Abstract
Objective
To compare human T-cell lymphotrophic virus type I (HTLV-I) seropositive and seronegative women for symptoms and signs of spasticity.
Background
Infection with HTLV-I causes tropical spastic paraparesis/ HTLV-I–associated myelopathy (TSP/HAM). Certain populations, including female commercial sex workers (FSW), are at increased risk of developing this infection. Fewer than 5% of HTLV-I–seropositive persons develop TSP/HAM, which is typically associated with spasticity.
Methods
Cross-sectional study of 255 registered FSW in Callao, Perú, involving a questionnaire detailing demographics and neurologic symptoms, standard neurologic examination, quantitative assessment of spasticity (QSA) of muscle tone, and serologic testing for HTLV-I. Participants and examiners were blinded to serology results.
Results
On the questionnaire and neurologic examination, none of the 32 HTLV-I–seropositive or 223 seronegative women had signs or symptoms of spasticity. However, mean values on QSA were significantly higher among seropositive women (27.1 Newton-meters/radian [N-m/r]) than among seronegative women (21.6 N-m/r, p = 0.01), indicating a subclinical increase in lower extremity tone. With values of QSA divided into tertiles, and the first tertile serving as the comparison group, the odds ratio for seropositivity was 1.4 (95% confidence interval [CI] 1.0 to 2.0) in the second and 3.1 (95% CI 2.2 to 4.3) in the third tertile, after adjusting for age and place of birth.
Conclusions
Although a standard neurologic evaluation could not distinguish between women with and without HTLV-I infection, QSA indicated significantly increased lower extremity tone in those with infection. Long-term follow-up will determine whether these subclinical findings in asymptomatic women progress to overt TSP/HAM.
Infection with human T-cell lymphotrophic virus type I (HTLV-I) causes tropical spastic paraparesis, also called HTLV-I–associated myelopathy (TSP/ HAM). TSP/HAM usually develops during the fifth decade of life, and typical symptoms include leg weakness, back pain, and bladder dysfunction. All affected individuals eventually demonstrate signs of spasticity in the lower limbs.1,2 The majority of HTLV-I–seropositive persons never seem to develop TSP/HAM. The prevalence of TSP/HAM in HTLV-I–seropositive individuals in Jamaica was 0.5%.3 In Japan, the lifetime incidence of TSP/HAM in HTLVI–seropositive individuals was calculated as only 0.25%.4 Some populations of HTLV-I–infected patients have a higher prevalence of TSP/HAM, perhaps reflecting a greater viral load at the time of initial infection or unique viral virulence or population susceptibility. For instance, a recent study noted TSP/HAM in 2.4% of HTLV-I–infected blood donors.5
Some populations experience an unusually high risk of HTLV-I infection. Several studies from Latin America and the United States have found strong associations between HTLV-I infection and number of sexual partners, duration of commercial sex work, and duration of homosexuality.6-8 Studies that have examined the relationship of HTLV-I to one or more concurrent sexually transmitted diseases (STD) have found relationships to both ulcerative STD (syphilis, herpes simplex virus type 2, chancroid) and nonulcerative STD (gonorrhea and chlamydial infection). The prevalence of HTLV-I infection in female commercial sex workers (FSW) has ranged from 3.2% in Kinshasa, Zaire,9 to 5.7% in Japan,10 to 21.8% in the port city of Callao, Perú.8 Cross-sectional studies of HTLV-I prevalence among FSW in two districts of Lima, Perú have shown a steady increment in prevalence of HTLV-I seropositivity of 1 to 2% per year after the onset of commercial sex work, with essentially all HTLV-I infection assumed due to sexual acquisition as adults. The risk of TSP/HAM in women infected through commercial sex is unknown.
The cardinal finding of TSP/HAM is spasticity, a state of increased muscle tone that results in a velocity-dependent resistance to muscle stretch. Measurement of spasticity involves stretching a muscle and then measuring the response to this stretch. The difficulty of applying a consistent stretch and accurately measuring the response to this stretch may compromise the reliability and accuracy of such measurements based on the routine neurologic examination. A device developed at the University of Washington in Seattle solves this problem by providing a quantitative spasticity assessment (QSA).11 In previous studies, this test has demonstrated a difference in muscle tone between adults with and without clinical evidence of spasticity, with a median value of 24 Newton-meters/radian (N-m/r) for normal adults and 98 N-m/r for adults with spasticity.12
In view of the widely varying risks of TSP/HAM in HTLV-I–infected persons, the high prevalence of HTLV-I infection in FSW in Callao, Perú, and the lack of data on TSP/HAM among individuals with sexually acquired TSP/HAM, we decided to measure the prevalence of symptoms and signs of spasticity or other neurologic dysfunction among HTLV-I–sero-positive FSW in Callao, Perú. We performed a standard neurologic examination and evaluated all women with the QSA.
Methods
Study design
All registered FSW visiting the public health clinic in Callao were invited to participate in the study. Each registered FSW must receive a health examination at the public health clinic every 2 weeks. This examination includes a gynecologic examination with cultures for Neisseria gonorrhoeae and Trichomonas vaginalis. Syphilis serologies (RPR) are performed four times a year and HIV tests are performed twice a year. FSW were recruited by a social worker hired for the study. Each woman completed a standard questionnaire, received a standard neurologic examination performed by a neurologist (S.M. or J.R.Z.), and underwent a QSA. All women and study personnel were blinded to HTLV-I serostatus. Informed consent was obtained from all participating women. The study protocol was approved by the human subjects committees of the University of Washington, Seattle, and the Universidad Nacional Mayor de San Marcos, Lima, Perú. Each woman received 25 condoms in exchange for her participation.
One of the study neurologists (S.M. or J.R.Z.) administered the questionnaire, which concentrated on possible clinical manifestations of HTLV-I infection as well as on other diseases that could confound the relationships being sought. The questionnaire included questions about stiffness, spasms, weakness of the lower limbs, and incontinence of bladder or bowel. In addition to questions regarding neurologic status, questions regarding constitutional symptoms, such as those that might occur with leukemia or lymphoma, were also asked. As HTLV-I has been associated with syndromes possibly attributed to dysfunction of autoimmunity, such as uveitis, arthritis, dermatitis, and Sjögren's syndrome, questions regarding the presence of related symptoms were asked. Finally, questions were asked concerning the existence of previous ankle injury or other neurologic diseases, such as cerebral palsy, and a detailed neurologic examination with quantitative assessment of spasticity was performed, as detailed in the following.
Clinical examination
The neurologic examination consisted of a detailed and standardized assessment of cranial nerve function, muscle strength and reflexes, sensory function (light touch, pinprick, and vibration), coordination, and gait. In addition, tone in the lower limbs was assessed using three maneuvers. In the seated position, the individual’s knee was rapidly abducted away from the other knee, and if the other knee followed, adductor tone at the hip was coded as increased. A similar test of adductor tone involved abduction of the extended lower limb in the supine position; if the other heel moved during the abduction of the limb, the tone was coded as increased. Finally, the extended lower limb was rapidly raised by pulling up at the knee. If the heel rose off of the examining table, the extensor tone at the knee was graded as increased.
A device developed at the University of Washington to provide QSA measures the variation in elastic and viscous stiffness of the gastroc-soleus-achilles tendon unit by oscillating the foot over precise sinusoidal displacements and measuring the resulting torque response of the tendon unit.11 The testing involved placing the individual supine on an examining table with her foot attached to the machine by means of a footplate. The frequency of the sinusoidal oscillation was varied by the examiner seated at the computer. Displacement and torque were measured simultaneously during the testing, and using Fourier analysis, the torsional stiffness was measured in N-m/r. This single summary variable was derived for the left ankle unless there was a previous injury or surgery to that ankle, in which case the right ankle was used. In a previous study, this system showed significant and reproducible differences between people with and without spasticity resulting from cerebral palsy.12
Laboratory testing
Serum for HTLV-I testing was obtained during routine blood draws at regular clinic visits. Testing was performed using an enzyme-linked immunosorbent assay (ELISA) for HTLV-I antibody (Cambridge Bioscience, Worcester, MA). ELISA positives were confirmed using an rp21e-enhanced Western blot assay (Cambridge Bioscience). An individual was considered seropositive for HTLV-I if the ELISA was positive and the Western blot revealed bands present at p24, gp46, or p21env(r) bands, or some combination.
Statistical analysis
We used t-tests for comparison of continuous variables and chi-square tests for comparison of discrete variables. These comparisons of HTLV-I–sero-negative or seropositive women yielded percents or means and standard deviations as well as p values. A multivariate logistic regression model was used to identify independent predictors of HTLV-I serostatus. Variables for this analysis included each reported symptom from the questionnaire and each clinical sign from the neurologic examination. Variables were coded as present or absent. Tallies of symptoms and signs were also included as variables. Other potential confounders included age and history of comorbid neurologic disease. These analyses yielded an adjusted odds ratio and 95% confidence interval (CI) for HTLV-I serostatus. For evaluating the QSA, which is a continuous measure, the data were grouped in tertiles based on results from all women. This allowed age-adjusted calculation of an odds ratio for HTLV-I seropositivity for persons in each tertile of muscle tone, as measured by the QSA. Statistical analyses used SPSS (Chicago, IL).13
Results
Between March 1997 and July 1997, 393 women attending the clinic were recruited to the study. Of 255 who completed all sections of the study, 32 (13%) were HTLV-I seropositive.
Compared to the HTLV-I–seronegative women, seropositive women were older (mean age, 34.8 versus 28.8 years, p = 0.001), were less well educated ( p < 0.001), had a longer duration of prostitution (110.6 versus 54.3 months, p < 0.001), were more likely to have been born in the Andes mountains (37 versus 21%, p = 0.06), and had fewer reported abortions (0.5 versus 0.8, p = 0.05) (table 1).
Table 1.
Baseline characteristics of human T-cell lymphotrophic virus type I (HTLV-I)–seropositive and seronegative women
| Variable* | HTLV-I–seropositive, n = 32 | HTLV-I–seronegative, n = 223 | p Value† |
|---|---|---|---|
| Age, y, mean (±SD) | 34.8 (±1.7) | 28.8 (±0.5) | 0.001 |
| Age at sexual debut, y, mean (±SD) | 16.9 (±0.5) | 16.5 (±0.6) | 0.44 |
| Age at onset of prostitution, y, mean (±SD) | 25.2 (±1.0) | 24.1 (±0.3) | 0.27 |
| Mean (±SD) number of abortions | 0.5 (±0.2) | 0.9 (±0.1) | 0.05 |
| Duration of prostitution, mo, mean (±SD) | 110.6 (±18.1) | 54.3 (±4.3) | <0.001 |
| Mean (±SD) number of clients in past week | 23.3 (±2.7) | 21.0 (±1.4) | 0.55 |
| Sex with foreigners, n (%) | 2 (7) | 54 (33) | 0.03 |
| Marital status, n (%) | |||
| Married/cohabitating | 5 (17) | 41 (18) | 0.74 |
| Divorced/separated | 1 (3) | 13 (6) | |
| Single | 24 (80) | 164 (74) | |
| Widow | 0 (0) | 4 (2) | |
| Education, n (%) | |||
| Grade school or less | 11 (37) | 23 (10) | <0.001 |
| High school | 19 (63) | 179 (81) | |
| Post high school | 0 (0) | 20 (9) | |
| Place of birth, n (%) | |||
| Jungle | 0 (0) | 19 (9) | 0.06 |
| Andes mountains | 11 (37) | 47 (21) | |
| Coast | 19 (63) | 156 (70) | |
For continuous variables, mean (± SD); for discrete variables, counts (%).
p Value based on t-test for continuous variables and chi-square for discrete variables.
The percent and mean number of specific neurologic symptoms and signs were similar in seropositive and sero-negative women (table 2). QSA values were significantly higher for seropositive women (mean 27.1 N-m/r) than for seronegative women (21.6 N-m/r, p = 0.002) (table 1 and figures 1 and 2). Increasing values on QSA were positively associated with increasing age ( p = 0.003) and duration of prostitution ( p = 0.007), and were negatively associated with history of sex with foreign clients ( p = 0.03).
Table 2.
Neurologic symptoms and signs in human T-cell lymphotrophic virus type I (HTLV-I)–seropositive and seronegative women
| Variable* | HTLV-I–seropositive, n = 32 | HTLV-I–seronegative, n = 223 | p Value† |
|---|---|---|---|
| Symptoms, n (%) | |||
| Back or leg pain | 21 (68) | 137 (61) | 0.75 |
| Difficulty walking | 4 (13) | 25 (11) | 0.79 |
| Weakness | 8 (25) | 40 (18) | 0.37 |
| Constipation | 9 (39) | 57 (26) | 0.78 |
| Bladder incontinence | 5 (16) | 22 (10) | 0.33 |
| Signs, n (%) | |||
| Cranial nerve abnormality | 0 (0) | 3 (1) | 0.51 |
| Reduced strength | 0 (0) | 1 (0.4) | 0.70 |
| Abnormal deep tendon reflexes | 1 (3) | 4 (2) | 0.62 |
| Increased tone | 0 (0) | 1 (0.4) | 0.70 |
| Abnormal sensation | 2 (6) | 7 (3) | 0.38 |
| Impaired coordination | 2 (6) | 4 (2) | 0.12 |
| Impaired gait | 1 (3) | 4 (2) | 0.28 |
| QSA, mean (±SD) | 27.1 (±1.5) | 21.6 (±0.6) | 0.002 |
| QSA values, range | |||
| First tertile | 13.1−22.2 | 9.5−16.2 | |
| Second tertile | 22.7−27.8 | 16.3−22.4 | |
| Third tertile | 28.8−53.7 | 22.6−62.6 | |
For continuous variables, mean (± SD); for discrete variables, counts (%).
p Value based on t-test for continuous variables and chi-square for discrete variables.
QSA = quantitative spasticity assessment.
Figure 1.
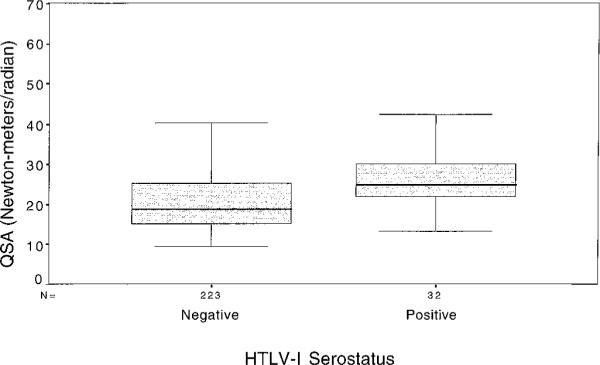
Boxplots of median values of quantitative spasticity assessment (QSA) in human T-cell lymphotrophic virus type I (HTLV-I)–seropositive and seronegative women.
Figure 2.
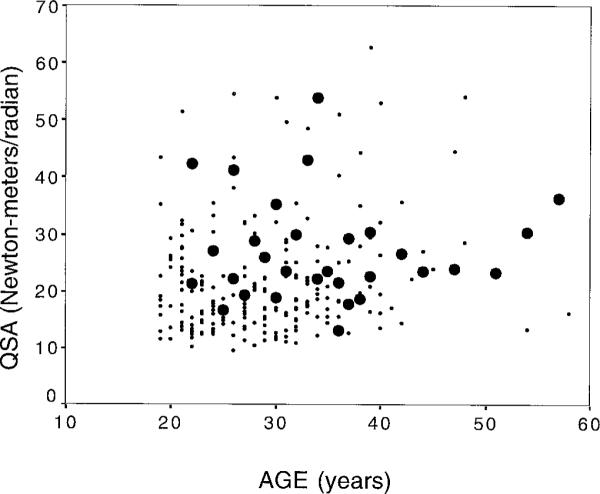
Scatterplot of quantitative spasticity assessment (QSA) by age and human T-cell lymphotrophic virus type I (HTLV-I) serostatus. ●, positive; •, negative.
Table 3 presents the odds ratios for HTLV-I serostatus by QSA divided into tertiles, the first tertile serving as the comparison group. Other potential confounders, such as duration of prostitution and history of sex with foreigners, which were both associated with HTLV-I infection and with QSA were no longer significant after adjusting for age, and were not added to the model. Controlling for number of abortions in addition to age and place of birth had little effect on the results.
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) for human T-cell lymphotrophic virus type I seropositivity for tertiles of quantitative spasticity assessment (QSA) before and after adjusting for age and place of birth
| QSA tertile | QSA range, N-m/r | OR (95% CI) | OR (95% CI), adjusting for age and place of birth |
|---|---|---|---|
| First | 9.5−16.7 | 1.0 (reference) |
1.0 (reference) |
| Second | 16.8−22.8 | 1.2 (0.8 to 1.7) |
1.4 (1.0 to 2.0) |
| Third | 23.3−62.6 | 3.2 (2.3 to 4.4) |
3.1 (2.2 to 4.3) |
N-m/r = Newton-meters/radian.
Discussion
Previous studies demonstrate a low prevalence of TSP/HAM in HTLV-I–seropositive persons.3-5 Spasticity eventually develops in persons with TSP/HAM but typically not as the first manifestation of the disease. More often, back or leg pain have been the first manifestations. In this study, which included 255 female commercial sex workers, these symptoms were not associated with HTLV-I infection. None of the 32 HTLV-I–seropositive women had evidence on history or neurologic examination of TSP/HAM. When comparing HTLV-I–sero-negative with seropositive women, no statistically significant differences in any specific neurologic symptoms or signs were noted. Using a sensitive method to quantitate muscle tone in the lower limb, we demonstrated an association of higher values on QSA with greater risk of HTLV-I seropositivity, indicating subclinical increased muscle tone in HTLV-I–seropositive compared with seronegative women. We know of no previous studies that have attempted to quantitate muscle tone in HTLV-I–seropositive persons.
Although some studies have shown an association between development of TSP/HAM and abnormal HTLV-I genotypes or host lymphocyte response to HTLV-I infection, studies are needed to determine which persons are most likely to develop neurologic disease when infected with HTLV-I. Our study demonstrated subclinical signs of increased muscle tone in a high proportion of HTLV-I–seropositive women, suggesting that HTLV-I infection may affect the CNS even during early stages of infection. If so, perhaps early intervention could lower the incidence of clinically overt TSP/HAM.
One of the limitations of this study is the limited experience in adults with QSA. QSA has demonstrated differences in muscle tone between both adults and children with and without spasticity. In children, variations in QSA values occur over time, and perhaps day-to-day variation in adults is larger. The range of QSA values in both groups of women in this study was similar to the range of adults without spasticity in the study by Price et al.12 Although the sensitivity and specificity of the QSA do not allow us to differentiate between seropositive and seronegative patients or between those who will remain asymptomatic versus those who will eventually develop myelopathy, the test does indicate that there is, on average, a higher level of muscle tone in those patients who are seropositive.
Long-term follow-up will determine the reproducibility of the QSA for measuring muscle tone in adults and whether the subtle findings found in the current study progress to become clinically manifest as TSP/HAM. Should the QSA prove reliable for measuring muscle tone in this population and a stable reproducible method for quantitating spasticity in symptomatic myelopathy, it could also be used to monitor objectively the effect of therapy on the spasticity of TSP/HAM and perhaps to allow early identification and early intervention for those HTLV-I–seropositive persons most likely to progress to TSP/HAM.
Acknowledgments
Supported by NIH grants TW00679 and AI0714P, Fogarty International grant T22-TW00001, and the University of Washington Center for AIDS and STD Research (CFAR) grant AI27757.
References
- 1.Román GC, Román LN. Tropical spastic paraparesis. A clinical study of 50 patients from Tumaco (Colombia) and review of the worldwide features of the syndrome. J Neurol Sci. 1988;87:121–138. doi: 10.1016/0022-510x(88)90059-7. [DOI] [PubMed] [Google Scholar]
- 2.Vernant JC, Maurs L, Gessain A, et al. Endemic spastic paraparesis associated with human T-cell lymphotrophic virus type I: a clinical and seroepidemiological study of 25 cases. Ann Neurol. 1987;21:123–130. doi: 10.1002/ana.410210204. [DOI] [PubMed] [Google Scholar]
- 3.Murphy EL, Wilks R, Morgan OSC, et al. Health effects of human T-lymphotropic virus type I (HTLV-I) in a Jamaican cohort. Int J Epidemiol. 1996;25:1090–1097. doi: 10.1093/ije/25.5.1090. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan JE, Osame M, Kubota H, et al. The risk of development of HTLV-I–associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr. 1990;3:1096–1101. [PubMed] [Google Scholar]
- 5.Murphy EL, Fridley J, Smith LW, et al. HTLV-associated myelopathy in a cohort of HTLV-I and HTLV-II–infected blood donors. Neurology. 1997;48:315–320. doi: 10.1212/wnl.48.2.315. [DOI] [PubMed] [Google Scholar]
- 6.Khabbaz RF, Darrow WW, Hartley TM, et al. Seroprevalence and risk factors for HTLV-I/II infection among female prostitutes in the United States. JAMA. 1990;263:60–64. [PubMed] [Google Scholar]
- 7.Gotuzzo E, Sánchez J, Escamilla J, et al. Human T-cell lymphotrophic virus type I infection among female sex workers in Peru. J Infect Dis. 1994;169:754–759. doi: 10.1093/infdis/169.4.754. [DOI] [PubMed] [Google Scholar]
- 8.Wignall FS, Hyams KC, Phillips IA, et al. Sexual transmission of human T-lymphotropic virus type I in Peruvian prostitutes. J Med Virol. 1992;38:44–48. doi: 10.1002/jmv.1890380110. [DOI] [PubMed] [Google Scholar]
- 9.Wiktor SZ, Piot P, Mann JM, et al. Human T-cell lymphotrophic virus type I (HTLV-I) among female prostitutes in Kinshasa, Zaire. J Infect Dis. 1990;161:1073–1077. doi: 10.1093/infdis/161.6.1073. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima K, Kashiwaga S, Kajiyama W, et al. Sexual transmission of human T-lymphotropic virus type I among female prostitutes and among patients with sexually transmitted diseases in Fukuoka, Kyushu, Japan. Am J Epidemiol. 1995;141:305–311. doi: 10.1093/aje/141.4.305. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann JF, Price R, deLateur BJ, Hinderer S, Traynor C. Spasticity: quantitative measurements as a basis for assessing effectiveness of therapeutic intervention. Arch Phys Med Rehabil. 1989;70:6–15. [PubMed] [Google Scholar]
- 12.Price R, Bjornson KF, Lehmann JF, McLaughlin JF, Hays RM. Quantitative measurement of spasticity in children with cerebral palsy. Dev Med Child Neurol. 1993;33:585–595. doi: 10.1111/j.1469-8749.1991.tb14928.x. [DOI] [PubMed] [Google Scholar]
- 13.SPSS for Macintosh . Advanced Statistics, Release 6.0. SPSS; Chicago, IL: 1993. [Google Scholar]


