-administration when compared low novelty responders [26, 42, 43]. These high responders also exhibit greater sensitization following repeated amphetamine [26, 27, 44]. However, this is a dose-dependent effect, since high and low responders show equal levels of sensitization at amphetamine doses higher than 1.0 mg/kg i.p. [26, 43]. While this variable should be examined in the future, the 2.5 mg/kg dose used in our study probably precludes novelty responses as a predictor of amphetamine sensitization. Furthermore, a significant difference between the current study and those examining the relationship between high/low responders and amphetamine is that rats in the current study were habituated to the testing enclosures prior to testing.
4.2 Open Field Behavior and Plasma Corticosterone
Sensitized rats took a significantly longer time to enter the center of the brightly-lit open field compared to saline-treated controls, indicating increased anxiety. While further testing in other anxiety paradigms is required, these results suggest that in addition to showing elevated stress responses to mild stressors [12, 16, 17], sensitized rats show increased anxiety-like behavior during the withdrawal period. However, the latency for non-sensitized animals to enter the center was not different from saline-treated controls or sensitized rats. Similarly, plasma corticosterone levels following drug treatment and withdrawal were increased in sensitized rats compared to saline-treated controls, but corticosterone levels in non-sensitized rats were not different from sensitized or saline-treated rats. These results highlight the importance of comparing amphetamine sensitized and non-sensitized rats to determine the effects of amphetamine sensitization on anxiety and endocrine measures. The findings suggest that for both anxiety-like behavior and plasma corticosterone, non-sensitized rats could represent a state intermediate to that of saline-treated and amphetamine-sensitized rats.
Previous studies have shown that amphetamine-treated rats exhibit increased plasma corticosterone, behavioral and neurochemical responses to stress when compared to saline controls [12, 13, 45], although some of these measures appear to be dependent on amphetamine dose [15]. Corticosterone has been proposed to facilitate the rewarding effects of amphetamine, and high circulating levels of corticosterone may potentiate amphetamine sensitization ([46]; although see [47]). Future research should examine whether pre-existing individual differences in anxiety behavior and corticosterone levels prior to drug exposure can predict the degree of amphetamine sensitization.
4.3 Neural Dopamine and DOPAC Concentrations
Following amphetamine treatment and withdrawal, no changes were seen in concentrations of the dopamine metabolite, DOPAC, in any brain region studied. This suggests that the changes in dopamine concentrations observed were a result of altered synthesis and storage of dopamine rather than alterations to dopaminergic activity. While both sensitized and non-sensitized rats had different dopamine concentrations in various striatal or limbic sites when compared to saline-treated controls, there were no instances where dopamine concentrations in a given brain region were significantly changed in both sensitized and non-sensitized rats. This suggests that the changes in dopamine concentrations were related to either the processes of behavioral habituation or sensitization to repeated amphetamine.
Higher dopamine concentrations in the nucleus accumbens shell of sensitized rats suggest that synthesis or storage of dopamine in this region increased during the sensitization process. This could explain why psychostimulant-sensitized rats show heightened amphetamine-induced nucleus accumbens shell dopamine release during withdrawal when measured by microdialysis [48 – 50]. The current results also show that repeated amphetamine treatment in the absence of sensitization does not appear to affect dopamine concentrations in the shell of the nucleus accumbens, which is in line with previous findings in an amphetamine sensitization-resistant line of rats [51, 52]. Our findings add support to the hypothesis that dopamine in the nucleus accumbens shell could underlie sensitization to repeated amphetamine treatment [53 – 55] and may be involved in increased reward and motivation leading to addiction [53, 54, 56]. In contrast to this hypothesis, Roman High-Avoidance rats sensitized to lower (1 mg/kg) doses of amphetamine show attenuated amphetamine-induced (0.2 mg/kg) dopamine release in the shell of the nucleus accumbens during withdrawal [52]. The contrasting results regarding sub-region changes to accumbal dopamine following sensitization highlight the impact of differences in amphetamine dose, sensitization methodology and rat strain on the study of the underlying mechanisms of amphetamine sensitization.
Dopaminergic activity in the ventral tegmental area is thought to be related to increased amphetamine-induced dopamine release in the nucleus accumbens in sensitized rats [1, 55, 57]. Following a period of withdrawal, dopamine responses to acute cocaine in the ventral tegmental area are diminished [1, 58]. The current study demonstrates that only sensitized rats had decreased dopamine concentrations in the ventral tegmental area, which could explain decreased drug-induced release of dopamine in this region as measured by microdialysis in the withdrawal period [1, 58]. In contrast, only non-sensitized rats had decreased dopamine concentrations in the dorsal striatum. Interestingly, rats that show reduced locomotion to repeated amphetamine treatment in a similar fashion to the non-sensitized rats of the current study have lower dopamine content in the striatum in the absence of amphetamine exposure, when compared to rats that sensitize to amphetamine [39]. Since dopamine in the dorsal striatum is required for some of the motor behaviors induced by amphetamine [31, 59, 60], future research should test the possibility that decreased dopamine concentrations in the dorsal striatum may play a role in habituation to the motor effects of amphetamine.
4.4 Neural Serotonin and 5-HIAA Concentrations
Similar to the dopaminergic systems, concentrations of the serotonin metabolite, 5-HIAA, did not significantly change in any of the brain regions studied as a response to amphetamine treatment and withdrawal. Therefore, the changes in serotonin concentrations appear to be a result of altered synthesis or storage of serotonin rather than alterations in serotonergic activity. With the exception of the medial prefrontal cortex, changes in serotonin concentrations detected in the sensitized and non-sensitized groups did not occur in the same direction (i.e., increase or decrease) within the same brain region. This again suggests that the majority of alterations to serotonin concentrations were related either to amphetamine habituation or to amphetamine sensitization.
Non-sensitized rats had decreased serotonin concentrations in the shell of the nucleus accumbens when compared to saline-treated and sensitized rats. Direct administration of serotonin to the nucleus accumbens inhibits acute amphetamine-induced locomotion [61, 62] via complex interactions with a number of serotonin receptor subtypes [63]. However, it is not known how serotonin function in the nucleus accumbens relates to chronic amphetamine treatment and withdrawal. Our current findings suggest that decreased serotonin concentrations in the nucleus accumbens may be an adaptation to repeated amphetamine treatment. Therefore, decreased serotonergic function in this region may inhibit sensitization to the locomotor activation effects of amphetamine, but this possibility requires testing.
Amphetamine-induced increases in serotonin are thought be responsible for amphetamine depression of glutamatergic excitatory transmission in the ventral tegmental area of drug-naive rats [64]. In our study, ventral tegmental area serotonin concentrations were lower in sensitized rats when compared to saline-treated controls. Conceivably, such reductions in serotonin could attenuate amphetamine-depression of excitatory transmission in the ventral tegmental area of these rats, leading to heightened responses to amphetamine.
Sensitized rats also had lower serotonin concentrations in the dorsal striatum compared to the saline-treated group. Central serotonin depletion results in increased locomotor responses to amphetamine [65]. While this effect cannot be attributed to serotonin depletion in a specific brain region, Rebec and colleagues [66] showed that enhanced amphetamine-locomotion in serotonin depleted rats was paralleled by altered neuronal responses in the dorsal striatum, and proposed that the effect of serotonin depletion on amphetamine-induced locomotion is mediated by the dorsal striatum. Our results add to this by suggesting that sensitization to the locomotor effects of amphetamine is associated with decreased serotonin levels in the dorsal striatum.
While no differences in serotonin concentrations in the ventral hippocampus were detected, serotonin in the dorsal hippocampus was higher in sensitized rats compared to non-sensitized rats. Our findings provide support for the hypothesis that serotonin in the dorsal hippocampus, but not ventral hippocampus, mediates amphetamine-induced locomotion [67]. Furthermore, it appears that sensitization to the locomotor effects of amphetamine is reflected by differences in dorsal hippocampal serotonin concentrations.
Decreased serotonin concentrations in the medial prefrontal cortex in both sensitized and non-sensitized rats suggest that these changes were due to repeated amphetamine treatment, and not associated with sensitization. Decreased serotonin activity in the frontal cortex during psychostimulant withdrawal has been shown previously [68]. Combined with our current results, this suggests that decreased serotonin concentrations and function in the frontal cortex results from the pharmacological actions of repeated amphetamine. Since elevations in medial prefrontal cortex serotonin activity are thought to be involved in reducing expression of acute fear behavior [69], reduced serotonin function in this region could result in prolonged fear behavior of amphetamine-treated rats. This speculation is partly supported by the finding that cocaine-treated rats undergoing withdrawal show poor extinction of fear behavior in a conditioned foot-shock paradigm [70].
4.5 Conclusions
Our results suggest that following repeated amphetamine (2.5 mg/kg) treatment, the changes in dopamine and serotonin concentrations in many limbic regions are associated with sensitization rather than with repeated amphetamine administration in the absence of sensitization. The findings also suggest that the specific alterations in limbic dopamine and serotonin concentrations detected in non-sensitized rats could reflect habituation to the effects of repeated amphetamine administration. Although it remains to be seen whether similar effects are observed with different doses of amphetamine, the current study should help direct future examination of the neural circuitry involved in the individual propensity for sensitization and stress-induced relapse. Future work should also examine the possibility that pre-existing individual differences in levels of anxiety-like behavior, plasma corticosterone or limbic monoamine markers could predispose animals to sensitize to the effects of amphetamine.
Acknowledgements
This work was supported by NIH grant P20 RR015567, which is designated as a Center of Biomedical Research Excellence (COBRE), and NIH grants R01 DA019921 and R03 MH068303 to GF, but is solely the responsibility of the authors and does not necessarily represent the official views of NIH. JS was a recipient of a University of South Dakota Graduate Student Research Grant. We would like to thank Ron Pringle and Brandon Kastein for technical assistance and Dr. Cliff Summers for helpful comments regarding these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- 1.Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav. Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- 2.Fowler SC, Birkestrand B, Chen R, Vorontsova E, Zarcone T. Behavioral sensitization to amphetamine in rats: changes in the rhythm of head movements during focused stereotypies. Psychopharmacol. (Berl) 2003;170:167–177. doi: 10.1007/s00213-003-1528-5. [DOI] [PubMed] [Google Scholar]
- 3.Olausson P, Engel JA, Soderpalm B. Effects of serotonergic manipulations on the behavioral sensitization and disinhibition associated with repeated amphetamine treatment. Pharmacol. Biochem. Behav. 2000;66:211–220. doi: 10.1016/s0091-3057(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 4.Wang YC, Hsiao S. Amphetamine sensitization: nonassociative and associative components. Behav. Neurosci. 2003;117:961–969. doi: 10.1037/0735-7044.117.5.961. [DOI] [PubMed] [Google Scholar]
- 5.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 6.Mendrek A, Blaha C, Phillips AG. Pre-exposure to amphetamine sensitizes rats to its rewarding properties as measured by a progressive ratio schedule. Psychopharmacol. 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- 7.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 8.Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res. Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur. J. Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 11.Stewart J. Pathways to relapse: the neurobiology of drug-and stress-induced relapse to drug-taking. J. Psych. Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, et al. Compulsive drug-seeking behavior and relapse. Ann. N.Y. Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 13.Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacol. 2002;26:286–294. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- 14.Vanderschuren , Louk JMJ, Schmidt ED, De Vries TJ, Van Moorsel CAP, Tilders FJH, Schoffelmeer ANM. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J. Neurosci. 1999a;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russig H, Pryce CR, Feldon J. Amphetamine withdrawal leads to behavioral sensitization and reduced HPA axis response following amphetamine challenge. Brain Res. 2006;1084:185–195. doi: 10.1016/j.brainres.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- 17.Eichler AJ, Antelman SM. Sensitization to amphetamine and stress may involve nucleus accumbens and medial frontal cortex. Brain Res. 1979;176:412–416. doi: 10.1016/0006-8993(79)90999-5. [DOI] [PubMed] [Google Scholar]
- 18.Levy AD, Baumann MH, Van de Kar LD. Monoaminergic regulation of neuroendocrine function and its modification by cocaine. Front. Neuroendocrinol. 1994;15:85–156. doi: 10.1006/frne.1994.1006. [DOI] [PubMed] [Google Scholar]
- 19.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotrophin-releasing factor in drug addiction. Pharmacol. Revi. 2001;53:209–243. [PubMed] [Google Scholar]
- 20.Aston-Jones G, Harris GC. Brain substrates for increased drug-seeking during protracted withdrawal. Neuropharmacol. 2004;47:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 22.Millan MJ. The neurobiology and control of anxious states. Prog. Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 23.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Dep. Anx. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 25.Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic d-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav. Brain. Res. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- 26.Hooks MS, Jones GH, Neill DB, Justice JB., Jr Individual differences in amphetamine sensitization: Dose-dependent effects. Pharmacol. Biochem. Behav. 1991;41:203–210. doi: 10.1016/0091-3057(92)90083-r. [DOI] [PubMed] [Google Scholar]
- 27.Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol. Biochem. Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- 28.Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J. Pharmacol. Exp. Therapeu. 1987;242:917–926. [PubMed] [Google Scholar]
- 29.Vanderschuren Louk JMJ, Schoffelmeer ANM, Mulder AH, De Vries TJ. Lack of cross-sensitization of the locomotor effects of morphine in amphetamine-treated rats. Neuropsychopharmacol. 1999b;21:550–559. doi: 10.1016/S0893-133X(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 30.Forster GL, Falcon AJ, Miller AD, Heruc GA, Blaha CD. Effects of laterodorsal tegmentum excitotoxic lesions on behavioral and dopamine responses evoked by morphine and d-amphetamine. Neurosci. 2002;114:817–823. doi: 10.1016/s0306-4522(02)00365-2. [DOI] [PubMed] [Google Scholar]
- 31.Miller AD, Forster GL, Metcalf KM, Blaha CD. Excitotoxic lesions of the pedunculopontine differentially mediate morphine and d-amphetamine evoked striatal dopamine efflux and behaviors. Neurosci. 2002;111:351–362. doi: 10.1016/s0306-4522(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 32.Ottoni EB. EthoLog 2.2 - a tool for the transcription and timing of behavior observation sessions. Behav. Res. Methods, Instrum, & Comp. 2000;32:446–449. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- 33.Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J. Neurochem. 1986;46:1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- 34.Kontur PJ, Al-Tikriti M, Innis RB, Roth RH. Postmortem stability of monoamines, their metabolites, and receptor binding in rat brain regions. J. Neurochem. 1994;62:282–290. doi: 10.1046/j.1471-4159.1994.62010282.x. [DOI] [PubMed] [Google Scholar]
- 35.Palkovits M, Brownstein MJ. Maps and guide to microdissection of the rat brain. New York: Elsevier Sciences Publishing Co., Inc.; 1988. [Google Scholar]
- 36.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd Edition. San Diego: Academic Press, Inc.; 1986. [Google Scholar]
- 37.Renner KJ, Krey LC, Luine VN. Effect of progesterone on monoamine turnover in the brain of the estrogen-primed rat. Brain Res. Bull. 1987;19:195–202. doi: 10.1016/0361-9230(87)90085-2. [DOI] [PubMed] [Google Scholar]
- 38.Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of the serotonergic raphe nuclei elicited by stress and the neuropeptide CRF. J. Neuroendocrinol. 2003;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- 39.Alttoa A, Eller M, Hern L, Rinken A, Harro J. Amphetamine-induced locomotion, behavioral sensitization to amphetamine, and striatal D2 receptor function in rats with high or low spontaneous exploratory activity: Differences in the role of the locus coeruleus. Brain Res. 2007;1131:138–148. doi: 10.1016/j.brainres.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol. Therapeu. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacol. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cain M, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp. Clin. Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- 43.Piazza PV, Deminiere J, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 44.Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol. Behav. 2005;86:347–355. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Hamamura T, Fibiger HC. Enhanced stress-induced dopamine release in the prefrontal cortex of amphetamine-sensitized rats. Eur. J. Pharmacol. 1993;237:65–71. doi: 10.1016/0014-2999(93)90094-x. [DOI] [PubMed] [Google Scholar]
- 46.Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc. Nat. Acad. Sci. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badiani A, Morano MI, Akil H, Robinson TE. Circulating adrenal hormones are not necessary for the development of sensitization to the psychomotor activating effects of amphetamine. Brain Res. 1995;673:13–24. doi: 10.1016/0006-8993(94)01365-o. [DOI] [PubMed] [Google Scholar]
- 48.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 49.Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J. Pharmacol. Exp. Therapeu. 1995;275:1019–1029. [PubMed] [Google Scholar]
- 50.Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J. Pharmacol. Exp. Therapeu. 1993;264:249–255. [PubMed] [Google Scholar]
- 51.Corda MG, Piras G, Lecca D, Fernandez-Teruel A, Driscoll P, Giorgi O. The psychogenetically selected Roman rat lines differ in the susceptibility to develop amphetamine sensitization. Behav. Brain Res. 2005;157:147–156. doi: 10.1016/j.bbr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Giorgi O, Piras G, Lecca D, Corda MG. Differential activation of dopamine release in the nucleus accumbens core and shell after acute or repeated amphetamine injections: A comparative study in the Roman high- and lowavoidance rat lines. Neurosci. 2005;135:987–998. doi: 10.1016/j.neuroscience.2005.06.075. [DOI] [PubMed] [Google Scholar]
- 53.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacol. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 54.Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Cur. Opin. Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Vezina P. Sensitization of midbrain dopamine neuron reacticity and the selfadministration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 57.Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res. 1993;605:332–337. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- 58.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. II. Dopamine perikarya. J. Neurosci. 1993;13:276–284. doi: 10.1523/JNEUROSCI.13-01-00276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacol. 1988;95:556–559. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- 60.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 61.Carter CJ, Pycock CJ. Differential effects of central serotonin manipulation on hyperactive and stereotyped behaviour. Life Sci. 1978;23:953–960. doi: 10.1016/0024-3205(78)90222-9. [DOI] [PubMed] [Google Scholar]
- 62.Costall B, Hui SC, Naylor RJ. The importance of serotonergic mechanisms for the induction of hyperactivity by amphetamine and its antagonism by intra-accumbens (3,4-dihydroxy-phenylamino)-2-imidazoline (DPI) Neuropharmacol. 1979;18:605–609. doi: 10.1016/0028-3908(79)90112-6. [DOI] [PubMed] [Google Scholar]
- 63.Layer RT, Uretsky NJ, Wallace LJ. Effect of serotonergic agonists in the nucleus accumbens on d-amphetamine-stimulated locomotion. Life Sci. 1992;50:813–820. doi: 10.1016/0024-3205(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 64.Jones S, Kauer JA. Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J. Neurosci. 1999;19:9790–9787. doi: 10.1523/JNEUROSCI.19-22-09780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segal DS. Differential effects of para-chlorophenylalanine on amphetamine-induced locomotion and stereotypy. Brain Res. 1976;116:267–276. doi: 10.1016/0006-8993(76)90904-5. [DOI] [PubMed] [Google Scholar]
- 66.Rebec GV, Alloway KD, Curtis SD. Apparent Serotonergic modulation of the dose-dependent biphasic response of neostriatal neurons produced by D-amphetamine. Brain Res. 1981;210:177–289. doi: 10.1016/0006-8993(81)90901-x. [DOI] [PubMed] [Google Scholar]
- 67.Kusljic S, van den Buuse M. Functional dissociation between serotonergic pathways in dorsal and ventral hippocampus in psychotomimetic drug-induced locomotor hyperactivity and prepulse inhibition in rats. Eur. J. Neurosci. 2004;20:3424–3432. doi: 10.1111/j.1460-9568.2004.03804.x. [DOI] [PubMed] [Google Scholar]
- 68.Dworkin SI, Co C, Smith JE. Rat brain neurotransmitter turnover rates altered during withdrawal from chronic cocaine administration. Brain Res. 1995;682:116–126. doi: 10.1016/0006-8993(95)00327-m. [DOI] [PubMed] [Google Scholar]
- 69.Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, et al. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neurosci. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Burke KA, Franz TM, Gugsa N, Schoenbaum G. Prior cocaine exposure disrupts extinction of fear conditioning. Learn. Mem. 2006;13:416–421. doi: 10.1101/lm.216206. [DOI] [PMC free article] [PubMed] [Google Scholar]