Abstract
Recent studies have suggested that mycophenolate mofetil (MMF) may offer advantages over intravenous cyclophosphamide (IVC) for the treatment of lupus nephritis, but these therapies have not been compared in an international randomized, controlled trial. Here, we report the comparison of MMF and IVC as induction treatment for active lupus nephritis in a multinational, two-phase (induction and maintenance) study. We randomly assigned 370 patients with classes III through V lupus nephritis to open-label MMF (target dosage 3 g/d) or IVC (0.5 to 1.0 g/m2 in monthly pulses) in a 24-wk induction study. Both groups received prednisone, tapered from a maximum starting dosage of 60 mg/d. The primary end point was a prespecified decrease in urine protein/creatinine ratio and stabilization or improvement in serum creatinine. Secondary end points included complete renal remission, systemic disease activity and damage, and safety. Overall, we did not detect a significantly different response rate between the two groups: 104 (56.2%) of 185 patients responded to MMF compared with 98 (53.0%) of 185 to IVC. Secondary end points were also similar between treatment groups. There were nine deaths in the MMF group and five in the IVC group. We did not detect significant differences between the MMF and IVC groups with regard to rates of adverse events, serious adverse events, or infections. Although most patients in both treatment groups experienced clinical improvement, the study did not meet its primary objective of showing that MMF was superior to IVC as induction treatment for lupus nephritis.
Lupus nephritis (LN) occurs in up to 60% of adults with systemic lupus erythematosus (SLE) and predicts poor survival.1,2 The prevalence of SLE and LN and treatment response vary by age, gender, location, and race/ethnicity; LN is especially common in black and Hispanic patients in the United States.3,4
Use of intravenous cyclophosphamide (IVC) is based on studies in the 1970s and 1980s at the National Institutes of Health (NIH).5,6 The subsequent induction regimen, widely considered the standard of care, requires monthly intravenous drug infusions.7 Response is often slow,8 and treatment fails to control LN fully and is associated with increased risks for adverse effects, including gonadal toxicity.9 Among other immunosuppressants, recent studies have focused on mycophenolate mofetil (MMF).10 Unlike IVC, MMF has not been associated with an increased risk for bladder or ovarian toxicity in LN10 or during long-term use after transplantation.11
MMF was at least as effective as IVC in induction treatment in previous trials in Hong Kong,12,13 Malaysia,14 China,15 and the United States.16 Meta-analyses of these and smaller trials suggested that MMF may offer advantages over IVC, but they have not yet been compared in an international randomized, controlled trial.17–19 We therefore undertook one of the largest studies to date in patients with LN, a two-part trial to assess the efficacy and safety of MMF as induction therapy and subsequently as maintenance therapy for LN. This report describes the comparison of MMF with IVC, both with corticosteroids, for the induction treatment of active classes III, IV, and V LN. The hypothesis was that more patients with LN would respond to MMF than to IVC during 24 wk.
RESULTS
Demographics
Of 460 patients screened, 370 were randomly assigned (Figure 1). Most patients excluded did not meet the study criteria for baseline disease. At baseline, all patients enrolled had active proliferative (class III/IV) and/or membranous (class V) LN. Demographics and baseline disease characteristics were similar between the treatment groups (Table 1). Six randomly assigned patients (one in the MMF group and five in the IVC group) were excluded from the safety analysis because they received no study drug. At week 24, 306 (82.7%) patients remained in the study. In the MMF group, 35 (18.9%) patients withdrew from the study, compared with 29 (15.7%) in the IVC group. The reasons for withdrawal before 24 wk in both groups are described in Table 2. There were no crossovers between treatments during the study.
Figure 1.
Patient disposition. *Hepatitis/cytomegalovirus infection (n = 16) or other illness (n = 4). ACR, American College of Rheumatology.
Table 1.
Demographics and baseline disease characteristicsa
| Characteristic | MMF (n = 185) | IVC (n = 185) | Total (N = 370) |
|---|---|---|---|
| Gender (n [%]) | |||
| male | 28 (15.1) | 29 (15.7) | 57 (15.4) |
| female | 157 (84.9) | 156 (84.3) | 313 (84.6) |
| Race (n [%]) | |||
| white | 75 (40.5) | 72 (38.9) | 147 (39.7) |
| Asian | 62 (33.5) | 61 (33.0) | 123 (33.2) |
| otherb | 48 (25.9) | 52 (28.1) | 100 (27.0) |
| Ethnicity (n [%]) | |||
| Hispanic | 64 (34.6) | 67 (36.2) | 131 (35.4) |
| non-Hispanic | 121 (65.4) | 118 (63.8) | 239 (64.6) |
| Region (n [%]) | |||
| Asia | 57 (30.8) | 60 (32.4) | 117 (31.6) |
| Latin America | 56 (30.3) | 50 (27.0) | 106 (28.6) |
| United States/Canada | 37 (20.0) | 38 (20.5) | 75 (20.3) |
| rest of world | 35 (18.9) | 37 (20.0) | 72 (19.5) |
| Renal biopsy class (n [%]) | |||
| III/III + V | 32 (17.3) | 26 (14.1) | 58 (15.7) |
| IV/IV + V | 124 (67.0) | 128 (69.2) | 252 (68.1) |
| V only | 29 (15.7) | 31 (16.8) | 60 (16.2) |
| Scarring on renal biopsy (n [%])c | 66 (35.7) | 56 (30.3)d | 122 (33.0)d |
| Serum creatinine (μmol/L [mg/dl]; mean ± SD) | 108.6 ± 1.2 (97.2 ± 1.1) | 92.7 ± 1.0 (56.9 ± 0.6)d | 100.6 ± 1.1 (80.0 ± 0.9)d |
| Urine protein/creatinine ratio (mean ± SD) | 4.1 ± 4.2e | 4.1 ± 3.2f | 4.1 ± 3.7e,f |
| Range of GFR (ml/min per 1.73 m2; n [%])d,g | |||
| ≥90 | 80 (43.2) | 86 (46.7) | 166 (45.0) |
| ≥60 to <90 | 53 (28.6) | 52 (28.3) | 105 (28.5) |
| ≥30 to <60 | 32 (17.3) | 34 (18.5) | 66 (17.9) |
| <30 | 20 (10.8) | 12 (6.5) | 32 (8.7) |
| Range of anti-dsDNA antibody titer (IU/ml; n [%])h | |||
| <30 (negative) | 32 (18.4) | 23 (13.5) | 55 (15.9) |
| 30 to 60 (low positive) | 25 (14.4) | 24 (14.0) | 49 (14.2) |
| >60 to 200 (positive) | 52 (29.9) | 40 (23.4) | 92 (26.7) |
| >200 (strong positive) | 65 (37.4) | 84 (49.1) | 149 (43.2) |
| Range of C3 concentration (g/L; n [%])i | |||
| >1.8 | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| 0.9 to 1.8 (normal) | 50 (28.4) | 34 (19.5) | 84 (24.0) |
| <0.9 (low) | 125 (71.0) | 139 (79.9) | 264 (75.4) |
| Range of C4 concentration (g/L; n [%])j | |||
| >0.47 | 2 (1.1) | 1 (0.6) | 3 (0.9) |
| 0.16 to 0.47 (normal) | 70 (39.8) | 47 (27.2) | 117 (33.5) |
| <0.16 | 104 (59.1) | 125 (72.3) | 229 (65.6) |
| Age at enrollment (yr; mean ± SD) | 32.4 ± 11.2 | 31.3 ± 10.3 | 31.9 ± 10.7 |
| Age at diagnosis of lupus nephritis (yr; mean ± SD) | 30.2 ± 11.0 | 28.8 ± 10.2 | 29.5 ± 10.6 |
| Time since diagnosis of lupus nephritis (yr; median [range])k | 1.0 (1 to 21) | 1.0 (1 to 23) | 1.0 (1 to 23) |
Anti-dsDNA, antibodies reactive to double-stranded DNA; C3, complement factor 3; C4, complement factor 4.
Race self-reported as black (46), Mexican-Mestizo (28), mixed race (9), Hispanic (3), North African (2), Chinese (1), South/Central America/Caribbean (3), Native American (1), Pacific Islander (1), Eritrean (1), East Indian (1), Middle Eastern (1), Latin (1), brown (1), or white (1).
Scarring defined according to ISN classification of class III/IV active/chronic lupus nephritis.20
Data missing for one patient in the IVC group.
n = 180.
n = 181.
GFR was estimated by the Modification of Diet in Renal Disease method.21
Data missing for 11 patients in the MMF group and for 14 patients in the IVC group.
Data missing for nine patients in the MMF group and for 11 patients in the IVC group.
Data missing for nine patients in the MMF group and for 12 patients in the IVC group.
Time since diagnosis was rounded up to 1.0 yr for patients whose time was <1 yr.
Table 2.
Summary of reasons for premature withdrawal from treatment (intention-to-treat population)
| Parameter | MMF (n = 185; n [%]) | IVC (n = 185; n [%]) |
|---|---|---|
| Completed 24-wk open-label induction phase | 150 (81.1) | 156 (84.3) |
| Total no. of patients withdrawn prematurely | 35 (18.9) | 29 (15.7) |
| Reasons for withdrawal from induction phase | ||
| adverse event | 21 (60.0) | 12 (41.4) |
| deterioration with respect to serum creatinine after 12 and 16 wk of treatment | 0 (0.0) | 2 (6.9) |
| dosage reduction of MMF <2 g/d for >14 d | 1 (2.9) | 0 (0.0) |
| lost to follow-up | 1 (2.9) | 2 (6.9) |
| patient died | 3 (8.6) | 1 (3.4) |
| patient withdrew consent | 6 (17.1) | 5 (17.2) |
| physician decision | 1 (2.9) | 3 (10.3) |
| sponsor decision | 2 (5.7) | 1 (3.4) |
| noncompliance | 0 (0.0) | 1 (3.4) |
| reason not noted | 0 (0.0) | 2 (6.9) |
Exposure
The median dosage was calculated for 179 patients in the MMF group as 2.6 g/d; median average dosage was similar for each of the racial groups (white 2.6 g/d; Asian 2.6 g/d; black 2.4 g/d; and other 2.8 g/d). The corresponding mean ± SD average dosage was 2.47 ± 0.58 g/d. A maximum MMF dosage of 2.5 to 3.0 g/d was achieved in 168 (91.3%) of 184 patients. For 180 patients in the IVC group and for patients in each of the self-reported racial groups, the median number of doses was 6.0. Overall, the median total dosage per infusion of IVC was 0.75 g/m2; the median total dosage per infusion was slightly higher in the “other” and black racial groups (0.840 and 0.875 g/m2, respectively) compared with the Asian and white groups (0.785 and 0.750 g/m2, respectively). The corresponding mean number of doses of IVC was 5.6 ± 1.1. The mean duration of treatment was 156.2 d for the MMF group and 162.5 d for the IVC group. Overall, the mean dosage of prednisone did not differ between groups (25.8 and 26.0 mg/d for the MMF and IVC groups, respectively), and the steady decrease in prednisone dosage in each group during the course of the 24-wk induction phase was consistent with the mandated steroid-tapering schedule (Supplemental Figure 1). The numbers of patients receiving concomitant medications were mostly similar between treatment groups.
Efficacy
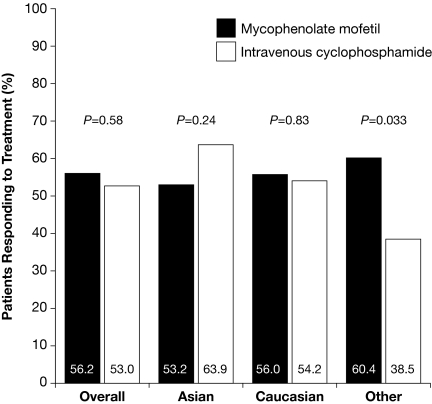
The primary efficacy end point was achieved in 104 (56.2%) patients receiving MMF, compared with 98 (53.0%) patients receiving IVC (odds ratio [OR] 1.2; 95% confidence interval [CI] 0.8 to 1.8; P = 0.58; Figure 2). There were statistically significant interactions between treatment group and race (P = 0.047) and between treatment group and region (P = 0.069). In the sensitivity analysis, the number of patients achieving the primary efficacy end point was not statistically significantly different between treatment groups, irrespective of adjustment for covariates. Data for the per-protocol population was supportive of that observed for the intention-to-treat population; the primary efficacy end point was achieved by 86 (63.7%) of 135 patients in the MMF group compared with 89 (57.1%) of 156 patients in the IVC group (OR 1.4; 95% CI 0.8 to 2.2; P = 0.32). Response rates according to self-classification of race with MMF and IVC were similar for Asian (33 [53.2%] of 62 versus 39 [63.9%] of 61; OR 0.6; 95% CI 0.3 to 1.3; P = 0.24) and white patients (42 [56.0%] of 75 versus 39 [54.2%] of 72; OR 1.1; 95% CI 0.6 to 2.1; P = 0.83; Figure 2); however, among patients grouped as “other,” a group mostly comprising black (n = 46) and mixed-race (n = 37) patients (see Table 1 for detailed racial breakdown), 29 (60.4%) of 48 patients responded with MMF and 20 (38.5%) of 52 patients with IVC (OR 2.4; 95% CI 1.1 to 5.4; P = 0.033). Post hoc analysis showed that response rates among Hispanic patients (n = 131) were 60.9% (39 of 64) for MMF and 38.8% (26 of 67) for IVC (OR 2.5; 95% CI 1.2 to 5.1; P = 0.011).
Figure 2.
Response rates of study population and by racial group.
There were no statistically significant differences between the scores of each treatment group on any of the secondary efficacy end points (Table 3). The response rates were similar between patients with renal biopsy class III or IV and those with renal biopsy class V, irrespective of treatment. At week 24, 130 (70.3%) patients in the MMF group had normal serum creatinine, compared with 125 (67.6%) in the IVC group; and 44 patients (23.8%) in the MMF group had ≤0.5 g/d proteinuria, compared with 50 (27.0%) patients in the IVC group. Only 16 (8.6%) patients in the MMF group and 15 (8.1%) in the IVC group achieved complete remission after 24 wk, with substantial urine protein persisting in many patients (Supplemental Figure 2). Overall, 32 patients had an estimated GFR <30 ml/min per 1.73 m2 at baseline.21 Of the 12 such patients in the IVC group, two (16.7%) responded and two (16.7%) died; of 20 in the MMF group, four (20.0%) responded and three (15.0% died). There was no progression on the Systemic Lupus International Collaborating Clinics damage index in either patient group.
Table 3.
Summary of results of secondary efficacy end pointsa
| Parameter | MMF (n = 185) | IVC (n = 185) | Odds Ratio (95% CI) |
|---|---|---|---|
| Responders with renal biopsy class III or IV | 88 (56.4)b | 83 (53.9)c | 1.1 (0.7 to 1.8) |
| Patients with renal biopsy class V | 16 (55.2)d | 15 (48.4)e | |
| Renal remission criterion met | Treatment difference (% [95% CI]) | ||
| serum creatinine | 130 (70.3) | 125 (67.6) | 2.7 (−6.7 to 12.1) |
| urine protein | 44 (23.8) | 50 (27.0) | −3.2 (−12.1 to 5.6) |
| urine sediment | 58 (31.4) | 44 (23.8) | 7.6 (−1.5 to 16.6) |
| all three criteria | 16 (8.6) | 15 (8.1) | 0.5 (−5.1 to 6.2) |
| Renal and extrarenal remission | |||
| complete absence of BILAG As and Bs | 54 (29.7)f | 45 (24.9)g | 4.8 (4.3 to 14.0) |
| SELENA-SLEDAI | Difference between means (95% CI) | ||
| change in score from baseline to end point (mean ± SD) | −6.2 ± 10.1h | −6.6 ± 8.0i | 0.41 (−1.48 to 2.30) |
| Anti-dsDNA | |||
| patients with dsDNA >60 IU/ml at baselinej | 117 (67.2)k | 124 (72.5)l | |
| patients with dsDNA >60 IU/ml at end point | 72 (41.4)k | 91 (53.2)l | |
| C3 | |||
| patients with low C3 at baselinem | 125 (71.0)n | 139 (79.9)k | |
| patients with low C3 at end pointm | 70 (39.8)n | 90 (51.7)k | |
| C4 | |||
| patients with low C4 at baselineo | 104 (59.1)n | 125 (72.3)p | |
| patients with low C4 at end pointo | 51 (29.0)n | 72 (41.6)p |
Data are n(%), unless specified otherwise. BILAG, British Isles Lupus Assessment Group Scale; SELENA-SLEDAI, Safety of Exogenous Estrogens in Lupus Erythematosus National Assessment / Systemic Lupus Erythematosus Disease Activity Index.
n = 156.
n = 154.
n = 29.
n = 31.
n = 182.
n = 181.
n = 179.
n = 178.
The threshold >60 IU/ml was twice the upper limit of the range defined as normal.
n = 174.
n = 171.
Low C3 was defined as <0.9 g/L.
n = 176.
Low C4 was defined as <0.16 g/L in one laboratory and <0.10 g/L in the other; each patient's baseline and end point samples were analyzed at the same laboratory.
n = 173.
Adverse Events
Of the 184 patients treated with MMF and 180 with IVC, the proportions reporting adverse events (AEs) were similar (96.2% for MMF versus 95.0% for IVC; treatment difference 1.20%; 95% CI −3.02 to 5.42%; P = 0.58; Table 4). There were 40.6% more AEs in the IVC group (2088) than in the MMF group (1485) during the 24-wk treatment period. In both treatment groups, the most common types of AE were infections (68.5% with MMF; 61.7% with IVC; treatment difference 6.81%; 95% CI −2.96 to 16.58%; P = 0.17; Supplemental Table 1) and gastrointestinal disorders (61.4% with MMF; 66.7% with IVC). The most commonly reported AEs are shown in Table 4. There were 24 withdrawals (13.0% of patients) as a result of AEs in the MMF group compared with 13 (7.2%) in the IVC group (treatment difference 5.82%; 95% CI −0.34 to 11.99%; P = 0.07).
Table 4.
Incidences of adverse events reported by >10% of patientsa
| Parameter | Patients Who Experienced at Least One AE
|
|
|---|---|---|
| MMF (n = 184) | IVC (n = 180) | |
| Deaths | 9 (4.9) | 5 (2.8) |
| Withdrawals as a result of AEs | 24 (13.0) | 13 (7.2) |
| All AEs | 177 (96.2) | 171 (95.0) |
| diarrhea | 52 (28.3) | 23 (12.8) |
| headache | 38 (20.7) | 47 (26.1) |
| peripheral edema | 35 (19.0) | 30 (16.7) |
| arthralgia | 29 (15.8) | 43 (23.9) |
| nausea | 27 (14.7) | 82 (45.6) |
| hypertension | 26 (14.1) | 25 (13.9) |
| nasopharyngitis | 25 (13.6) | 29 (16.1) |
| vomiting | 25 (13.6) | 68 (37.8) |
| cough | 24 (13.0) | 16 (8.9) |
| anemia | 23 (12.5) | 12 (6.7) |
| alopecia | 20 (10.9) | 64 (35.6) |
| abdominal pain | 19 (10.3) | 13 (7.2) |
| back pain | 19 (10.3) | 16 (8.9) |
| muscle spasms | 19 (10.3) | 17 (9.4) |
| rash | 19 (10.3) | 21 (11.7) |
| urinary tract infection | 19 (10.3) | 17 (9.4) |
In the safety population, 51 (27.7%) patients in the MMF group and 41 (22.8%) in the IVC group had at least one serious AE (treatment difference 4.90%; 95% CI −4.01 to 13.81%; P = 0.28). The most commonly reported types of serious AEs in both groups were infections, occurring in 22(12.0%) patients with MMF and 18 (10.0%) patients with IVC; gastrointestinal disorders, occurring in eight (4.3%) patients with MMF and three (1.7%) patients with IVC; and renal and urinary disorders, occurring in eight (4.3%) patients with MMF and three (1.7%) patients with IVC.
There were nine deaths in the MMF group and five in the IVC group. In the MMF group (treatment difference 2.11%; 95% CI −1.82 to 6.04%; P = 0.29), seven deaths were due to infection and none were due to SLE, compared with two that were due to infections and two that were due to SLE in the IVC group. In the MMF group, there were two deaths in Latin America and seven in Asia. In the IVC group, there were two deaths in North America, two in Asia, and one in Europe.
DISCUSSION
In this study, MMF did not show superiority over IVC for the induction therapy of LN, as measured by renal response rate after 24 wk of treatment (95% CI 0.8 to 1.8; P = 0.58). Interestingly, there was a statistically significant interaction between treatment group and race (P = 0.047) and between treatment group and region (P = 0.069). Results did not differ between treatment groups for any secondary renal or nonrenal efficacy end points. Serious AEs were reported at similar rates in the two treatment groups (95% CI −3.02 to 5.42%; P = 0.58), whereas the most common types of AE were infections (95% CI −2.96 to 16.58%; P = 0.17). A total of 14 patients died during the study: Nine in the MMF group and five in the IVC group (95% CI −1.82 to 6.04%; P = 0.29). Seven deaths in the MMF group were due to infection and none were due to SLE, compared with two that were due to infections and two that were due to SLE in the IVC group. Treatment discontinuation owing to AEs was responsible for 24 study withdrawals in the MMF group and 13 in the IVC group.
Although meta-analyses of smaller studies17–19 have suggested that more patients respond to MMF than to IVC, results from the large and racially diverse population of this study indicate that these drugs in combination with prednisone have similar efficacy in short-term induction therapy. The open-label design of this study was chosen because the AE profiles of the two study drugs would interfere with attempted blinding; the randomization of patients to treatment groups should mitigate any potential bias produced as a result of this design.
Although the primary objective was not met, there were important differences across racial and ethnic groups, with more patients in the high-risk, nonwhite, non-Asian group responding to MMF than to IVC. Statistically significant interactions between treatment and race and between treatment and region were not explained by differences in disease characteristics at baseline between the subgroups. Subanalyses revealed that statistically significantly fewer patients responded to IVC than to MMF in the “other” group, most of whom were black or Latin American mixed race. Similarly, fewer Hispanic patients responded to IVC than to MMF. It has been reported that black and Hispanic patients are at an increased risk for aggressive disease.4,22 Furthermore, a greater prevalence of renal failure has been reported among black patients,23 with genetic rather than socioeconomic factors believed to be a more likely cause. Conversely, environmental, socioeconomic/demographic, psychosocial, genetic, and clinical factors were thought to play an important role as determinants of ethnic differences observed in LN outcome.24 Socioeconomic and medical factors were largely controlled in the trial setting, and socioeconomic data were not recorded as part of this study; consequently, the impact of socioeconomic factors on the efficacy of therapeutic interventions for LN remains to be determined. The results of this study may support the clinical impression that the efficacy of IVC varies between racial and ethnic groups and that IVC is less effective in patients of African or Hispanic descent.3,22–26 In contrast, MMF has seemed to be consistently effective in all racial/ethnic groups. The wide variation in response by race/ethnicity for IVC may have been confounded by regional variations, possibly as a result of differences in clinical practice.
Data on response to any previous therapies were unavailable, so investigation was not possible into this potential source of bias; however, >50% of patients had received a diagnosis of LN <1 yr before enrollment. Complete remission rates were low for both treatments in comparison with previous randomized studies in Hong Kong and the United States, although the definitions of remission vary between studies.12,16 Persistent urine protein is common after 6 mo of treatment for severe LN, regardless of treatment regimen, and usually decreases further with continued follow-up.16,27 The low remission rates across all of the racial/ethnic groups studied in the Aspreva Lupus Management Study (ALMS) highlight the need to investigate the ideal regimen and its duration in patients with LN. Parameters such as the duration of the induction phase and the response criteria were based on previously reported trials12,16,25,27,28; however, 24 wk may be too short to differentiate between the treatments, because the disease may continue to improve and AEs may continue to emerge.8 After the induction phase, patients who responded to treatment were randomly assigned again to receive double-blinded MMF or azathioprine in the maintenance phase, which remains in progress.
The overall AE profiles of both MMF and IVC in this study are consistent with previous studies12,16–19,25; however, differences in the relative proportions of patients reporting specific AEs in the two treatment groups in this study were apparent when compared with previously reported rates. For both treatments, the most common AEs were infections and gastrointestinal disorders, occurring in approximately two thirds of patients. Nausea and vomiting were the most common gastrointestinal disorder in patients with IVC, but diarrhea predominated with MMF. Alopecia, an undesirable AE in a disease that mainly affects young women, occurred mostly in the IVC group. It should be noted that, unlike the renal outcome measures, patients’ reports of some AEs may have been influenced by the open-label trial design. During 24 wk, increased malignancy risk or gonadal toxicity with IVC was not expected.29–31 These results will be examined in the ongoing maintenance phase of ALMS.
There were more deaths in the MMF group, contrasting with previous trials.12,14,16 There seemed to be no single factor that led to death of these patients; however, especially in the Asian patients, a complex interaction between severe underlying lupus disease and the rapid deterioration in and late presentation of respiratory signs and symptoms may have led to particularly adverse outcomes. In some patients, this led to continued aggressive treatment of lupus with MMF and high-dosage corticosteroids, which we speculate may have contributed to the overwhelming infection in patients whose immune status was unstable at study entry. The different infection rates and mortality data in ALMS compared with previous trials remain unexplained, but, clearly, these agents are potent immunosuppressants and infectious complications must be expected.
Examination of the data did not reveal an association of region, race, body weight, or body surface area with safety outcomes of either treatment group. Most patients who died had severe renal disease at baseline. Unlike previous studies, which excluded patients with substantially reduced GFR, ALMS included 32 patients with GFR <30 ml/min per 1.73 m2.
In this induction phase of ALMS, one of the largest and most racially diverse studies of treatments for LN to date, the efficacy and tolerability of oral MMF during 6 mo did not differ from those of IVC, the current standard treatment; however, other measures of clinical effectiveness are important to clinicians and patients and may influence prescribing decisions. These include the convenience of twice-daily oral medication (MMF) versus a monthly infusion (IVC) and the impact on women of child-bearing age as a result of the potential of IVC to cause ovarian dysfunction. Neither of these was objectively addressed in this study because of the length of follow-up and limited health economic assessments undertaken. On the basis of these study data, physicians may consider MMF as an alternative therapy to IVC in induction treatment of LN.
CONCISE METHODS
Design Overview
This was a prospective, randomized, open-label, parallel-group, multicenter study. Detailed methods of ALMS (protocol WX17801, registered at ClinicalTrials.gov, NIH registration no. NCT00377637) has been published.32 The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. The institutional review boards at all participating centers approved the protocol, and all patients provided written informed consent.
Setting and Participants
Patients (n = 370) were enrolled at 88 centers in 20 countries in North America, Latin America, Asia, Australia, and Europe. Patients who were aged 12 to 75 yr and had a diagnosis of SLE (by American College of Rheumatology criteria33) were enrolled between July 27, 2005, and October 6, 2006. LN (active or active/chronic) was confirmed by kidney biopsy within 6 mo before randomization as International Society of Nephrology/Renal Pathology Society 2003 class III, IV-S or IV-G, V, III+V, or IV+V.20 Patients with class III or V LN must have had proteinuria (at least 2 g/d), which was considered a clinically significant level of proteinuria, and might indicate a recent deterioration in renal function. Reasons for exclusion were treatment with MMF or IVC within the previous year, continuous dialysis for >2 wk before randomization or anticipated duration longer than 8 wk, pancreatitis, gastrointestinal hemorrhage within 6 mo or active peptic ulcer within 3 mo, severe viral infection, severe cardiovascular disease, bone marrow insufficiency with cytopenias not attributable to SLE, or current infection requiring intravenous antibiotics. Pulse intravenous corticosteroids were prohibited within 2 wk before first randomization and throughout the study. During the study, any drugs affecting the angiotensin system were taken at stable dosage. Detailed inclusion and exclusion criteria are given in Supplemental Table 2.
Randomization and Interventions
Patients were randomly assigned (1:1, stratified by race and biopsy class, nonblocked) to treatment with MMF or IVC by a central, computerized, interactive voice response system. Oral MMF was given twice daily, titrated from 0.5 g twice daily in week 1 and 1.0 g twice daily in week 2, to a target dosage of 1.5 g twice daily in week 3. Reduction was permitted to 2 g/d in response to AEs. IVC was given in monthly pulses of 0.5 to 1.0 g/m2, according to the modified NIH protocol.34 Both groups received oral prednisone, with a defined taper from a maximum starting dosage of 60 mg/d. The induction phase was defined as 24 wk, because 24-wk response can predict disease outcome25,27 and minimize the risks for long-term AEs of IVC.30,34 After screening, randomization, and treatment initiation, patients were assessed at weeks 2 and 4 and then every 4 wk.
Patients were withdrawn at week 12 when their serum creatinine was ≥30% above baseline on two successive measurements separated by at least 4 wk or when they required other immunosuppressive treatment. Patients could be withdrawn if the MMF dosage fell below 2 g/d for >14 d or was stopped for >7 d.
Outcome, Measurements, and Follow-up
The objective of this study was to test whether MMF was superior to IVC in the primary end point, namely the proportion of patients responding to treatment. Response was defined as a decrease in urine protein/creatinine ratio (P/Cr), calculated from a 24-h urine collection, to <3 in patients with baseline nephrotic range P/Cr (≥3), or by ≥50% in patients with subnephrotic baseline P/Cr (<3), and stabilization (±25%) or improvement in serum creatinine at 24 wk as adjudicated by a blinded Clinical Endpoints Committee. The 24-h urine collections were obtained at baseline and every 4 wk thereafter until completion of the 24-wk induction phase. Any patient who did not complete the 24-wk induction phase for any reason or who received pulse methylprednisolone therapy for major renal or extrarenal flare was classified as a nonresponder. Reasons for early withdrawal and hence nonresponse included AEs leading to withdrawal, intolerance of either therapy, or requirement to receive prohibited treatments. Assuming a 70% response rate, on the basis of previous studies,12,16 a population of 358 patients was predicted to provide 90% power to detect a 15% difference between the groups, with a 0.05 level of significance.
Key secondary end points included the proportion of patients who achieved complete remission, defined as return to normal serum creatinine, urine protein ≤0.5 g/d, and inactive urinary sediment (≤5 white blood cells per high-power field and ≤5 red blood cells per high-power field, and a reading of lower than 2+ on dipstick and absence of red cell casts); proportion of patients who achieved any one of these renal outcomes; combined renal and extrarenal remission, defined as absence of A and B scores on the British Isles Lupus Assessment Group system; mean change on the Safety of Exogenous Estrogens in Lupus Erythematosus National Assessment/Systemic Lupus Erythematosus Disease Activity Index; and mean change on the Systemic Lupus International Collaborating Clinics–American College of Rheumatology damage index.
Safety assessments included the assessment of laboratory tests, vital signs, and spontaneous reporting of AEs. An independent Data and Safety Monitoring Board, comprising two physicians and one biostatistician, was convened every 3 mo to review study data on an ongoing basis.
Statistical Analysis
The primary end point analysis was performed on the intention-to-treat population. Odds ratios were calculated using logistic regression models for response. Models included a term for treatment group and covariates of race (Asian, white, or other), class of disease (V or other), and location (United States/Canada, Asia, Latin America, or rest of world). In the initial, prospectively planned, primary efficacy analysis, interactions between treatment and these covariates were added and assessed at the 0.1 level. When the P value of the interaction term was ≤0.10, the interaction for that term was explored. In a sensitivity analysis, the analysis of the primary efficacy end point was adjusted for age, gender, and nephrotic/subnephrotic proteinuria at entry (P/Cr ≥3 versus <3). Two-sided 95% CIs were calculated for secondary efficacy end points as descriptive analyses. Statistical analysis was performed using SAS software (SAS Institute, Cary, NC). Post hoc safety analyses were performed using a χ2 test.
DISCLOSURES
This study was sponsored by the Aspreva Pharmaceuticals Corporation as part of the Roche-Aspreva collaboration agreement. G.B.A. has received honoraria (for lecturing) from Aspreva, served as a consultant for Aspreva, and received grants for ALMS. G.C. has received honoraria for traveling and lecturing from Roche. M.A.D. has served as a consultant for Teva and Aspreva; received honoraria from Aspreva; provided expert testimony for UCB; and received grants from Bristol-Myers Squibb, Aspreva, Amgen, and Roche. E.M.G. has received honoraria and grants from Aspreva. D.J. has received grants from Aspreva. N.S. is an employee of Aspreva.
Supplementary Material
Acknowledgments
This study was sponsored by F Hoffman-La Roche Ltd./Inc./AG as part of the Aspreva Pharmaceuticals Corporation Rare Disease Program. The members of the ALMS Group take full responsibility for the contents of this article.
We thank Steven Nettler, MPH (Aspreva Pharmaceuticals Inc., Basking Ridge, NJ), for the statistical analysis, and Tim Koder and Phillippa Curran (Caudex Medical Ltd., Oxford, UK [supported by Aspreva Pharmaceuticals Inc.]) for editorial assistance with the preparation of the manuscript.
Data from the ALMS study have been published in abstract form at the following congresses: American Society of Nephrology, San Francisco, CA, October 31 through November 5, 2007 (abstract 555038); Abstracts of the American College of Rheumatology and Association of Rheumatology Health Professionals annual scientific meeting, Boston, MA, November 6 through 11, 2007, Arthritis Rheum Sep;56(9 Suppl): S35–834, 2007; European Renal Association-European Dialysis and Transplant Association annual meeting, 2008, Stockholm, Sweden, May 10 through 13, 2008 (abstract 550516); EUROLUPUS 2008, Amsterdam, The Netherlands, May 7 through 10, 2008, Lupus 2008;17(5):455–456 (abstract 023); European League Against Rheumatism 2007, Barcelona, Spain, June 13 through 16, 2007, Ann Rheum Dis 2007;66(Suppl II):606 (abstract 0757); European League Against Rheumatism 2008, Paris, France, June 11 through 14, 2008, Ann Rheum Dis 2008;67(Suppl II):493 (abstract 0794); and Asia Pacific League of Associations for Rheumatology 2008, Yokohama, Japan, September 23 through 27, 2008, Int J Rheum Dis 2008;11:A347 P2P007.
The ALMS investigators are Carlos Abud, Hospital Central Dr Ignacio Morones Prieto, Mexico; Sharon Adler, Harbor-UCLA Medical Center, Torrance, CA; Graciela Alarcón, University of Alabama, Birmingham, AL; Elisa Albuquerque, Hospital Universitário Pedro Ernesto-UERJ, Rio de Janeiro, Brazil; Fernando Almeida, Centro de Ciências Médicas e Biológicas de Sorocaba, São Paulo, Brazil; Alejandro Alvarellos, Hospital Privado SA, Córdoba, Argentina; Gerald Appel, Columbia University Medical Center, New York, NY; Hilario Avila, Hospital Civil de Guadalajara Dr Juan I. Menchaca, Guadalajara, Mexico; Cornelia Blume, Universitätsklinikum Düsseldorf, Düsseldorf, Germany; Ioannis Boletis, Laiko General Hospital, Athens, Greece; Alain Bonnardeaux, Polyclinique Medicale Concorde, Montreal, Canada; Alan Braun, Mercy Arthritis and Osteoporosis Center, Urbandale, IA; Jill Buyon, NYU Hospital for Joint Diseases, New York, NY; Ricard Cervera, Hospital Clinic i Provincial, Barcelona, Spain; Nan Chen, Ruijin Hospital, Shanghai, China; Shunle Chen, Renji Hospital, Shanghai, China; Gabriel Contreras, University of Miami, Miami, FL; António Gomes Da Costa, Hospital Santa Maria, Lisbon, Portugal; Razeen Davids, Tygerberg Academic Hospital, Cape Town, South Africa; David D'Cruz, St. Thomas’ Hospital, London, UK; Enrique De Ramón, Hospital Universitário Carlos Haya, Malaga, Spain; Atul Deodhar, Oregon Health & Science University, Portland, OR; Mary Anne Dooley, University of North Carolina, Chapel Hill, NC; Andrea Doria, Università di Padova, Padova, Italy; Bertrand Dussol, Hôpital de la Conception, Marseille, France; Paul Emery, University of Leeds, Leeds, UK; Justus Fiechtner, Lansing, MI; Jürgen Floege, Universitätsklinikum Aachen, Aachen, Germany; Hilda Fragoso-Loyo, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico; Richard Furie, North Shore-LIJ Health System, Lake Success, NY; Rozina Ghazalli, Penang Hospital, Penang, Malaysia; Cybele Ghossein, Northwestern University, Chicago, IL; Gary Gilkeson, Medical University of South Carolina, Charleston, SC; Ellen Ginzler, SUNY Downstate Medical Center, Brooklyn, NY; Caroline Gordon, University of Birmingham, Birmingham, UK; Jennifer Grossman, UCLA Medical Center, Los Angeles, CA; Jieruo Gu, The 3rd Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China; Loïc Guillevin, Hôpital Cochin, Paris, France; Pierre-Yves Hatron, Hôpital Claude Huriez, Lille, France; Gisela Herrera, CIMA, San Jose, Costa Rica; Falk Hiepe, Charité-Universitätsmedizin Berlin, Berlin, Germany; Frederic Houssiau, Université Catholique de Louvain, Brussels, Belgium; Osvaldo Hübscher, Centro de Educación Médica e Investígaciones Clínicas, Buenos Aires, Argentina; Claudia Hura, San Antonio Kidney Disease Center Physicians Group, San Antonio, TX; Joshua Kaplan, University of Medicine and Dentistry of New Jersey, Newark, NJ; Gianna Kirsztajn, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil; Emese Kiss, University of Debrecen Medical and Health Science Center, Debrecen, Hungary; Ghazali Ahmad Kutty, Hospital Selayang, Selangor, Malaysia; Maurice Laville, Hôpital Édouard Herriot, Lyon, France; Maria Lazaro, Fundacion CIDEA, Buenos Aires, Argentina; Oliver Lenz, University of Miami, Miami, FL; Leishi Li, Jinling Hospital, Nanjing, China; Liz Lightstone, Imperial College, Hammersmith Hospital, London, UK; Sam Lim, Emory University School of Medicine, Atlanta, GA; Michel Malaise, Centre Hospitalier Universitaire de Liège, Domaine Universitaire du Sart Tilman, Liege, Belgium; Susan Manzi, University of Pittsburgh, Pittsburgh, PA; Juan Marcos, Hospital Interzonal General de Agudos General San Martin, Buenos Aires, Argentina; Olivier Meyer, Hôpital Bichat-Claude Bernard, Paris, France; Pablo Monge, Centro Medico Integral, San Jose, Costa Rica; Saraladev Naicker, Johannesburg Hospital, Johannesburg, South Africa; Nathaniel Neal, Valerius Medical Group & Research Center of Greater Long Beach, Long Beach, CA; Michael Neuwelt, C. Michael Neuwelt, MD, Inc., San Leandro, CA; Kathy Nicholls, Royal Melbourne Hospital, Victoria, Australia; Nancy Olsen, University of Texas Southwestern Medical School, Dallas, TX; Jose Ordi-Ros, Hospital del Vall d'Hebron, Barcelona, Spain; Barbara Ostrov, Milton S. Hershey Medical Center, Penn State University School of Medicine, Hershey, PA; Manuel Pestana, Hospital Sao João, Porto, Portugal; Michelle Petri, Johns Hopkins University School of Medicine, Baltimore, MD; Gyula Pokorny, Albert Szent-Györgyi Medical University, Szeged, Hungary; Jacques Pourrat, Hôpital de Rangueil, Toulouse, France; Jiaqi Qian, Shanghai Second Medical University, Renji Hospital, Shanghai, China; Jai Radhakrishnan, Columbia-Presbyterian Medical Center, New York, NY; Brad Rovin, Ohio State University, Columbus, OH; Jorge Sánchez-Guerrero, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, México; Julio Sanchez Roman, Hospital Virgen del Rocio, Sevilla, Spain; Joseph Shanahan, Duke University Medical Center, Durham, NC; William Shergy, Rheumatology Associates of North Alabama, Huntsville, AL; Fotini Skopouli, Euroclinic of Athens, Athens, Greece; Alberto Spindler, Centro Medico Privado de Reumatologia, Tucuman, Argentina Christopher Striebich, University of Colorado Health Sciences Center, Aurora, CO; Robert Sundel, Children's Hospital, Boston, MA; Charles Swanepoel, Groote Schuur Hospital, Cape Town, South Africa; Si Yen Tan, University Malaya Medical Centre, Kuala Lumpur, Malaysia; Guillermo Tate, Organización Médica de Investigación, Buenos Aires, Argentina; Vladimír Tesaŕ, Klinika Nefrologie, Všeobecná Fakultní Nemocnice, Prague, Czech Republic; Mohamed Tikly, Chris Hani Baragwanath Hospital, Johannesburg, South Africa; Haiyan Wang, The First Hospital Peking University, Beijing, China; Rosnawati Yahya, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; Xueqing Yu, The 3rd Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China; Fengchun Zhang, Peking Union Medical College Hospital, Beijing, China; Diana Zoruba, Hospital Municipal de Vicente Lopez Prof. Dr. Bernardo Houssay, San Isidro, Argentina.
Published online ahead of print. Publication date available at www.jasn.org.
Re print requests: Dr. Neil Solomons, c/o Medical Information Department, Aspreva Pharmaceuticals, The Old Stables, Bagshot Park, Bagshot, Surrey, GU19 SPJ, UK
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Waldman M, Appel GB: Update on the treatment of lupus nephritis. Kidney Int 70: 1403–1412, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Font J, Ramos-Casals M, Cervera R, García-Carrasco M, Torras A, Sisó A, Darnell A, Ingelmo M: Cardiovascular risk factors and the long-term outcome of lupus nephritis. QJM 94: 19–26, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Dooley MA, Hogan S, Jennette C, Falk R: Cyclophosphamide therapy for lupus nephritis: Poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int 51: 1188–1195, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Barr RG, Seliger S, Appel GB, Zuniga R, D'Agati V, Salmon J, Radhakrishnan J: Prognosis in proliferative lupus nephritis: The role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 18: 2039–2046, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Austin HA III, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL: Therapy of lupus nephritis: Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 314: 614–619, 1986 [DOI] [PubMed] [Google Scholar]
- 6.Steinberg AD, Decker JL: A double-blind controlled trial comparing cyclophosphamide, azathioprine and placebo in the treatment of lupus glomerulonephritis. Arthritis Rheum 17: 923–937, 1974 [DOI] [PubMed] [Google Scholar]
- 7.McCune WJ, Golbus J, Zeldes W, Bohlke P, Dunne R, Fox DA: Clinical and immunologic effects of monthly administration of intravenous cyclophosphamide in severe systemic lupus erythematosus. N Engl J Med 318: 1423–1431, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis JP, Boki KA, Katsorida ME, Drosos AA, Skopouli FN, Boletis JN, Moutsopoulos HM: Remission, relapse, and re-remission of proliferative lupus nephritis treated with cyclophosphamide. Kidney Int 57: 258–264, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Petri M: Cyclophosphamide: New approaches for systemic lupus erythematosus. Lupus 13: 366–3671, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Kuiper-Geertsma DG, Derksen RH: Newer drugs for the treatment of lupus nephritis. Drugs 63: 167–180, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Robson R, Cecka JM, Opelz G, Budde M, Sacks S: Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 5: 2954–2960, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN: Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med 343: 1156–1162, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Chan TM, Tse KC, Tang CS, Mok MY, Li FK: Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 16: 1076–1084, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G, Ghazalli R, Teo SM, Wong HS, Tan SY, Shaariah W, Tan CC, Morad Z: Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology (Carlton) 10: 504–510, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Liu Z, Chen H, Tang Z, Wang Q, Shen K, Li L: Mycophenolate mofetil vs cyclophosphamide therapy for patients with diffuse proliferative lupus nephritis. Chin Med J (Engl) 115: 705–709, 2002 [PubMed] [Google Scholar]
- 16.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, Appel GB: Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 353: 2219–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Moore RA, Derry S: Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis. Arthritis Res Ther 8: R182, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh M, James M, Jayne D, Tonelli M, Manns BJ, Hemmelgarn BR: Mycophenolate mofetil for induction therapy of lupus nephritis: A systematic review and meta-analysis. Clin J Am Soc Nephrol 2: 968–975, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Zhu B, Chen N, Lin Y, Ren H, Zhang W, Wang W, Pan X, Yu H: Mycophenolate mofetil in induction and maintenance therapy of severe lupus nephritis: A meta-analysis of randomized controlled trials. Nephrol Dial Transplant 22: 1933–1942, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004. [published erratum in J Am Soc Nephrol 15: 835–836, 2004] [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, Nahar N, de La Cuesta C, Hurtado A, Fornoni A, Beltran-Garcia L, Asif A, Young L, Diego J, Zachariah M, Smith-Norwood B: Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 69: 1846–1851, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Adler M, Chambers S, Edwards C, Neild G, Isenberg D: An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology 45: 1144–1147, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Fernández M, Alarcón GS, Calvo-Alén J, Andrade R, McGwin GJ, Vilá LM, Reveille JD, LUMINA Study Group: A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 57: 576–584, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O'Nan P, Roth D: Sequential therapies for proliferative lupus nephritis. N Engl J Med 350: 971–980, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Illei GG, Takada K, Parkin D, Austin HA, Crane M, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, Pando J, Steinberg AD, Gourley MF, Klippel JH, Balow JE, Boumpas DT: Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: Long-term followup of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum 46: 995–1002, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: Lessons from long-term followup of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum 50: 3934–3940, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis Trial, a randomized trial of low-dose high-dose intravenous cyclophosphamide. Arthritis Rheum 46: 2121–2131, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Raptopoulou A, Sidiropoulos P, Boumpas DT: Ovarian failure and strategies for fertility preservation in patients with systemic lupus erythematosus. Lupus 13: 887–890, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Boumpas DT, Austin HA, Vaughan EM, Yarboro CH, Klippel JH, Balow JE: Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med 119: 366–369, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Bernatsky S, Boivin JF, Joseph L, Gordon C, Urowitz M, Gladman D, Fortin PR, Ginzler E, Bae SC, Barr S, Edworthy S, Isenberg D, Rahman A, Petri M, Alarcón GS, Aranow C, Dooley MA, Rajan R, Sénécal JL, Zummer M, Manzi S, Ramsey-Goldman R, Clarke AE: The relationship between cancer and medication exposures in systemic lupus erythematosus: A case-cohort study. Ann Rheum Dis 67: 74–79, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Sinclair A, Appel G, Dooley MA, Ginzler E, Isenberg D, Jayne D, Wofsky D, Solomons N: Mycophenolate mofetil as induction and maintenance therapy for lupus nephritis: Rationale and protocol for the randomized, controlled Aspreva Lupus Management Study (ALMS). Lupus 16: 972–980, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Boumpas DT, Austin HA III, Vaughn EM, Klippel JH, Steinberg AD, Yarboro CH, Balow JE: Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 340: 741–745, 1992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.