Abstract
Current protocols for myeloma patients require more than one autologous transplant. We performed a retrospective study to determine cost effectiveness of large volume leukapheresis (LVL) compared to standard volume leukapheresis (SVL) collection when two transplants are required. We evaluated 87 patients who underwent a cumulative total of 260 LVL and SVL collections. The median product volume per collection was 356ml for LVL and this was significantly higher than the median product volume per collection for SVL (median 149.5ml, p <0.001). The median total CD34+ cell yield/kg was 6.4 × 106 for LVL and 5.2 ×106 for SVL. This difference was statistically significant (p = 0.005). Since the target CD34+ cell dose for a single transplant was 3 × 106/Kg at our institution, overall the LVL yields enough CD34+ cells that could allow for two transplants. Therefore, more patients in the LVL group were able to undergo a potential 2nd transplant. Because of the reserved cells for a second transplant, LVL patients received significantly less CD34+ cell/Kg per transplant than the patients in SVL group (p = <0.001). As a result, LVL group had statistically significant but clinically insignificant delay in neutrophil (p = <0.001) and platelet (p = 0.02) engraftments. Additionally, using LVL instead of SVL to collect ≥6 × 106/Kg CD34+ cells may potentially save $7,497/patient. We therefore conclude that LVL is the method of choice for collection of multiple myeloma patients when two transplants are anticipated.
Introduction
Autologous hematopoietic stem cell transplantation has become an integral part of multiple myeloma treatment1–4. Usually a hematopoietic stem cell transplant is performed with a minimum of 2.5 to 3× 106 peripheral blood CD34 cells per kilogram of body weight5.
Because of its high relapse rate many patients may require more than one transplant. Therefore, it is a common practice to collect sufficient Peripheral Blood Stem Cells (PBSC) for two transplants prior to initiating myeloablative therapy.
PBSC collection using standard volume leukapheresis (SVL) involves processing 10 – 16 litres of blood through the apheresis machine. This results in 1 – 2 ×106/Kg autologous CD34+ cell yield per collection. A standard collection takes approximately 4 hours to complete. Therefore, to get enough cells for two transplants, autologous donors with no significant prior chemotherapy or radiation therapy undergo 1 –5 daily collections before reaching their target cell dose6. Some heavily pretreated patients, particularly those who received thalidomide and or its derivatives (lenalidomide) undergo more than average number of collections to reach their target cell dose7. In general, about 20 – 30% of all mobilized autologous transplant candidates do not reach their target cell dose.8,9
Large volume leukapheresis (LVL) involves processing more than 20 liters of blood through the apheresis machine. Total mononuclear cell and CD34+ cell yield per collection was reported to be higher in LVL than SVL.6,10
We conducted a retrospective study to determine if large volume leukapheresis (LVL) is superior and more cost effective than standard volume leukapheresis (SVL) in yielding sufficient human progenitor cells (HPC) for two transplants.
Material and Methods
We reviewed records of 87 multiple myeloma patients who received a cumulative total of 260 collections. 35 of the patients underwent LVL collections and 52 underwent SVL collections. HPC collections at Mayo Clinic in Jacksonville Florida were performed using SVL and Mayo Clinic in Scottsdale, Arizona using LVL. The donor cohort consisted of 35 females and 52 males with median age of 63 years (range 33 to 76).
PBSC collection
Donors were treated with 10ug/kg filgrastim once daily subcutaneously for 4 to 5 days to mobilize the stem cell pool. Peripheral blood CD34+cells were monitored daily and peripheral blood stem cell collection was started on the day their peripheral CD34+ cell count reached 10/μl or higher. There was about 18 to 24 hours interval between the last filgrastim injection and the start of stem cell collection. Daily filgrastim injection continued to be given right before LVL collection and right after each SVL collection. Both SVL and LVL were performed using a Cobe Spectra (Gambro BCT, Lakewood, CO) machine. The machine was run using operating software version 7. A summary of the leukapheresis machine parameters are shown in Table 1. The venous access for all donors was via a triple lumen central venous catheter (Pheresis-flow® or Mahukar).
Table 1.
Leukapheresis instrument parameters
| Parameter | SVL | LVL |
|---|---|---|
| Duration (minutes) | 260 | 210–335 (mean=276) |
| Blood flow rate (ml/min) | 60 – 70 | 90 – 110 |
| Collect/replacement pump rate (ml/min) | 1.0 | 1.0 – 1.5 |
| AC ratio* | 12:1 | 15:1 – 40:1 |
| Blood Volume processed (litres) | 12 – 20 | ≥20 |
Note for LVL, 6,000 units of heparin is added to every liter of ACD-A.
The same randomly assigned operator and machine were used for all collections from each donor. Operators had passed their annual competency evaluations and all apheresis machines were validated and received routine biannual preventive maintenance.
SVL was defined as a PBSC collection that involves processing between 12 to 20 liters of whole blood through the apheresis machine. LVL was defined as PBSC collection that involved processing more than 20 liters of blood. Patients were initially examined by a physician prior to onset of procedure closely monitored with vital signs every 15 minutes during the collection. Electrolytes (K+, Mg++, Ca++) were obtained at the onset and toward the end of the procedure and corrected IV or PO as necessary. Fluid balance was closely monitored and corrected if necessary Anticoagulation for LVL collection consisted of 6000 Units of Heparin in 1000 ml of acid citrate dextrose-A (ACD-A). 60 ml of that solution was then injected in the product collection bag. . The AC ratio ranged from 15:1 to 40:1 depending on platelet counts.. For SVL collection, ACD-A is used without the addition of heparin
Adequate mobilization was defined as the ability of a donor to have ≥ 2 million CD34+ cell/Kg collection yield. In general, the target total CD34+ cell/Kg was 3 –5 × 106 for one transplant and 6 – 10 × 106 for 2 transplants. Those who failed to mobilize the first time were mobilized with a combination of G-CSF and either GM-CSF or cyclophosphamide.
CD34+ cell enumeration
The stem cell product was assayed for the total CD34+ cells. Total CD34+ cell yield was defined as cumulative total number of CD34+ cells from all collections. Mononuclear cells were stained with anti-CD45-FITC (Pharmingen, San Diego CA) and anti-CD34-PE (Becton Dickinson Immunocytometry Systems, San Jose CA) and analyzed using FACS Calibur flow cytometer (BD Biosciences, Waltham, MA). The number of CD34+ cells was measured using fresh products, just before freezing. The data analysis was performed using Cell Quest (BD Biosciences Waltham, MA) software following Procount™ acquisition layouts. The percentage of CD34+ cells in each sample was determined and the total number CD34+ cells infused was calculated.
Statistical analysis
Associations between continuous and categorical variables were assessed by the two sample t-test or the Wilcoxon rank-sum test. The linear correlation between two continuous factors was assessed by Spearman’s rank correlation. No statistical adjustment was made for performing multiple tests. All probability values are 2-sided.
Results
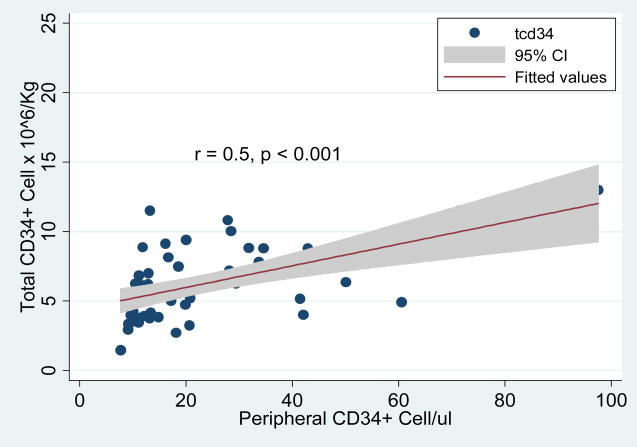
The median age for LVL group was 66 years while for the SVL was 60. The age distribution among the two groups was therefore statistically similar (p = 0.5). In addition, the ratio of females to males was similar between the two groups (LVL: Females 14, Males 21 and SVL: Females 21, Males 31). The median weight for LVL group was 77Kg and for SVL was 78.2Kg (p = 0.1). Median peripheral CD34+ cell count on first day of collection was 16.7/μl for LVL and 17.2/μl for SVL (p = 0.6). There was a statistically significant linear correlation between peripheral CD34 count and total number of CD34+ cell/Kg among the SVL group (r = 0.69, p=0.004) and LVL group (r = 0.52, p = 0.001), though the strength of correlation was slightly higher in SVL group than LVL group (data not shown). Overall, when both groups were considered, there was a direct linear correlation between peripheral blood CD34+ cell count and the total CD34+ cell yield (Table 2, Figure 1).
Table 2.
Patient characteristics and apheresis collection outcomes
| Variable | LVL Median(range) | SVL Median(range) | P value |
|---|---|---|---|
| Number of patients | 35 | 52 | |
| Age (years) | 66 (33 – 74) | 60 (43 – 76) | 0.5 |
| Weight (kg) | 77 (51 – 110) | 78.2 (53 – 143 | 0.1 |
| Peripheral CD34+ cell/μl | 16.7 (7.7 – 97.5) | 17.2 (9.1 – 60.5) | 0.6 |
| Collections | 3 (1 – 5) | 3 (1 – 9) | 0.7 |
| Total CD34+ cell yield × 106/Kg | 6.4 (1.5 – 13) | 5.2 (2 – 22.8) | 0.005 |
| Product Volume (mls) | 356 (229 – 478) | 149.5 (54 – 556) | <0.001 |
| CD34+ cells Infused ×106/Kg | 3.9 (2.7 – 6.5) | 5.2 (2 – 22.8) | <0.001 |
Figure 1.
Scatter diagram that demonstrates the correlation of pre-leukapheresis peripheral CD34+ cell count and total number of CD34+ cell yield. The line represented fitted values from linear regression model and the shade area represents the 95% confidence interval.
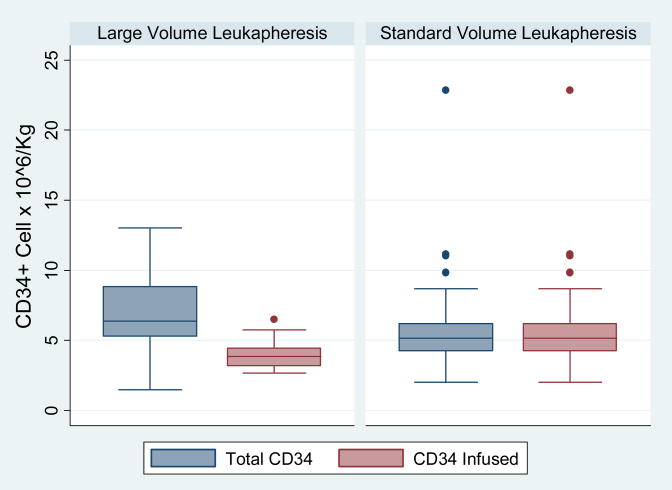
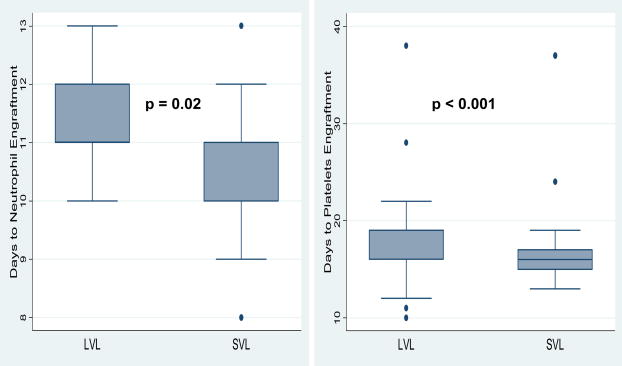
The median number of collections for both group was 3 (p = 0.7). The median HPC product volume per collection was 356ml for LVL group and this was significantly higher than the median HPC product volume per collection obtained from SVL group (median 149.5ml, p <0.001) (Table 2). The median total CD34+ cell yield/kg was 6.4 × 106 for LVL and 5.2 × 106 for SVL. This difference was statistically significant (p = 0.005) (Figure 2) even though the number of collections between the two groups was not significantly different. Because some patients in the LVL group only received half their cell numbers, the median CD34+ cell infused (3×106/kg) was significantly lower than the CD34+ cell/kg received by patients in SVL group. (5.2 ×106/kg p = <0.001). (Figure 2) As a result, patient in the LVL group had statistically significant higher number of days to neutrophil (p = <0.001) and platelet (p = 0.02) engraftments. However, since the difference represents only 1 – 2 day delay in engraftment, this might not be clinically significant. (Figure 3)
Figure 2.
Box plot the shows the distribution of total number of CD34+ cell for all collections and number of CD34+ cell infused between LVL and SVL groups. The upper border of the the box represents the 75 percentile and lower border represents the 25 percentile of the distribution. The median is represented by a line within the box. The whiskers of the plot represent the 99 percentile range and the solid dots represent the CD34+ cell dose that are beyond the 99 percentile of the distribution.
Figure 3.
Box plot demonstrating engraftment outcomes between LVL and SVL groups. LVL patients had statistically significant delayed neutrophil and platelet engraftment than SVL patients.
To estimate the cost savings of performing LVL collection compared to SVL collection, we performed a hypothetical cost-benefit analysis. We hypothetically assigned 100 patients in each group and all patients in both groups required enough cells for two transplants and this has to be achieved in 1 or 2 collection series. Since the median number of collections from our analysis was 3 for both groups, all patients were assumed to have 3 collections. Also all patients would have 7 filgrastim treatments; 4 treatments before collection and 3 treatments during collection days. From our data analysis we have determined the chance of a patient to have enough CD34+ cell for two transplants using SVL method was 29% while that of LVL was 69%. Using these assumptions, we have estimated the cost savings for using LVL instead of SVL is $7,497 per patient. A detail of the cost benefit analysis is shown in table 3.
Table 3.
Cost-effectiveness analysis for LVL compared to SVL method of hematopoietic stem cell collection. The assumption was that each patient would reach target cell dose in 1 or 2 series of collections. Each series would consist of 3 collections. For each collection series, 7 treatments of filgrastim would be administered. Cost of laboratory test may include complete blood count, ionized calcium, CD34+ cell enumeration, coagulation test etc. The patient cost may include loss wages, transportation, accommodation etc.
| Scenario | SVL | LVL |
|---|---|---|
| Number of patients | 100 | 100 |
| Probability of having enough CD34+ cells for two transplants ≥6 ×106/Kg) | 15/52 = 29% | 24/35 = 69% |
| Cost of 1st leukapheresis × 3 ($1300/SVL procedure and $1500/LVL procedure) | 3900 × 100 = $390,000 | 4500 × 100 = $450,000 |
| Cost of 2nd leukapheresis ×3 ($1300/SVL procedure and $1500/LVL procedure) | 3900 × 71 = $276,900 | 4500 × 31 = $139,200 |
| Cost of 1st set of filgrastim treatments ×7 ($1200/treatment) | 8400 × 100 = $840,000 | 8400 × 100 = $840,000 |
| Cost of 2nd set of filgrastim treatments ×7 ($1200/treatment) | 8400 × 71 = $596,400 | 8400 × 31 = $260,400 |
| Cost of 1st set of labs ($200 × 7) | 1400 × 100 = $140,000 | 1400 × 100 = $140,000 |
| Cost of 2nd set of labs | 1400 × 71 = $99,400 | 1400 × 31 = $43,400 |
| Patient cost 1st set of visits ×7 ($1000/visit) | 7000 × 100 = $700,000 | 7000 × 100 = $700,000 |
| Patient cost 2nd set of visits ×7 | 7000 × 71 = $497,000 | 7000 × 31 = $217,000 |
| Total cost | $3,539,700 | $2,790,000 |
| Total Savings | $749,700 | |
| Savings per patient | $7,497 | |
Discussion
PBSC collection by Leukapheresis has become a common practice and is mostly performed as outpatient procedure in most hospitals or community centers.
The goal of every PBSC leukapheresis is to reach target CD34+ cell dose with the smallest number of collections and with minimal or no donor inconvenience. We and others previously reported a statistically significant linear correlation between pre-leukapheresis peripheral blood CD34+ cell counts and cumulative number of CD34+ cell/Kg yield from all collections11–13.
Our SVL collections processed 12 – 16 liters, which was slightly higher than reported averages6,14. As a result, our median CD34+ cell/kg was slightly higher than the previously reported averages for SVL6,10. Recipients of SVL products had more CD34+ cells for one transplant. Consequently, neutrophil and platelet engraftments were faster in recipients of SVL than recipient of LVL products. When compared with recipients of SVL products, patients receiving LVL products had less CD34+ cells/Kg even though the total CD34+ cell yield was higher than SVL. This led to relatively slower neutrophil and platelet engraftment. Overall, more LVL collections resulted in two or more transplants than SVL PBSC collections.
Our study shows LVL PBSC collections from multiple myeloma patients yield more CD34+ cells/Kg without significant increase in the number of collections. This agrees with a previous study by Desikan et al that reported higher PBSC collection yield using LVL in patient with multiple myeloma in fewer than average collection days 15. However, in that report they defined LVL as processing of at least 3 volumes or 15 liter total blood volume, which means some of their collections would have been categorized as SVL based on our criteria. In addition, some of their patients were mobilized with both Cyclophosphamide and G-CSF from steady-state while our patients received this regimen only if they failed to mobilize with G-CSF alone. When compared to SVL collections, despite the differences between the two studies, LVL collections increase CD34+ cell yield with comparable or less number of collection days.
Contrary to previous report by Humpe et al16 and in agreement with Reik et al17, we did not observe significant increase in number of adverse events during LVL collections (data not shown). Common reported adverse event associated with LVL include prolonged partial thromboplastin time, significant drop in platelet count and electrolyte imbalance such as hypocalcemia and hypomagnesemia and metabolic alkalosis18. To prevent these adverse events some centers monitor ionized calcium and magnesium and pH before, during and after the procedure. In addition as was previously reported19–21, our LVL donors were given supplemental calcium and magnesium throughout the duration of the procedure. We reduce the amount of citrate used during the procedure by adding heparin. No heparin supplementation was given during SVL collections. None of our patients experienced heparin induced thrombocytopenia as a side effect of heparin use.
The old dogma that suggests the more cells infused in a given transplant, the better the transplant outcome may not currently be true. In the autologous transplants, there is a threshold of engraftment beyond which more cells does not significantly improve the engraftment rate.22,23 Therefore, infusing slightly less CD34+ cells in the LVL collection recipients may not be detrimental. LVL was rather beneficial since it allowed more patients to have double transplants, which had been shown to improved survival in multiple myeloma patients24.
Challenges arise when a cost-benefit analysis of LVL compared to SVL method is to be performed. It is not uncommon for patients with enough CD 34+ cells for two transplants to not get a second transplant due to a variety of reasons. The most significant are adverse events during the neutropenic period, and other medical conditions that are a consequence of the first transplant. Also only a small number of patients that failed to yield enough CD34+ cell/Kg for two transplants comes back for a second collection. The only option for some patients is allogeneic hematopoietic stem cell transplants.
Several significant morbidities are associated with allogeneic stem cell transplants. Most prominent is graft-versus-disease (GVHD) and it can lead to death25. The cost of managing GVHD significantly exceeds the cost of a second collection. Using a hypothetical scenario and standardized assumptions, we have shown that using LVL method to collect ≥6 × 106/Kg, the cost savings could be up to or greater than $7,597.
We therefore concluded that LVL collection for multiple myeloma patients can cost effectively and efficiently yield enough CD34+ cell/Kg that may allow two transplants.
Since the target CD34+ cell dose for a single transplant was 3.0 × 106/Kg, most patients that underwent LVL collection had enough CD34+ cells that could allow for two transplants. This was attainable without significantly increasing the number of collections. Therefore, more patients in the LVL group had enough cryopreserved cells for a potential 2nd transplant when compared to the patients in SVL group.
Acknowledgments
Supported by NIH grants CA102824
References
- 1.Anderson KC, Barut BA, Ritz J, Freedman AS, Nadler LM. Autologous bone marrow transplantation therapy for multiple myeloma. European Journal of Haematology Supplementum. 1989;51:157–163. doi: 10.1111/j.1600-0609.1989.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 2.Sirohi B, Powles R, Treleaven J, et al. The role of autologous transplantation in patients with multiple myeloma aged 65 years and over. Bone Marrow Transplantation. 2000;25:533–539. doi: 10.1038/sj.bmt.1702188. [DOI] [PubMed] [Google Scholar]
- 3.Tosi P, Zamagni E, Ronconi S, et al. Safety of autologous hematopoietic stem cell transplantation in patients with multiple myeloma and chronic renal failure. Leukemia. 2000;14:1310–1313. doi: 10.1038/sj.leu.2401819. [DOI] [PubMed] [Google Scholar]
- 4.Gahrton G, Bjorkstrand B. Progress in haematopoietic stem cell transplantation for multiple myeloma. SO - Journal of Internal Medicine. 2000 Sep;248(3):185–201. doi: 10.1046/j.1365-2796.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 5.Gianni AM. Where do we stand with respect to the use of peripheral blood progenitor cells? Ann Oncol. 1994;5:781–784. doi: 10.1093/oxfordjournals.annonc.a059003. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsen JF, Stamnesfet S, Liseth K, Hervig T, Bruserud O. Large-volume leukapheresis yields more viable CD34+ cells and colony-forming units than normal-volume leukapheresis, especially in patients who mobilize low numbers of CD34+ cells. Transfusion. 2005;45:248–253. doi: 10.1111/j.1537-2995.2004.04210.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 8.Sugrue MWWK, Pollock BH, Khan, Paracha S, Wingard JR, Moreb JS. Characterization and outcome of “Hard to Mobilize” lymphoma patients undergoing autologous stem cell transplantation. Leukemia and Lymphoma. 2001;39:509–519. doi: 10.3109/10428190009113381. [DOI] [PubMed] [Google Scholar]
- 9.Watts MJ, Ings SJ, Flynn M, Dodds D, Goldstone AH, Linch DC. Remobilization of patients who fail to achieve minimal progenitor thresholds at the first attempt is clinically worthwhile. Br J Haematol. 2000;111:287–291. doi: 10.1046/j.1365-2141.2000.02346.x. [DOI] [PubMed] [Google Scholar]
- 10.Sohn SK, Kim JG, Chae YS, et al. Large-volume leukapheresis using femoral venous access for harvesting peripheral blood stem cells with the Fenwal CS 3000 Plus from normal healthy donors: predictors of CD34+ cell yield and collection efficiency. J Clin Apher. 2003;18:10–15. doi: 10.1002/jca.10044. [DOI] [PubMed] [Google Scholar]
- 11.Zubair AC, Grant R, Wu W, et al. Platelet count is a sensitive predictor of autologous peripheral blood progenitor cell collection yield in previously treated plasma cell disease patients. Transfusion. 2008;48:1106–1114. doi: 10.1111/j.1537-2995.2008.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottler-Fox M, Lapidot T. Mobilizing the older patient with myeloma. Blood Rev. 2006;20:43–50. doi: 10.1016/j.blre.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Cottler-Fox MH, Lapidot T, Petit I, et al. Stem cell mobilization. Hematology. Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 14.Gidron A, Singh V, Egan K, Mehta J. Significance of low peripheral blood CD34+ cell numbers prior to leukapheresis: what should the threshold required for apheresis be? Bone Marrow Transplant. 2008;42:439–442. doi: 10.1038/bmt.2008.187. [DOI] [PubMed] [Google Scholar]
- 15.Desikan KR, Jagannath S, Siegel D, et al. Collection of more hematopoietic progenitor cells with large volume leukapheresis in patients with multiple myeloma. Leuk Lymphoma. 1998;28:501–508. doi: 10.3109/10428199809058357. [DOI] [PubMed] [Google Scholar]
- 16.Humpe A, Riggert J, Munzel U, Kohler M. A prospective, randomized, sequential crossover trial of large-volume versus normal-volume leukapheresis procedures: effects on serum electrolytes, platelet counts, and other coagulation measures. Transfusion. 2000;40:368–374. doi: 10.1046/j.1537-2995.2000.40030368.x. [DOI] [PubMed] [Google Scholar]
- 17.Reik RA, Noto TA, Fernandez HF. Safety of large-volume leukapheresis for collection of peripheral blood progenitor cells. J Clin Apher. 1997;12:10–13. doi: 10.1002/(sici)1098-1101(1997)12:1<10::aid-jca3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Mcleod B, editor. Apheresis: Principle and Practice. Bethesda, MD: AABB Press; 2003. Mobilization and Collection of Peripheral Blood Progenitor Cells. [Google Scholar]
- 19.Bolan CD, Leitman SF. Management of anticoagulation-associated toxicity during large-volume leukapheresis of peripheral blood stem cell donors. Blood. 2002;99:1878. doi: 10.1182/blood.v99.5.1878. [DOI] [PubMed] [Google Scholar]
- 20.Bolan CD, Carter CS, Wesley RA, et al. Prospective evaluation of cell kinetics, yields and donor experiences during a single large-volume apheresis versus two smaller volume consecutive day collections of allogeneic peripheral blood stem cells. Br J Haematol. 2003;120:801–807. doi: 10.1046/j.1365-2141.2003.04157.x. [DOI] [PubMed] [Google Scholar]
- 21.Bolan CD, Cecco SA, Wesley RA, et al. Controlled study of citrate effects and response to i.v. calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion. 2002;42:935–946. doi: 10.1046/j.1537-2995.2002.00151.x. [DOI] [PubMed] [Google Scholar]
- 22.Zubair A, Zahrieh D, Daley H, et al. Early neutrophil engraftment following autologous BMT provides a functional predictor of long-term hematopoietic reconstitution. Transfusion. 2003;43:614–621. doi: 10.1046/j.1537-2995.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 23.Zubair AC, Zahrieh D, Daley H, et al. Engraftment of autologous and allogeneic marrow HPCs after myeloablative therapy. Transfusion. 2004;44:253–261. doi: 10.1111/j.1537-2995.2004.00666.x. [DOI] [PubMed] [Google Scholar]
- 24.Attal MHJ, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 25.Kroger N, Perez-Simon JA, Myint H, et al. Relapse to prior autograft and chronic graft-versus-host disease are the strongest prognostic factors for outcome of melphalan/fludarabine-based dose-reduced allogeneic stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2004;10:698–708. doi: 10.1016/j.bbmt.2004.06.002. [DOI] [PubMed] [Google Scholar]