Abstract
Globalization has produced an increase in the number of people at risk for contracting parasitic infection. Central nervous system infection by nematodal parasites can be devastating. Early recognition and treatment of infection can significantly decrease morbidity of the parasitic infection, as well as the risk of secondary superinfection. The clinical presentation, diagnosis, and treatment for five of the more common nematodal infections of the nervous system—Angiostrongylus spp., Baylisacaris procyonis, Gnathostoma spinigerum, Strongyloides stercoralis, and Toxocara spp.—is reviewed.
Objectives
On completion of this article, the reader should be able to summarize the clinical presentation, diagnosis, and treatment of the common nematodal infections of the nervous system.
Accreditation
The Indiana University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit
The Indiana University School of Medicine designates this educational activity for a maximum of 1 Category 1 credit toward the AMA Physicians Recognition Award. Each physician should claim only those credits that he/she actually spent in the educational activity.
Disclosure
Statements of disclosure have been obtained regarding the authors’ relevant financial relationships. The authors have nothing to disclose.
Keywords: Parasite, nervous system, nematode
Nematodes, commonly known as “roundworms” because of their round cross section, comprise the second largest phylum in the animal kingdom. Nematodes can live freely but many parasitize humans, most often as accidental hosts. With increasing globalization and exotic travel, parasitic infection of the central nervous system (CNS), once considered a “tropical” infection, is becoming increasingly more prevalent in all parts of the world. In addition, immunosuppression increases susceptibility to opportunistic parasitic infection. Although infected individuals may remain asymptomatic for many years, a higher parasite burden is correlated with greater morbidity and mortality. The epidemiology, pathophysiology, clinical presentation, and recommended diagnostic evaluation and treatment for selected nematode infections are reviewed in this article (Table 1).
Table 1.
Imaging Findings of Selected Nematode Infections of the CNS
| Parasite | Type of Lesion | Location | Other Features |
|---|---|---|---|
| Angiostrongylus | High T1 signal in basal ganglia and/or cerebral peduncles | White matter, deep gray matter of cortex | Meningeal enhancement, edema; CT may show ring-enhancing lesions |
| Baylisascaris | Global atrophy, patchy regions of T2 hyperintensity | White matter of cerebral cortex and cerebellum | Ventriculomegaly, more common in children than adults |
| Gnathostomiasis | Small clusters of rounded hyperintensities on FLAIR and T2 | Near gray-white junction of cerebral cortex | Usually bilateral; CT may show intracerebral hemorrhage |
| Strongyloides | Global atrophy, periventricular white matter hyperintensities on T2 and FLAIR | White matter, spinal cord | Abscess may occur, usually unilateral; meningeal enhancement may be seen especially in spinal lesions; can superinfect tumors and other lesions |
| Toxocara | Single or multiple lesions, which are low-signal on T1, hyperintense on T2-weighted MR images, and homogeneously enhancing | Gray and white matter, cortical and subcortical; may occur in the spinal cord | Hypodense lesions on CT with variable enhancement patterns, may be mistaken for gliomas |
For additional information and references, please refer to text.
ANGIOSTRONGYLUS (ALICATA'S DISEASE)
Epidemiology
The earliest reported infection with Angiostrongylus cantonensis occurred in the rat population of Canton, China in 1933 and went virtually unnoticed until the first human case was reported in Taiwan in 1945.1 Human infection is caused by ingestion of infected aquatic or terrestrial snails (usually Lymnaea catascopium), of slime produced by an infected snail or slug, or of certain shrimp and freshwater fish.2 Infection with Angiostrongylus spp. is often asymptomatic and remains undetected for years, so recall of dietary history is often problematic. Abdominal disease due to A. costaricensis infection was recently reported in the Caribbean and Central America, but concomitant involvement of the CNS was not noted.3,4
Pathophysiology
Mature Angiostrongylus worms reside in the pulmonary arteries of rodents and are thus commonly called “rat lung worms.”5 After entry into a host rat, adult female parasites lay eggs in the pulmonary vasculature. The eggs then hatch into young larvae that migrate to the pharynx, where they are swallowed and eventually excreted in the feces. Freshwater scavengers, such as shrimp, snails, crabs, and some fish, are invaded by the larvae and harbor the developing larvae. Mucus produced by infected snails also can be infective. Once ingested by a human host, the larvae migrate to the lungs or brain and die. A. costaricensis, unlike A. cantonensis, usually remains in the intestines. Tissue damage is common during migration, in part due to the host's inflammatory response to proteins secreted by the worm. Host immune status does not appear to affect disease severity.6
Clinical Findings
Symptoms typically develop after an incubation period of up to 2 weeks. Although systemic infection is rare, acute abdominal discomfort similar to appendicitis has been reported.7 Most infections produce neurological symptoms, most often a persistent headache. Additional symptoms are common and depend upon the extent of invasion and host inflammatory response.2 There may be involvement of the cranial nerves, spinal cord, or the eye.8 Ocular involvement can occur with or without invasion of the CNS.9 Intraocular invasion can affect vision through destruction of tissue during migration or by causing retinal detachment. Although most infections present acutely, a chronic pain syndrome due to Angiostrongylus infection has also been reported.10 In addition, one-third of patients will develop hyperesthesia involving a limb or the trunk.11
Diagnosis
Angiostrongylus spp. is one of several parasites that causes eosinophilic meningitis.12 Cerebrospinal fluid (CSF) pleocytosis is common, with pronounced eosinophilia, increased protein concentration, and elevated opening pressure.13 Spinal fluid eosinophilia is usually present 2 to 4 weeks after symptoms develop, then wanes, returns again between weeks 6 and 8, then declines toward the end of the third month.11 Definitive diagnosis is achieved by detecting larvae in biopsy tissue, or, more rarely, in the CSF. The diagnosis is more often based on clinical findings and exposure history. Detection of anti–A. cantonensis antibodies is both sensitive and specific, with sensitivity higher in CSF than in serum.14,15
Neuroimaging
Computed tomography (CT) imaging may reveal hyper-intensities in the basal ganglia or contrast enhancement of the meninges.16 T1-weighted magnetic resonance imaging (MRI) postcontrast administration often demonstrates leptomeningeal enhancement and thickening, increased signal in the basal ganglia, as well as small hemorrhages seen with gradient imaging (Fig. 1).16 Chronic infection often produces a granulomatous lesion that can be mistaken for tuberculosis.
Figure 1.
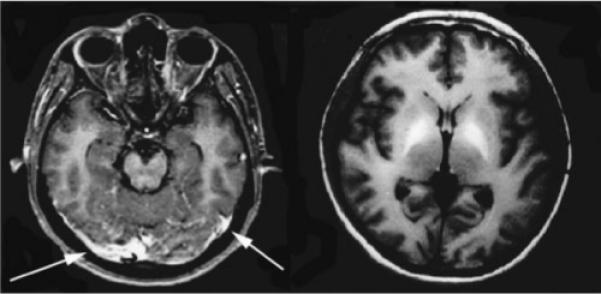
Patient with Angiostrongylus infection. Axial T1 contrast-enhanced images demonstrate meningeal enhancement (left, arrows) and markedly increased signal intensity within the globus pallidus (right). (Reprinted with permission from Tsai HC, Liu YC, Kunin CM, et al. Eosinophilic meningitis caused by Angiostrongylus cantonensis associated with eating raw snails: correlation of brain magnetic resonance imaging scans with clinical findings. Am J Trop Med Hyg 2003;68:281−285.)
Treatment
Treatment is supportive, with most infections being self-limited. Steroids and antiparasitic medications are ineffective. Older studies recommended periodic drainage of CSF to remove the nematode and any eggs that might be present; however, this therapy is no longer widely practiced.17 Recovery is usually complete, with children faring slightly better than adults.
BAYLISASCARIS
Epidemiology
The raccoon roundworm, Baylisacaris procyonis, is endemic in raccoon populations of North America and present in up to 80% of raccoons in the bicoastal and Midwestern United States.18 In a recent study of California communities reporting high densities of raccoons, 28 to 49% of surveyed properties had raccoon scat containing B. procyonis eggs.19 Only mild intestinal infection occurs in the raccoon, but parasites can reside within the small bowel of the raccoon for many years. Female adult procyonids produce millions of eggs per day, which are shed with the feces. These eggs are very resilient and can remain viable in the environment for years.20 Ingestion of eggs by species other than the raccoon results in extraintestinal migration of the larvae, with 5 to 7% of migration leading to the brain, causing “neural larval migrans.”21 Children with pica, developmental delay, or exposure to raccoons are at highest risk for contracting Baylisascaris infection and resultant CNS infection.22 Severity of human disease is directly proportional to the number of larvae migrating to the brain, thus to the number of eggs ingested.23
Pathophysiology
B. procyonis eggs are ingested by humans in the adult form. Larvae are infrequent in human infection, and unlike other parasites (such as Toxocara), the parasite grows as it migrates from the gastrointestinal tract to the CNS, with the adult size reaching up to 2 mm in length.24 During migration to the CNS, neurotoxins are secreted by the developing procyonid and contribute to the formation of eosinophilic granulomas.
Clinical Findings
B. procyonis infection has been associated with eosinophilic meningoencephalitis, cardiac pseudotumor, and retinitis.25 Children may develop retinitis, with or without concomitant encephalopathy.26 Although adults rarely develop clinical signs of infection, mild cases of ocular larva migrans have been reported.27 Children occasionally have a slowly progressive disease course, and the presence of a profound developmental delay without explanation should raise concern for this infection. Most infections are fatal or neurologically devastating.
Diagnosis
Definitive diagnosis is obtained through identification of B. procyonis larvae in a tissue sample.28 CSF and peripheral eosinophilia are sometimes present but nonspecific. Stool examination is not helpful because eggs and larvae are not shed in stool. Anti–B. procyonis antibodies in CSF and serum can be detected via enzyme-linked immunosorbent assay or indirect fluorescent antibody, but neither test is available commercially.18 Both tests are specific for procyonids and do not cross-react with other ascarids (e.g., Toxocara).
Neuroimaging
Although often initially normal, with progression of infection the MRI eventually reveals deep white matter abnormalities and global atrophy (Fig. 2).29 Head CT is usually normal.
Figure 2.
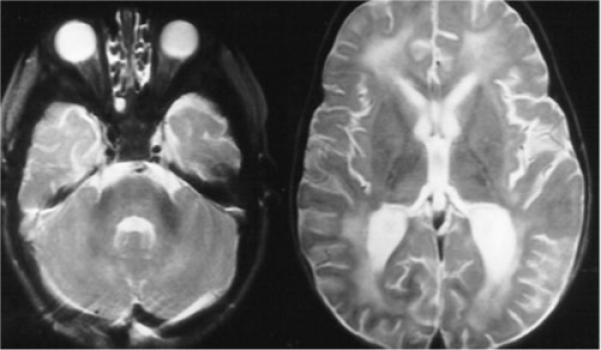
Thirteen month-old patient with Baylisascaris infection. Bilateral, patchy T2 hyperintensity is seen predominantly in the white matter, including the periventricular regions and corpus medullaris of the cerebellum. (Images courtesy of Dr. Howard Rowley.)
Treatment
Unfortunately, Baylisascaris infection does not respond to treatment with antihelminthics. Early diagnosis is uncommon and when infection does become clinically evident, the extent of neurological damage is usually severe and, for the most part, irreversible. Ivermectin can reduce CNS eosinophilia but does not alter disease course.30 Anti-inflammatory medications may be useful early in the course of disease but have not improved outcome. As treatment is largely ineffective, prevention is the best approach to reducing Baylisascaris infection.30 Raccoons should be discouraged from nesting or eating pet food around children's play areas or residential backyards. Toys exposed to animal excrement should be destroyed because Baylisascaris is resistant to formalin and other typical decontamination efforts.31
GNATHOSTOMIASIS
Epidemiology
The definitive hosts for Gnathostoma spp. infection include dogs, cats, lions, leopards, minks, and raccoons.32 Travel through or residence in areas of endemic G. spinigerum infection, such as Southeast Asia and South America, increases the risk of contracting infection. Certain regional specialties are more likely to contain the parasite, including dishes containing raw fish (ceviche) in Latin America, sashimi in Japan, or fresh water eel (sumfak) in Thailand.33,34 Food handlers in endemic regions are at especially high risk of contracting infection through skin penetration. The parasite is not killed by soaking in lime, even after 5 days. Effective methods for eradication of larva include boiling in water for 5 minutes and soaking in vinegar for at least 6 hours or in soy sauce for 12 hours.
Pathophysiology
Adult worms reside within the gastric wall of the definitive host and discharge large quantities of eggs into the stomach. Eggs are excreted with the feces and, if exposed to water, hatch within 7 days. The gnathostome larva matures within the intermediate host, a tiny crustacean of the genus Cyclops. Cyclops is then ingested by a variety of waterborne organisms, including fish, crawfish, and eels.35 Hearty gnathostomes can encyst within the host until conditions are appropriate for migration or reproduction. Human infection occurs by ingestion of infected water or animals or by introduction of the parasite through skin wounds. Adult gnathostomes reach 2 to 3 cm in length within definitive hosts but are often much smaller in accidental hosts, such as humans. In humans, larval forms do not migrate to the gastric wall as they do in other mammals but continue migrating through subcutaneous and visceral structures. The parasite can also enter the human host through skin wounds produced by rodents, fish, or cats. Once in subcutaneous tissue, the parasite usually migrates to the liver but can migrate to other areas.
Clinical Findings
Initial symptoms depend upon the mode of infection. If the organism was ingested, mild gastrointestinal distress usually occurs.36 When skin penetration is the cause of infection, a migrating cutaneous swelling will develop.37 Clinical symptoms are produced by parasite migration, host inflammatory response, and proteolytic and hemolytic toxins secreted by the gnathostome.38 The classic manifestation of CNS gnathostomiasis is radiculomyelitis, presenting as severe radicular pain and paresthesia.39 The pain usually lasts from 1 to 5 days and likely represents the time it takes for the organism to migrate from the nerve root to the CNS. Cranial nerves can also be affected but usually only later in the course of disease after the spinal cord has been traversed.39 Meningitis is uncommon, especially during early infection, but can occur in patients who are less severely affected or who have rapid rates of organism migration. Subarachnoid hemorrhage occurs in up to 25% of infected patients, and in Thailand, some experts suggest that almost one-quarter of hemorrhagic strokes may be caused by gnathostomiasis infection.40-42
Diagnosis
CSF examination often reveals a mild eosinophilic pleocytosis with xanthochromia or elevated red blood cell count.42 History of migratory subcutaneous swellings in a patient with eosinophilic meningoencephalitis or hemorrhagic stroke should suggest the diagnosis of gnathostomiasis. Extraction of the worm from infected tissue can provide the definitive diagnosis and treatment.28 Serological tests, such as immunoblot detection of the 24-kDa band, are both sensitive and specific.43
Neuroimaging
Neuroimaging often reveals intracranial hemorrhage or resultant obstructive hydrocephalus.40,41 Scattered foci of hyperintensity on T2 MRI are also described, usually bilaterally (Fig. 3). Contrast-enhanced imaging usually demonstrates meningeal involvement.
Figure 3.
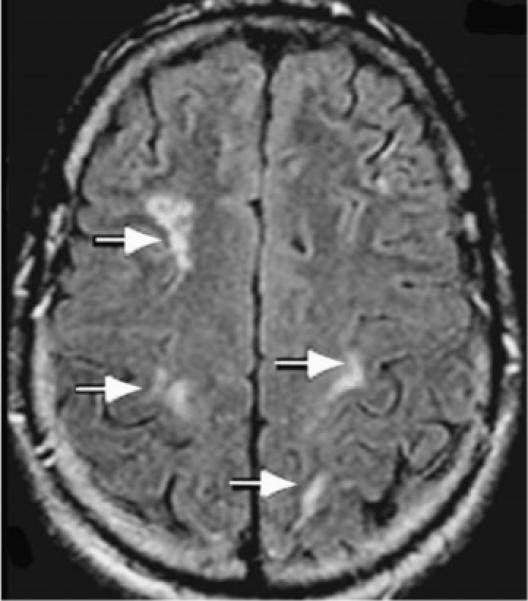
Patient with Gnathostoma infection. Axial FLAIR MRI of the brain showing bilateral clusters of small rounded hyperintensities in deep white matter (arrows). (Reprinted with permission from Hughes AJ, Biggs BA. Parasitic worms of the central nervous system: an Australian perspective. Intern Med J 2002;32:541−553.)
Treatment
Mebendazole and ivermectin are equally effective treatments for gnathostomiasis.44 Gnathostomiasis can persist for up to a decade and may require multiple treatments to achieve total eradication.36,45 Surgical resection should be performed if an organism is identified. Adjunctive steroids have been useful in some cases.41 An increase in serum antibodies suggests treatment failure.
STRONGYLOIDES
Epidemiology
Strongyloidiasis is a human intestinal infection most often caused by Strongyloides stercoralis. Other subspecies, such as S. fulleborni, although pathogenic in primates, typically cause only minor infections in humans. Historically, strongyloidiasis had been confined to tropical and subtropical regions, but infection is increasingly common in Europe and the United States.46 In the United States, cases are encountered primarily in tertiary medical centers, especially in the Mid-Atlantic region. In addition, with the advent of HIV infection and more frequent use of immunosuppressant medications, more people in the developed and developing world are at risk for contracting this infection and for developing hyperinfection.47,48
Pathophysiology
Strongyloides spp. are capable of living as parasites or free-living organisms. There are three developmental stages: filariform (infective), rhabditiform, and adult. Rhabditoid larvae can become filariform or differentiate into male or female and maintain the rhabditiform cycle indefinitely. Strongyloidiasis is most prevalent in areas of poor sanitation and is usually acquired through contact with the parasite in contaminated water or by direct penetration of the skin by the filariform larvae. In addition, host autoinfection can occur when the parasite completes its life cycle within the host. After entering a human host, the parasite enters the venous circulation, migrates through the lung alveoli, and eventually burrows into the small intestine, where it can reside for up to 50 years.49 From this site, worms can be released into the stool or develop into the filariform state and reinfect the host. Infection also facilitates coinfection with other agents, sometimes resulting in overwhelming bacteremia with dissemination to the CNS and other organs.47 Disseminated S. stercoralis infection is more likely to occur in the immunocompromised host.50 Massive worm burden, known as hyperinfection syndrome, may occur when the usual parasitic life cycle is accelerated.51 Hyperinfection is usually limited to the gastrointestinal tract or lungs and is rare in the CNS.
Clinical Findings
Infection may persist for many decades without producing symptoms. Acute disease is limited to the gastrointestinal tract and lungs. Patients often develop wheezing, diarrhea, and postprandial abdominal pain.52 Transient low-grade fever is common. Chronic disease develops over days to weeks and usually includes a dermatological manifestation called larva currens.53 The perianal region is the initial site of involvement, but most patients do not notice this early manifestation. The larvae migrate at a rate of up to 5 cm/d and travel subcutaneously or internally to other organs. Disseminated disease can produce infection in other organ systems, including the CNS.46,54 Alteration in mental status and meningismus are the most common manifestations of CNS involvement, but penetration of vessel walls can produce mycotic aneurysm and intracranial hemorrhage, even vasculitis.55 If bacterial hyperinfection develops, brain abscess, caused by Escherichia coli in ∼30% of cases, may produce focal neurological symptoms.56
Diagnosis
Serum eosinophilia is common during primary infection but wanes with dissemination of infection.28 Diagnosis can be confirmed by identification of Strongyloides spp. rhabditiform larvae in stool, serum, CSF, or peritoneal fluid. Larvae do not appear in the stool until approximately 1 month after initial infection. In patients with disseminated infection, larvae may also appear in the sputum. Larvae can be detected in duodenal secretions with the Entero-test (Hedero, Palo Alto, CA); a weighted gelatin capsule is swallowed, the gelatin dissolves allowing the string to pass into the duodenum and 4 hours later the string is removed and examined for larvae. Due to the low sensitivity of direct identification tests, testing of serial samples is recommended, especially for stool specimens. If strongyloidiasis is suspected but not detected by direct identification tests, antibody detection testing should be performed.57,58 Unfortunately, antibody detection tests cannot distinguish between past or present infection, can be negative in patients with disseminated infection, and may cross-react with other helminthic and filarial infections. Of the available antibody tests, enzyme immunoassay has the highest sensitivity (∼90%).58
Neuroimaging
In patients with chronic infection, neuroimaging is often nonspecific, but atrophy may be prominent (Fig. 4). Additional abnormalities include abscess formation or mycotic aneurysms, either of which may occur along any vascular distribution but usually spare the extracranial vascular system.59
Figure 4.
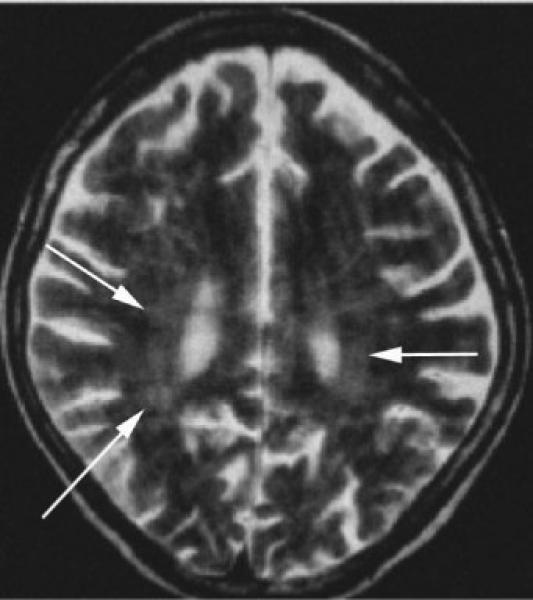
Thirty-five-year-old immunosuppressed patient with Strongyloides infection. Axial T2-weighted images reveal global atrophy and patchy periventricular white matter hyperintensities (arrows). (Reprinted with permission from Kothary NN, Muskie JM, Mathur SC. Strongyloides stercoralis hyperinfection. Radio-graphics 1999;19:1077−1081.)
Treatment
Ivermectin is the treatment of choice, but thiabendazole, albendazole, and mebendazole are also effective.60 Steroids should not be used during acute infection, as they may promote dissemination. Disseminated disease carries a mortality rate of almost 80%, so early detection and treatment is imperative.47,61
TOXOCARA
Epidemiology
Toxocariasis is endemic in all parts of the world.62 Most human Toxocara spp. infections are caused by T. canis, but T. cati, and T. leonina infections also occur. Recent studies suggest that living in a rural area, ownership of dogs, and dementia are associated with a higher risk for CNS T. canis infection.63 Children who eat earth (geophagia, pica) are also at higher risk of becoming infected. An infected dog or cat can excrete up to one million eggs each day, and eggs can survive in the environment for many years.64 Some experts have disputed the role of dogs as vectors of transmission, noting that up to half of patients do not own a pet and cannot recall any close animal contact.63 In northern industrialized countries, seroprevalence of this infection is 5% in urban adults and up to 40% in children and rural farmers. In the West Indies and Bali, seroprevalence rates approach 80%.65,66
Pathophysiology
Introduction into a human host is accidental and occurs by ingestion, most often on contaminated hands.67 Once in the human gastrointestinal tract, eggs remain in the small bowel for a short period, hatch into second-stage larvae, and then migrate to the liver. Larvae then enter the portal circulation and migrate through small-caliber vessels to the viscera, producing a mild inflammatory response along the migratory path.28 Chronic inflammation can eventually induce granuloma formation. Migration to the brain is uncommon but usually produces a more dramatic inflammatory response than migration through the periphery.68
Clinical Findings
Toxocariasis is almost always a benign self-limited disease, but ocular or cerebral involvement can cause significant morbidity, and infection in the elderly can be fatal.69 Symptoms depend on the disease burden and system infected, but weakness, lethargy, fever, and headache are common.68 Visceral larva migrans occurs mainly in children with disseminated infection and can produce granulomas in the liver, lungs, kidneys, heart, muscle, brain, or eye.70 Ocular involvement, known as ocular larva migrans, can produce symptoms of optic neuritis or blindness and occurs when the parasite migrates to the optic nerve head.71 Infection can also produce ocular findings similar to retinoblastoma; as retinoblastoma is treated by enucleation, toxocariasis should be excluded as an etiology before such treatment is considered.72 Although the majority of human Toxocara spp. infections are asymptomatic, subtle cognitive symptoms may not be appreciated or attributed to other behavioral conditions.73 Unlike other human nematode infections, cognition is affected during almost all chronic Toxocara spp. infections and can range from hyperactive behavior in children to dementia in elderly adults.73
Diagnosis
Toxocara spp. larvae are only rarely identified in clinical specimens. Because the parasite enters the human host as a mature adult, eggs are not isolated in stool.28 Detection of eggs from other organisms, such as Ascaris and Trichuris, suggests exposure to fecal material where Toxocara may also reside.74 Serum and CSF eosinophilia is a frequent finding, but treatment can reverse this abnormality, and chronic infections may have a blunted eosinophilic response.67,68 Antibody testing for second-stage T. canis excretory-secretory larval antigens (TESAg) is sensitive and specific for visceral larva migrans provided the serum is pretreated to remove cross-reacting antibodies to organisms such as Ascaris suum.75 TES-Ag testing can be performed on blood or CSF samples.
Neuroimaging
Contrast-enhanced head CT often demonstrates vasogenic edema and a heterogeneous enhancement pattern resembling malignant gliomas (Fig. 5; personal communication, Dr. Nezih Oktar). MRI imaging can reveal subcortical and white matter disease, resembling small vessel vasculitis. Imaging abnormalities suggestive of small infarctions on FLAIR and T2 sequences often demonstrate microhemorrhages on gradient-echo sequences.76
Figure 5.
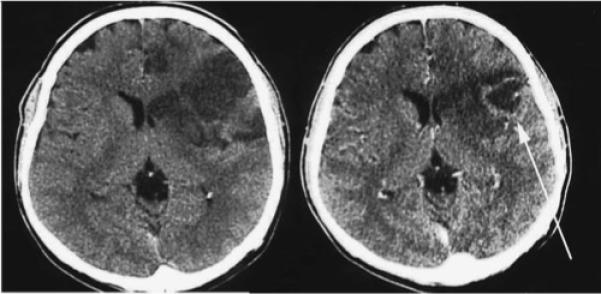
Patient with Toxocara infection. Noncontrast CT imaging demonstrates left frontal hypodensity consistent with edema (left), and contrast-enhanced imaging reveals a ring enhancing lesion (arrow on right). (Reprinted with permission from Oktar N, Barçin E, Kazandi A, Korkmaz M. Cerebral Toxocara mimicking a malignant glioma. Norol Bil D 2002;19:#12.)
Treatment
Although diethylcarbamazine is the treatment of choice, mebendazole and albendazole are also effective against toxocariasis.77 Suggested length of treatment is 3 to 4 weeks. In patients with ocular involvement, steroids should be administered and an ophthalmologist consulted.78
CONCLUSION
Nematodal infection of the CNS includes a large and diverse variety of parasites. Although many of the published cases are from tropical and subtropical countries, the incidence of many parasitic infections is increasing throughout the world, due to a combination of increased global travel and immunosuppression. In addition, some nematodal infections, such as Baylisacaris procyonis, have caused infections mainly within the United States. The indolent course of many of the nematodal infections make identification difficult, but prompt recognition and diagnosis of some of these infections can prevent additional morbidity and mortality.
Treatment for nematodal infection varies according to the organism (Table 2) but is often limited to management of symptoms. Once a nematodal infection has been identified, a tropical medicine expert should be consulted to assist with determining the appropriate treatment, as treatment regimens do change and newer investigative medications may be available. Several online references are useful for obtaining assistance with diagnosis and expert consultation:
The World Health Organization Fact Sheet, available at http://www.who.int/inf-fs/en/index.html
Centers for Disease Control, Division of Parasitic Diseases, available at http://www.dpd.cdc.gov/dpdx/
The Medical Letter, available at http://www.medletter.com/index.html
Table 2.
Treatment of Selected Nematode Infections of the CNS
| Parasite | Medication | Dosage | Precautions | Potential Side Effects |
|---|---|---|---|---|
| Angiostrongyliasis | Symptomatic care | |||
| Baylisascariasis | Symptomatic care | |||
| Gnathostomiasis | Albendazole | 400 mg PO bid for 21 days; steroid use controversial | Use of concurrent steroids or praziquantel may cause toxicity | Abdominal pain, jaundice, alopecia |
| Strongyloidiasis | Ivermectin; steroids may cause dissemination | 200 mcg/kg/d PO for 2 days; may repeat in 14 d | Avoid use in first term of pregnancy | Mild; generally very well tolerated |
| Toxocariasis | Mebendazole | 25 mg/kg/d PO single dose for 4 weeks | Caution in patients on anticonvulsants or medications metabolized by the p450 system | Jaundice, abdominal pain, headaches, alopecia |
For treatment options and further information, please consult The Medical Letter or contact the CDC.
ACKNOWLEDGMENTS
Supported by NIH Grants K23-AI01600 and University of Washington Center for AIDS Research (CFAR) Grant AI27757.
REFERENCES
- 1.Jindrak K. Angiostrongyliasis cantonensis (eosinophilic meningitis, Alicata's disease). Contemp Neurol Ser. 1975;12:133–164. [PubMed] [Google Scholar]
- 2.Chau TT, Thwaites GE, Chuong LV, et al. Headache and confusion: the dangers of a raw snail supper. Lancet. 2003;361:1866. doi: 10.1016/s0140-6736(03)13506-4. [DOI] [PubMed] [Google Scholar]
- 3.Andersen E, Gubler DJ, Sorensen K, et al. First report of Angiostrongylus cantonensis in Puerto Rico. Am J Trop Med Hyg. 1986;35:319–322. doi: 10.4269/ajtmh.1986.35.319. [DOI] [PubMed] [Google Scholar]
- 4.Slom TJ, Cortese MM, Gerber SI, et al. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med. 2002;346:668–675. doi: 10.1056/NEJMoa012462. [DOI] [PubMed] [Google Scholar]
- 5.Weir E. Travel warning: eosinophilic meningitis caused by rat lungworm. CMAJ. 2002;166:1184. [PMC free article] [PubMed] [Google Scholar]
- 6.Prociv P, Spratt DM, Carlisle MS. Neuro-angiostrongyliasis: unresolved issues. Int J Parasitol. 2000;30:1295–1303. doi: 10.1016/s0020-7519(00)00133-8. [DOI] [PubMed] [Google Scholar]
- 7.Hulbert TV, Larsen RA, Chandrasoma PT. Abdominal angiostrongyliasis mimicking acute appendicitis and Meckel's diverticulum: report of a case in the United States and review. Clin Infect Dis. 1992;14:836–840. doi: 10.1093/clinids/14.4.836. [DOI] [PubMed] [Google Scholar]
- 8.Patikulsila D, Ittipunkul N, Theerakittikul B. Intravitreal angiostrongyliasis: report of 2 cases. J Med Assoc Thai. 2003;86:981–985. [PubMed] [Google Scholar]
- 9.Alto W. Human infections with Angiostrongylus cantonensis. Pac Health Dialog. 2001;8:176–182. [PubMed] [Google Scholar]
- 10.Clouston PD, Corbett AJ, Pryor DS, Garrick R. Eosinophilic meningitis: cause of a chronic pain syndrome. J Neurol Neurosurg Psychiatry. 1990;53:778–781. doi: 10.1136/jnnp.53.9.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punyagupta S, Bunnag T, Juttijudata P, Rosen L. Eosinophilic meningitis in Thailand: epidemiologic studies of 484 typical cases and the etiologic role of Angiostrongylus cantonensis. Am J Trop Med Hyg. 1970;19:950–958. [PubMed] [Google Scholar]
- 12.Lo Re V, III, Gluckman SJ. Eosinophilic meningitis. Am J Med. 2003;114:217–223. doi: 10.1016/s0002-9343(02)01495-x. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Huang H, Dong Q, et al. A clinical study of eosinophilic meningoencephalitis caused by angiostrongyliasis. Chin Med J (Engl) 2002;115:1312–1315. [PubMed] [Google Scholar]
- 14.Chye SM, Yen CM, Chen ER. Detection of circulating antigen by monoclonal antibodies for immunodiagnosis of angiostrongyliasis. Am J Trop Med Hyg. 1997;56:408–412. doi: 10.4269/ajtmh.1997.56.408. [DOI] [PubMed] [Google Scholar]
- 15.Ellis-Pegler R, Parry G. Eosinophilic meningitis due to Angiostrongylus cantonensis. Clin Infect Dis. 2002;35:777–778. doi: 10.1086/342569. [DOI] [PubMed] [Google Scholar]
- 16.Tsai HC, Liu YC, Kunin CM, et al. Eosinophilic meningitis caused by Angiostrongylus cantonensis associated with eating raw snails: correlation of brain magnetic resonance imaging scans with clinical findings. Am J Trop Med Hyg. 2003;68:281–285. [PubMed] [Google Scholar]
- 17.Kuberski T, Wallace GD. Clinical manifestations of eosinophilic meningitis due to Angiostrongylus cantonensis. Neurology. 1979;29:1566–1570. doi: 10.1212/wnl.29.12.1566. [DOI] [PubMed] [Google Scholar]
- 18.Sorvillo F, Ash LR, Berlin OG, Morse SA. Baylisascaris procyonis: an emerging helminthic zoonosis. Emerg Infect Dis. 2002;8:355–359. doi: 10.3201/eid0804.010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roussere GP, Murray WJ, Raudenbush CB, et al. Raccoon roundworm eggs near homes and risk for larva migrans disease, California communities. Emerg Infect Dis. 2003;9:1516–1522. doi: 10.3201/eid0912.030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SY, Glaser C, Murray WJ, et al. Raccoon roundworm (Baylisascaris procyonis) encephalitis: case report and field investigation. Pediatrics. 2000;106:E56. doi: 10.1542/peds.106.4.e56. [DOI] [PubMed] [Google Scholar]
- 21.Kazacos KR. Raccoon ascarids as a cause of larva migrans. Parasitol Today. 1986;2:253–255. doi: 10.1016/0169-4758(86)90010-4. [DOI] [PubMed] [Google Scholar]
- 22.Gavin PJ, Shulman ST. Raccoon roundworm (Baylisascaris procyonis). Pediatr Infect Dis J. 2003;22:651–652. doi: 10.1097/01.inf.0000078168.78131.1e. [DOI] [PubMed] [Google Scholar]
- 23.Fox AS, Kazacos KR, Gould NS, et al. Fatal eosinophilic meningoencephalitis and visceral larva migrans caused by the raccoon ascarid Baylisascaris procyonis. N Engl J Med. 1985;312:1619–1623. doi: 10.1056/NEJM198506203122507. [DOI] [PubMed] [Google Scholar]
- 24.Kazacos KR, Boyce WM. Baylisascaris larva migrans. J Am Vet Med Assoc. 1989;195:894–903. [PubMed] [Google Scholar]
- 25.Boschetti A, Kasznica J. Visceral larva migrans induced eosinophilic cardiac pseudotumor: a cause of sudden death in a child. J Forensic Sci. 1995;40:1097–1099. [PubMed] [Google Scholar]
- 26.Huff DS, Neafie RC, Binder MJ, et al. Case 4: the first fatal Baylisascaris infection in humans—an infant with eosinophilic meningoencephalitis. Pediatr Pathol. 1984;2:345–352. doi: 10.3109/15513818409022268. [DOI] [PubMed] [Google Scholar]
- 27.Mets MB, Noble AG, Basti S, et al. Eye findings of diffuse unilateral subacute neuroretinitis and multiple choroidal infiltrates associated with neural larva migrans due to Baylisascaris procyonis. Am J Ophthalmol. 2003;135:888–890. doi: 10.1016/s0002-9394(02)01539-8. [DOI] [PubMed] [Google Scholar]
- 28.CDC . DPDx Laboratory diagnosis of parasites of public health concern. Vol. 2003. Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 29.Rowley HA, Uht RM, Kazacos KR, et al. Radiologicpathologic findings in raccoon roundworm (Baylisascaris procyonis) encephalitis. AJNR Am J Neuroradiol. 2000;21:415–420. [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham CK, Kazacos KR, McMillan JA, et al. Diagnosis and management of Baylisascaris procyonis infection in an infant with nonfatal meningoencephalitis. Clin Infect Dis. 1994;18:868–872. doi: 10.1093/clinids/18.6.868. [DOI] [PubMed] [Google Scholar]
- 31.Ching HL, Leighton BJ, Stephen C. Intestinal parasites of raccoons (Procyon lotor) from southwest British Columbia. Can J Vet Res. 2000;64:107–111. [PMC free article] [PubMed] [Google Scholar]
- 32.Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33–50. doi: 10.1093/clinids/16.1.33. [DOI] [PubMed] [Google Scholar]
- 33.Ogata K, Nawa Y, Akahane H, et al. Short report: gnathostomiasis in Mexico. Am J Trop Med Hyg. 1998;58:316–318. doi: 10.4269/ajtmh.1998.58.316. [DOI] [PubMed] [Google Scholar]
- 34.Sugaroon S, Wiwanitkit V. Gnathostoma infective stage larvae in swamp eels (Fluta alba) at a metropolitan market in Bangkok, Thailand. Ann Clin Lab Sci. 2003;33:94–96. [PubMed] [Google Scholar]
- 35.Chen QQ, Lin XM. A survey of epidemiology of Gnathostoma hispidum and experimental studies of its larvae in animals. Southeast Asian J Trop Med Public Health. 1991;22:611–617. [PubMed] [Google Scholar]
- 36.Moore DA, McCroddan J, Dekumyoy P, Chiodini PL. Gnathostomiasis: an emerging imported disease. Emerg Infect Dis. 2003;9:647–650. doi: 10.3201/eid0906.020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley JJ, Kim YH. Cutaneous gnathostomiasis. J Am Acad Dermatol. 1995;33:825–828. doi: 10.1016/0190-9622(95)91841-8. [DOI] [PubMed] [Google Scholar]
- 38.Saksirisampant W, Chawengkiattikul R, Kraivichain K, Nuchprayoon S. Specific IgE antibody responses to somatic and excretory-secretory antigens of third stage G. spinigerum larvae in human gnathostomiasis. J Med Assoc Thai. 2001;84(suppl 1):S173–S181. [PubMed] [Google Scholar]
- 39.Boongird P, Phuapradit P, Siridej N, et al. Neurological manifestations of gnathostomiasis. J Neurol Sci. 1977;31:279–291. doi: 10.1016/0022-510x(77)90113-7. [DOI] [PubMed] [Google Scholar]
- 40.Brant-Zawadzki M, Wofsy CB, Schechter G. CT-evidence of subarachnoid hemorrhage due to presumed gnathostomiasis. West J Med. 1982;137:65–67. [PMC free article] [PubMed] [Google Scholar]
- 41.Germann R, Schachtele M, Nessler G, et al. Cerebral gnathostomiasis as a cause of an extended intracranial bleeding. Klin Padiatr. 2003;215:223–225. doi: 10.1055/s-2003-41401. [DOI] [PubMed] [Google Scholar]
- 42.Punyagupta S, Bunnag T, Juttijudata P. Eosinophilic meningitis in Thailand: clinical and epidemiological characteristics of 162 patients with myeloencephalitis probably caused by Gnathostoma spinigerum. J Neurol Sci. 1990;96:241–256. doi: 10.1016/0022-510x(90)90136-b. [DOI] [PubMed] [Google Scholar]
- 43.Nopparatana C, Setasuban P, Chaicumpa W, Tapchaisri P. Purification of Gnathostoma spinigerum specific antigen and immunodiagnosis of human gnathostomiasis. Int J Parasitol. 1991;21:677–687. doi: 10.1016/0020-7519(91)90079-m. [DOI] [PubMed] [Google Scholar]
- 44.Nontasut P, Bussaratid V, Chullawichit S, et al. Comparison of ivermectin and albendazole treatment for gnathostomiasis. Southeast Asian J Trop Med Public Health. 2000;31:374–377. [PubMed] [Google Scholar]
- 45.Parola P. Gnathostomiasis. Lancet. 2001;358:332. doi: 10.1016/S0140-6736(01)05508-8. [DOI] [PubMed] [Google Scholar]
- 46.Morgello S, Soifer FM, Lin CS, Wolfe DE. Central nervous system Strongyloides stercoralis in acquired immunodeficiency syndrome: a report of two cases and review of the literature. Acta Neuropathol (Berl) 1993;86:285–288. doi: 10.1007/BF00304143. [DOI] [PubMed] [Google Scholar]
- 47.Simpson WG, Gerhardstein DC, Thompson JR. Disseminated Strongyloides stercoralis infection. South Med J. 1993;86:821–825. doi: 10.1097/00007611-199307000-00022. [DOI] [PubMed] [Google Scholar]
- 48.McLarnon M, Ma P. Brain stem glioma complicated by Strongyloides stercoralis. Ann Clin Lab Sci. 1981;11:546–549. [PubMed] [Google Scholar]
- 49.Thompson JR, Berger R. Fatal adult respiratory distress syndrome following successful treatment of pulmonary strongyloidiasis. Chest. 1991;99:772–774. doi: 10.1378/chest.99.3.772. [DOI] [PubMed] [Google Scholar]
- 50.Gompels MM, Todd J, Peters BS, et al. Disseminated strongyloidiasis in AIDS: uncommon but important. AIDS. 1991;5:329–332. doi: 10.1097/00002030-199103000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Kothary NN, Muskie JM, Mathur SC. Strongyloides stercoralis hyperinfection. Radiographics. 1999;19:1077–1081. doi: 10.1148/radiographics.19.4.g99jl171077. [DOI] [PubMed] [Google Scholar]
- 52.Robinson J, Ahmed Z, Siddiqui A, et al. A patient with persistent wheezing, sinusitis, elevated IgE, and eosinophilia. Ann Allergy Asthma Immunol. 1999;82:144–149. doi: 10.1016/S1081-1206(10)62588-4. [DOI] [PubMed] [Google Scholar]
- 53.Arthur RP, Shelley WB. Larva currens: a distinctive variant of cutaneous larva migrans due to Strongyloides stercoralis. AMA Arch Derm. 1958;78:186–190. doi: 10.1001/archderm.1958.01560080044007. [DOI] [PubMed] [Google Scholar]
- 54.Igra-Siegman Y, Kapila R, Sen P, et al. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis. 1981;3:397–407. doi: 10.1093/clinids/3.3.397. [DOI] [PubMed] [Google Scholar]
- 55.Wachter RM, Burke AM, MacGregor RR. Strongyloides stercoralis hyperinfection masquerading as cerebral vasculitis. Arch Neurol. 1984;41:1213–1216. doi: 10.1001/archneur.1984.04050220115030. [DOI] [PubMed] [Google Scholar]
- 56.Smallman LA, Young JA, Shortland-Webb WR, et al. Strongyloides stercoralis hyperinfestation syndrome with Escherichia coli meningitis: report of two cases. J Clin Pathol. 1986;39:366–370. doi: 10.1136/jcp.39.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva LP, Barcelos IS, Passos-Lima AB, et al. Western blotting using Strongyloides ratti antigen for the detection of IgG antibodies as confirmatory test in human strongyloidiasis. Mem Inst Oswaldo Cruz. 2003;98:687–691. doi: 10.1590/s0074-02762003000500017. [DOI] [PubMed] [Google Scholar]
- 58.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 59.Masdeu JC, Tantulavanich S, Gorelick PP, et al. Brain abscess caused by Strongyloides stercoralis. Arch Neurol. 1982;39:62–63. doi: 10.1001/archneur.1982.00510130064019. [DOI] [PubMed] [Google Scholar]
- 60.Gann PH, Neva FA, Gam AA. A randomized trial of single- and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. J Infect Dis. 1994;169:1076–1079. doi: 10.1093/infdis/169.5.1076. [DOI] [PubMed] [Google Scholar]
- 61.Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11:755–767. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 62.Magnaval JF, Galindo V, Glickman LT, Clanet M. Human Toxocara infection of the central nervous system and neurological disorders: a case-control study. Parasitology. 1997;115:537–543. doi: 10.1017/s0031182097001558. [DOI] [PubMed] [Google Scholar]
- 63.Overgaauw PA. Aspects of Toxocara epidemiology: human toxocarosis. Crit Rev Microbiol. 1997;23:215–231. doi: 10.3109/10408419709115137. [DOI] [PubMed] [Google Scholar]
- 64.Wolfe A, Wright IP. Human toxocariasis and direct contact with dogs. Vet Rec. 2003;152:419–422. doi: 10.1136/vr.152.14.419. [DOI] [PubMed] [Google Scholar]
- 65.Chomel BB, Kasten R, Adams C, et al. Serosurvey of some major zoonotic infections in children and teenagers in Bali, Indonesia. Southeast Asian J Trop Med Public Health. 1993;24:321–326. [PubMed] [Google Scholar]
- 66.Bundy DA, Thompson DE, Robertson BD, Cooper ES. Age-relationships of Toxocara canis seropositivity and geohelminth infection prevalence in two communities in St. Lucia, West Indies. Trop Med Parasitol. 1987;38:309–312. [PubMed] [Google Scholar]
- 67.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Del Prete G, Ricci M, Romagnani S. T cells, IgE antibodies, cytokines and allergic inflammation. Allerg Immunol (Paris) 1991;23:239–243. [PubMed] [Google Scholar]
- 69.Pawlowski Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol. 2001;75:299–305. doi: 10.1017/s0022149x01000464. [DOI] [PubMed] [Google Scholar]
- 70.Schochet SS. Human Toxocara canis encephalopathy in a case of visceral larva migrans. Neurology. 1967;17:227–229. doi: 10.1212/wnl.17.3.227. [DOI] [PubMed] [Google Scholar]
- 71.Komiyama A, Hasegawa O, Nakamura S, et al. Optic neuritis in cerebral toxocariasis. J Neurol Neurosurg Psychiatry. 1995;59:197–198. doi: 10.1136/jnnp.59.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shields JA, Parsons HM, Shields CL, Shah P. Lesions simulating retinoblastoma. J Pediatr Ophthalmol Strabismus. 1991;28:338–340. doi: 10.3928/0191-3913-19911101-12. [DOI] [PubMed] [Google Scholar]
- 73.Hill IR, Denham DA, Scholtz CL. Toxocara canis larvae in the brain of a British child. Trans R Soc Trop Med Hyg. 1985;79:351–354. doi: 10.1016/0035-9203(85)90378-5. [DOI] [PubMed] [Google Scholar]
- 74.Boes J, Helwigh AB. Animal models of intestinal nematode infections of humans. Parasitology. 2000;121(suppl):S97–S111. doi: 10.1017/s003118200000648x. [DOI] [PubMed] [Google Scholar]
- 75.Magnaval JF, Fabre R, Maurieres P, et al. Evaluation of an immunoenzymatic assay detecting specific anti-Toxocara immunoglobulin E for diagnosis and post treatment follow-up of human toxocariasis. J Clin Microbiol. 1992;30:2269–2274. doi: 10.1128/jcm.30.9.2269-2274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xinou E, Lefkopoulos A, Gelagoti M, et al. CT and MR imaging findings in cerebral toxocaral disease. AJNR Am J Neuroradiol. 2003;24:714–718. [PMC free article] [PubMed] [Google Scholar]
- 77.Magnaval JF. Comparative efficacy of diethylcarbamazine and mebendazole for the treatment of human toxocariasis. Parasitology. 1995;110:529–533. doi: 10.1017/s0031182000065240. [DOI] [PubMed] [Google Scholar]
- 78.Taylor MR. The epidemiology of ocular toxocariasis. J Helminthol. 2001;75:109–118. [PubMed] [Google Scholar]


