Abstract
Agents that inhibit glycation end products by reducing the carbonyl load from glycation and glycoxidation are an emerging pharmacologic approach to treat complications of diabetes. We previously demonstrated that antibodies generated to the glycoprotein keyhole limpet hemocyanin (KLH) can cross-link with reactive carbonyl residues on protein conjugates. Here, we immunized streptozotocin-induced diabetic rats with KLH to assess the capacity of the elicited antibodies to intercept carbonyl residues on glycated proteins and to mitigate glycation-related pathology. Compared with diabetic rats immunized with adjuvant alone, KLH-immunized diabetic rats had decreased levels of glycated peptides in sera and demonstrated a reduction in albuminuria, proteinuria, deposition of glycation end products in the kidney, and histologic damage. In vitro, low molecular weight glycated peptides from rat serum reacted with anti-KLH antibodies at a faster rate than normal IgG and selectively modified the λ chains. The reaction products contained peptide sequences from type I collagen α chain, albumin, and LDL receptor–related protein. These adduction reactions were inhibited by free KLH and by reduction of glycated peptides with borohydride. In summary, these results suggest that inherent reactivity of Ig light chains provides a natural mechanism for the removal of cytotoxic glycation products. This reactivity can be augmented by glycoprotein-specific reactive immunization, a potential biopharmaceutical approach to glycation-related pathology.
Glycation of proteins by free glucose and their further modifications, collectively termed the Maillard reaction, generate a heterogeneous array of advanced glycation end products (AGE), characterized by alkylated amino acids, fluorescence residues, and a variety of intra- and intermolecular cross-linkages. These products are elevated in diabetes as a result of chronic or recurring hyperglycemia. The AGE hypothesis suggests that the accumulation of these adducts in various tissues contributes to diabetic vascular complications including nephropathy, retinopathy, neuropathy, and atherosclerosis.1–3 The diversity of AGE species poses a major challenge in the investigation of their composition and pathogenic properties. Cell membrane and extracellular matrix proteins are principal targets of glycation as indicated by extensive AGE accumulation in collagen matrix of skin, vasculature, and kidney. Furthermore, a reservoir of soluble AGE, derived from proteins in circulation and degradation of tissue-bound AGE, contributes to pathogenesis by interacting with vascular tissues via specific receptors, including receptor of AGE (RAGE), galectin-3, scavenger receptor,4 and megalin.5 Ligation to the receptors can mediate uptake and trigger cellular activation or proliferation, leading to inflammation and tissue destruction.4 Furthermore, AGEs can also act as neoantigens to initiate proinflammatory cellular immune responses.
The degree of glycation, oxidation state, and molecular mass are important determinants of cross-linking activity, receptor binding, and toxicity of soluble AGE. A number of studies indicate that low molecular weight AGE (LMW-AGE) have increased cytotoxic potential6 and that their levels in circulation correlate with accumulation in nephrons and impaired kidney function.7,8 Facile diffusion through microvasculature and interstitial space, resistance to proteolytic degradation, and a high ratio of glycation to peptide mass could enhance their capacity to promote vascular pathology. Accordingly, LMW-AGE are of interest as potential biomarkers in progression of complications and as targets for therapy.9
Incorporation of reactive carbonyl species at basic residues on proteins (carbonyl stress) defines a mechanistic crossroads in the Maillard pathway that has been exploited in therapeutic strategies. Pharmacologic agents, including aminoguanidine, carnosine, and pyridoxamine, that provide an alternative base to intercept the reactive species as Schiff base adducts can relieve carbonyl stress and ameliorate diabetic complications.10 Protein-based AGE blockade, including soluble RAGE11 or mAbs specific for glycated albumin,12 has also been shown to reduce vascular pathologies in diabetic models.
Nonenzymatic glycation is in principle indiscriminate; however, certain proteins are more susceptible to AGE formation. IgG L chain was one of three major glycated proteins recovered from human diabetic serum.13 Enhanced reaction at L chains could also account for increased mass of Fab prepared from glycated IgG of individuals with diabetes.14 A previous report demonstrated that the reaction of LMW-AGE of diabetic rat serum with normal IgG to under physiologic conditions generated modified L chains.15 Moreover, vascular clearance of glycated IgG16 and renal filtration of L chains are efficient routes for their elimination. These observations suggested a possible natural role of circulating IgG as buffers of glycation, serving to thwart formation of alternative toxic AGE. This hypothesis predicts that specific immunization to augment reactive Abs could accelerate scavenging of circulating glycation intermediates in vivo and thus attenuate AGE-related pathology. Along these lines, Abs that react with diverse carbonyl substrates are inducible by experimental “reactive immunization.”17 Recently, we described enhanced reaction in vitro of Abs elicited against the glycoprotein keyhole limpet hemocyanin (KLH) with a carbonyl-bearing protein conjugate.18 Here we examined the effect of KLH immunization on AGE formation and development of nephropathy in streptozotocin (STZ)-induced diabetic rats. Reaction rates of Abs with glycated peptides from diabetic sera were studied and the products were characterized to elucidate further the unconventional activity and assess the immunotherapeutic potential.
RESULTS
Induction of Diabetes, Serum Protein Glycation, and AGE Formation
Rats that were previously immunized with KLH/complete Freund's adjuvant (CFA) or PBS/CFA received two injections 5 d apart of STZ to induce moderate diabetes. Hyperglycemia developed within 7 d after the last dose and remained elevated throughout the course of the experiment. Average values at wk 6 were 339 ± 50 and 316 ± 90 mg/dl, respectively, for the two groups compared with 140 ± 15 mg/dl for age-matched, nondiabetic normal rats.
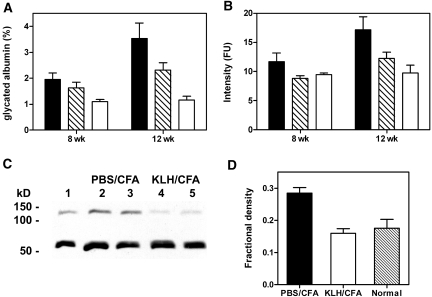
Differences in serum glycated albumin levels were evident at 8 wk from the last STZ dose. At 12 wk, these differences were magnified further. Levels in diabetic rats immunized with PBS/CFA were approximately 3.5- to four-fold greater than in nondiabetic normal controls, whereas levels in KLH/CFA-immunized diabetic rats were two- to 2.5-fold above normal (Figure 1A). LMW-AGE is elevated in serum of patients with diabetes, presenting a possible correlate for development of renal disease.19 Compared with normal controls, LMW-AGE fluorescence in the PBS/CFA-immunized diabetic group was increased by 17% at 8 wk and 67% at 12 wk. By contrast, fluorescence levels were essentially normal at 8 wk and only 27% above normal at 12 wk in KLH/CFA-immunized diabetic rats (Figure 1B). In addition, staining of a macromolecular AGE by Western blot was significantly greater in sera of PBS/CFA-immunized diabetic animals relative to KLH/CFA-immunized diabetic or normal rat sera (Figure 1C). A 68-kD band detected in all sera was attributed to cross-reactivity of the antiserum raised against AGE-modified human/bovine albumins with nonglycated rat albumin. Accordingly, addition of 0.5% human albumin to the blotting buffer substantially blocked this staining (data not shown). Thus, by three different methods of assessment, AGE levels were significantly reduced in diabetic rats immunized with KLH/CFA relative to diabetic rats immunized with adjuvant alone.
Figure 1.
Glycation levels in diabetic rats. (A) Fractional glycated albumin in sera of diabetic rats preimmunized with PBS/CFA (▪), KLH/CFA ( ), and age-matched normal control rats (□) were determined at 8 and 12 wk from the time of last STZ injection. (B) Similarly, LMW-AGE fluorescence values were determined from standard volumes of sera for three groups with legends as in A. Data are means ± SEM of five rats per group. Differences between PBS/CFA and KLH/CFA groups at 12 wk were statistically significant for both glycated albumin (P = 0.0102) and peptide fluorescence (P = 0.0188) as established by t test. (C) Western blot of AGE in whole serum (5 μl/lane) of age-matched control (lane 1) and two representative diabetic rats from groups preimmunized with PBS/CFA (lanes 2 and 3) or KLH/CFA (lanes 4 and 5) as stained with commercial anti-AGE antiserum. The common band at 68 kD was due to cross-reaction of the reagent antiserum with rat albumin. (D) Relative serum AGE levels were estimated by quantitative densitometry of the upper band in C, expressed as a fraction of total density of upper and lower bands. Bars are means ± SD for five rats in each group.
), and age-matched normal control rats (□) were determined at 8 and 12 wk from the time of last STZ injection. (B) Similarly, LMW-AGE fluorescence values were determined from standard volumes of sera for three groups with legends as in A. Data are means ± SEM of five rats per group. Differences between PBS/CFA and KLH/CFA groups at 12 wk were statistically significant for both glycated albumin (P = 0.0102) and peptide fluorescence (P = 0.0188) as established by t test. (C) Western blot of AGE in whole serum (5 μl/lane) of age-matched control (lane 1) and two representative diabetic rats from groups preimmunized with PBS/CFA (lanes 2 and 3) or KLH/CFA (lanes 4 and 5) as stained with commercial anti-AGE antiserum. The common band at 68 kD was due to cross-reaction of the reagent antiserum with rat albumin. (D) Relative serum AGE levels were estimated by quantitative densitometry of the upper band in C, expressed as a fraction of total density of upper and lower bands. Bars are means ± SD for five rats in each group.
Assessment of Diabetic Nephropathy
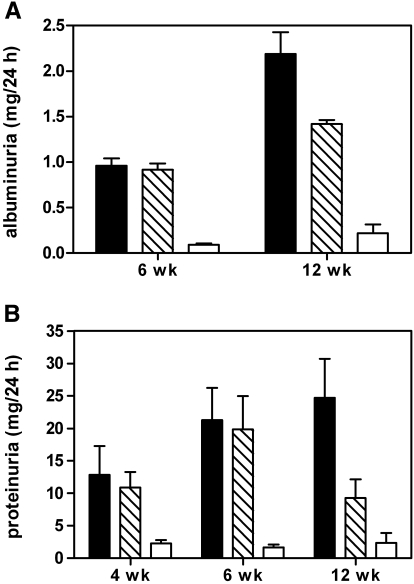
Elevated proteinuria was induced in PBS/CFA- and KLH/CFA-immunized diabetic groups, for which values at 6 wk were similar in the two groups; however, during the subsequent 6-wk period, a reduction by nearly 50% was seen in the latter group, whereas the levels remained elevated in the former (Figure 2A). Albuminuria values were also ameliorated in the KLH-immunized rats (Figure 2B). Although the magnitude of these changes was less dramatic than that seen in proteinuria, the differences were statistically significant.
Figure 2.
(A) Albuminuria and (B) proteinuria values of diabetic rats immunized with PBS/CFA (▪) or with KLH/CFA ( ) and unimmunized age-matched control rats (□). Differences between PBS/CFA and KLH/CFA groups at 12 wk were statistically significant for both albuminuria (P = 0.0201) and proteinuria (P = 0.0295).
) and unimmunized age-matched control rats (□). Differences between PBS/CFA and KLH/CFA groups at 12 wk were statistically significant for both albuminuria (P = 0.0201) and proteinuria (P = 0.0295).
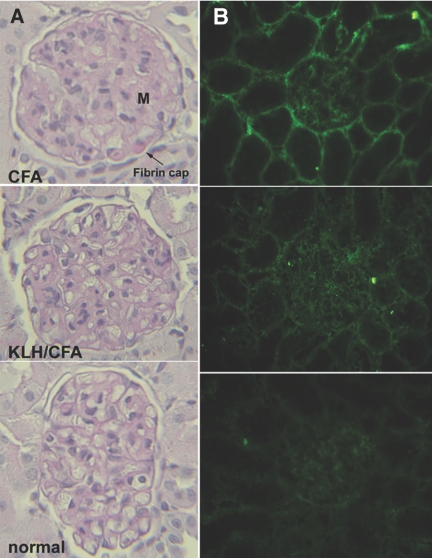
Histology of kidney cortex obtained at 12 wk showed evidence for renal pathology consistent with impaired kidney function. Diabetic rats preimmunized with PBS/CFA alone showed expanded mesangial matrix, thickened glomerular basement membrane, and appearance of narrow capillary loops typical of diabetic nephropathy. Glomerular area and mesangial area were increased as compared with the normal group. In contrast, glomerular changes of KLH/CFA-immunized diabetic rats were milder (Figure 3A, middle), with significantly reduced glomerular and mesangial areas relative to the CFA-immunized diabetic group, indicating amelioration of the glomerular hypertrophy and mesangial expansion (Table 1). Fibrin cap lesions generally associated with advanced diabetic nephropathy, as seen in occasional glomeruli of PBS/CFA-immunized diabetic rats (Figure 3A, top), were absent in kidneys of KLH/CFA-immunized diabetic rats.
Figure 3.
(A) PAS-stained renal sections of diabetic and control rats. Typical glomerular changes of diabetic nephropathy were seen in representative sections from PBS/CFA-preimmunized rats (top), consisting of expanded mesangial matrix (M), thickened glomerular basement membrane, and narrow capillary loops. The fibrin cap seen in this section is generally associated with advanced disease. The glomerular changes in KLH/CFA-preimmunized diabetic rats (center) were more similar to those of age-matched normal rat (bottom). (B) Direct immunofluorescence on renal sections of rats as described in A with anti-AGE antiserum. Staining is seen in the mesangium, Bowman capsule, tubular basement membrane, and peritubular capillaries (top). The staining in KLH/CFA-preimmunized diabetic rat kidney (center) is considerably reduced and only mild compared with background staining of control rat kidney (bottom).
Table 1.
Glomerular hypertrophy and mesangial expansion in rat kidney as assessed by mean glomerular and mesangial areas.a
| Group | PBS/CFA | KLH/CFA | Normal |
|---|---|---|---|
| MGA (μm2) | 13,110 ± 2618b | 11,535 ± 2176c | 9452 ± 1870 |
| MMA (μm2/glomerulus) | 1659 ± 196b | 1319 ± 213d | 1138 ± 81 |
Data are means ± SD. MGA, mean glomerular area; MMA, mean mesangial area.
P < 0.0001 versus normal controls.
P = 0.026 versus PBS/CFA diabetic group.
P = 0.0045 versus PBS/CFA diabetic group.
Immunofluorescence microscopy revealed significant staining of AGE in kidneys of PBS/CFA-immunized diabetic rats in tubules, glomeruli, and peritubular capillaries in interstitium, scored as 3 to 3+ on a semiquantitative scale. Tubular staining seemed to localize in the basement membrane, whereas glomerular staining was seen in the mesangium and Bowman capsule (Figure 3B, top). Staining of kidney sections from KLH/CFA-immunized diabetic rats showed markedly reduced fluorescence, scored as 2 to 2+ (Figure 3B, middle), although diffuse fluorescence was greater than background staining (0 to 1+) of age-matched nondiabetic rats (Figure 3B, bottom).
Modification of IgG by Glycated Peptides
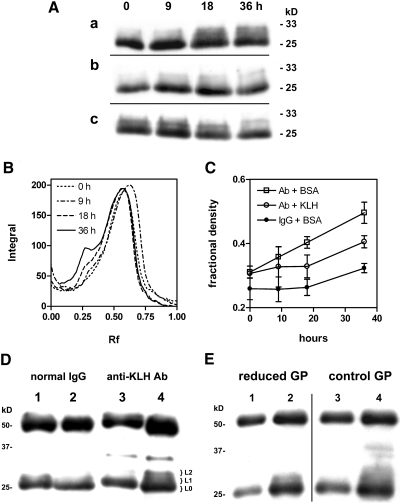
Reaction in vitro of normal rat IgG with glycated peptides of diabetic rat serum generated L chain products with apparent size ranging from 28 to 33 kD as previously reported.15 Significant product formation required 3 to 5 d of incubation, whereas products in reaction of anti-KLH Ab were detected within 18 h. The enhanced reaction of L chains of anti-KLH Ab could be inhibited in the presence of KLH to a rate similar to that observed with normal IgG (Figure 4). Under these conditions, the relative rates of anti-KLH Ab and normal IgG seemed to equalize upon prolonged reaction (Figure 4C). Furthermore, reactive Ab was recovered from the bound fraction (0.36) from a KLH-Sepharose affinity matrix, whereas the unbound fraction (0.64) was depleted of activity. The bound (0.05) and unbound (0.95) fractions of similarly adsorbed normal IgG at normalized concentration failed to react with glycated peptides under these conditions (Figure 4D). Although reactive Ab could be inhibited and adsorbed by KLH, anti-KLH titers of antisera were similar before and after reaction with glycated peptides, suggesting that high-affinity binding sites were not destroyed in the process.
Figure 4.
(A) Affinity-purified rat anti-KLH Ab (0.5 mg/ml) in PBS containing 0.2 mg/ml of either BSA (a) or KLH (b), and normal rat IgG (c) in PBS/BSA were reacted with glycated peptides for 0 to 36 h at 37°C. Aliquots collected at four time points were separated by SDS-PAGE, and immunoblots were stained with anti-rat IgG (H + L). Bands in the 23- to 33-kD range are shown. (B) Densitometry of bands in the 20- to 36-kD range in A (a) showed increases in higher mass product as a peak at Rf 0.25 relative to the L chain (Rf 0.6). (C) Rates of adduct formation in the 30- to 32-kD region for reactions shown in A were estimated from normalized band densities. Reactions were performed in triplicate, and fractional densities are plotted as means ± SEM. The difference in adduct amount formed in the presence of BSA (□) or KLH (○) at 18 h was statistically significant (P = 0.035). (D) Normal IgG (lanes 1 and 2) and anti-KLH Ab (lanes 3 and 4) in unbound (lanes 1 and 3) and nonspecifically (lane 2) or specifically (lane 4) bound fractions eluted from KLH-Sepharose were normalized to 0.5 mg/ml and incubated with glycated peptides for 48 h at 37°C, and products were analyzed as in A. Gel slices excised from lane 4 corresponding to L-chain (L0) and higher mass adducts (L1 and L2) were submitted for mass spectrometry/mass spectrometry (MS/MS) analysis. The immunoreactive band at 35 kD was not characterized. (E) Anti-KLH Ab was reacted with sodium cyanoborohydride–reduced (lanes 1 and 2, reduced GP) or with mock-reduced glycated peptides (lanes 3 and 4, control GP) as in D. Aliquots collected at time of mixing (lanes 1 and 3) and at 48 h (lanes 2 and 4) were analyzed as in A.
Serum IgG concentrations at 3 wk after immunization to the conclusion of the study were similar in PBS/CFA- and KLH/CFA-immunized diabetic groups, varying from 8.4 ± 0.5 to 12.5 ± 2.4 mg/ml and from 8.2 ± 0.9 to 13.6 ± 2.6 mg/ml, respectively. The levels in unimmunized age-matched controls varied from 5.5 ± 0.3 to 4.9 ± 0.3 mg/ml. Thus, depletion of glycation products as a result of nonspecific scavenging by total IgG was unlikely to be significantly different in immunized groups.
To probe the chemistry of adduct cross-linkage, we treated the glycated peptides with sodium cyanoborohydride to reduce aldehyde or other carbonyl groups before incubation with anti-KLH Ab. Levels of L chain adducts were significantly diminished relative to those in a control reaction with glycated peptides treated by mock reduction (Figure 4E). In addition, reaction of anti-KLH Ab with a protein fraction from diabetic serum obtained by phenylboronate affinity (PBA) produced similar adducts, suggesting that the reactive molecules were co-purified with glycated proteins.
Characterization of L-Chain AGE Adducts
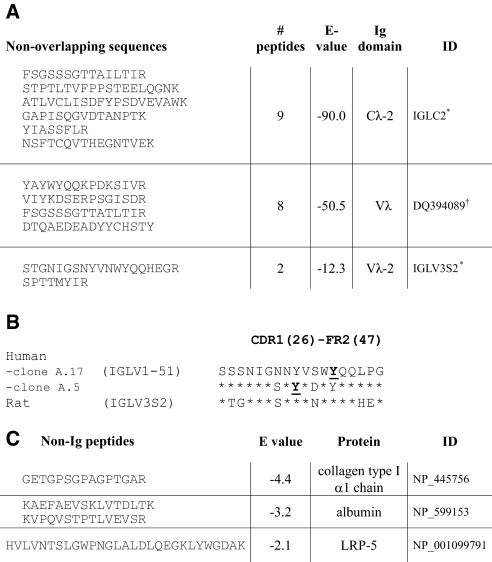
The relative staining of L chains and L-chain adducts in SDS-PAGE by Coomassie blue was proportional to their immunoreactivity in Western blot. Peptides eluted from the respective excised bands by in-gel tryptic digest were analyzed by liquid chromatography–mass spectrometry/mass spectrometry. Proteomic analysis of L chains before incubation with glycated peptides identified 20 unique Vκ and 2 Vλ sequences, in addition to CL peptides. After the reaction, polypeptides migrating in the size range of native L chains included 19 Vκ and no Vλ sequences. Products in the 28- to 30-kD size range (Figure 4D, lane 4, L1) included peptides from 9 Vκ and 2 Vλ sequences. In contrast, only Vλ and CL peptides were detected in adducts resolved in the 30- to 33-kD range (L2). Nine peptides mapped to the CL-2 sequence, whereas others identified contiguous 61-residue and 27-residue sequences of two distinct rat Vλ (Figure 5). A peptide of type I collagen α1 chain was represented in all three fractions of the 48-h products but not in L chains isolated from a reaction mixture before incubation. Peptides found only in the higher mass adducts (bands L1 and L2) were derived from serum albumin and an LDL receptor–related protein (Figure 5C).
Figure 5.
L chains and associated proteins identified by MS/MS and proteomic analysis of covalent adducts generated from anti- KLH Ab, IgG and glycated peptides. (A) Tryptic peptides identified by ESI MS/MS from SDS-PAGE lane 4, band L2 (Figure 4D) matched to one CL domain and two Vλ domains. Proteins were defined by conceptual translation of genes indicated. Rat Ig genes are identified by accession ID for the IMGT (*) or NCBI (†) databases. (B) Similarity between sequences of human Vλ peptides in CDR1/FR2 that were modified in scFv clones identified by reactive selection25 and the corresponding peptide identified in L-chain adducts and encoded by the germline rat Vλ. Underlined residues denote nucleophiles that react covalently with phosphonates.25 (C) Proteins identified from non-Ig peptides found by MS/MS in bands L1 and L2 of Figure 4D, lane 4. Significance of E values is given in the Concise Methods section. Accession IDs are for the NCBI protein database.
DISCUSSION
AGE levels in diabetic rats immunized with KLH or adjuvant alone correlated with severity of diabetic nephropathy as scored by albuminuria, proteinuria, and renal histology. A renoprotective effect mediated by blockade of low-level nonenzymatic glycation of proteins in vasculature is indirectly supported by several observations. Markers of both high and low mass AGE levels in serum were diminished in rats with high titers of anti-KLH Abs that also had milder nephropathy. Differences in circulating AGE seen in the diabetic rats were mirrored by their renal tissue–associated AGE. The striking stain pattern at the tubular basement membrane suggested potential cellular AGE uptake at proximal tubules.5,20 Modification of the collagen matrix of renal peritubular capillaries might also account for this. These observations are in agreement with previous determinations of AGE accumulation in the renal extracellular matrix and their correlation with circulating AGE peptides.8 Reduced staining in kidney of the KLH-immunized diabetic rats corroborated the association between circulating AGE levels and development of nephropathy. Immunization of some murine strains21 as well as Lewis rat22 with CFA can exacerbate diabetes in the subdiabetogenic STZ protocol. This is unlikely to be a factor in determining disease outcome in this study because we used the same adjuvant and standard high STZ dosage in both immunized groups.
L chains were previously shown to be a major component of AGE in patients with diabetes.13 Gugliucci and Menini15 first described the selective modification of L chains by LMW-AGE. In our study, reactions of L chains from anti-KLH Ab were favored, supporting the contention that the inherent chemical function was recruited. A significant rate enhancement was evident in the initial 18 h as compared with the reaction of normal IgG, and the reaction was specifically inhibited by KLH (Figure 4C). Although the IgG concentrations were normalized for in vitro reactions, the more reactive component presumably constitutes a minor fraction. Its reactivity could therefore provide for efficient sequestration of glycated peptides or other glycotoxins in circulation. It is noteworthy that the higher mass products that formed in vitro were not detected in IgG or glycated IgG of immunized diabetic rats (data not shown). The failure of the L-chain adducts to accumulate in sera of diabetic rats is consistent with previous reports of accelerated vascular clearance of glycated or chemically modified IgG.16 Release of L chains and excretion in renal filtrate could also account for their rapid clearance.23
Nonenzymatic glucosylation of many serum proteins, including IgG, is a much slower process.24 These results are consistent with Maillard chemistry, involving condensation between nucleophilic groups on proteins and electrophilic carbonyl groups of glycated polypeptides. The carbonyls in glycated peptides survived in serum stored for several weeks, suggesting relative stability compared with low mass reactive aldehydes such as methylglyoxal and malondialdehyde. This stability could also prolong survival in circulation, contributing to their cytotoxicity. Glycated peptides correlate with AGE accumulation in nephrons,8 have higher toxic potential on cultured renal proximal tubule,6 and are elevated in patients with diabetes and impaired renal function.7 Nevertheless, a causal role for these peptides in nephropathy remains to be established.
Differences in V region sequences, particularly in hypervariable residues that determine antigen binding, most likely account for the variations in chemical reactivities of induced Abs. The residues modified in L chains of IgG were not identified in this study; however, in related work, we noted that Abs induced against phosphonate esters also use L chains for covalent binding.18 Furthermore, reactive human single-chain fragments (scFvs) obtained from a phage display library used nucleophilic tyrosine residues at the CDR1-FR2 junction of Vλ sequences.25 Interestingly, we found that a germline gene IGLV1-51 encoded the VL region of the most reactive scFv clone. In this study, two reactive rat Vλ sequences identified by proteomic analysis diverged minimally from germline-encoded sequences. One of these Vλ polypeptides is a direct translation from germline IGLV3S2. The second, identified by contiguous peptides spanning CDR1 through FR3, is also encoded by genomic DNA. This rat sequence has 79% identity in 59-residue overlap to the human VL expressed by the germline IGLV3-25, and its deduced VL is identical in length and shares 54 to 55% sequence identity in 97-residue overlap with the reactive human Vλ encoded by IGLV1-51.26 Tyrosines identified as nucleophiles on λ L chains of reactive human scFv are represented in both rat sequences (Figure 5B). Even though κ chains are more highly expressed in rat,26 no κ chains were found in adducts with the greater size shift; however, κ chains resolved at the 28- to 30-kD range may also have been chemically modified. These results support the contention that reactivity is a conserved rather than an acquired function of the L chains.
Conventional antigen affinity could guide Abs containing reactive L chains to carbohydrate-like structures of AGE. Indeed, anti-KLH Abs have been reported to cross-react with a range of carbohydrate structures.27,28 This is also the proposed basis for the therapeutic efficacy of KLH in clinical applications.29 Inhibition of L chain adducts by KLH in solution without reduction of titer suggests reactive Abs of moderate affinities, as could be expected in carbohydrate recognition. KLH is hyperimmunogenic in various species, and we described a similar carbonyl reactivity of Abs elicited in several mouse strains.18 Nevertheless, the response to determinants for reactive Abs specific for glycated peptides could vary in rodent strains or other species. The nature of these determinants remains to be investigated.
These considerations collectively suggest a possible new role of reactive Abs in moderating toxicities of glycated or oxidized polypeptides, which bear carbonyl residues on the attached carbohydrates. Our results demonstrate a beneficial effect of the inducible reactive Abs in diabetic nephropathy, which can be competitive with the use of glycation inhibitors and AGE breakers as potential pharmacologic agents.30 This approach could be more aptly compared with biopharmaceutical strategies for antiglycation therapy, which have also shown promise. For example, diabetic mice were protected from nephropathy by administration of a mAb to glycated albumin.31 More recently, the use of soluble RAGE for blockade of AGE uptake has generated great interest.32 A vaccine approach represents a new modality with obvious merits in management of diabetic complications. Whereas pharmacologic intervention would require lifelong dosing and raises serious concerns for long-term harmful effects from the agents, simple vaccination could sustain immunologic blockade lasting for years at far less cost and negligible risk for toxicity.
CONCISE METHODS
Reagents, Proteins, and Animals
KLH, bovine albumin, STZ, PBA, sodium cyanoborohydride, and diethylaminoethylcarbodiimide were obtained from Sigma-Aldrich Chemical Co (St. Louis, MO). Horseradish peroxidase (HRP)-conjugated goat anti-rat IgG was obtained from Cappel Laboratories (Cochranville, PA). Rabbit anti-AGE antiserum was procured from Abcam (Cambridge, MA). Protein concentrations were determined by BCA assay (Bio-Rad, Hercules, CA) or by 280-nm absorption. Lewis rats at 6 wk of age were purchased from Charles River Laboratories (Wilmington, MA). Rats were housed, cared for, and used in accordance with institutional animal welfare assurances and National Institutes of Health guidelines for care and use of laboratory animals. Death was by CO2 asphyxiation.
Immunizations, Induction of Diabetes, and Assessment of Disease
Female Lewis rats were immunized in groups of five by subcutaneous injection with 100 μg of KLH in PBS emulsified 1:1 in CFA (Difco Laboratories, Detroit, MI) or PBS/CFA. All rats received a booster 10 d later with the same dosage in incomplete Freund's adjuvant. Diabetes was induced by intraperitoneal injection of streptozotocin (45 mg/kg) in citrate buffer (pH 5.0) on days 15 and 20. Blood and 24-h urine samples were collected at 2-wk intervals from fasting rats.
Plasma glucose values were determined on test strips read by glucose meter (Advantage; Roche Diagnostics, Indianapolis, IN). Total urinary protein was estimated by turbidometry in 5% sulfosalicylic acid, using BSA as standard. Albuminuria values were determined using the Nephrat ELISA kit according to the product specifications (Exocell, Philadelphia, PA). Similarly, serum IgG concentrations were determined by competition ELISA on plates coated with normal rat IgG, using goat anti-rat IgG-HRP (1:5000 in TBS, 2% BSA). Glycated proteins were separated from whole serum on PBA chromatography as described previously.33 Glycated albumin was reported as the ratio of PBA-bound and total serum albumin as determined by Nephrat ELISA.
Quantification of AGE by Fluorescence and Immunoblot Analysis
Glycated peptides were obtained as previously described15 from diabetic rat sera by dialysis in 1-kD membrane against PBS, followed by ultrafiltration on 10-kD cutoff membrane. AGE fluorescence was measured on a Cary Eclipse spectrofluorimeter at 320/480 nm excitation/emission.
For detection of high-mass AGE, 5 μl of whole sera of diabetic or normal rats was resolved by SDS-PAGE and blotted on polyvinylidene difluoride. Membranes were stained with rabbit anti-AGE serum (1:5000 in PBST) followed by goat anti-rabbit HRP conjugate (1:10,000 in PBST). Blots were developed using ECL substrates (GE Biosciences, Piscataway, NJ). Quantitative densitometry was performed on a Chemidoc EQ system using Quantity One software (Bio-Rad Laboratories). Average band density was used to calculate fractional densities relative to bands for L chain or rat albumin [e.g., L2/(L2+LC)].
Assay for Antibody Reactivity and L-Chain Adduct Formation
Glycated peptides were used as obtained from sera or after reduction with 20 mM sodium cyanoborohydride for 1 h at 23°C. Borohydride reactions were quenched with 5% acetic acid and dialyzed against PBS. IgG was purified from normal or immune serum by protein G–Sepharose affinity chromatography (Pierce Biotechnology, Rockford, IL) and dialyzed against PBS. KLH-Sepharose, prepared by diethylaminoethylcarbodiimide coupling of KLH to AH Sepharose 4B (GE Healthcare Biosciences, Piscataway, NJ), was used to adsorb IgG in PBS. The unbound fraction was collected, the column was washed with 3 × 5 ml of 100 mM phosphate buffer (pH 7.5), and bound Ab was eluted with 0.1 M glycine (pH 2.7) neutralized with 1 M Tris (pH 8.5) and dialyzed against PBS. Bound and unbound fractions were concentrated, normalized to 0.5 mg/ml, and mixed 1:1 with glycated peptides (200 μl). Reactions were incubated at 37°C, and aliquots collected over 48 h were kept at −20°C. All samples were then analyzed by reducing SDS-PAGE and stained with Coomassie blue. A duplicate gel was used for Western blotting using anti-rat IgG (H + L)-HRP at 1:5000 dilution.
Analysis of L-Chain Adducts and Sequence Identification
Gel slices from SDS-PAGE containing isolated bands corresponding to L-chain and higher mass adducts were analyzed by mass spectrometric protein identification at the Proteomics Core Facility of the University of California, Davis. Proteins in the gel were reduced, alkylated, and digested with trypsin for release of peptides by standard procedures. Eluted peptides were analyzed by capillary LC coupled to a Finnegan LTQ ion trap tandem mass spectrometer. Proteomic analysis was performed using the open source search engine X! Tandem and sequences searched in the Global Proteome Machine Database (http://gpmdb.proteomics.ucdavis.edu). Protein matches were ranked on the basis of log(e) value, which corresponds to the expectation value of matching the protein randomly, and their log(l) score, which is a base 10 log of the sum of the intensities of the fragment ion spectra. Protein and gene identifiers (accession) refer to databases of the National Center for Biotechnology Information (NCBI) or the international Immunogenetics information system (IMGT) http://imgt.org.
Histochemistry and Immunofluorescence Microscopy
Paraformaldehyde-fixed sections were stained with periodic acid-Schiff (PAS). Frozen 4-μM sections were stained with rabbit anti-AGE antiserum (1:32 dilution) followed by goat anti-rabbit IgG-FITC (1:64 dilution). Sections were viewed on a Zeiss inverted microscope under epifluorescence, and images were captured with a digital camera. Four to six sections from each group were scored for fluorescence intensity by two observers on a semiquantitative scale: 0 to 1+ (background or dispersed faint fluorescence), 2 to 2+ (mild to moderate focused fluorescence), and 3 to 3+ (moderate fluorescence of glomerular mesangium with strong fluorescence in interstitium). Mean glomerular and mean mesangial (PAS-positive) areas were quantified from images of PAS-stained sections taken on 10 glomeruli per section. Average values were derived from five sections from each group.
Statistical Analysis
Graphical analysis and t test were performed using GraphPad Prism 4.02 for Windows (GraphPad Software, San Diego, CA). Glomerular areas in sections were selected in Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) and measured using ImageJ 1.41 (National Institutes of Health, Bethesda, MD).
DISCLOSURES
None.
Acknowledgments
This work was supported by American Diabetes Association grant 1-05-RA-136; National Institutes of Health grant CA90564; and UC Davis, Medical School, Children's Miracle Network.
Mass spectrometry was performed at the UC Davis Proteomics Facility. We thank Rich Eigenheer for assistance with MS analysis.
Published online ahead of print. Publication date available at www.jasn.org.
T.S.'s current affiliation is Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
REFERENCES
- 1.Vlassara H, Palace MR: Diabetes and advanced glycation endproducts. J Intern Med 251: 87–101, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M: Lilly Lecture 1993. Glycation and diabetic complications. Diabetes 43: 836–841, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Sensi M, Pricci F, Andreani D, Di Mario U: Advanced nonenzymatic glycation endproducts (AGE): Their relevance to aging and the pathogenesis of late diabetic complications. Diabetes Res 16: 1–9, 1991 [PubMed] [Google Scholar]
- 4.Vlassara H: The AG: E-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev 17: 436–443, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Saito A, Nagai R, Tanuma A, Hama H, Cho K, Takeda T, Yoshida Y, Toda T, Shimizu F, Horiuchi S, Gejyo F: Role of megalin in endocytosis of advanced glycation end products: Implications for a novel protein binding to both megalin and advanced glycation end products. J Am Soc Nephrol 14: 1123–1131, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Deuther-Conrad W, Franke S, Sommer M, Henle T, Stein G: Differences in the modulating potential of advanced glycation end product (AGE) peptides versus AGE proteins. Kidney Int Suppl 78: S63–S66, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Thomas MC, Tsalamandris C, MacIsaac R, Medley T, Kingwell B, Cooper ME, Jerums G: Low-molecular-weight AGEs are associated with GFR and anemia in patients with type 2 diabetes. Kidney Int 66: 1167–1172, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Verbeke P, Perichon M, Borot-Laloi C, Schaeverbeke J, Bakala H: Accumulation of advanced glycation endproducts in the rat nephron: Link with circulating AGEs during aging. J Histochem Cytochem 45: 1059–1068, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Monnier VM, Sell DR, Genuth S: Glycation products as markers and predictors of the progression of diabetic complications. Ann N Y Acad Sci 1043: 567–581, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Cho SJ, Roman G, Yeboah F, Konishi Y: The road to advanced glycation end products: A mechanistic perspective. Curr Med Chem 14: 1653–1671, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM: Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4: 1025–1031, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Cohen MP, Hud E, Wu VY: Amelioration of diabetic nephropathy by treatment with monoclonal antibodies against glycated albumin. Kidney Int 45: 1673–1679, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Mitsuhashi T, Li YM, Fishbane S, Vlassara H: Depletion of reactive advanced glycation endproducts from diabetic uremic sera using a lysozyme-linked matrix. J Clin Invest 100: 847–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapolla A, Tonani R, Fedele D, Garbeglio M, Senesi A, Seraglia R, Favretto D, Traldi P: Non-enzymatic glycation of IgG: An in vivo study. Horm Metab Res 34: 260–264, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gugliucci A, Menini T: Circulating advanced glycation peptides in streptozotocin-induced diabetic rats: Evidence for preferential modification of IgG light chains. Life Sci 62: 2141–2150, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Kennedy DM, Skillen AW, Self CH: Glycation increases the vascular clearance rate of IgG in mice. Clin Exp Immunol 94: 447–451, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner J, Lerner RA, Barbas CF 3rd: Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science 270: 1797–1800, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Armentano F, Knight T, Makker S, Tramontano A: Induction of covalent binding antibodies. Immunol Lett 103: 51–57, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Thomas MC, Forbes JM, MacIsaac R, Jerums G, Cooper ME: Low-molecular weight advanced glycation end products: markers of tissue AGE accumulation and more? Ann N Y Acad Sci 1043: 644–654, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gugliucci A, Bendayan M: Renal fate of circulating advanced glycated end products (AGE): Evidence for reabsorption and catabolism of AGE-peptides by renal proximal tubular cells. Diabetologia 39: 149–160, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Richens ER, Tungekar MF, Behbehani K: Complete Freund's adjuvant has a differential amplification action on the induction of diabetes by streptozotocin in various murine strains: CFA amplifies STZ in murine diabetes. Pathology 19: 351–357, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Kohnert KD, Odselius R, Falt K, Ziegler B, Ziegler M, Falkmer S: Destruction of pancreatic beta cells in rats by complete Freund's adjuvant combined with non-diabetogenic doses of streptozotocin. Diabetes Res 5: 1–11, 1987 [PubMed] [Google Scholar]
- 23.Ledingham JG: Tubular toxicity of filtered proteins. Am J Nephrol 10[Suppl 1]: 52–57, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Vrdoljak A, Trescec A, Benko B, Hecimovic D, Simic M: In vitro glycation of human immunoglobulin G. Clin Chim Acta 345: 105–111, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Reshetnyak AV, Armentano MF, Ponomarenko NA, Vizzuso D, Durova OM, Ziganshin R, Serebryakova M, Govorun V, Gololobov G, Morse HC 3rd, Friboulet A, Makker SP, Gabibov AG, Tramontano A: Routes to covalent catalysis by reactive selection for nascent protein nucleophiles. J Am Chem Soc 129: 16175–16182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar BA, Gutman GA: Transcription and diversity of immunoglobulin lambda chain variable genes in the rat. Immunogenetics 37: 39–48, 1992 [DOI] [PubMed] [Google Scholar]
- 27.May RJ, Beenhouwer DO, Scharff MD: Antibodies to keyhole limpet hemocyanin cross-react with an epitope on the polysaccharide capsule of Cryptococcus neoformans and other carbohydrates: Implications for vaccine development. J Immunol 171: 4905–4912, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Wirguin I, Suturkova-Milosevic L, Briani C, Latov N: Keyhole limpet hemocyanin contains Gal(beta 1–3)-GalNAc determinants that are cross-reactive with the T antigen. Cancer Immunol Immunother 40: 307–310, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris JR, Markl J: Keyhole limpet hemocyanin (KLH): A biomedical review. Micron 30: 597–623, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi M, Yamagishi S, Iwaki M, Nakamura K, Imaizumi T: Advanced glycation end product (age) inhibitors and their therapeutic implications in diseases. Int J Clin Pharmacol Res 24: 95–101, 2004 [PubMed] [Google Scholar]
- 31.Cohen MP: Intervention strategies to prevent pathogenetic effects of glycated albumin. Arch Biochem Biophys 419: 25–30, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Hudson BI, Bucciarelli LG, Wendt T, Sakaguchi T, Lalla E, Qu W, Lu Y, Lee L, Stern DM, Naka Y, Ramasamy R, Yan SD, Yan SF, D'Agati V, Schmidt AM: Blockade of receptor for advanced glycation endproducts: a new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch Biochem Biophys 419: 80–88, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Cohen MP, Vasselli JR, Neuman RG, Witt J: Treatment with acarbose, an alpha-glucosidase inhibitor, reduces increased albumin excretion in streptozotocin-diabetic rats. Gen Pharmacol 26: 1355–1361, 1995 [DOI] [PubMed] [Google Scholar]