Abstract
T cells infiltrate the kidney in both human and experimental glomerulonephritis, and several lines of evidence indicate that T cell-mediated tissue damage plays an important role in the immunopathogenesis of renal inflammatory diseases. However, the functions of the different T cell subsets, particularly the recently identified interleukin-17 (IL-17)-producing T cells (Th17 cells), are incompletely understood in glomerulonephritis. Here, we identified renal IL-17-producing T cells in the T cell-mediated model of nephrotoxic nephritis in mice. In vitro, IL-17 enhanced the production of the proinflammatory chemokines CCL2/MCP-1, CCL3/MIP-1α, and CCL20/LARC, which are implicated in the recruitment of T cells and monocytes, in mouse mesangial cells. To determine the function of Th17 cells in renal inflammation, we induced nephrotoxic nephritis in IL-23 p19−/− mice, which have reduced numbers of Th17 cells, and in IL-17−/− mice, which are deficient in the effector cytokine IL-17 itself. In comparison with nephritic wild-type mice, IL-23 p19−/− mice demonstrated less infiltration of Th17 cells, and both IL-23 p19−/− and IL-17−/− mice developed less severe nephritis as measured by renal function, albuminuria, and frequency of glomerular crescent formation. These results demonstrate that the IL-23/IL-17 pathway significantly contributes to renal tissue injury in experimental glomerulonephritis. Targeting the IL-23/Th17 axis may be a promising therapeutic strategy for the treatment of proliferative and crescentic glomerulonephritis.
The recruitment of T cells into the kidney is a hallmark of human and experimental glomerulonephritis and is closely correlated with renal function. Infiltrating T cells can induce tissue injury, either directly by cytotoxic functions and cytokine secretion or indirectly by activating macrophages.1,2 In particular, infiltrating effector T cells of the Th1 type are supposed to initiate and perpetuate renal tissue damage in crescentic and proliferative forms of glomerulonephritis, which eventually leads to progressive loss of renal function.3 This pathophysiological concept is mainly based on findings from experimental rodent models of glomerulonephritis. Renal T cell and monocyte recruitment and subsequent tissue damage are attenuated in mice with genetic deletion of Th1 cytokines such as IL-12 p404 and IFNγ5 and by blocking of Th1 cytokines with inhibitory antibodies including anti-IL-12 p40(p35)6 and anti-IFNγ.7 Furthermore, exogenous administration of IL-12 augments Th1 responses and crescentic glomerulonephritis.8 In contrast, mice genetically deficient in the Th2 cytokines IL-49 or IL-1010 have more pronounced Th1 responses and develop more severe glomerulonephritis. Consistent with this, elevated serum levels of IL-10 ameliorate acute11 and chronic12 renal inflammation.
Recently, the Th1/Th2 paradigm has been challenged by identification of a third IL-17-producing CD4+ effector T cell subset termed Th17.13 Th17 cells not only differ from Th1 and Th2 cells by their cytokine expression profile but also by the cytokines that drive their differentiation. The combination of IL-6 plus TGFβ (plus IL-1) and subsequent activation of the transcription factor RORγt have recently been described to be essential for the initial differentiation of Th17 cells in mice.14 IL-23, a member of the IL-12 family, is dispensable for differentiation but important for Th17-cell expansion and survival.15 Interestingly, IL-23 is a heterodimer consisting of a unique p19 subunit and a p40 subunit that is also part of the Th1 cytokine IL-12. Therefore, the beneficial effects of p40 deficiency on experimental glomerulonephritis, which have been attributed to a lack of the IL-12/Th1 pathway, might at least in part be due to the previously unrecognized blockade of the IL-23/Th17 pathway.
The potential function of Th17 cells in autoimmune disease was first shown in IL-23 p19 gene-deficient mice. IL-23 p19−/− knockout animals had an unaltered capacity to produce the Th1 cytokine IFNγ but demonstrated a substantial decrease in Th17-polarized cells. Most importantly, these mice were resistant to the development of experimental autoimmune encephalomyelitis,16 collagen-induced arthritis,17 experimental induction of multiple sclerosis, and rheumatoid arthritis. Taken together, these lines of evidence support the conclusion that Th17 cells represent a unique T cell type that plays a central role in inflammatory and autoimmune reactions.
The function of Th17 cells in glomerulonephritis has not yet been determined. This study was therefore performed to define the roles of Th17 cells and IL-23 in glomerulonephritis. We induced nephrotoxic serum nephritis (NTN) in C57BL/6 wild-type, C57BL/6 IL-23 p19−/−, and C57BL/6 IL-17−/− mice to address two major issues: (1) Are IL-17-producing effector T cells detectable in the kidneys of nephritic mice? (2) What is the potential impact of Th17 cells on the clinical course of experimental glomerulonephritis?
RESULTS
Detection of Renal Th17 Cells in Experimental Glomerulonephritis
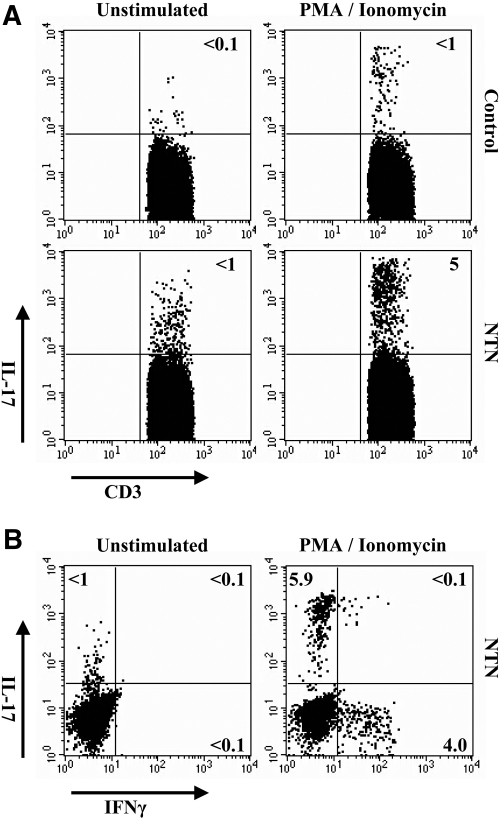
In a first step, we analyzed whether Th17 cells are detectable in the kidneys of nephritic mice. Nephrotoxic serum nephritis in C57BL/6 wild-type mice was induced by intraperitoneal injection of nephrotoxic sheep serum. On day 10 after induction of nephritis, when histologic and functional signs of kidney damage were evident, renal T cells were isolated and stimulated with PMA/ionomycin for 5 h. Intracellular cytokine staining with subsequent FACS analysis revealed that 3 to 7% of the infiltrating CD3+ T cells from kidneys of nephritic animals produced IL-17, whereas less than 1% were IL-17+ in control mice (Figure 1A).
Figure 1.
Detection of renal Th17 cells in experimental glomerulonephritis. (A) Representative intracellular cytokine staining assessed by flow cytometry for production of IL-17 in renal T cells 10 d after induction of nephritis. T cells were isolated from the kidneys of nephritic mice (NTN) or controls and cultured with or without PMA (5 ng/ml)/ionomycin (1 μg/ml) for 5 h. Dot plots show intracellular IL-17 staining of CD3+ T cells. (B) Intracellular IL-17/IFNγ double staining of renal T cells from nephritic mice. Results are representative of three independent experiments.
Interestingly, combined intracellular staining with IL-17 and IFNγ demonstrated that no double-positive IL-17- and IFNγ-producing renal T cells were detectable in the kidneys of nephritic mice, underscoring the dichotomy of these T cell subtypes (Figure 1B).
IL-17 Induces Proinflammatory Chemokine Expression in Mouse Mesangial Cells
To study the potential role of IL-17 in glomerular inflammation, we analyzed its regulatory effect on the expression of the chemokines CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, and CCL20/LARC in mouse mesangial cells (mMCs).
The biological effects of IL-17 are mediated via activation of IL-17 receptors A and C (IL-17RA and IL-17RC).18 IL-17RA and IL-17RC mRNA expression was detected in mMCs by real-time PCR (RT-PCR), identifying them as a putative target for IL-17 in the kidney (Figure 2A).
Figure 2.
Effects of IL-17 on chemokine expression in mMCs. (A) Detection of IL-17RA and IL-17RC mRNA expression in mouse mMCs by RT-PCR. (B) mMCs were incubated with IL-17 (10 ng/ml) in the absence or presence of TNFα (10 ng/ml) for 4 h. The mRNA chemokine expression was assessed by RT-PCR. Data are expressed as x-fold of basal mRNA expression (n = 6). (C) Chemokine protein production was measured by specific ELISA in the supernatants after 24 h of stimulation (n = 4). Bars represent means ± SD (*P < 0.05, **P < 0.01).
Next, mMCs were incubated with IL-17, either with or without TNFα, for 4 h to test potential synergistic effects. RT-PCR analysis revealed that renal mRNA expression of CCL2/MCP-1 (2.9-fold), CCL3/MIP1α (4.5-fold), and CCL20/LARC (20-fold) was induced by IL-17 (each P < 0.05 compared with unstimulated cells), whereas CCL5/RANTES expression (1.3-fold) was not affected by IL-17.
TNFα-mediated upregulation of CCL3/MIP1α expression (ΤΝFα, 2.7-fold; TNFα + IL-17, 24.2-fold; P < 0.05) and CCL20/LARC expression (ΤΝFα, 410-fold; TNFα + IL-17, 2006-fold; P < 0.01; Figure 2B) were synergistically increased by IL-17. In contrast, TNFα-induced mRNA expression of CCL2/MCP-1 and CCL5/RANTES was not further increased by application of IL-17 (Figure 2B).
In a second step, protein production of CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, and CCL20/LARC was analyzed by ELISA using supernatants from mMCs stimulated with IL-17 for 24 h in the absence or presence of TNFα (Figure 2C). In line with the results from RT-PCR analysis, addition of IL-17 alone significantly induced secretion of CCL2/MCP-1 (basal, 1294 ± 102 pg/ml; IL-17, 1646 ± 100 pg/ml; P < 0.05) and CCL20/LARC (basal, 0.84 ± 0.82 pg/ml; IL-17, 7.45 ± 1.55 pg/ml; P < 0.05). CCL5/RANTES protein secretion was stimulated to a lesser extent by IL-17 (basal, 5414 ± 293 pg/ml; IL-17, 6230 ± 398 pg/ml; P < 0.05), whereas CCL3/MIP1α production was only marginally induced by IL-17 (basal, not detectable; IL-17, 0.70 ± 0.32 pg/ml). The combination of IL-17 and TNFα synergistically amplified the protein secretion of CCL2/MCP-1 (TNFα, 1693 ± 36 pg/ml; TNFα + IL-17, 1858 ± 59 pg/ml; P < 0.05), CCL3/MIP-1α (TNFα, not detectable; TNFα + IL-17, 47.17 ± 15.42 pg/ml; P < 0.01), and CCL20/LARC (TNFα, 11.5 ± 0.91 pg/ml; TNFα + IL-17, 34.15 ± 5.66 pg/ml; P < 0.05). CCL5/RANTES protein secretion, in contrast, was not further increased by IL-17 (TNFα, 8011 ± 177 pg/ml; TNFα + IL-17, 7914 ± 183 pg/ml).
Experimental Glomerulonephritis in IL-23 p19−/− Mice
To test whether Th17 cells contribute to T cell-mediated tissue damage in experimental glomerulonephritis, we induced nephrotoxic nephritis in C57BL/6 wild-type and C57BL/6 IL-23 p19−/− mice. IL-23 p19−/− mice have reduced numbers of Th17 cells.17 Specific glomerular binding and deposition patterns of the nephrotoxic sheep antibody did not differ between C57BL/6 wild-type and IL-23 p19−/− mice (data not shown).
Examination of periodic acid–Schiff (PAS)-stained kidney sections of nephritic wild-type mice at day 10 showed severe focal glomerular and tubular damage with destruction of regular tissue structures. Glomerular changes included hypercellularity and formation of cellular crescents, capillary aneurysms, and intraglomerular deposition of PAS-positive material (Figure 3A). In addition to massive leukocyte infiltrates, the tubulointerstitial compartment showed tubular dilation, necrosis and atrophy, and protein casts and tubular protein reuptake due to proteinuria. Glomerular and tubulointerstitial tissue damage was less severe in nephritic IL-23 p19−/− mice as shown by representative PAS staining (Figure 3A).
Figure 3.
Attenuated glomerulonephritis in IL-23 p19−/− mice. (A) Representative photographs of PAS-stained kidney sections of control, nephritic wild-type, and nephritic IL-23 p19−/− mice at day 10 (original magnification, 400×). (B) Nephritic IL-23 p19−/− mice (n = 13) developed less renal tissue injury than nephritic wild-type mice (n = 14) in terms of glomerular crescent formation, glomerular sclerosis, and tubulointerstitial tissue damage. (C) Renal dysfunction was assessed by determination of the serum BUN level and albumin-to-creatinine ratio in non-nephritic control (n = 7 to 11), nephritic wild-type (n = 11 to 13), and nephritic IL-23 p19−/−mice (n = 9 to 13) at day 10. Symbols represent individual data points, and the horizontal lines indicate mean values (*P < 0.05, **P < 0.01).
To quantify renal tissue damage, PAS-stained kidney sections were evaluated for the presence of crescents, glomerular sclerosis, and tubulointerstitial injury (Figure 3B). The frequency of glomerular crescents at day 10 was significantly decreased in nephritic IL-23 p19−/− mice compared with nephritic wild-type mice (nephritic WT, 29.1 ± 9.7%; nephritic IL-23 p19−/−, 14.6 ± 14.5%; P < 0.01). Furthermore, nephritic kidneys of IL-23 p19−/− mice showed a significantly lower glomerulosclerosis score (nephritic WT, 31.4 ± 14.5; nephritic IL-23 p19−/−, 16.8 ± 16.7; P < 0.05) and reduced tubulointerstitial injury as indicated by a significant decrease in the interstitial area (nephritic WT, 22.3 ± 6.5; nephritic IL-23 p19−/−, 15.2 ± 6.6; P < 0.01).
IL-23 p19 Deficiency Ameliorates Renal Dysfunction in Nephrotoxic Nephritis
Ten days after induction of NTN, mice were euthanized for assessment of renal function (Figure 3C). The blood urea nitrogen levels (BUN) of nephritic wild-type mice were elevated compared with non-nephritic controls (nephritic WT, 61.8 ± 15.4 mg/dl; non-nephritic controls, 41.6 ± 9.2 mg/dl; P < 0.01). BUN levels in nephritic IL-23 p19−/− mice showed a nonsignificant tendency to decrease compared with nephritic wild-type mice (nephritic WT, 61.8 ± 15.4 mg/dl; nephritic IL-23 p19−/−, 50.1 ± 7.9 mg/dl; P > 0.05 NS). Nephritic wild-type and nephritic IL-23 p19−/− mice showed markedly increased albuminuria 10 d after induction of nephritis. However, the albumin-to-creatinine ratio was significantly reduced in nephritic IL-23 p19−/− mice compared with nephritic wild-type mice (nephritic WT: 140.8 ± 120.3, nephritic IL-23 p19−/−: 57.4 ± 51.3, non-nephritic controls: 0.2 ± 0.1; P < 0.05 for nephritic WT versus nephritic IL-23 p19−/−).
Renal T Cell and Monocyte Recruitment in IL-23 p19−/− Mice
To investigate the effects of IL-23 p19 deficiency on renal T cell and monocyte recruitment, kidney sections were immunohistochemically stained for tubulointerstitial and glomerular T cells (CD3). Tubulointerstitial monocytes/dendritic cells (F4/80) and glomerular monocytes (MAC-2) were assessed by immunohistochemistry. Representative staining patterns of control, nephritic wild-type, and nephritic IL-23 p19−/− animals are shown in Figure 4A.
Figure 4.
Renal T cell and monocyte recruitment in IL-23 p19−/− mice. (A) Representative photographs of kidney sections immunohistochemically stained for CD3, F4/80, and MAC-2 at day 10 after induction of nephritis (original magnification, 400×). (B) Quantification of tubulointerstitial (left) and glomerular (right) CD3+ T cells and F4/80+ or MAC-2+ monocytes in control (n = 11), nephritic wild-type (n = 14), and nephritic IL-23 p19−/− mice (n = 13) at day 10 of NTN. Symbols represent individual data points, and the horizontal lines indicate mean values (*P < 0.05, **P < 0.01).
Quantification of tubulointerstitial and glomerular CD3+ T cells at day 10 after induction of NTN (Figure 4B) revealed a significant decrease in renal T cell infiltration in nephritic IL-23 p19−/− mice compared with nephritic wild-type mice (tubulointerstitial compartment: nephritic WT, 18.7 ± 3.8/high-power field (hpf); nephritic IL-23 p19, 10.8 ± 5.1/hpf, non-nephritic controls, 2.0 ± 2.2; P < 0.01 for nephritic WT versus nephritic IL-23 p19−/−; glomerular compartment: nephritic WT, 0.5 ± 0.2/glomerular cross section (gcs), nephritic IL-23 p19−/−, 0.3 ± 0.2/gcs, non-nephritic controls, 0.1 ± 0.1/gcs; P < 0.05 for nephritic WT versus nephritic IL-23 p19−/−).
Furthermore, glomerular MAC-2+ monocytes were significantly reduced in nephritic IL-23 p19−/− mice compared with nephritic wild-type mice (nephritic WT, 1.3 ± 0.6/gcs; nephritic IL-23 p19−/−, 0.6 ± 0.4/gcs, non-nephritic controls, 0.3 ± 0.2/gcs; P < 0.01 for nephritic WT versus nephritic IL-23 p19−/−). Also, the number of F4/80+ monocytes/dendritic cells in the tubulointerstitial compartment appeared reduced in nephritic IL-23 p19−/− mice, but it was not significantly different from nephritic wild-type mice (nephritic WT, 33.4 ± 26.2/hpf; nephritic IL-23 p19−/−, 13.7 ± 9.8/hpf, non-nephritic controls, 3.0 ± 0.8; P > 0.05 NS for nephritic WT versus nephritic IL-23 p19−/−).
Renal and Systemic Immune Responses in IL-23 p19−/− Mice
To characterize the renal T cell response in more detail, leukocytes were isolated from the kidneys of wild-type and IL-23 p19−/− mice at day 10 of NTN and analyzed by flow cytometry. IL-17- or IFNγ-producing CD3+ T cells were identified by intracellular cytokine staining after stimulation with PMA/ionomycin. Nephritic IL-23 p19−/− mice showed a similar percentage of Th1-polarized IFNγ+ T cells (9.7% versus 7.7% of all CD3+ T cells) but a substantial decrease in Th17-polarized IL-17+ T cells as compared with nephritic wild-type mice (1.3% versus 3.1%) (Figure 5A).
Figure 5.
Renal and systemic immune responses in IL-23 p19−/− mice. (A) Flow cytometric analysis of isolated renal T cells from nephritic wild-type and nephritic IL-23 p19−/− mice for production of IL-17 and IFNγ after stimulation with PMA/ionomycin. Numbers are counts in percentage of CD3+ T cells (mean values of three independent experiments). (B, C) RT-PCR analysis of renal cytokine and chemokine mRNA expression in nephritic wild-type (n = 14), nephritic IL-23 p19−/− (n = 13), and non-nephritic wild-type control (n = 11) mice. mRNA expression is indicated as x-fold of non-nephritic wild-type controls. Symbols represent individual data points, and the horizontal lines indicate mean values. (D) Representative RT-PCR of the Th17-cell differentiation marker RORγt in two control, two nephritic wild-type, and two nephritic IL-23 p19−/− mice and quantification by densitometry. (E) Circulating titers of mouse anti-sheep total IgG, IgG1 isotype, IgG2a isotype, and IgG2b isotype 10 days after induction of nephritis were measured by ELISA in nephritic wild-type (n = 11) and nephritic IL-23 p19−/− mice (n = 12). Serum dilutions as indicated. Bars represent means ± SD (*P < 0.05).
Renal IL-17 mRNA expression was upregulated in nephritic mice when compared with controls (nephritic WT, 4.3-fold; nephritic IL-23 p19−/−, 2.9-fold) and showed a tendency to decrease in nephritic IL-23 p19−/− mice. The expression of the Th1 cytokine IFNγ (nephritic WT, 6.3-fold; nephritic IL-23 p19−/−, 8.6-fold) and the major proinflammatory cytokine TNFα (nephritic WT, 9.2-fold; nephritic IL-23 p19−/−, 6.4-fold), by comparison, was not substantially different between nephritic wild-type and IL-23 p19−/− mice (Figure 5B). RNA expression of the Th17-related cytokine IL-21 (nephritic WT, 2.4-fold; nephritic IL-23 p19−/−, 2.8-fold) was also similar in nephritic wild-type and IL-23 p19−/− mice. IL-22, another proinflammatory cytokine produced by Th17 cells, was below the detection level in nephritic wild-type and IL-23 p19−/− mice. The expression of IL-23 was upregulated in nephritic wild-type mice (3.6-fold) and not detectable in nephritic IL-23 p19−/− mice, as was expected.
Renal mRNA expression of the chemokines CCL20/LARC (nephritic WT, 126.6-fold; nephritic IL-23 p19−/−, 52.4-fold; P < 0.05; Figure 5C) and CCL5/RANTES (nephritic WT, 15.1-fold; nephritic IL-23 p19−/−, 6.6-fold; P < 0.05) was significantly reduced in IL-23 p19−/− mice. CCL2/MCP-1 (nephritic WT, 65.0-fold; nephritic IL-23 p19−/−, 22.6-fold) and CCL3/MIP-1α (nephritic WT, 5.2-fold; nephritic IL-23 p19−/−, 3.8-fold) mRNA expression tended to be reduced in nephritic IL-23 p19−/− mice when compared with nephritic wild-type mice but failed to reach statistical significance because of considerable variability among the animals. Basal renal Th1 cytokines and chemokine mRNA expression levels were comparable between control wild-type and control IL-23 p19−/− mice (data not shown).
As shown by representative RT-PCR and densitometry (Figure 5D), the mRNA expression of the transcription factor RORγt, which is predominantly expressed by Th17-polarized cells, was significantly lower in kidneys of nephritic IL-23 p19−/− mice compared with nephritic wild-type mice (non-nephritic controls, 1.0 ± 0.6 densitometric arbitrary units [AU]; nephritic WT, 7.0 ± 2.2 AU; nephritic IL-23 p19−/−, 3.3 ± 1.5 AU), indicating a reduced number of Th17 cells in kidneys of IL-23 p19−/− mice.
To address the question of whether IL-23 p19 deficiency induces alterations in immunoglobulin G (IgG) production directed against the nephritogenic antigen, we performed immunohistochemistry for mouse IgG on kidney sections 10 days after induction of nephritis. The amount of glomerular deposition of mouse IgG and the distribution patterns were similar in wild-type and IL-23 p19−/− kidneys (data not shown). For a more precise description of antigen-specific humoral immune responses, we analyzed by ELISA the isotype pattern of IgG antibody response directed against sheep IgG in the serum of nephritic mice (Figure 5E). There was no significant difference in sheep IgG-specific antibody titers of total mouse IgG at day 10 of NTN. Furthermore, the analysis of IgG isotypes revealed no bias for either Th1 (IgG2a) or Th2 type (IgG1) antibody production in nephritic IL-23 p19−/− mice compared with nephritic wild-type mice. However, there was a tendency toward increased production of all IgG subclasses in nephritic IL-23 p19−/− mice. The sheep IgG-specific antibodies of the IgG2b isotype were significantly increased in nephritic IL-23 p19−/− mice compared with nephritic wild-type mice.
Experimental Glomerulonephritis Is Ameliorated in IL-17−/− Mice
After showing that lower levels of Th17 cells in IL-23 p19−/− mice are associated with less severe renal disease, we next examined the role of IL-17, one of the major effector cytokines of Th17 cells. To this end, nephrotoxic nephritis was induced in C57BL/6 wild-type and C57BL/6 IL-17−/− mice. At day 10 of NTN, IL-17−/− mice were partly protected from immune-mediated kidney injury, as demonstrated by substantially reduced renal T cell and monocyte recruitment and reduced tissue damage in comparison to nephritic wild-type mice. Quantification of tubulointerstitial and glomerular CD3+ T cells revealed a significant decrease in renal T cell infiltration in nephritic IL-17−/− mice compared with nephritic wild-type mice (tubulointerstitial compartment: nephritic WT, 20.7 ± 6.0 cells/hpf; nephritic IL-17−/−, 10.8 ± 4.1 cells/hpf, non-nephritic controls, 2.9 ± 1.6 cells/hpf; P < 0.05 for nephritic WT versus nephritic IL-17−/−; glomerular compartment: nephritic WT, 0.8 ± 0.3 cells/gcs, nephritic IL-17−/−, 0.5 ± 0.2 cells/gcs, non-nephritic controls, 0.1 ± 0.1 cells/hpf; P < 0.05 for nephritic WT versus nephritic IL-17−/−; Figure 6A).
Figure 6.
Attenuated glomerulonephritis in IL-17−/− mice. (A, B) Glomerular and tubulointerstitial T cell and monocyte infiltration in nephritic wild-type (n = 5), nephritic IL-17−/− (n = 7), and non-nephritic wild-type control (n = 4) mice. (C) Nephritic IL-17−/− mice (n = 7) developed less renal tissue injury than nephritic wild-type mice (n = 5) in terms of glomerular crescent formation. (D) Renal dysfunction was assessed by determination of the serum BUN and albumin-to-creatinine ratio in non-nephritic control (n = 4), nephritic wild-type (n = 5), and nephritic IL-17−/− mice (n = 7) at day 10. (E) Flow cytometric analysis of isolated renal T cells from nephritic wild-type and nephritic IL-17−/− mice for production of IL-17 after stimulation with PMA/ionomycin. Numbers are counts in percentage of CD3+ T cells (mean values of three independent experiments). (F) RT-PCR analysis of renal chemokine mRNA expression in nephritic wild-type (n = 5), nephritic IL-17−/− (n = 7), and non-nephritic wild-type control (n = 4) mice. mRNA expression is indicated as x-fold of non-nephritic wild-type controls. Symbols represent individual data points, and the horizontal lines indicate mean values (*P < 0.05, **P < 0.01).
Furthermore, the numbers of F4/80+ monocytes/dendritic cells in the tubulointerstitial compartment and glomerular infiltration of MAC-2+ monocytes were significantly reduced in nephritic IL-17−/− mice compared with their wild-type counterparts (tubulointerstitial compartment: nephritic WT, 25.8 ± 4.4 cells/hpf, nephritic IL-17−/−, 15.7 ± 4.8 cells/hpf, non-nephritic controls, 1.2 ± 0.4 cells/hpf; P < 0.05 for nephritic WT versus nephritic IL-17−/−; glomerular compartment: nephritic WT, 3.4 ± 0.5 cells/gcs, nephritic IL-17−/−, 1.8 ± 0.4 cells/gcs, non-nephritic controls, 0.2 ± 0.2 cells/gcs; P < 0.01 for nephritic WT versus nephritic IL-17−/−; Figure 6B). In addition, glomerular crescent formation was reduced in IL-17−/− mice compared with nephritic wild-type mice (nephritic WT, 58.8 ± 8.8 (glomeruli in %); nephritic IL-17−/−, 29.0 ± 19.7 (glomeruli in %), non-nephritic controls, 0.8 ± 1.5 (glomeruli in %); P < 0.01 for nephritic WT versus nephritic IL-17−/−; Figure 6C). In line with reduced renal leukocyte infiltration and tissue damage, IL-17−/− mice showed a tendency toward decreased BUN levels (nephritic WT, 49.2 ± 5.2 mg/dl; nephritic IL-17−/−, 41.0 ± 8.5 mg/dl; non-nephritic controls, 35.0 ± 2.6 mg/dl; P > 0.05 NS for nephritic WT versus nephritic IL-17−/−; Figure 6D) and a reduced albumin-to-creatinine ratio (nephritic WT, 77.2 ± 20.7 mg/dl; nephritic IL-17−/−, 41.8 ± 31.3 mg/dl; non-nephritic controls, 0.1 ± 0.02 mg/dl; P > 0.05 NS for nephritic WT versus nephritic IL-17−/−; Figure 6D).
FACS analysis of renal leukocytes after intracellular cytokine staining demonstrated the absence of IL-17 production in T cells of nephritic IL-17−/− mice (Figure 6E).
Renal mRNA expression of CCL2/MCP-1 was significantly reduced in IL-17−/− mice compared with nephritic wild-type mice (nephritic WT, 199.1-fold; nephritic IL-17−/−, 30.5-fold; P < 0.05 for nephritic WT versus nephritic IL-17−/−; Figure 6F), whereas CCL20/LARC (nephritic WT, 75.3-fold; nephritic IL-17−/−, 42.3-fold) showed an insignificant tendency toward a reduction in IL17−/− mice. CCL3/MIP-1α (nephritic WT, 3.1-fold; nephritic IL-17−/−, 5.0-fold) and CCL5/RANTES (nephritic WT, 10.5-fold; nephritic IL-17−/−, 14.2-fold) mRNA expression, in contrast, was not reduced in nephritic IL17−/− mice.
DISCUSSION
The Th1/Th2 paradigm was first proposed by Mosmann et al. 20 years ago19 and has shaped our view, not only of anti-infectious immunity but also of immune-mediated diseases, because these, too, can be classified as Th1- or Th2-associated conditions. Th1 cells produce large quantities of IFNγ and have been considered to be almost exclusively responsible for driving cell-mediated tissue damage in several autoimmune diseases, including proliferative and crescentic glomerulonephritis.3 Th2 cells, however, produce IL-4, IL-5, and IL-13 and are of central importance to IgE production and to the immunopathogenesis of allergic diseases. Their role in glomerular inflammation is less well characterized. Although it has long been known that the Th1/Th2 paradigm is not an absolute dichotomy, this concept has been enormously useful in the past and is still used today. However, the Th1/Th2 paradigm has recently been challenged by the identification of a third population of T helper cells producing IL-17, TNFα, IL-21, and IL-22, which are termed Th17 cells.20,21
Th17 cells appear to be critical to the enhancement of host protection against extracellular bacteria and fungi, which are not efficiently cleared by Th1 and Th2 responses.22 In addition, Th17 cells can very potently promote tissue inflammation by inducing proinflammatory cytokines and chemokines that attract and activate macrophages and other immune cells.23 The novel hypothesis that Th17 cells, in addition to Th1 cells, play an important role in cell-mediated autoimmune inflammatory diseases is based on findings in mice lacking important cytokines and receptors involved in the Th1 immune response such as IFNγ, IFNγ receptor, IL-12p35, and IL-18. Surprisingly, these “Th1-deficient” mice were not protected from experimentally induced autoimmune encephalomyelitis or collagen-induced arthritis. These models have so far been believed to be exclusively Th1 cell mediated. However, IL-23 (expanding Th17-cell population), but not IL-12 (promoting Th1-cell responses), was crucial for initiating an autoaggressive T cell response in the central nervous system.16 Finally, Th17 cells were more potent than Th1 cells in transferring experimental autoimmune encephalomyelitis to naïve wild-type recipient animals.24 Collectively, these data suggest an important role for Th17 cells in the immunopathogenesis of autoimmune tissue inflammation.
Although the role of Th17 cells in renal inflammation has not been studied so far,25 a report by Kitching et al. provided indirect evidence for a role of the Th17 immune response in anti-glomerular basement membrane glomerulonephritis. IFNγ-deficient mice developed aggravated nephritis, but IL-12 p40−/− animals were partly protected from renal tissue damage, suggesting a role for the IL-23/Th17 pathway.26
For the first time, we were able to demonstrate the presence of IL-17-producing T cells (in addition to IFNγ+ Th1 cells) in the kidneys of nephritic mice. Interestingly, no double-positive IL-17 and IFNγ renal T cells were detectable, underscoring the dichotomy of these T cell types. IL-17 (and TNFα) secretion is one of the principal effector mechanisms of Th17 cells. To investigate the potential contribution of IL-17 to glomerular inflammation, we studied the influence of IL-17 on the expression of the chemokines CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, and CCL20/LARC by mMCs. IL-17 and TNFα synergistically induced chemokine mRNA and protein production in mesangial cells, which are known to play an important role in renal leukocyte recruitment.27,28 These results are in line with a study by Woltman et al. showing that tubular cells also respond to IL-17 with increased protein production of the chemokines CXCL8/IL-8 and CCL2/MCP-1.29 Overall, these findings suggest a general proinflammatory effect of IL-17 on resident kidney cells. Of special interest is the finding that IL-17 induces the expression of CCL20/LARC in mesangial cells implicated in the recruitment of CCR6-positive Th17 cells,30 suggesting a positive feedback loop. It remains to be determined whether intervention in Th17-cell trafficking via CCR6/CCL20 is useful to treat and prevent renal Th17-cell infiltration and consequent tissue damage.
In a next step, we examined the functional role of the Th17 immune response in experimental glomerulonephritis. Nephrotoxic nephritis was induced in IL-23 p19−/− mice, which display reduced Th17 immune responses.31 As expected, we found a reduction in renal Th17-cell infiltration in IL-23 p19−/− mice during the autologous phase (day 10) of nephrotoxic nephritis. In agreement with our in vitro results described above, the reduced recruitment of IL-17-producing T cells was paralleled by decreased expression of proinflammatory chemokines, which might contribute to the reduced recruitment of inflammatory cells observed in IL-23 p19−/− mice. Most importantly, C57BL/6 IL-23 p19−/− mice developed less severe nephrotoxic nephritis than C57BL/6 wild-type mice in terms of renal function, albuminuria, and histologic injury (including glomerular crescent formation and sclerosis). It is noteworthy that the beneficial effects seen in nephritic IL-23 p19−/− animals were evident despite a comparable humoral systemic anti-sheep IgG immune response and an unaltered capacity of infiltrating T cells to produce IFNγ.
Next, we aimed at investigating whether the lower levels of renal Th17 cells in IL-23 p19−/− animals lead to amelioration of nephritis in these mice. Because IL-17 secretion is thought to be a principal effector mechanism of Th17 cells, we analyzed nephrotoxic serum nephritis in IL-17−/− mice. The results showed that IL-17−/− mice had less severe nephritis in terms of renal tissue damage, leukocyte recruitment, BUN level, and proteinuria. These data are in line with the decreased susceptibility of IL-23 p19−/− mice, providing additional evidence for a contribution of the IL-23/Th17 axis to immune-mediated renal tissue injury.
The main finding of this study is the detection of IL-17-producing Th17 cells in the kidneys of nephritic mice. Furthermore, we were able to demonstrate that interference with the Th17 immune response either at the level of Th17-cell survival and expansion or at the level of IL-17 effector cytokine production is associated with a mitigated course of experimental nephritis in terms of renal tissue injury, proteinuria, and renal function. These data indicate that, in addition to Th1 cells, the IL-23/Th17 immune response contributes to the immunopathogenesis of glomerulonephritis.
CONCISE METHODS
Animals
IL-23 p19−/− mice (C57BL/6 background) were provided by N. Ghilardi (Genentech, San Francisco, CA),31 and IL-17A-deficient (IL-17−/−) mice were provided by Y. Iwakura (Center for Experimental Medicine, Institute of Medical Science, University of Tokyo, Japan).32 The p19−/− and IL-17−/− genotypes were confirmed by PCR analysis for each animal. Age-matched C57BL/6 wild-type controls (8- to 10-wk-old) were obtained from Charles River (Sulzfeld, Germany). All animals were raised under specific pathogen-free conditions. Animal experiments were performed according to national and institutional animal care and ethical guidelines and were approved by local committees.
Animal Experiments
Nephrotoxic serum nephritis was induced in 8- to 10-wk-old male C57BL/6 wild-type, C57BL/6 IL-23 p19−/−, and C57BL/6 IL-17−/− mice by intraperitoneal injection of 2.5 mg of nephrotoxic sheep serum per gram of mouse body weight, as described.33 Controls were injected intraperitoneally with an equal amount of nonspecific sheep IgG.
Functional Studies
For urine sample collection, mice were housed in metabolic cages for 6 h. Albuminuria was determined by standard ELISA analysis (Mice-Albumin Kit, Bethyl, Montgomery, TX). Blood samples for BUN measurement and assessment of systemic antibody response were obtained at the time of euthanasia. Urinary creatinine and blood BUN levels were measured by standard laboratory methods.
RT-PCR Analysis
Total RNA of renal cortex was prepared according to standard laboratory methods. RT-PCR was performed with 1.5 μl of cDNA samples in the presence of 2.5 μl (0.9 μM) of specific murine primers (primer sequences are available upon request) and 12.5 μl of 2× Platinum SYBR Green qPCR Supermix (Invitrogen, Karlsruhe, Germany) using an AbiPrism Sequence Detection System 7000 (Applied Biosystems, Foster City, CA). All samples were run in duplicate and normalized to 18S rRNA to account for small RNA and cDNA variability.34
Morphologic Examinations
Light microscopy and immunohistochemistry were performed using routine procedures. Crescent formation and glomerular sclerosis (deposition of PAS-positive material) were assessed in 30 glomeruli per mouse in a blinded fashion in PAS-stained paraffin sections. Cumulative score for glomerular sclerosis was calculated as follows: 0 = no sclerosis, 1 = <25% sclerosis, 2 = 26 to 50% sclerosis, 3 = 51 to 75% sclerosis, 4 = 76 to 100% sclerosis. As a measure of tubulointerstitial injury, the interstitial area was estimated by point-counting three independent areas of renal cortex per mouse at low magnification (200×), as described previously.35 Paraffin-embedded sections (2 μm) were stained with either an antibody directed against the pan-T cell marker CD3 (A0452, Dako, Germany) or the monocyte and renal dendritic cell marker F4/80 (BM8; BMA, Hamburg, Germany) and the monocyte-specific MAC-2 (M3/38, Cedarlane, ON, Canada). Tissue sections were developed with the Vectastain ABC-AP kit (Vector Laboratories, Burlingame, CA). MAC-2- and CD3-positive cells in 30 glomerular cross sections and F4/80- and CD3-positive cells in 30 tubulointerstitial hpf per kidney were counted by light microscopy in a blinded fashion.
Antigen-Specific Humoral Immune Response
Mouse anti-sheep IgG antibody titers were measured by ELISA using sera collected 10 d after induction of nephritis, as recently described.33 In brief, ELISA microtiter plates were coated with 100 μl of 100 μg/ml sheep IgG (Sigma, St. Louis, MO) in carbonate–bicarbonate buffer overnight at 4°C. After being blocked with 1% BSA in Tris-buffered saline (Sigma), the plates were incubated with serial dilutions of mouse serum (1:100 to 1:12,500) for 1 h at room temperature. Bound mouse IgG was detected using peroxidase-conjugated goat anti-mouse IgG (Biozol, Eching, Germany) at 1:1000, 3,3′,5,5′-tetramethylbenzidine peroxidase substrate, and absorbance readings (at 450 nm) on a spectrophotometer. Lack of cross-reactivity of the secondary antibody with sheep IgG was demonstrated by omitting the primary antibody. Ig isotypes (IgG1, IgG2a, and IgG2b) were measured using the ELISA technique, as described previously.33
Renal Single-Cell Suspension
Previously described methods for renal cell isolation from murine kidneys were used.33 In brief, kidneys were finely minced and digested with 0.4 mg/ml collagenase D (Roche, Mannheim, Germany) and 0.01 mg/ml DNAse I in Dulbecco modified Eagle medium (DMEM; Roche) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen) for 45 min at 37°C. Cell suspensions were sequentially filtered through 70- and 40-μm nylon meshes and washed with HBSS without Ca2+ and Mg2+ (Invitrogen). Single-cell suspensions were separated using Percoll density gradient (70% and 40%) centrifugation.36 The leukocyte-enriched cell suspension was aspirated from the Percoll interface. Viability of the cells was assessed by trypan blue staining before flow cytometry.
Flow Cytometry
For T cell differentiation, renal single-cell suspension was stained with fluorochrome-labeled antibodies specific for CD3 (APC; 17A2, R&D Systems, Wiesbaden, Germany) and CD4 (PE; GK1.5, Miltenyi, Bergisch Gladbach, Germany) for 25 min at 4°C. Before antibody incubation, unspecific staining was blocked with normal mouse serum (Sigma). Staining of intracellular IFNγ and IL-17 was performed as recently described by Korn et al.37 In brief, isolated renal leukocytes were activated by incubation at 37°C, 5% CO2 for 5 h with PMA (5 ng/ml; Sigma) and ionomycin (1 μg/ml; Calbiochem-Merck, Darmstadt, Germany) in RPMI 1640 (Gibco, Grand Island, NY) with 10% FCS. After 30 min of incubation, Brefeldin A (10 μg/ml; Sigma) was added. After several washing steps and staining of cell surface markers, cells were incubated with Cytofix/Cytoperm (BD Biosciences, Franklin Lakes, NJ) for 20 min at 4°C to permeabilize cell membranes. Then, intracellular IL-17 and IFNγ were stained using rat anti-mouse IL-17 antibody (PE; TC11–18H10, BD Biosciences) and IFNγ antibody (FITC; XMG1.2, BD Biosciences). Experiments were performed with a Becton Dickinson FACScalibur System using the Cell Quest Professional software.
Mesangial Cell Culture Stimulation
Mouse mesangial cells38 were cultured in DMEM (Life Technologies-BRL/Invitrogen) containing 10% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin (Life Technologies-BRL/Invitrogen, Karlsruhe, Germany) at 37°C, 5% CO2. Before stimulation, confluent cells were incubated in serum-free DMEM for 24 h. mMCs were stimulated with IL-17 and TNFα (RD Systems, Wiesbaden Germany). After 4 h of incubation, the expression of CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, and CCL20/LARC mRNA was analyzed. The production of CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, and CCL20/LARC protein from mMCs into the medium was determined after 24 h by removing 50 μl of supernatants for measurement by specific mouse ELISA according to the manufacturer's instructions (RD Systems).
Statistical Analysis
Results are expressed as mean ± SD. Differences between individual experimental groups were compared by Kruskal–Wallis test with post hoc analysis by Mann–Whitney test. Experiments yielding insufficient independent data for statistical analysis due to the experimental setup were repeated at least three times.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank C. Meyer-Schwesinger for her excellent technical support. This work was supported by grants from the Deutsche Forschungsgemeinschaft (PA 754/6-3).
Published online ahead of print. Publication date available at www.jasn.org.
H.-J.P. and J.-E.T. contributed equally to this work.
See related editorial, “17 and 23: Prime Numbers that Matter,” on pages 925–927.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Kurts C, Heymann F, Lukacs-Kornek V, Boor P, Floege J: Role of T cells and dendritic cells in glomerular immunopathology. Semin Immunopathol 29: 317–335, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Kluth DC, Erwig LP, Rees AJ: Multiple facets of macrophages in renal injury. Kidney Int 66: 542–557, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Tipping PG, Holdsworth SR: T cells in crescentic glomerulonephritis. J Am Soc Nephrol 17: 1253–1263, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR, O'Sullivan KM, Tipping PG, Takeda K, Akira S, Holdsworth SR: IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol 16: 2023–2033, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Kitching AR, Holdsworth SR, Tipping PG: IFN-γ mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol 10: 752–759, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Kitching AR, Tipping PG, Holdsworth SR: IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol 29: 1–10, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Huang XR, Tipping PG, Shuo L, Holdsworth SR: Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 51: 94–103, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Ruth AJ, Kitching AR, Li M, Semple TJ, Timoshanko JR, Tipping PG, Holdsworth SR: An IL-12-independent role for CD40-CD154 in mediating effector responses: studies in cell-mediated glomerulonephritis and dermal delayed-type hypersensitivity. J Immunol 173: 136–144, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Kitching AR, Tipping PG, Mutch DA, Huang XR, Holdsworth SR: Interleukin-4 deficiency enhances Th1 responses and crescentic glomerulonephritis in mice. Kidney Int 53: 112–118, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Kitching AR, Tipping PG, Timoshanko JR, Holdsworth SR: Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int 57: 518–525, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR: Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol 27: 530–537, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, Hauswirth WW, Flotte TR, Rodriguez-Iturbe B, Johnson RJ: IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol 16: 3651–3660, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Steinman L: A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med 13: 139–145, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR: The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B: TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD: Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421: 744–748, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ: Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 198: 1951–1957, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver CT, Hatton RD, Mangan PR, Harrington LE: IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25: 821–852, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL: Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357, 1986 [PubMed] [Google Scholar]
- 20.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT: Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6: 1123–1132, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C: A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6: 1133–1141, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzaki G, Umemura M: Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol 51: 1139–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP: IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J Immunol 160: 3513–3521, 1998 [PubMed] [Google Scholar]
- 24.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ: IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201: 233–240, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurts C: Th17 cells: A third subset of CD4+ T effector cells involved in organ-specific autoimmunity. Nephrol Dial Transplant 23: 816–819, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kitching AR, Turner AL, Semple T, Li M, Edgtton KL, Wilson GR, Timoshanko JR, Hudson BG, Holdsworth SR: Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: A protective role for IFN-γ. J Am Soc Nephrol 15: 1764–1774, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Panzer U, Steinmetz OM, Stahl RA, Wolf G: Kidney diseases and chemokines. Curr Drug Targets 7: 65–80, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Segerer S, Schlondorff D: Role of chemokines for the localization of leukocyte subsets in the kidney. Semin Nephrol 27: 260–274, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Woltman AM, de Haij S, Boonstra JG, Gobin SJ, Daha MR, van Kooten C: Interleukin-17 and CD40-ligand synergistically enhance cytokine and chemokine production by renal epithelial cells. J Am Soc Nephrol 11: 2044–2055, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S: Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 204: 2803–2812, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney AL, De Sauvage FJ: Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol 172: 2827–2833, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y: Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17: 375–387, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Panzer U, Steinmetz OM, Paust HJ, Meyer-Schwesinger C, Peters A, Turner JE, Zahner G, Heymann F, Kurts C, Hopfer H, Helmchen U, Haag F, Schneider A, Stahl RA: Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol 18: 2071–2084, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Panzer U, Steinmetz OM, Reinking RR, Meyer TN, Fehr S, Schneider A, Zahner G, Wolf G, Helmchen U, Schaerli P, Stahl RA, Thaiss F: Compartment-specific expression and function of the chemokine IP-10/CXCL10 in a model of renal endothelial microvascular injury. J Am Soc Nephrol 17: 454–464, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Steinmetz OM, Sadaghiani S, Panzer U, Krebs C, Meyer-Schwesinger C, Streichert T, Fehr S, Hamming I, van Goor H, Stahl RA, Wenzel U: Antihypertensive therapy induces compartment-specific chemokine expression and a Th1 immune response in the clipped kidney of Goldblatt hypertensive rats. Am J Physiol Renal Physiol 292: F876–F887, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Kursar M, Hopken UE, Koch M, Kohler A, Lipp M, Kaufmann SH, Mittrucker HW: Differential requirements for the chemokine receptor CCR7 in T cell activation during Listeria monocytogenes infection. J Exp Med 201: 1447–1457, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK: Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 13: 423–431, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf G, Haberstroh U, Neilson EG: Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol 140: 95–107, 1992 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.