Abstract
Stimulation of the bone morphogenetic protein (BMP) pathway protects the kidney from acute and chronic injury. Numerous regulators in the kidney control BMP signaling, offering many targets for therapeutic manipulation. Here, we screened for modulators of BMP signaling in the ischemia-sensitive S3 segment and found that Chordin-like 1 is expressed in this segment of both the mouse and human nephron. Chordin-like 1 specifically antagonizes BMP7, which is expressed in the neighboring distal nephron, and this depends on the presence of the protein Twisted gastrulation. Upon ischemia-induced degeneration of the S3 segment, we observed a reduction in Chordin-like 1 expression coincident with intense BMP signaling in tubules of the recovering kidney. Restored expression accompanied proximal tubule epithelia redifferentiation, again coincident with decreased BMP signaling. We propose that Chordin-like 1 reduces BMP7 signaling in healthy proximal tubules, and the loss of this activity upon sloughing of injured epithelia promotes BMP7 signaling in repopulating, dedifferentiated epithelia. As regenerating epithelia differentiate, Chordin-like 1 is again expressed, antagonizing BMP7. These data suggest a mechanism for dynamic regulation of renoprotective BMP7 signaling in the S3 segment of the proximal tubule.
Bone morphogenetic protein (BMP) signaling plays crucial roles in kidney development and homeostasis. BMP7 is required for maintenance of embryonic nephron progenitors,1–3 protects adult nephrons from acute injury,4–6 and prevents and possibly reverses chronic kidney injury.7–9 Studies indicate diverse mechanisms of BMP7 action. For example, BMP7 counteracts and reverses epithelial-to-mesenchymal transition in renal epithelia by regulating E-cadherin.9,10 BMP7 also reduces proximal tubule expression of cytokines such as IL-6 and macrophage chemoattractant protein, modifying inflammatory infiltration.11 In addition, BMP7 changes the conformation of cell-surface hyaluronic acid on proximal tubule cells, altering monocyte binding and preventing increased TGF-β production.12,13
BMPs signal by binding serine-threonine kinase receptors, which phosphorylate Smad transcription factors. Association of Smads with cell type–specific transcription factors determines transcriptional outcomes, enabling context-dependent responses.14 Pathway inhibitors have evolved at numerous levels to limit the potent effects of BMPs. Secreted antagonists such as Noggin and Chordin bind BMPs extracellularly, preventing receptor association. Opposing ligand and antagonist gradients establish fields of graded signaling strengths known as morphogenetic gradients.15–18
Considering the potent effects of BMP7, it is not surprising that BMP signaling is tightly regulated in the kidney. BMP7 expression is limited to podocytes, the distal nephron, and collecting duct.11,19 Furthermore, the antagonist Sostdc1 (Usag1) is expressed in distal tubule.20 Conversely, the agonist Crim2 (Kcp) is expressed upon kidney injury, amplifying signaling in the damaged nephron.21 Thus, BMP signaling is suppressed in the healthy kidney and elevated in the diseased kidney by extracellular modulators.
Little is known about BMP regulation by the proximal tubule. Here, we used experimental acute injury to screen for antagonists expressed in this region of the nephron. Upon severe ischemic injury, epithelial cells of the straight (S3) segment are lost, and segment-specific transcripts can be identified by comparison with healthy kidneys. We identified Chordin-like 1 (CHRDL1) as a secreted BMP7 modulator expressed in the S3 segment of the mouse and human nephron. Furthermore, we showed that although CHRDL1 can both amplify and antagonize BMP7, it functions as a strong antagonist in conjunction with the extracellular protein Twisted gastrulation. Transgenic overexpression indicates that CHRDL1 functions as an antagonist in the kidney, which is potentially explained by overlapping expression with Twisted gastrulation in both the embryo and the adult. Expression of Chrdl1 is lost after severe ischemic injury and returns as nephrons regenerate. On the basis of these findings, we propose that CHRDL1 limits BMP responsiveness of the healthy proximal tubule and that its loss in injury sensitizes the regenerating nephron to pro-nephrogenic BMP7 signaling.
RESULTS
To identify BMP antagonists unique to the S3 proximal tubule segment, we compared candidate gene expression in healthy and ischemically injured kidneys. Tubular degeneration after injury is proportional to the duration of ischemia,22 and nephron segments respond differently: The S3 segment is most susceptible23,24 yet possesses a high regenerative capacity.25–27 Thus, a severe ischemic insult can be used to probe expression of S3-specific candidate genes, because their expression will be significantly reduced upon loss of that nephron segment. Moreover, expression of such genes should reemerge concomitant with S3 regeneration.
Cells of the S3 Segment are Lost after 45 Min of Ischemia-Reperfusion Injury
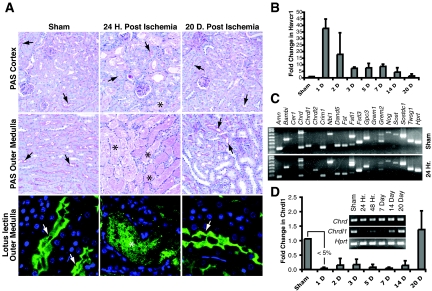
After 45 min of ischemia, the vast majority of S3 segment cells were lost, whereas most cells of the S1 and S2 segments remained intact after 24 h (Figure 1A). Moreover, 5 to 7 d after injury, cells could be seen lining the denuded S3 segment, and within 20 d, brush border formation detected by lotus lectin28 and periodic acid-Schiff staining29 indicated extensive S3 regeneration (Figure 1A). Previous studies have shown similar dynamics of injury and regeneration.26 Consistent with this, expression of the kidney injury molecule 1 (KIM1) ortholog Havcr1 peaked upon injury and regressed to preinjury levels along with the appearance of differentiated S3 segments (Figure 1B). KIM1 and Havcr1 are markers of proximal tubule injury correlating closely with serum creatinine and blood urea nitrogen in ischemia-reperfusion injury (IRI).30
Figure 1.
IRI reduces Chrdl1 expression. (A) Periodic acid-Schiff (PAS) staining shows tubule injury in cortex (top) and outer medulla (middle) after 45 min of renal ischemia at 24 h after surgery and after 20 d, when a substantial amount of tubule regeneration has taken place. The majority of tubular degeneration takes place in the outer medulla. In the cortex and outer medulla of the sham-operated control, proximal tubules show strong lumenal PAS staining (arrows), whereas at 24 h after ischemia, degenerated tubules of the outer medulla are filled with protein casts (*). After 20 d, large numbers of tubules with luminal PAS staining can be seen in the recovering kidney (arrows). This is reflected by staining with the proximal tubule marker lotus lectin in the outer medulla (bottom): Numerous proximal tubules display lumenal staining in the sham and 20 d after ischemia (arrows), whereas only trapping in protein casts of degenerated tubules (*) can be seen 24 h after ischemia. (B) Injury and recovery is reflected by dynamic expression of the injury marker Havcr1, the mouse ortholog of human KIM1. QPCR for Havcr1 was normalized to β-actin expression, and all assays were performed in triplicate. (C) RT-PCR screen of BMP antagonists expressed in the kidney 24 h after induction of ischemic injury indicates that levels of Chrdl1 expression are uniquely reduced. (D) RT-PCR showing dynamic expression of Chrdl1 compared with Chordin (inset) and QPCR of Chrdl1 expression (normalized to β-actin), both during a 20-d period after ischemic injury (n = 3). At 24 h after ischemia, expression of Chrdl1 is reduced to <5% of the level detected in the sham sample. The cDNA used in C and D (inset) was generated using RNA pooled from three animals. Loss of Chrdl1 expression comparable to that seen in the assay of pooled RNA was confirmed on five individual samples from injured kidneys (data not shown).
Chrdl1 Expression Is Dramatically Reduced after IRI and Returns to Normal Levels as Tubules Regenerate
We assessed expression of 19 verified or predicted BMP antagonists by reverse transcription–PCR (RT-PCR) in total kidney RNA from pools of three sham operated and three injured kidneys 24 h after injury (Figure 1C). Interestingly, only expression of Chrdl1 displayed a gross reduction. Increased transcription of Chrdl2 after ischemic injury was noted, but we chose to focus our study on Chrdl1, whose expression was reduced upon S3 segment loss, suggesting expression specifically in that nephron segment. Quantitative PCR of total kidney cDNA from three biological replicates confirmed that Chrdl1 expression 24 h after injury was reduced to <5% of uninjured levels (Figure 1D). Upon tubular repair, expression of Chrdl1 returned to levels comparable to those seen in healthy kidneys (Figure 1D). The loss of Chrdl1 expression after IRI indicates either that it may be expressed specifically in the S3 segment or that its expression is regulated by hypoxia or injury. To resolve these questions, we analyzed Chrdl1 expression in healthy and injured mouse kidneys as well as in cell lines representing distinct tubules of the kidney.
Chrdl1 Is Expressed in the S3 Segment of the Proximal Tubule
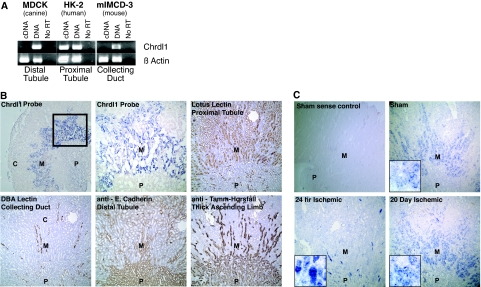
RT-PCR for Chrdl1 in cell lines representing distal tubule, proximal tubule, and collecting duct reveals expression exclusively in proximal tubule cells (Figure 2A). Chrdl1 expression in tissue is limited to the outer stripe of the outer medulla: When compared with proximal tubule, collecting duct, distal tubule, and thick ascending limb markers, Chrdl1 co-localizes exclusively with the proximal tubule marker (Figure 2B). RNA analysis thus conclusively demonstrates that Chrdl1 is expressed in proximal tubules in the outer medulla. Tubules found in this region are predominantly the S3 segments, and we therefore conclude that Chrdl1 is expressed specifically in this tubule segment. To ascertain whether expression remains limited to the S3 segment during nephron regeneration, we assayed Chrdl1 expression before and after IRI. As anticipated from quantitative analysis of Chrdl1 after injury (Figure 1, C and D), no expression can be seen after 24 h (Figure 2C); however, after 20 d, when S3 segments have regenerated, the intensity and distribution of Chrdl1 expression is comparable to that seen in the healthy kidney (Figure 2C). We conclude that Chrdl1 expression is lost after injury, concomitant with loss of cells in the S3 proximal tubule, and that it is regained in this tubule segment upon regeneration.
Figure 2.
Chrdl1 is expressed in the medullary proximal tubule of the nephron. (A) RT-PCR for Chrdl1 was performed on cDNA from cells representing distal tubule (MDCK), proximal tubule (HK-2), and collecting duct (mIMCD-3). Only the HK-2 proximal tubule cell line expresses Chrdl1. The PCR primers were designed to amplify within a single exon, allowing genomic DNA to serve as a positive control. The template for the negative control was synthesized using DNAsed RNA in a reverse transcription reaction in the absence of reverse transcriptase (No RT). (B) Chrdl1 is shown by in situ hybridization to be expressed in the outer medulla (M) but not in the papilla (P) or the cortex (C) (first panel); boxed region enlarged in the second panel. This expression pattern overlaps with the expression of the proximal tubule marker Lotus Tetragonolobus lectin but not with markers for distal tubule (E-cadherin), collecting duct (Dolichos Bifloris Agglutinin lectin [DBA]), or thick ascending limb (Tamm-Horsfall antigen). (C) Consistent with quantitative PCR data (Figure 1D), in situ hybridization for Chrdl1 shows that expression is reduced 24 h after ischemic injury and returns to approximately normal levels by 20 d after injury. The high-magnification insets show signal in tubular epithelial cells in the sham and at 20 d after ischemia, whereas at 24 h after ischemia, background signal results from nonspecific binding to cellular debris in lumenal protein casts (inset and Supplemental Figure S1). A control tested with a sense probe showed no specific staining (first panel).
CHRDL1 Protein Is Produced in the Proximal Tubules of the Outer Medulla in the Human Kidney
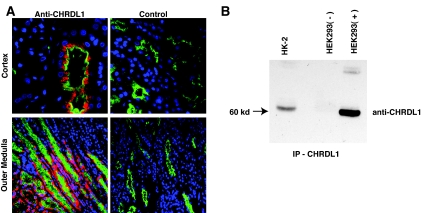
To understand the translational value of our findings, we immunolocalized CHRDL1 in normal human kidney tissue. Using an antiserum specific for human CHRDL1 (Figure 3 and data not shown), we performed co-localization studies with lotus lectin as a proximal tubule marker. CHRDL1 is indeed expressed in proximal tubules of the outer medulla, correlating well with the localization of expression in the mouse (Figure 3A). In human, we also observed a domain of CHRDL1 expression in some proximal convoluted tubules adjacent to glomeruli. In addition, we determined by immunoprecipitation that CHRDL1 is secreted by HK-2 human proximal tubule cells (Figure 3B).
Figure 3.
CHRDL1 protein is expressed in proximal tubules of the human renal medulla and is secreted by proximal tubule cells. (A) Proximal tubules are identified in paraffin sections of the human renal cortex (top) and outer medulla (bottom) by staining with lotus lectin (green). Co-staining with a polyclonal antiserum specific for CHRDL1 (red) in the left panels demonstrates co-localization with lotus lectin in tubules of the medullary region of the human kidney. CHRDL1 can also be seen, however, in some cortical convoluted tubules, possibly representing secreted protein or a protein expression pattern somewhat different from mouse. The right panels show negative control sections treated in an identical manner except for the omission of the CHRDL1 antibody. (B) HK-2 proximal tubule cells secrete CHRDL1. A goat polyclonal antiserum specific for CHRDL1 was used to precipitate protein from HK-2 conditioned medium (HK-2). Precipitated protein was immunodetected using a mouse mAb specific for CHRDL1, revealing a protein of approximately 60 kD. Although the predicted molecular weight of CHRDL1 is approximately 51 kD, previous immunoblotting studies with the recombinant protein have shown the molecular weight to be approximately 60 kD (data not shown), possibly as a result of extensive posttranslational processing. To control for the specificity of the assay and to predict the molecular weight accurately, HEK293 cells transfected with a control vector or a CHRDL1 expression vector were immunoprecipitated using the same conditions as HK-2 cells and immunoblotted on the same filter. The negative control (HEK293−) shows that the antibody does not precipitate confounding protein species, whereas the positive control (HEK293+) demonstrates that the approximately 60-kD band is indeed CHRDL1.
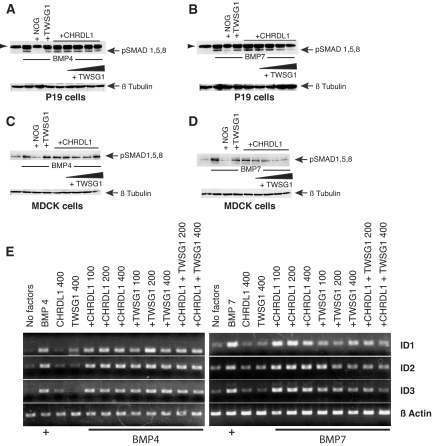
CHRDL1 Alone Amplifies BMP4 and 7 Signaling but Selectively Antagonizes BMP7 in the Presence of Twisted Gastrulation
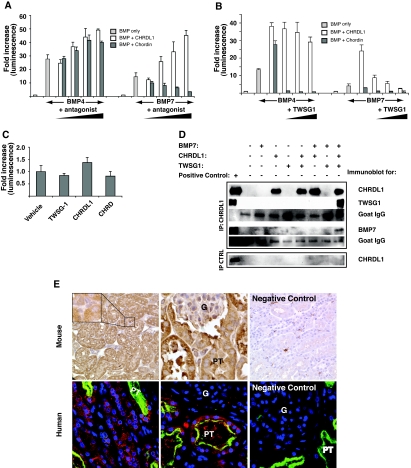
To evaluate the effect of CHRDL1 on BMP signaling, we used the BRE-Luc transcriptional reporter.31 Because the BMP-antagonistic properties of the related protein Chordin are, in some contexts, dependent on Twisted gastrulation as an accessory protein,15,32,33 we included recombinant Twisted gastrulation homolog 1 (TWSG1) in our analysis. Surprisingly, CHRDL1 or Chordin alone amplified BMP4-induced signaling. In contrast, BMP7 signaling was significantly antagonized by Chordin alone and strongly amplified by CHRDL1 (Figure 4A). As expected, the BMP inhibitory properties of Chordin were potentiated in a dosage-dependent manner by the addition of TWSG1.32,34 In contrast, addition of TWSG1 did not convert CHRDL1 to an antagonist of BMP4, and CHRDL1 amplified BMP4 signaling in the presence of TWSG1; however, TWSG1 converted CHRDL1 to a selective BMP7 antagonist in a dosage-dependent manner (Figure 4B). No significant reporter activity was seen in the presence of Chordin, CHRDL1, or TWSG1 alone (Figure 4C). In protein binding assays, CHRDL1 co-precipitates with BMP7 and TWSG1, showing that the inhibitory complex is physically associated (Figure 4D). Surprisingly, binding of CHRDL1 to BMP7 alone cannot be detected in this assay, raising the interesting possibility that the amplifying effect of CHRDL1 may be independent of physical association. In summary, CHRDL1 functions as a specific antagonist of BMP7 in the presence of TWSG1 through physical association and alone is an agonist of both BMP4 and BMP7 signaling.
Figure 4.
CHRDL1 functions as a general BMP-signaling amplifier but specifically antagonizes BMP7 in the presence of TWSG1. (A) P19 embryonal carcinoma cells transfected with the BMP transcriptional reporter pBRE-Luc were incubated overnight with BMP4 (5 ng/ml) or BMP7 (10 ng/ml) and increasing amounts of CHRDL1 (50 to 400 ng/ml) or Chordin (100 to 800 ng/ml). Both CHRDL1 and Chordin amplify BMP4 signaling; however, in contrast to Chordin, which antagonizes BMP7 signaling, CHRDL1 amplifies BMP7 signaling in a dosage-responsive manner. (B) In the presence of TWSG1 (100 to 400 ng/ml), CHRDL1 (200 ng/ml) continues to act as an amplifier of BMP4 signaling unlike Chordin (400 ng/ml), which becomes a potent BMP4 antagonist. In contrast, CHRDL1 amplification of BMP7 signaling is converted to antagonism by addition of TWSG1 in a dosage-dependent manner. (C) In the P19 pBRE-luc reporter assay, the addition of TWSG1, CHRDL1, or Chordin (400 ng/ml) alone does not affect transcriptional activation. (D) CHRDL1 binds BMP7 and TWSG1 only in a trimolecular complex. A co-immunoprecipitation experiment in which CHRDL1 (500 ng/ml) is incubated in the presence of TWSG1 (500 ng/ml) and/or BMP7 (250 ng/ml) demonstrates that CHRDL1 binds TWSG1 and BMP7 only when all three proteins are present. No evidence of binding is detected when TWSG1 or BMP7 alone is incubated with CHRDL1. Recombinant proteins were immunoprecipitated with a goat polyclonal antibody against CHRDL1, and blots of goat IgG serve as loading controls. The last panel, a CHRDL1 immunoblot of an identical immunoprecipitation substituting an irrelevant goat antibody, demonstrates the absence of nonspecific binding by CHRDL1 to either the goat antibody or Protein G beads used in the experiment. The first lane of all blots contains recombinant protein as a positive control. (E) TWSG1 is strongly expressed in tubule epithelia of the mouse and human kidney. (Top) Strong immunohistochemical staining for TWSG1 in tubule epithelia of adult mouse kidney but weak staining in the glomerulus (G) and interstitial cell population (inset). (Right) Negative control using an antibody of the same species specific for macrophage. (Bottom) Immunofluorescent micrographs of an adult human kidney showing TWSG1 expression (red) in both proximal tubules (PT), marked green with Lotus lectin, and other tubules. As in the mouse, glomerular expression of TWSG1 is much weaker than seen in tubular epithelia. (Right) Negative control for TWSG1.
Previous studies showed ubiquitous Twsg1 expression in the kidney, with the highest expression in outer medulla.20,35 TWSG1 protein expression was confirmed by immunostaining in both mouse and human kidney. In both species, protein expression is strong in proximal and other tubule epithelia and weak in interstitial cells and glomeruli (Figure 4E). Moreover, strong expression of TWSG1 was detected by immunoblot of HK-2 cells and primary human proximal tubule cells (Supplemental Figure S1). We therefore conclude that CHRDL1 predominantly antagonizes BMP7 signaling in the healthy kidney because it is expressed in an overlapping manner with TWSG1.
Functional Discrimination between BMP4 and BMP7 by CHRDL1 Associates Directly with Activation of the BMP-Induced Smad Transcription Factors
We performed immunoblots for phosphorylated Smads (pSmad1/5/8) in P19 and MDCK cells treated with BMP, CHRDL1, and TWSG1 alone or in combination (Figure 5, A through D). pSmad1/5/8 immunodetection correlates closely with transcriptional assays, demonstrating that CHRDL1 functions upstream of receptor activation, modulating ligand binding in a manner dependent on both BMP ligand identity and the presence of TWSG1.
Figure 5.
CHRDL1 reduces BMP7-stimulated Smad activation and ID gene expression in the presence of TWSG1 but has no effect on BMP4 signaling. (A and B) In the presence of TWSG1, CHRDL1 inhibits Smad phosphorylation by BMP7 but not by BMP4. P19 cells were serum starved for 2 h and then incubated with BMPs (10 ng/ml) and antagonists for 2 h before lysis and Western blotting. Blots were probed for phosphorylated Smads 1, 5, and 8 and β-tubulin. In lanes 3, 4, and 5, Noggin (200 ng/ml), TWSG1, and CHRDL1 alone (400 ng/ml) were added. In lanes 6 through 9 CHRDL1 (400 ng/ml) was added together with increasing concentrations of TWSG1 (50, 100, 200, and 400 ng/ml). BMPs were incubated at 37 °C for 1 h with or without antagonists before application. Note that the arrow points to the band corresponding to pSmad1/5/8. The top band (arrowhead) is a contaminating band specific to P19 lysates and is not present in lysates from MDCK cells (compare C and D). (C and D) The effects of CHRDL1 and TWSG1 on BMP signaling can be reproduced in the MDCK kidney cell line. (E) RT-PCR showing expression of ID1, 2, and 3 genes in HK-2 cells stimulated with BMP4 or 7 (25 ng/ml) for 6 h in the presence of varying concentrations (100 to 400 ng/ml) of CHRDL1 and/or TWSG1. ID expression by HK-2 cells in response to BMP4 is unaffected by the presence of TWSG1. In cells incubated with BMP7, the addition of TWSG1 reduces ID gene expression.
To assess the role of CHRDL1 in modulating BMP responses in the proximal tubule, where it is endogenously expressed, we assayed expression of the BMP responsive ID genes in HK-2 cells treated with BMP, CHRDL1, and TWSG1 (Figure 5E). As predicted from our previous analyses, CHRDL1 and TWSG1, added separately or together, had little effect on BMP4-induced ID transcription; however, both CHRDL1 and TWSG1 affected BMP7 signaling: Alone, CHRDL1 potentiated ID transcription slightly, whereas TWSG1 reduced transcription. The latter is consistent with HK-2–producing CHRDL1 cells’ being rendered antagonistic to BMP7 signaling by addition of TWSG1. Predictably, CHRDL1 and TWSG1 together reduced transcriptional responses to BMP7 in HK-2 cells. Attempts to knock down CHRDL1 using siRNAs were unsuccessful, and we were thus unable to measure cellular responses to BMP stimulation in an environment where CHRDL1 was reduced or absent. Nonetheless, our data show that CHRDL1 specifically antagonizes BMP7 in proximal tubule cells in the presence of increased concentrations of TWSG1.
CHRDL1 Antagonizes BMP Signaling In Vivo
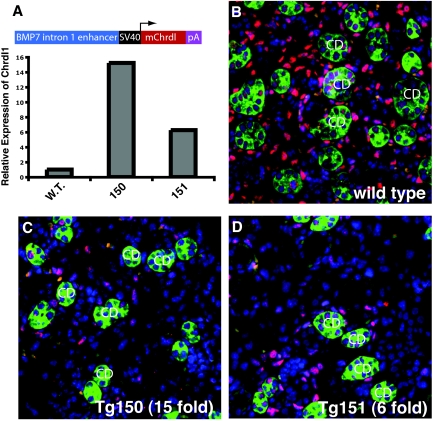
To understand whether CHRDL1 antagonizes BMP7 in vivo, we generated a transgene driving Chrdl1 expression in collecting ducts using an enhancer element derived from intron 1 of Bmp7.36 We chose a collecting duct specific enhancer for three reasons: (1) Interference with BMP signaling in the collecting duct should result in only subtle disturbances during kidney development,37,38 minimizing the risk for confounding developmental defects; (2) CHRDL1 is secreted, and paracrine effects of secretion from the collecting ducts are anticipated; and (3) the collecting duct is a major site of Bmp7 expression in the developing kidney. Quantitative PCR confirmed up to 15-fold elevated expression of Chrdl1 in transgenic kidneys (Figure 6A). pSmad1/5/8 immunostaining showed that Chrdl1 overexpression significantly reduces BMP signaling in the developing kidney (Figure 6, B through D). Previous studies report that Twsg1 is widely expressed in the embryonic day 17.5 metanephros,39 and we conclude that CHRDL1 antagonizes BMP7 in the embryonic kidney and likely retains this function in the adult kidney.
Figure 6.
Overexpression of mouse Chrdl1 in the collecting duct substantially reduces BMP signaling in vivo. (A) Diagram of mouse Chrdl1 transgene construct driven by a collecting duct–specific enhancer element from intron 1 of the Bmp7 gene; quantitative PCR assay shows Chrdl1 overexpression in kidneys of two embryonic day 17.5 transgenic embryos (150 and 151) compared with wild-type. (B through D) Immunolocalization of phosphorylated Smads (red) in the embryonic day 17.5 wild-type kidney (B), and kidneys from transgenic embryos 150 and 151 (C and D) show that BMP signaling is substantially reduced by expression of the Chrdl1 transgene. Collecting ducts (CD) were localized by staining with the lectin Dolichos Biflorus Agglutinin (green), and nuclei were counterstained with DAPI (blue).
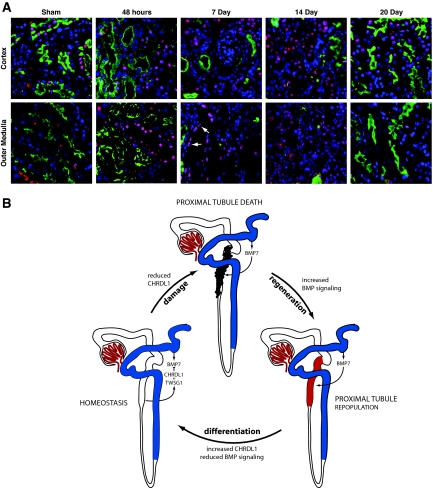
Having established the role of CHRDL1 as a BMP7-specific antagonist in the outer medulla, we fluorescently immunostained for pSmad1/5/8 during regeneration after IRI (Figure 7A). In the uninjured kidney, there was a low basal level of pSmad1/5/8 in the cortex, and no active signaling was detectable in the outer medulla. Within 48 h of injury, BMP signaling increased in both cortex and outer medulla and was maintained for the remainder of the regenerative phase. Interestingly, cells displaying the most intense activation were those repopulating tubules. By day 20, whereas some baseline signaling remained in the cortex, BMP signaling was virtually absent from the outer medulla (Figure 7A). The level of BMP signaling was thus inversely proportional to expression of Chrdl1.
Figure 7.
BMP signaling in the regenerating postischemic kidney is dynamic. (A) BMP signaling visualized by immunofluorescent staining for pSmad1/5/8 (red) shows the increase in signaling associated with recovery from ischemic injury. Proximal tubules are marked with lotus lectin (green) and nuclei with DAPI (blue). In the sham control, limited nuclear pSmad1/5/8 is seen in the cortex, and none can be seen in the outer medulla. After injury, nuclear pSmad1/5/8 can be seen in degenerating proximal tubules of the outer medulla. Extensive BMP signaling is maintained at 7 and 14 d after ischemia, at which point the majority of proximal tubule cells have been sloughed off and are being replaced. pSmad1/5/8 staining is prominent in cells repopulating proximal tubules of the outer medulla (arrows). At 20 d after injury, the level of signaling approaches baseline in the cortex and is again undetectable in the medulla. The sensitivity of the pSmad1/5/8 antibody in immunofluorescence is lower than in immunohistochemical assays, allowing clearer visualization of changes in signaling. (Supplemental Figure S3). (B) In homeostasis, BMP7 ligand emanating from the distal tubule and thick ascending limb is antagonized in the S3 segment of the proximal tubule by CHRDL1 in conjunction with Twisted gastrulation. In ischemic injury, the epithelium of the S3 segment is particularly prone to injury and death. As a result, CHRDL1 expression is reduced, with a concomitant reduction in the antagonism of BMP7 signaling. The resulting increase in BMP signaling is part of the regenerative process that rebuilds the tubule epithelia. As the cells of the recovering epithelia fully redifferentiate, a state of homeostasis is again achieved with BMP7 signaling antagonized by the presence of CHRDL1 and Twisted gastrulation.
DISCUSSION
In many cases, a process of self-repair begins almost immediately after acute ischemic injury. Within 24 h, epithelial cells begin to dedifferentiate, divide, and repopulate damaged tubules.25,27,40 Developmental pathways are reactivated in this process,24,40,41 and BMP7, a potent regulator of renal development,1,3 displays protective effects in IRI as well as in other models of acute and chronic kidney injury.4,5,7,9 Although there is a paucity of published data on the expression of BMPs other than BMP7 in the adult kidney, overlapping expression of multiple BMPs is anticipated, as has been demonstrated in the embryonic organ.42 Furthermore, circulating BMP943 may contribute to BMP signaling in the kidney. A complex system for the control of BMP signaling by both BMP antagonists and agonists is therefore required.44 These modulators are essential to BMP function, and their inactivation strongly influences the kidney's response to acute and chronic injury.21,45 The response of the S3 segment to BMPs is of particular relevance to acute ischemic injury because this is the site of the most profound cellular damage. Using the IR model, we identified CHRDL1 as an S3 segment–specific modulator that selectively antagonizes BMP7.
Previous reports identified CHRDL1 as a BMP antagonist in Xenopus.46–48 Although no comparison of CHRDL1 orthologs has been published, substantial species differences seem likely. For example, physical association studies showed that chick CHRDL1 binds to BMP4 but not to BMP7,46 whereas mouse and human CHRDL1 bind to BMP449 and mouse CHRDL1 to BMP5 and BMP6.47 In a functional osteoblast differentiation assay, rat CHRDL1 binds to and antagonizes BMP7 but not BMP2.50 In our studies, we demonstrate that human CHRDL1 binds BMP7 in the presence of TWSG1 and ascribe a function to human CHRDL1 similar to that reported for rodent CHRDL1. Furthermore, we define for the first time a role for TWSG1 in CHRDL1-mediated antagonism of BMP7, suggesting the intriguing possibility that distinct fields of BMP signaling in the adult kidney may be established through interaction of these three proteins. Chordin, in association with TWSG1, binds with and antagonizes BMP4. This inactive complex can subsequently be reactivated by metalloproteases such as tolloid that cleave Chordin between its cysteine-rich domains.16,51,52 CHRDL1, in conjunction with TWSG1, may establish a similar gradient for BMP7: CHRDL1 and TWSG1 would antagonize BMP7 (Figure 4) while allowing diffusion and possibly metalloprotease reactivation of BMP signaling at remote sites. Indeed, CHRDL1 contains cleavage sites between its cysteine-rich domains, indicating that it may be subject to metalloprotease cleavage similar to chordin.47 BMP7 is produced by the distal tubule and collecting duct,11,19 whereas CHRDL1 is secreted by the adjacent proximal tubule (Figures 2 and 3), establishing opposing gradients of these two proteins. Both in situ hybridization20,35 and immunostaining (Figure 4E) assays indicate that Twsg1 is expressed in the renal epithelia. Thus, it is anticipated that the proximal tubule of the healthy kidney is subject to the greatest BMP7 antagonism by CHRDL1/TWSG1. Extracellular matrix is rich in metalloproteases, including the Tolloid ortholog BMP1,53 and it is possible that the BMP7-antagonistic properties of CHRDL1 and TWSG1 originating in the proximal tubule are counteracted in the interstitium. Thus fibroblasts, endothelia, pericytes, and inflammatory cells may experience degrees of BMP7 signaling differing significantly from those in the proximal tubule.
Our analysis demonstrates that CHRDL1/TWSG1 antagonism is specific for BMP7, indicating a strong physiologic requirement for quenching BMP7 signaling in the homeostatic proximal tubule. BMP7 signaling limits excessive tissue injury by suppressing initiation of the inflammatory response by tubular epithelial cells.4,11–13 Immune surveillance is required for maintenance of the healthy kidney, and antagonism of BMP7 may therefore be necessary for tissue homeostasis. Upon ischemic injury, release of antagonism by degeneration of the CHRDL1-expressing S3 segment would facilitate BMP7 signaling required to counteract the inflammatory response.
In summary, we show that the healthy kidney expresses BMP7-antagonistic Chrdl1 in the straight segment of the proximal tubule and demonstrates little active BMP signaling in this region. Upon injury, the epithelium of the straight segment is sloughed off and Chrdl1 activity is lost, correlating with increased BMP signaling during the regenerative phase of the injury response. Finally, Chrdl1 expression is regained upon differentiation of regenerated epithelia, and BMP signaling is again reduced to basal levels (Figure 7B). Together with positive and negative modulators of BMP signaling such as Crim221 and Sostdc1,20 we propose that Chrdl1 contributes to the complex multifactorial regulation of BMP signaling in the healthy and injured kidney.
CONCISE METHODS
Cell Culture and Transfection
HK-2 human proximal tubule epithelial cells (ATCC, Manassas, VA) were cultured at 37°C with 5% CO2 in keratinocyte serum free media supplemented with bovine pituitary extract and EGF (Life Technologies, Carlsbad, CA), penicillin, streptomycin, and Amphotericin B according to the manufacturer's instructions. P19 mouse embryonal carcinoma cells (ATCC) were cultured at 37°C with 5% CO2 in DMEM supplemented with 7.5% bovine serum and 2.5% FBS, penicillin/streptomycin, and Amphotericin B. HEK293 (human embryonic kidney cells), MDCK (canine distal tubule cells), and MIMCD-3 (mouse inner medullary collecting duct cells; all from ATCC) were cultured at 37°C with 5% CO2 in DMEM supplemented with 10% bovine serum, glutamine, penicillin/streptomycin, and Amphotericin B. Transfection of HEK293 cells with the expression construct for human CHRDL1 (Origene, Rockville, MD) was performed in 12-well plates (1.8 μg of DNA/well) during a 6-h period with Lipofectamine 2000 according to the manufacturers’ instructions. Reporter plasmids were transfected in 48-well plates during a 6-h period with Lipofectamine 2000 according to the manufacturer's instructions. Cells were co-transfected with the BMP reporter pBRE-Luc (provided by Peter ten Dijke, Leiden University, Leiden, Netherlands) at 0.3 μg/well and the Renilla control pRL-CMV (Promega, Madison, WI) at 0.02 μg/well to normalize for transfection efficiency. Ligands were preincubated for 1 h in serum-free medium before addition to the cells. After incubation of the transfected cells with ligands for 16 h, luciferase activity was measured using the Dual Luciferase Assay kit (Promega), and readings were taken on a Femtomaster FB12 luminometer (Zylux, Huntsville, AL) using FB12/Sirius software. Activity was normalized to Renilla readings, and all experiments were done in triplicate. The results depicted are means ± SD.
Renal IRI
All ischemia-reperfusion experiments were performed using 6- to 8-wk-old male ICR mice (Taconic, Hudson, NY). All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the Maine Medical Center Research Institute Institutional Animal Care and Use Committee. Mice were anesthetized with ketamine (105 mg/kg) and xylazine (16 mg/kg) by intraperitoneal injection. The right kidney was exposed by a flank incision, and the renal pedicle was occluded with a micro-aneurysm clamp for 45 min. Upon removal of the clamp, blood flow to the kidney was reestablished and visually confirmed, and incisions were closed with surgical staples. Sham-operated mice were submitted to the same surgical procedure and conditions but without clamping of the renal pedicle. After surgery, buprenorphine (0.01 mg/kg) was administered as an analgesic by subcutaneous injection. At the stated time points after surgery, mice were killed by cervical dislocation after anesthesia with isofluorane, and kidneys were harvested for RNA and processed for sectioning. Sham kidneys were harvested 24 h after surgery.
Histology
Kidneys were fixed in 4% paraformaldehyde overnight, dehydrated in methanol, and paraffin embedded for sectioning and preparation with periodic acid-Schiff.
Immunohistochemistry
Lectin staining was accomplished using biotinylated Lotus Tetragonolobus and Dolichos Biflorus Agglutinin (Vector Laboratories, Burlingame, CA) followed by incubation with RTU Vectastain (Vector Laboratories) and development using DAB (Sigma, St. Louis, MO). E-cadherin was marked with a mouse mAb (BD Biosciences, San Jose, CA) after antigen retrieval (DAKO Target Retrieval Solution, Dako, Carpinteria, CA). Mouse TWSG1 was detected with rat anti-TWSG1 (R&D Systems, Minneapolis, MN), and pSmad 1,5,8 was localized with a rabbit primary antibody (Cell Signaling Technology, Danvers, MA) after antigen retrieval. Primary antibody was followed with biotinylated appropriate secondary antibody (Jackson Immunoresearch, West Grove, PA) and RTU Vectastain (Vector Laboratories) and developed with DAB (Sigma). Tamm-Horsfall protein was localized with a sheep antibody (BioDesign Int., Saco, ME) followed with biotinylated donkey anti-sheep secondary antibody (Jackson Immunoresearch), RTU Vectastain (Vector Laboratories), and DAB (Sigma). Sections were counterstained with hematoxylin.
Immunofluorescence
Deparaffinized mouse kidney sections prepared as described already were incubated with biotinylated lectins Lotus Tetragonolobus or Dolichos Biflorus Agglutinin (Vector Laboratories). Slides were then washed and incubated with streptavidin Alexa Fluor 488 or 568 conjugates and DAPI (Molecular Probes, Eugene, OR) to visualize the proximal tubule brush border, collecting ducts, and nuclei, respectively. PhosphoSmads 1, 5, and 8 were detected after antigen retrieval (DAKO Target Retrieval Solution) with a rabbit antibody from Cell Signaling Technology and goat anti-rabbit Alexa Fluor 568 (Molecular Probes).
Formalin-fixed human kidney sections were purchased from the Biochain Institute (Hayward, CA) and Zyagen Laboratories (San Diego, CA). Proximal tubules and nuclei were marked as described already. After antigen retrieval (DAKO Target Retrieval Solution), CHRDL1 was visualized with a goat polyclonal antibody (R&D Systems) and TWSG1 was marked with a rabbit antibody (Atlas Antibodies, Stockholm, Sweden). Primary antibodies were followed with chicken anti-goat and goat anti-mouse secondary antibodies conjugated to Alexa Fluors (Molecular Probes). The negative controls were processed in the same manner, except for the omission of the primary antibody.
In Situ Hybridization
The RNA probe for Chrdl1 was synthesized from a 1-kb portion of the Chrdl1 coding sequence inserted into the pBluescript II KS vector. Sense and antisense probes were transcribed from the linearized plasmid using T7 and T3 RNA polymerases and the DIG RNA Labeling Kit (Roche Diagnostics). For paraffin sections, tissue was fixed in 4% paraformaldehyde, dehydrated, and paraffin embedded for sectioning. Samples were prepared, probed, and stained as described previously.37
PCR and RT-PCR
For RT-PCR on kidney tissue, a portion of harvested kidney was mechanically homogenized in Trizol Reagent (Invitrogen) and RNA was extracted according to the manufacturer's protocol. Genomic DNA was removed using DNA-Free (Ambion, Austin, TX), and cDNA was synthesized using oligo-dT primers and the SuperScript-II kit (Invitrogen, Carlsbad, CA). Amplification was performed using Hot Start MasterMix (Eppendorf, Hamburg, Germany). Primers are listed in Supplemental Table S1. For control PCR on cell lines, genomic DNA was extracted and used as template. For RT-PCR, cDNA from cell lines was synthesized as described already, except cells were dissolved directly into Trizol without homogenizing. Amplification was accomplished as described already. Primers are listed in Supplemental Table S1.
Quantitative PCR
RNA and cDNA were prepared as described already. All quantitative PCR was run in triplicate wells on an iCycler IQ (BioRad, Hercules, CA) using RT2 qPCR Master Mix from SuperArray Biosciences. Primers used for quantitative PCR are listed in Supplemental Table S1 and were analyzed for efficiency and melting curves before use. Primer efficiency was incorporated into the fold analysis using the Pfaffl method.54
Recombinant Proteins
Human BMP4 and 7, human CHRDL1, mouse Chordin, mouse Noggin, and mouse Twisted gastrulation 1 were purchased from R&D Systems.
Immunoblot Analysis and Immunoprecipitation
SDS-PAGE was performed in polyacrylamide gels, and proteins were transferred to nitrocellulose membranes. PhosphoSmads 1, 5, and 8 were detected with a rabbit antibody from Cell Signaling Technology and secondary goat anti-rabbit horseradish peroxidase (HRP)-conjugated antibody (Jackson ImmunoResearch). β-Tubulin was detected with rabbit anti–β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA). Human CHRDL1 was detected with mouse anti-CHRDL1 (R&D Systems), and a sheep anti-mouse secondary HRP conjugate (Amersham, Piscataway, NJ). BMP7 was detected with mouse anti-BMP7 (R&D Systems) and a sheep anti-mouse HRP conjugate (Amersham). Goat IgG was detected with a donkey anti-goat HRP conjugate (Jackson Immunoresearch). ECL chemiluminescent substrate (Amersham) was used for signal detection. The immunoprecipitation of CHRDL1 from conditioned medium was accomplished by incubating 48-h-conditioned medium from HK-2 cells or from HEK293 cells (transfected and not transfected with the human CHRDL1 expression construct [Origene]) with a goat polyclonal antibody against CHRDL1 (R&D Systems). The co-immunoprecipitation of CHRDL1, BMP7, and TWSG1 was accomplished by incubating recombinant proteins in Keratinocyte-SFM medium (Life Technologies) with the same goat polyclonal antibody against CHRDL1. Incubations were done at 4°C overnight on a rotator and followed by incubating samples at 4°C on a rotator with washed Protein G Sepharose beads (GE Healthcare, Piscataway, NJ) for 2 h. The bead complexes were then spun down, the medium was aspirated, and lysis buffer was added. After boiling for 5 min in a reducing buffer (nonreducing buffer for BMP7 immunoblots), the sample was subjected to SDS-PAGE and the membrane probed with the antibodies described already.
Embryonic Transgene Expression
The mouse Chrdl1 cDNA was cloned by PCR (forward primer CACCATGGATGGCATGAAATACATCATTT; reverse primer CTAACAGTGGTCCTTTTCAGGTCT) using a cDNA library prepared from the kidney of an adult male ICR mouse. The cDNA product was cloned into the pcDNA3.1 Topo v-5 His vector (Invitrogen) and verified by sequencing. For construction of the transgene driving overexpression of mouse Chrdl1 in the collecting duct, the 480-bp enhancer element from intron 1 of the Bmp7 gene36 was inserted upstream of the SV40 promoter of the pGL3-Promoter Vector (Promega), and the Chrdl1 cDNA was subcloned downstream of the promoter in place of the luciferase sequence. This construct was linearized before pronuclear injection into oocytes of FVB/NTac females implanted into the oviduct of Swiss Webster pseudopregnant females.55 Embryos were harvested at embryonic day 17.5.
DISCLOSURES
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Center for Research Resources grant 2P20RR18789-06 (project 1, L.O.). Additional support was provided by Maine Medical Center Research Institute core facilities for Bioinformatics, Histopathology (both supported by 2P20RR18789), Mouse Transgenics (supported by 2P20RR15555), and the Animal Facility.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Dudley AT, Lyons KM, Robertson EJ: A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Dudley AT, Godin RE, Robertson EJ: Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13: 1601–1613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G: BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9: 2808–2820, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK: Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102: 202–214, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruska KA, Guo G, Wozniak M, Martin D, Miller S, Liapis H, Loveday K, Klahr S, Sampath TK, Morrissey J: Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol 279: F130–F143, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S: Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 13[Suppl 1]: S14–S21, 2002 [PubMed] [Google Scholar]
- 7.Wang S, de Caestecker M, Kopp J, Mitu G, Lapage J, Hirschberg R: Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol 17: 2504–2512, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Muller GA, Kalluri R: Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol 285: F1060–F1067, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R: BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Zeisberg M, Shah AA, Kalluri R: Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem 280: 8094–8100, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Gould SE, Day M, Jones SS, Dorai H: BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 61: 51–60, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Selbi W, de la Motte C, Hascall V, Phillips A: BMP-7 modulates hyaluronan-mediated proximal tubular cell-monocyte interaction. J Am Soc Nephrol 15: 1199–1211, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Zhang XL, Selbi W, de la Motte C, Hascall V, Phillips AO: Bone morphogenic protein-7 inhibits monocyte-stimulated TGF-beta1 generation in renal proximal tubular epithelial cells. J Am Soc Nephrol 16: 79–89, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Massagué J: How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1: 169–178, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM: Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development 128: 4439–4447, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM: BMP-binding modules in chordin: A model for signalling regulation in the extracellular space. Development 127: 821–830, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balemans W, Van Hul W: Extracellular regulation of BMP signaling in vertebrates: A cocktail of modulators. Dev Biol 250: 231–250, 2002 [PubMed] [Google Scholar]
- 18.Massagué J, Chen YG: Controlling TGF-beta signaling. Genes Dev 14: 627–644, 2000 [PubMed] [Google Scholar]
- 19.Wetzel P, Haag J, Campean V, Goldschmeding R, Atalla A, Amann K, Aigner T: Bone morphogenetic protein-7 expression and activity in the human adult normal kidney is predominantly localized to the distal nephron. Kidney Int 70: 717–723, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Yanagita M, Okuda T, Endo S, Tanaka M, Takahashi K, Sugiyama F, Kunita S, Takahashi S, Fukatsu A, Yanagisawa M, Kita T, Sakurai T: Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J Clin Invest 116: 70–79, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Patel SR, Cheng X, Cho EA, Levitan I, Ullenbruch M, Phan SH, Park JM, Dressler GR: Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat Med 11: 387–393, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Faarup P, Holstein-Rathlou NH, Norgaard T, Hegedus V: Early segmental changes in ischemic acute tubular necrosis of the rat kidney. APMIS 112: 192–200, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG: Ischemic damage and repair in the rat proximal tubule: Differences among the S1, S2, and S3 segments. Kidney Int 14: 31–49, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Sheridan AM, Bonventre JV: Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens 9: 427–434, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney: Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Faraggiana T, Malchiodi F, Prado A, Churg J: Lectin-peroxidase conjugate reactivity in normal human kidney. J Histochem Cytochem 30: 451–458, 1982 [DOI] [PubMed] [Google Scholar]
- 29.Schulte BA, Spicer SS: Histochemical evaluation of mouse and rat kidneys with lectin-horseradish peroxidase conjugates. Am J Anat 168: 345–362, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV: Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Korchynskyi O, ten Dijke P: Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem 277: 4883–4891, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Blitz IL, Cho KW, Chang C: Twisted gastrulation loss-of-function analyses support its role as a BMP inhibitor during early Xenopus embryogenesis. Development 130: 4975–4988, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O'Connor MB: The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev Biol 267: 374–386, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH: Twisted gastrulation can function as a BMP antagonist. Nature 410: 483–487, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Graf D, Timmons PM, Hitchins M, Episkopou V, Moore G, Ito T, Fujiyama A, Fisher AG, Merkenschlager M: Evolutionary conservation, developmental expression, and genomic mapping of mammalian Twisted gastrulation. Mamm Genome 12: 554–560, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Adams D, Karolak M, Robertson E, Oxburgh L: Control of kidney, eye and limb expression of Bmp7 by an enhancer element highly conserved between species. Dev Biol 311: 679–690, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oxburgh L, Chu GC, Michael SK, Robertson EJ: TGFb superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development 131: 4593–4605, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, Rosenblum ND: BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol 19: 117–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosaka T, Morita S, Kitamura H, Nakajima H, Shibata F, Morikawa Y, Kataoka Y, Ebihara Y, Kawashima T, Itoh T, Ozaki K, Senba E, Tsuji K, Makishima F, Yoshida N, Kitamura T: Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol Cell Biol 23: 2969–2980, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonventre JV: Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14[Suppl 1]: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Dudley AT, Robertson EJ: Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 208: 349–362, 1997 [DOI] [PubMed] [Google Scholar]
- 43.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S: Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109: 1953–1961, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R: Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134: 2397–2405, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Yanagita M: Modulator of bone morphogenetic protein activity in the progression of kidney diseases. Kidney Int 70: 989–993, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, Noda M: Ventroptin: A BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science 293: 111–115, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Nakayama N, Han CE, Scully S, Nishinakamura R, He C, Zeni L, Yamane H, Chang D, Yu D, Yokota T, Wen D: A novel chordin-like protein inhibitor for bone morphogenetic proteins expressed preferentially in mesenchymal cell lineages. Dev Biol 232: 372–387, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Ueki T, Tanaka M, Yamashita K, Mikawa S, Qiu Z, Maragakis NJ, Hevner RF, Miura N, Sugimura H, Sato K: A novel secretory factor, Neurogenesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. J Neurosci 23: 11732–11740, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kane R, Godson C, O'Brien C: Chordin-like 1, a bone morphogenetic protein-4 antagonist, is upregulated by hypoxia in human retinal pericytes and plays a role in regulating angiogenesis. Mol Vis 14: 1138–1148, 2008 [PMC free article] [PubMed] [Google Scholar]
- 50.Chandra A, Itakura T, Yang Z, Tamakoshi T, Xue X, Wang B, Ueki T, Sato K, Uezato T, Miura N: Neurogenesin-1 differentially inhibits the osteoblastic differentiation by bone morphogenetic proteins in C2C12 cells. Biochem Biophys Res Commun 344: 786–791, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Oelgeschlager M, Larrain J, Geissert D, De Robertis EM: The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature 405: 757–763, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS: Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature 410: 475–478, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Hopkins DR, Keles S, Greenspan DS: The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 26: 508–523, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagy G: Manipulating the Mouse Embryo, Cold Spring Harbor, Cold Spring Harbor Laboratory Press, 2003
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.