Abstract
Despite the high prevalence of chronic kidney disease (CKD), relatively few individuals with CKD progress to ESRD. A better understanding of the risk factors for progression could improve the classification system of CKD and strategies for screening. We analyzed data from 65,589 adults who participated in the Nord-Trøndelag Health (HUNT 2) Study (1995 to 1997) and found 124 patients who progressed to ESRD after 10.3 yr of follow-up. In multivariable survival analysis, estimated GFR (eGFR) and albuminuria were independently and strongly associated with progression to ESRD: Hazard ratios for eGFR 45 to 59, 30 to 44, and 15 to 29 ml/min per 1.73 m2 were 6.7, 18.8, and 65.7, respectively (P < 0.001 for all), and for micro- and macroalbuminuria were 13.0 and 47.2 (P < 0.001 for both). Hypertension, diabetes, male gender, smoking, depression, obesity, cardiovascular disease, dyslipidemia, physical activity and education did not add predictive information. Time-dependent receiver operating characteristic analyses showed that considering both the urinary albumin/creatinine ratio and eGFR substantially improved diagnostic accuracy. Referral based on current stages 3 to 4 CKD (eGFR 15 to 59 ml/min per 1.73 m2) would include 4.7% of the general population and identify 69.4% of all individuals progressing to ESRD. Referral based on our classification system would include 1.4% of the general population without losing predictive power (i.e., it would detect 65.6% of all individuals progressing to ESRD). In conclusion, all levels of reduced eGFR should be complemented by quantification of urinary albumin to predict optimally progression to ESRD.
Since the publication of the Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines on the classification of chronic kidney disease in 2002,1 several studies based on this classification system have shown very high prevalence estimates of chronic kidney disease (CKD) in the general population (10 to 13%).2,3 Screening for CKD is therefore increasingly suggested1,4; however, only a small proportion of patients with stage 3 to 4 CKD progress to ESRD.5 There is an ongoing discussion on whether the current CKD criteria are appropriate.6–8 Developing a risk score to identify better the patients who are at increased risk for ESRD would be of major importance for the current efforts to establish clinical guidelines and public health plans for CKD.4,9,10
Several predictors of progression to ESRD have been identified,9 but their independent predictive power has not been well studied either in the general population or in high-risk subgroups. Intuitively, a low estimated GFR (eGFR) is an important risk factor for ESRD, and eGFR is the backbone of the current CKD classification. High urine albumin is a well-established major risk factor for progression.9 Only a few studies have examined the renal risk as a function of the combination of eGFR and albuminuria.11–14 These studies are of restricted value, however, because of exclusion of patients with diabetes14; inclusion of men only12; inclusion of only patients with diabetes13; or absence of information on potentially important risk factors, such as smoking, obesity, dyslipidemia, and cardiovascular disease.11,14
CKD screening beyond patients with known hypertension or diabetes has been proposed,1,4 but such screening programs have remained unsatisfactory because of their limited predictive power. We used the data of the Second Nord-Trøndelag Health Study (HUNT 2), Norway, to improve such prediction. HUNT 2 is a large population-based study with a high participation rate.15 Our aim was to examine how accurately subsequent progression to ESRD could be predicted by a combined variable of baseline eGFR and urine albumin. We also tested whether further potential renal risk factors provided additional independent prediction.
RESULTS
After 10.3 yr of follow-up of 65,589 patients, 58 started on renal replacement therapy (RRT) and 132 others died of advanced CKD. We excluded four patients who died of acute-on-chronic renal failure, nine of acute renal failure, 38 with stages 3 to 4 CKD, and 15 for whom adequate information on renal function was missing; therefore, 124 patients with documented progression to ESRD were included. Baseline characteristics of our study participants are summarized in Table 1. The prevalence of stages 3 and 4 CKD at baseline was 4.4 and 0.1%, respectively, in patients who did not progress and 46.0 and 23.4%, respectively, in patients who did progress to ESRD.
Table 1.
Characteristics of participants in the HUNT 2 study at baseline (1995 through 1997) by future progression or no progression to ESRDa
| Characteristic | All (n = 65,589) | No Progression to ESRD (n = 65,465) | Progression to ESRD (n = 124) | P |
|---|---|---|---|---|
| Age (yr; mean [SD]; n = 65,589) | 50.1 (17.3) | 50.1 (17.3) | 70.8 (13.4) | <0.001 |
| Male gender (%; n = 65,589) | 46.8 | 46.8 | 62.9 | <0.001 |
| Low education (%; n = 61,369)b | 70.7 | 70.6 | 86.5 | <0.001 |
| Depression (%; n = 58,423)c | 10.6 | 10.6 | 26.5 | <0.001 |
| Current smoking (%; n = 64,395) | 28.0 | 28.0 | 17.6 | 0.012 |
| Low physical activity (%; n = 57,881)d | 20.1 | 20.1 | 42.7 | <0.001 |
| Diabetes (%; n = 64,693) | 3.3 | 3.3 | 18.2 | <0.001 |
| CVD (%; n = 64,624)e | 7.9 | 7.9 | 25.8 | <0.001 |
| BMI (kg/m2; mean [SD]; n = 64,306) | 26.4 (4.1) | 26.4 (4.1) | 28.0 (5.0) | <0.001 |
| Waist circumference (cm; mean [SD]; n = 64,022) | 86.5 (11.8) | 86.5 (11.8) | 94.1 (13.6) | <0.001 |
| Systolic BP (mmHg; mean [SD]; n = 64,708) | 137.9 (21.8) | 137.9 (21.8) | 160.6 (24.9) | <0.001 |
| Diastolic BP (mmHg; mean [SD]; n = 64,708) | 80.3 (12.3) | 80.3 (12.2) | 87.3 (15.8) | <0.001 |
| Antihypertensive medication (%; n = 64,649) | 11.1 | 11.0 | 46.7 | <0.001 |
| Cholesterol (mg/dl; mean [SD]; n = 65,158) | 228.2 (48.9) | 228.2 (50.3) | 252.4 (58.0) | <0.001 |
| HDL cholesterol (mg/dl; mean [SD]; n = 65,155) | 53.2 (15.1) | 54.1 (15.5) | 46.4 (15.4) | <0.001 |
| Triglycerides (mg/dl; median [range]; n = 65,158) | 131 (12–696) | 131 (12–696) | 187 (35–363) | <0.001 |
| Glucose (mg/dl; median [range]; n = 65,158)f | 93 (41–558) | 94 (41–558) | 102 (61–348) | <0.001 |
| Creatinine (mg/dl; mean [SD]; n = 65,158) | 1.0 (0.2) | 1.0 (0.2) | 1.7 (0.6) | <0.001 |
| eGFR (ml/min per 1.73 m2; mean [SD]; n = 65,158) | 94.2 (21.5) | 94.3 (21.5) | 49.8 (23.5) | <0.001 |
| ACR (mg/g; median [range]; n = 9703) | 7 (0.4–2437) | 7 (0.4–1558) | 136 (94.4–2437) | <0.001 |
The “no progression” and “progression” groups are compared using χ2 test or two-samplet test. BMI, body mass index; CVD, cardiovascular disease.
Fewer than 12 yr in school.
Eight points or more on the depression part of the Hospital Anxiety and Depression Score.
Less than 1 h of light activity per week in participants’ leisure time.
Self-reported history of myocardial infarction, angina pectoris, or stroke.
Measured at random.
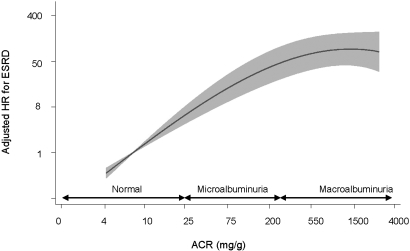
Table 2 shows the age-adjusted hazard ratios (HRs) of all potential risk factors for progression to ESRD available. Increased risk was significantly associated with male gender, low physical activity, presence of diabetes, higher values of body mass index and waist circumference, higher values of systolic and diastolic BP, treatment with antihypertensive drugs, lower values of HDL cholesterol concentration, and higher values of triglyceride and glucose concentration. No significantly increased risk was associated with low education, depression, current smoking, prevalent cardiovascular disease, or higher values of total cholesterol. A “best clinical model” was thenidentified by manual forward selection of the significant age-adjusted variables. In this multivariable analysis, age, gender, physical activity, diabetes, systolic BP, antihypertensive medication, and HDL cholesterol remained as significant predictors (P ≤ 0.016); however, they all turned out to be NS after inclusion of eGFR and albumin-to-creatinine ratio (ACR). For example, patients with diabetes had an age-adjusted HR of 2.68 and of 1.80 in the multivariable “best clinical model,” but after accounting for eGFR and ACR, the HR was not different from 1.00. Evidently, the major predictors of future ESRD were low eGFR and high ACR. Patients with eGFR 45 to 59 ml/min per 1.73 m2 had a multiadjusted HR of 6.67 compared with those with eGFR ≥60 ml/min per 1.73 m2. The HR associated with eGFR 30 to 44 and 15 to 29 ml/min per 1.73 m2 was 18.8 and 65.7, respectively. Compared with patients with normoalbuminuria, the presence of micro- or macroalbuminuria conferred a 13.0 and 47.2 times higher risk for progressing to ESRD, respectively. Figure 1 shows that after adjustment for age, gender, and eGFR, the risk associated with increasing ACR was continuous with no lower limit (i.e., the association continued even into the range of normoalbuminuria).
Table 2.
HRs of potential risk factors for progression to ESRDa
| Parameter | Age-Adjusted Variables
|
Best Clinical Model
|
Best Clinical + eGFR + ACR
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age (per 10 yr) | 1.72 | 1.48 to 1.99 | <0.001 | 1.15 | 0.98 to 1.35 | 0.073 | |||
| Male gender | 2.13 | 1.50 to 3.07 | <0.001 | 2.22 | 1.49 to 3.31 | <0.001 | 1.24 | 0.83 to 1.86 | 0.296 |
| Low educationb | 1.09 | 0.61 to 1.94 | 0.768 | ||||||
| Depressionc | 1.32 | 0.91 to 1.92 | 0.139 | ||||||
| Current smoking | 0.92 | 0.56 to 1.49 | 0.735 | ||||||
| Low physical activityd | 1.74 | 1.15 to 2.63 | 0.009 | 1.71 | 1.13 to 2.60 | 0.012 | 1.23 | 0.81 to 1.87 | 0.337 |
| Diabetes | 2.68 | 1.68 to 4.27 | <0.001 | 1.80 | 1.11 to 2.90 | 0.016 | 0.87 | 0.53 to 1.43 | 0.585 |
| CVDe | 1.26 | 0.82 to 1.93 | 0.288 | ||||||
| BMI (per 1-kg/m2) | 1.06 | 1.02 to 1.10 | 0.004 | ||||||
| Waist circumference (per 10-cm) | 1.38 | 1.19 to 1.61 | <0.001 | ||||||
| Systolic BP (per 10-mmHg) | 1.17 | 1.09 to 1.26 | <0.001 | 1.16 | 1.07 to 1.25 | <0.001 | 1.04 | 0.97 to 1.12 | 0.245 |
| Diastolic BP (per 10-mmHg) | 1.16 | 1.02 to 1.32 | 0.018 | ||||||
| Antihypertensive treatment | 2.98 | 2.08 to 4.32 | <0.001 | 2.38 | 1.62 to 3.50 | <0.001 | 1.08 | 0.69 to 1.68 | 0.731 |
| Cholesterol (per 40-mg/dl) | 1.09 | 0.96 to 1.25 | 0.197 | ||||||
| HDL cholesterol (per 4-mg/dl) | 0.85 | 0.81 to 0.91 | <0.001 | 0.90 | 0.85 to 0.95 | <0.001 | 0.96 | 0.91 to 1.02 | 0.129 |
| Triglycerides (per 80-mg/dl) | 1.25 | 1.16 to 1.35 | <0.001 | ||||||
| Glucose (per 20-mg/dl) | 1.10 | 1.04 to 1.16 | <0.001 | ||||||
| Renal function (ml/min per 1.73 m2) | |||||||||
| eGFR ≥60 | 1.00 | 1.00 | |||||||
| eGFR 45 to 59 | 11.50 | 6.56 to 20.20 | <0.001 | 6.70 | 3.78 to 11.90 | <0.001 | |||
| eGFR 30 to 44 | 52.60 | 29.60 to 93.40 | <0.001 | 18.80 | 10.30 to 34.40 | <0.001 | |||
| eGFR <30 | 299.50 | 165.60 to 541.80 | <0.001 | 65.60 | 35.20 to 122.10 | <0.001 | |||
| ACR | |||||||||
| normal | 1.00 | 1.00 | |||||||
| microalbuminuriaf | 18.50 | 9.90 to 34.70 | <0.001 | 13.00 | 6.76 to 25.1 | <0.001 | |||
| macroalbuminuria | 193.70 | 94.60 to 396.70 | <0.001 | 47.50 | 19.8 to 109.0 | <0.001 | |||
Age-adjusted HRs associated with all available potential risk factors based on Cox regression analysis are given. A best clinical model was then identified by manual forward selection among the significant variables. Finally, eGFR and ACR were added to the best clinical model.
Fewer than 12 yr in school.
Eight points or more on the depression part of the Hospital Anxiety and Depression Score.
Less than 1 h of light activity per week in participants’ leisure time.
Self-reported history of myocardial infarction, angina pectoris, or stroke.
ACR ranging from 20 to 200 mg/g in men and 30 to 300 mg/g in women.
Figure 1.
HR (95% CI) for ESRD associated with urine albumin excretion. The fractional polynomial analysis was adjusted for age, gender, and eGFR, and the reference (HR 1) was set to the median ACR (8.6 mg/g).
A positive interaction between eGFR and ACR was observed. Patients with eGFR <60 ml/min per 1.73 m2 and normoalbuminuria had an age-adjusted HR of 34.8 (95% confidence interval [CI] 11.4 to 107.1) compared with the reference group with eGFR ≥60 ml/min per 1.73 m2 and normoalbuminuria. Patients with eGFR ≥60 ml/min per 1.73 m2 and micro- or macroalbuminuria had an HR of 36.4 (95% CI 12.4 to 107.0). The expected HR for patients with both risk factors should be 71.2, but the observed HR was 570.5 (95% CI 199.7 to 1630.0). Therefore, the relative excess risk due to interaction was 500.3 (95% CI 59.8 to 940.6), and an attributable portion of 0.877 (95% CI 0.826 to 0.928) indicate that 88% of the joint effect of low eGFR and increased albuminuria was due to interaction between the two risk factors. This strong synergistic effect indicates that eGFR and ACR give better risk stratification when used in combination.
Table 3 shows the HRs of progression to ESRD associated with a new 12-category variable based on the combined effect of eGFR and ACR. Cox regression analysis showed that within each ACR category, lower eGFR categories were associated with a higher risk. Likewise, progressively higher ACR categories were associated with a progressively higher risk within each eGFR category. Patients with macroalbuminuria and eGFR 15 to 29 ml/min per 1.73 m2 had a 6957 times higher risk for ESRD compared with the reference category of patients with eGFR ≥60 ml/min per 1.73 m2 and normal ACR. After multivariable adjustment, these patients had a 4146 times higher risk. Categories of low, medium, and high risk for progression to ESRD are indicated in Table 3 with footnote symbols (b, c, and d, respectively).
Table 3.
HRs for progression to ESRD by categories of eGFR and ACRa
| Parameter | eGFR (ml/min per 1.73 m2)
|
|||
|---|---|---|---|---|
| ≥60 | 45 to 59 | 30 to 44 | 15 to 29 | |
| Normal ACR | ||||
| unadjusted | 1.0b | 30.8 (9.3 to 102.2)b | 76.0 (18.5 to 313.2)b | 583.1 (120.5 to 2822.0)c |
| adjusted | 1.0b | 23.4 (6.7 to 82.1)b | 51.9 (11.5 to 233.5)b | 368.7 (69.2 to 1964.0)c |
| Microalbuminuria | ||||
| unadjusted | 33.9 (11.2 to 102.6)b | 227.4 (72.8 to 710.2)c | 740.6 (246.7 to 2222.0)c | 3833.0 (1265.0 to 11,611.0)d |
| adjusted | 27.3 (8.8 to 84.5)b | 146.5 (42.7 to 502.7)c | 448.9 (133.7 to 1508.0)c | 2202.0 (632.5 to 7669.0)d |
| Macroalbuminuria | ||||
| unadjusted | 306.6 (50.3 to 1871.0)c | 1108.0 (285.8 to 4297.0)c | 3167.0 (1066.0 to 9403.0)d | 6957.0 (2286.0 to 21,165.0)d |
| adjusted | 196.3 (27.6 to 1397.0)c | 641.1 (143.6 to 2862.0)c | 2036.0 (594.3 to 6973.0)d | 4146.0 (1187.0 to 14,482.0)d |
Numbers are unadjusted HR (95% CI) and HR after adjustment for age, gender, systolic BP, antihypertensive medication, diabetes, HDL cholesterol, and physical activity in a Cox regression analysis. Microalbuminuria was ACR ranging from 20 to 200 mg/g in men and 30 to 300 mg/g in women.
Low risk for progression to ESRD.
Medium risk for progression to ESRD.
High risk for progression to ESRD.
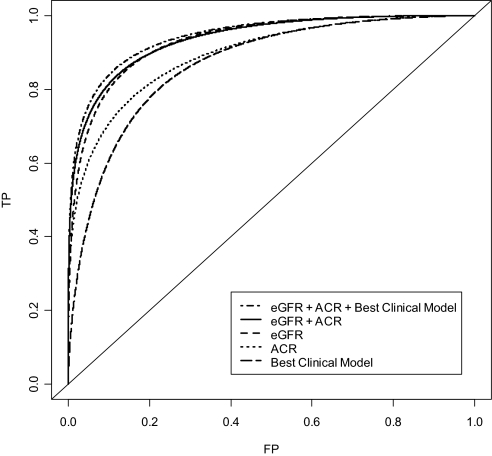
Figure 2 shows that the best clinical model (age, gender, physical activity, diabetes, systolic BP, antihypertensive medication, and HDL cholesterol) had a total area under the receiver operating characteristic (ROC) curve (AUC) of 0.864 (i.e., classified correctly 86.4% of pairs of patients in the general population who did and did not progress to ESRD). ACR alone performed substantially better (AUC 0.893), but eGFR alone had the best prediction (AUC 0.933). Combining ACR and eGFR increased the total AUC only marginally (0.936); however, using total AUC for evaluating the diagnostic accuracy of a test implies equal importance of true-positive and false-positive rates. For screening-related situations, the clinical relevant region of the curve is at very low false-positive rates. Table 4 therefore displays the partial area under the clinically relevant part of the ROC curve (false-positive rates between 0.00 and 0.10). It also shows the true-positive rates at a fixed false-positive rate of 0.03. For all screening populations evaluated, combining ACR and eGFR substantially improved discrimination, whereas the additional effect of adding the best clinical model variables was marginal. For example, when screening patients with diabetes, hypertension, or age >55 yr, adding ACR to eGFR improved the true-positive rate from 0.464 to 0.596. Including the best clinical model variables increased the true-positive rate to 0.616.
Figure 2.
Diagnostic accuracy of prognostic markers for future ESRD in the general population. The ROC curves show true-positive (TP) rates (i.e., sensitivity) for all possible false-positive (FP) rates (i.e., 1 − specificity). Increasing AUC indicates better diagnostic accuracy, and the clinical relevant region of the curve is at low FP rates (<0.10).
Table 4.
Diagnostic accuracy of prognostic markers for progression to ESRD in different populations selected for screeninga
| Prognostic Marker | Screening Strategies
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Diabetes/Hypertension
|
Diabetes/Hypertension/Age >55 yr
|
UK CKD Guidelinesb
|
Everybody
|
|||||
| TPR0.03 | pAUC | TPR0.03 | pAUC | TPR0.03 | pAUC | TPR0.03 | pAUC | |
| Best clinical model | 0.155 | 0.603 | 0.191 | 0.623 | 0.225 | 0.644 | 0.339 | 0.704 |
| ACR | 0.459 | 0.752 | 0.520 | 0.776 | 0.544 | 0.786 | 0.535 | 0.786 |
| eGFR | 0.453 | 0.754 | 0.464 | 0.757 | 0.526 | 0.787 | 0.605 | 0.821 |
| eGFR + ACR | 0.579 | 0.807 | 0.596 | 0.813 | 0.639 | 0.834 | 0.660 | 0.844 |
| eGFR + ACR + best clinical model | 0.612 | 0.820 | 0.616 | 0.822 | 0.660 | 0.842 | 0.692 | 0.858 |
TPR0.03, true-positive rate (i.e., sensitivity) at a fixed false-positive rate (FPR) of 0.03; pAUC, partial area under the clinically relevant part of the ROC curve (FPR 0.00 to 0.10) transformed to values between 0.5 and 1.0.35 Analogous to ordinary ROC analysis, a perfect test would have pAUC = 1.0, whereas a test with no ability to discriminate between those progressing to ESRD and those not progressing would have pAUC = 0.5. Best clinical model includes age, gender, physical activity, diabetes, systolic BP, antihypertensive treatment, and HDL cholesterol.
British CKD guidelines recommend screening of individuals with hypertension, diabetes, autoimmune diseases, CVD, or postrenal obstruction.4
Table 5 shows the diagnostic effectiveness of CKD screening by screening strategy (“whom to screen”) and type of CKD classification system used for referral to nephrologists or other forms of intensified follow-up (“how to screen”). The currently recommended practice is to examine patients with diabetes or hypertension for renal damage. If all patients who were found to have stages 1 through 4 CKD in this subpopulation were referred to nephrologists, then we would place under specialist care 50.2% of all patients in the general population who would progress to ESRD within the next 10.3 yr; however, 38.4 patients who would not progress would also be referred and followed per one case of ESRD (i.e., number needed to follow [NNTF] = 38.4). More restrictive referral (i.e., only patients with stages 3 to 4 CKD or stage 4 CKD alone) would substantially reduce the NNTF, but detection rate of future ESRD would be low (44.2 and 11.3%, respectively). If a wider screening strategy were used (including those with diabetes, hypertension, or age >55 yr), then referral of patients who were found to have stages 1 through 4 CKD would include 87.6% of all future ESRD cases, but a very high number of patients who would not progress to ESRD would also be referred (NNTF = 51.5). In contrast, a new classification system whereby all eGFR levels are modified by information on ACR would improve performance substantially. If patients with moderate or high risk (marked with “c” and “d” in Table 3) were referred, then detection rate would be 63.5% and NNTF only 11.4. If only those at high risk (marked with “d” in Table 3) were referred, then NNTF would be 2.6 and still 31.5% of all future ESRD cases would be detected.
Table 5.
Diagnostic effectiveness of different algorithms for detecting patients progressing to ESRDa
| Referral Criteria | Screening Strategy
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes/Hypertension
|
Diabetes/Hypertension/Age >55 yr
|
UK Guidelines
|
Everybody
|
|||||||||
| DR (%) | Pop. (%) | NNTF | DR (%) | Pop. (%) | NNTF | DR (%) | Pop. (%) | NNTF | DR (%) | Pop. (%) | NNTF | |
| CKD | ||||||||||||
| stages 1 to 4 | 50.2 | 3.7 | 38.4 | 87.6 | 8.6 | 51.5 | 70.3 | 6.5 | 48.4 | 91.9 | 12.0 | 68.4 |
| stages 3 to 4 | 44.2 | 2.1 | 25.3 | 66.9 | 4.3 | 33.8 | 57.1 | 3.2 | 28.9 | 69.4 | 4.7 | 35.4 |
| stage 4 | 11.3 | 0.1 | 4.2 | 21.8 | 0.2 | 3.8 | 18.6 | 0.1 | 4.0 | 23.4 | 0.2 | 3.6 |
| eGFR-ACR | ||||||||||||
| moderate or high risk | 39.2 | 0.9 | 11.4 | 63.5 | 1.4 | 11.4 | 52.6 | 1.2 | 11.6 | 65.6 | 1.4 | 11.4 |
| high risk only | 21.0 | 0.1 | 3.1 | 31.5 | 0.2 | 2.6 | 28.3 | 0.2 | 2.8 | 32.4 | 0.2 | 2.6 |
DR, detection rate (i.e., the proportion of all participants in the HUNT 2 study experiencing ESRD during the next 10.3 yr included for intensive follow-up); Pop., proportion of the total adult population included for intensive follow-up. eGFR-ACR moderate risk = GFR 15 to 29 and normal ACR, or eGFR 30 to 59 and microalbuminuria, or eGFR ≥60 and macroalbuminuria in Table 3; eGFR-ACR high risk = eGFR 15 to 29 and microalbuminuria, or eGFR 15 to 44 and macroalbuminuria in Table 3.
Sensitivity analyses showed that all results were stable against primary outcome misclassification originating from the low-risk groups (i.e., participants who progressed to eGFR <15 ml/min per 1.73 m2 but did not start RRT or died with ESRD during the observation period). For example, even if the number of ESRD cases from the reference group tripled (from seven to 21 cases), patients with macroalbuminuria and eGFR 15 to 29 ml/min per 1.73 m2 would still have an HR of 730 (95% CI 170 to 3103; Table 3). Likewise, the detection rates for future ESRD in the general population were still similar for stages 3 to 4 CKD versus moderate/high-risk eGFR-ACR model for referral (62.2 versus 58.8%), whereas the number of patients referred to a specialist were more than three-fold lower with the latter referral criteria (Table 5).
DISCUSSION
In this large population-based sample, we identified the combination of eGFR and albuminuria as a powerful predictor of progression to ESRD. This model did not improve significantly by addition of further variables. Our predictor was more effective than the current K/DOQI CKD classification system because the latter does not include albumin as a predictor in stages 3 to 4 CKD.
Several methodologic aspects of our study deserve discussion. First, the validity of ESRD as a primary outcome may pose problems in epidemiologic research. Many patients with ESRD die from cardiovascular causes before being taken on dialysis, but we were able to include these by linkage to the Cause of Death Registry. In addition, a few patients with eGFR <15 ml/min per 1.73 m2 remained alive without dialysis throughout a 10.3-yr study period. They pose a potential cause of misclassification bias because most cases would come from the low-risk groups16; however, sensitivity analyses showed that our results would still be valid even if the reference category contributed >200% extra cases of ESRD, an extent of misclassification that is very unlikely. We were also able to validate the diagnosis of ESRD in all cases included—a major strength of this study—and the incidence of study participants who started RRT was identical to the mean incidence for Norway.17 Another limitation is that only a subgroup of HUNT 2 participants were invited to deliver urine for albumin testing. To avoid bias and loss of statistical power, however, we used multiple imputation proposed by statisticians for this particular purpose rather than using the traditional complete-case analysis.18–21 In large populations (n > 1000) and with 20 databases imputed, as in our study, this technique has been shown to handle up to 90% missing values with a validity and precision of the imputed values almost identical to the “true” values.22,23 To avoid potential artifacts, we measured ACR in three nonfrozen urine samples within 5 d. This is highly recommended24,25 and could give more accurate results than a single measurement. Other limitations may be attributed to the imprecision of the Modification of Diet in Renal Disease (MDRD) formula in the range of near-normal values leading to risk category misclassification of some participants.26 The homogeneous study population decreases the generalizability of our results to other ethnic groups. In addition, selection bias is always a potential problem in observational studies, but the participation rate of HUNT 2 was among the highest ever reported in such large-scale studies.
The combination of eGFR and proteinuria was assessed in some studies that used small cohorts, specific populations, or dipstick testing,11,12,14 but studies specifically measuring albuminuria and covering the complete range of urine albumin values in a white general population with hard end points have not yet been reported. Iseki et al.11 studied 95,255 Japanese patients and found that dipstick proteinuria and eGFR 30 ml/min per 1.73 m2 were associated with a 1000 times higher risk for future dialysis compared with no proteinuria and eGFR ≥60 ml/min per 1.73 m2. In a small cohort of black patients (n = 1094) with hypertension and nephrosclerosis, Lea et al.14 documented that the risk for progression extends down into the range of microalbuminuria. None of these studies adjusted for other variables. Ishani et al.12 were the first to explore the eGFR–proteinuria combination in a multivariable analysis using data from 12,866 men at high risk for heart disease. Patients with macroalbuminuria and eGFR <60 ml/min per 1.73 m2 had 33 times higher risk for progressing to RRT compared with those with negative dipstick and eGFR ≥60 ml/min per 1.73 m2. Verhave et al.27 studied 6022 individuals who were from the general population and had eGFR >60 ml/min per 1.73 m2 and found that increasing albumin excretion, even in the normal range, was associated with increasing risk for renal function loss. Using the same population, Brantsma et al.28 showed that even for eGFR 30 to 59 ml/min per 1.73 m2 microalbuminuria or worse was associated with higher annual loss of renal function. Our study confirms that urine albumin is a continuous risk factor for progression to ESRD—a much more relevant end point—with no lower limit and at all levels of eGFR.
How does the predictive power of this study compare with that of past studies? Previously suggested screening strategies can detect 44 to 100% of all patients with stages 3 to 4 CKD in the general population, but, of these, only a small minority will progress to ESRD.5 Consequently, using these algorithms, the health system would be overwhelmed with an excessive number of patients who actually do not need treatment by a nephrologist. It has therefore been increasingly questioned whether the currently available CKD classification system is appropriate.6–8 Inclusion of albuminuria even at eGFR <60 ml/min per 1.73 m2 for the assessment of the risk for progression of CKD has been suggested4,8,28; however, our study is the first to address and quantify systematically the predictive power of such a classification system using a large population-based cohort with hard end points. Our study documents that adding information on urine albumin to patients with stages 3 to 4 CKD substantially increases the predictive power to identify individuals at high risk for progression. We emphasize that a large proportion of patients with stage 3 CKD are at low risk for progression as long as the urine albumin concentration is low. At the same level of eGFR, the risk rises substantially when micro- or macroalbuminuria is present.
Our study shows that including additional variables beyond eGFR and ACR in a renal risk model will not substantially improve risk prediction and can therefore be omitted for cost's and simplicity's sake. In addition, eGFR and ACR complement each other very well, leading to a strong interaction and a strong predictive power. Roughly, ACR is a marker of the rate of progression, whereas eGFR is a marker of how advanced the disease process is. This by no means negates the importance of, for example, hypertension, diabetes, and smoking as modifiable factors in the progression of CKD, because ability to predict future ESRD should not be equated with causality. As an added benefit of this approach—although not directly tested in this analysis—a previous study using the same data set showed that the combination of eGFR and ACR significantly improved cardiovascular risk prediction at all age levels but particularly in the elderly, for whom the predictive power of traditional risk factors is attenuated.29
We conclude that in the general population, eGFR and urine albumin excretion, even in the range of microalbuminuric values, are the most powerful predictors of ESRD known to date. They exhibit strong interaction, and all levels of eGFR should be complemented by information on urine albumin to improve classification and prognostication. Future renal risk scores and CKD classification systems based on these two variables will be a simple and powerful tool improving our ability to handle efficiently the large group of patients with CKD.
CONCISE METHODS
The HUNT 2 study is a Norwegian large-scale general health study. From 1995 through 1997, all individuals who resided in Nord-Trøndelag county and were aged ≥20 yr were invited. The population is stable (net out migration of 0.3% per year) and ethnically homogeneous (97% white). The participants answered a comprehensive questionnaire, underwent clinical examination, and donated a blood sample. A planned missing data design was used for expensive or bothersome examinations (e.g., repeated measurements of urine albumin concentration).30 A more detailed description of the objectives, methods, and participation in the HUNT 2 study has been given elsewhere.15 The participants gave informed consent, including linkage to central national registries. This study was approved by the Regional Committee for Medical Research Ethics, the Norwegian Data Inspectorate, and the Ministry of Health.
Of 92,939 individuals invited, 27,350 did not respond; thus, 70.6% of the entire adult population participated. All participants reported on current and former health, illness in the family, education, and risk factors such as smoking and physical inactivity. Three consecutive standardized BP measurements were recorded in the sitting position at 1-min intervals using an automatic oscillometric method (Dinamap 845XT; Critikon, Tampa, FL). Blood was obtained from all participants, immediately centrifuged and refrigerated, and analyzed within 2 d using a Hitachi 911 autoanalyzer (Hitachi, Mito, Japan).
GFR was estimated with the MDRD Study equation for standardized serum creatinine values31: eGFR = 175× [serum creatinine (mg/dl)]−1.154 × age−0.203 (×0.742 for women). The original Jaffé-based creatinine values were recalibrated providing isotope dilution mass spectrometry traceable values. By doing so, eGFR values for the HUNT 2 study have been shown to be unbiased in the normal range and for all age groups.32 Individuals with eGFR <15 ml/min per 1.73 m2 at baseline (n = 15) were excluded, and individuals with eGFR values ≥160 ml/min per 1.73 m2, which is unlikely to be physiologic, were given a value of 160 ml/min per 1.73 m2 (n = 541).
Among the 65,589 participants, 8360 had hypertension and were taking BP-lowering drugs or were patients with diabetes. These patients were asked to deliver spot urine samples on three consecutive mornings, and 88.6% returned all requested urine samples. Nonresponders were not statistically different from those who delivered all urine samples regarding important variables such as age, gender, cardiovascular disease, weight, BP, lipids, and serum creatinine. A random 5% sample of individuals without diabetes and hypertension (n = 2 861) were also asked to deliver urine samples; 75.6% returned all requested urine samples. Nonresponders were not statistically different regarding the variables mentioned already except for a younger age (44 versus 49 yr; P = 0.001) Urine albumin concentration in refrigerated urine samples (5°C) was measured within 5 d using an immunoturbidimetric method (Dako A/S, Glostrup, Denmark; lower detection level 1 mg/L), and the mean urine ACR was used as an expression for albumin excretion.
The study outcome was ESRD. In Norway, all live births are given a national identification number, enabling record linkage of all study participants to the Norwegian Renal Registry, which is >99.9% complete regarding start of RRT (T. Leivestad, registry director, personal communication, May 2008) and to the Cause of Death Registry. We used CKD death, defined as those who had a CKD diagnosis (International Classification of Diseases, 10th Revision diagnosis N00 through N19 excluding N10 and N17) as the immediate cause of death or underlying cause of death on their death certificate to find individuals who had ESRD and did not start RRT. Two experienced nephrologists manually searched the deceased individuals’ records available from the hospital and/or the general practitioner. Cases with “acute-on-chronic” renal failure were excluded. ESRD was defined as starting RRT or death with advanced renal failure (i.e., a documented CKD diagnosis and a documented stable eGFR <15 ml/min per 1.73 m2 or other indications for RRT before death).
Statistical analyses were performed using Stata 10.0 (Stata Corp., College Station, TX). In general, there were few missing data (<2% for most variables; Table 1), but repeated measurements of ACR were, by study design, available only in a subgroup. Multiple imputation is now considered the standard method for handling this type of data,18–21 whereas complete case analysis would yield too imprecise as well as biased results. The multiple imputation technique estimates the mean and uncertainty of the missing data in individuals using all information from the actually observed data in a proper way. Using the Stata command “ice,” we created 20 complete data sets to achieve maximum accuracy.22,23 Subsequently, the Stata command “micombine” was used together with standard statistical methods, giving unbiased risk estimates with correct CIs. For most individuals without diabetes and hypertension, data were missing completely at random, and for those who did not return urine samples as requested, data were assumed to be missing at random, thus meeting the assumptions of the method. ACR was log-transformed and not used as predictor in the imputation of other missing variables,19 study outcome (ESRD) was included in the imputation model,33 and the time variable was log-transformed.19 Regression modeling revealed interactions between gender and both BP and diabetes; therefore, these two interactions were included in the imputation model.
Cox proportional hazard regression analysis was used to evaluate the influence of renal risk factors on progression to ESRD. We checked for crossover and other nonproportional survival patterns and for linearity in continuous variables. Binary variables were coded as 0/1, and ordinal variables were coded as integer values. All individuals were passively observed (i.e., further clinical examinations or blood samples were not performed) until January 1, 2006; death; or starting RRT, whichever occurred first. Age-adjusted associations for all available variables were evaluated, and a best clinical model was built by entering significant variables into a multivariable model using stepwise forward selection. Finally, eGFR and ACR were added to the previous best clinical model, and, in all further analyses, we consistently adjusted the eGFR-ACR results for the best clinical model variables. Interaction on an additive scale was used to evaluate whether eGFR and ACR are more effective for risk stratification when used together than separately.34 Two-sided P < 0.05 was considered significant. A composite variable with 12 categories combining four eGFR categories and three ACR categories was used to evaluate the combined effect of these two variables. Microalbuminuria was defined as ACR 20 to 200 mg/g in men and 30 to 300 mg/g in women, and macroalbuminuria was defined as ACR >200 mg/g in men and >300 mg/g in women.25 The continuous relationship of higher ACR with future ESRD was explored using fractional polynomial models adjusted for eGFR, gender, and age. Because relative risk–based methods are not well suited for classifying or predicting risk in individuals, we used time-dependent ROC curves for survival data.35 In population-based screening, the clinical relevant region of the ROC curve is at low false-positive rates. We calculated the partial area under the ROC curve for false-positive rates between 0.00 and 0.10.36 For diseases with a low prevalence and/or with no confirmative test available, false-positive rates of 0.03 and lower are often recommended to avoid the financial, medical, and psychological costs inflicted by a large number of false-positive cases.37,38 Different models were therefore also compared at a fixed false-positive rate of 0.03.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The HUNT Study is a collaboration among the HUNT Research Center, Faculty of Medicine, Norwegian University of Science and Technology; The Norwegian Institute of Public Health; Nord-Trøndelag County Council; and Central Norway Regional Health Authority.
We thank the health service and people of Nord-Trøndelag for endurance and participation.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “CKD Classification: Time to Move Beyond KDOQI,” on pages 929–930.
REFERENCES
- 1.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 39: S1–S246, 2002 [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275–2284, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Crowe E, Halpin D, Stevens P, Guideline Development Group. Early identification and management of chronic kidney disease: summary of NICE guidance. BMJ 337: a153, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Hallan SI, Dahl K, Oien CM, Grootendorst DC, Aasberg A, Holmen J, Dekker FW: Screening strategies for chronic kidney disease in the general population: Follow-up of cross sectional health survey. BMJ 333: 1047, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer C, Melamed ML, Hostetter TH: Staging of chronic kidney disease: Time for a course correction. J Am Soc Nephrol 19: 844–846, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Couser WG: Chronic kidney disease the promise and the perils. J Am Soc Nephrol 18: 2803–2805, 2007 [DOI] [PubMed] [Google Scholar]
- 8.de Jong PE, Gansevoort RT: Fact or fiction of the epidemic of chronic kidney disease: Let us not squabble about estimated GFR only, but also focus on albuminuria. Nephrol Dial Transplant 23: 1092–1095, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Taal MW, Brenner BM: Renal risk scores: Progress and prospects. Kidney Int 73: 1216–1219, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives—A position statement from Kidney Disease: Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Iseki K, Kinjo K, Iseki C, Takishita S: Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis 44: 806–814, 2004 [PubMed] [Google Scholar]
- 12.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD: Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17: 1444–1452, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Keane WF, Zhang Z, Lyle PA, Cooper ME, de Zeeuw D, Grunfeld JP, Lash JP, McGill JB, Mitch WE, Remuzzi G, Shahinfar S, Snapinn SM, Toto R, Brenner BM, RENAAL Study Investigators: Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: The RENAAL Study. Clin J Am Soc Nephrol 1: 761–767, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Lea J, Greene T, Hebert L, Lipkowitz M, Massry S, Middleton J, Rostand SG, Miller E, Smith W, Bakris GL: The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: Results of the African American Study of Kidney Disease and Hypertension. Arch Intern Med 165: 947–953, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Holmen J, Midthjell K, Kruger O, Langhammer A, Lingaas Holmen T, Bratberg G, Vatten L, Lund-Larsen P: The Nord-Trondelag Health Study 1995–97 (HUNT 2): Objectives, contents, methods and participation. Norsk Epidemiol 13: 19–32, 2003 [Google Scholar]
- 16.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE: Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 22: 1955–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Norwegian Renal Registry, annual reports. Available at: http://www.nephro.no/nnr.html. Accessed May 22, 2008
- 18.Rassler S, Rubin DB, Zell ER: Incomplete data in epidemiology and medical statistics. In: Handbook of Statistics, vol. 27 edited by Rao CR, Miller J, Rao DC, Amsterdam, Elsevier, 2008, pp 569–601
- 19.van Buuren S, Boshuizen HC, Knook DL: Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 18: 681–694, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Donders AR, van der Heijden GJ, Stijnen T, Moons KG: Review: A gentle introduction to imputation of missing values. J Clin Epidemiol 59: 1087–1091, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Schafer JL, Graham JW: Missing data: Our view of the state of the art. Psychol Methods 7: 147–177, 2002 [PubMed] [Google Scholar]
- 22.Newgard CD, Haukoos JS: Advanced statistics: Missing data in clinical research—Part 2: Multiple imputation. Acad Emerg Med 14: 669–678, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB: Multiple Imputations for Nonresponse in Surveys, New York, J. Wiley & Sons, 1987
- 24.Brinkman JW, de Zeeuw D, Lambers Heerspink HJ, Gansevoort RT, Kema IP, de Jong PE, Bakker SJ: Apparent loss of urinary albumin during long-term frozen storage: HPLC vs immunonephelometry. Clin Chem 53: 1520–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 25.de Jong PE, Curhan GC: Screening, monitoring, and treatment of albuminuria: Public health perspectives. J Am Soc Nephrol 17: 2120–2126, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function: Measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De Zeeuw D, de Jong PE, The PREVEND study group. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl 2004 Nov(92): S18–21 [DOI] [PubMed]
- 28.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT: Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: The importance of urinary albumin excretion. Nephrol Dial Transplant 23: 3851–3858, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Hallan SI, Astor BC, Romundstad S, Aasarod K, Kvenild K, Coresh J: Association of kidney function and albuminuria with cardiovascular mortality in older versus younger individuals: The HUNT II study. Arch Intern Med 167: 2490–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Graham JW, Taylor BJ, Olchowski AE, Cumsille PE: Planned missing data designs in psychological research. Psychol Methods 11: 323–343, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hallan S, Astor BC, Lydersen S: Estimating glomerular filtration rate in the general population: The Second Health Survey of Nord Trondelag (HUNT II). Nephrol Dial Transplant 21: 1525–1533, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Moons KG, Donders RA, Stijnen T, Harrell FE: Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 59: 1092–1101, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Rothman KJ, Greenland S: Modern Epidemiology, 2nd Ed., Philadelphia, Lippincott Williams & Wilkins, 2002
- 35.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P: Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 159: 882–890, 2004 [DOI] [PubMed] [Google Scholar]
- 36.McClish DK: Analyzing a portion of the ROC curve. Med Decis Making 9: 190–195, 1989 [DOI] [PubMed] [Google Scholar]
- 37.Alfirevic Z, Neilson JP: Antenatal screening for Down's syndrome. BMJ 329: 811–812, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokol J, Hyde M: Hearing screening. Pediatr Rev 23: 155–162, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.