Abstract
Previous studies suggested that activation of the innate immune system impairs the induction of transplantation tolerance, but the responsible inflammatory mediators have not been identified. In this study, we examined whether IL-6 and TNF-α promote resistance to transplantation tolerance. Using a highly immunogenic murine skin allograft model, we found that the absence of both IL-6 and TNF-α in the graft recipient synergized with co-stimulatory blockade to induce tolerance. Furthermore, IL-6 and TNF-α acted together to promote T cell alloimmune responses both in vitro and in vivo and to impair the ability of regulatory T cells to suppress effector T cell alloimmunity. In addition, deficiency of recipient IRAK-M, a negative regulator of certain innate immune pathways, augmented cellular IL-6 and TNF-α responses and impaired the ability of co-stimulatory blockade to extend allograft survival. In summary, IL-6 and TNF-α synergistically impair the efficacy of therapies that promote allograft acceptance.
Studies have demonstrated that innate immunity and inflammation are involved in acute allograft rejection and transplantation tolerance.1–4 Specifically, activation of Toll-like receptors (TLRs), innate immune receptors, on dendritic cells (DCs) is critical for acute allograft rejection in certain experimental models.2 Furthermore, the administration of TLR ligands prevents the induction of transplantation tolerance,3,4 whereas the genetic deletion of MyD88, a key TLR signal adaptor, leads to allograft acceptance induced by co-stimulatory blockade.1 Importantly, humans who exhibit operational tolerance of kidney transplants exhibit reduced peripheral expression of MyD88 compared with transplant recipients with evidence of chronic rejection.5 Thus, high expression of MyD88 predisposes patients to a phenotype of resistance to transplant tolerance, whereas low expression indicates a greater chance that transplant tolerance will occur; however, to test these findings in clinical transplantation trials, it is necessary to elucidate the inflammatory mediators downstream of TLR/MyD88 activation that leads to the resistance of transplant tolerance.
Previous work demonstrated that inflammatory cytokines, in particular IL-6, can impair the function of regulatory T cells (Tregs) by promoting the proliferation of effector T cells.6 This is supported by a study demonstrating that IL-6 is necessary for the induction of experimental allergic encephalitis7; however, other inflammatory cytokines play important roles on immune regulation.8 Indeed, TNF-α, like IL-6, promotes T cell proliferation9 and also impairs peripheral tolerance of islets.10 It is likely that there are redundant pathways between inflammatory cytokines in immune regulation,11 but it is not clear whether IL-6 and TNF-α cooperate in preventing transplant tolerance.
Our previous work demonstrated that DCs produce both IL-6 and TNF-α during acute allograft rejection in a MyD88-dependent manner.1 In particular, the administration of co-stimulatory blockade and the absence of MyD88 led to an abrogated ability of DCs to produce these cytokines during acute allograft rejection.1 We further demonstrated that inflammatory cytokines produced by TLR-activated DCs augment T cell alloimmune responses and subsequently impair the function of Tregs.1 Given these findings and previous work indicating that IL-6 and TNF-α impair immune regulation, we examined whether the absence of IL-6, TNF-α, or both is necessary for allograft acceptance induced by co-stimulatory blockade antibody treatment. We provide in vivo evidence that the absence of both IL-6 and TNF-α but neither alone is critical for the ability of co-stimulatory blockade treatment to induce indefinite allograft survival. These data demonstrate that the inflammatory cytokines IL-6 and TNF-α cooperate to prevent immune tolerance of allografts.
RESULTS
Absence of IL-6 and TNF-α Synergizes with Co-stimulatory Blockade to Induce Allograft Acceptance
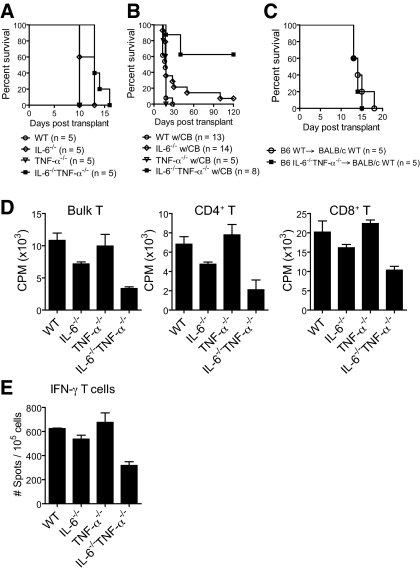
We used a highly immunogenic, in vivo experimental skin allograft model to examine the impact of IL-6 and TNF-α on the ability of co-stimulatory blockade to enhance allograft survival. Without co-stimulatory blockade, we found that wild-type (WT), IL-6–deficient (IL-6−/−), TNF-α–deficient (TNF-α−/−), and double-deficient (IL-6−/−TNF-α−/−) recipients rejected fully MHC-mismatched skin allografts at a similar rate (Figure 1A); however, when we administered co-stimulatory blockade (CTLA4-Ig and anti-CD154) agents that induce donor-specific acceptance of allografts,1,12,13 we found that most IL-6−/−TNF-α−/− skin allograft recipients accepted their allografts indefinitely (Figure 1B). In contrast, only a minority of IL-6−/− recipients manifested delayed allograft rejection, although IL-6−/− recipients did have a significant delay compared with WT recipients (Figure 1B); however, TNF-α−/− recipients rejected their allografts at a similar rate as WT recipients (Figure 1B). In addition, we found that perioperative administration of a TNF-α–inhibiting mAb to IL-6−/− skin allograft recipients extended the allograft-prolonging effects of co-stimulatory blockade, whereas administration of this mAb had no effect on WT transplant recipients treated with co-stimulatory blockade (data not shown); however, IL-6 and TNF-α signaling within the allograft was not important for the rate of allograft rejection, in this model, because IL-6−/−TNF-α−/−-deficient allografts were rejected at a similar rate compared with WT allografts when transplanted into WT recipients, even in the presence of co-stimulatory blockade (Figure 1C). Thus, these data indicate that recipient IL-6 and TNF-α are the functionally relevant mediators of allograft rejection and act synergistically to inhibit the allograft-prolonging effects of co-stimulatory blockade in this experimental system.
Figure 1.
Absence of both recipient IL-6 and TNF-α synergizes with the administration of co-stimulatory blockade to induce permanent allograft acceptance. (A and B) C57BL/6 WT, IL-6−/−, TNF-α−/−, and IL-6−/−TNF-α−/− recipients received a BALB/c skin allograft and were untreated (A) or administered co-stimulatory blockade (CB; CTLA4-Ig and anti-CD154) (B). Without co-stimulatory blockade, all experimental groups rejected their allografts at a similar rate. When co-stimulatory blockade was administered, most IL-6−/−TNF-α−/− recipients accepted their allograft for >100 d (P < 0.0001, log rank versus WT; P = 0.004 versus IL-6−/−). Only a minority of IL-6−/− recipients exhibited extended allograft survival compared with the WT group, although this reached statistical significance (P = 0.01). TNF-α−/− rejected their allografts at a similar rate to WT. (C) Full-thickness C57BL/6 WT and IL-6−/−TNF-α−/− skin was transplanted to BALB/c recipients, which were administered CTLA4-Ig and anti-CD154. There was no difference in allograft survival times between the groups. (D and E) C57BL/6 WT, IL-6−/−, TNF-α−/−, and IL-6−/− TNF-α−/− recipients (n > 3 per group) received a BALB/c skin allograft and were treated with co-stimulatory blockade (CTLA4-Ig and anti-CD154). At day 21 after transplantation, bulk T cells, CD4+ and CD8+ T cells, were purified from spleens and stimulated ex vivo with irradiated donor spleen cells for <24 h. T cell proliferation (D) and IFN-γ responses were then measured (E). T cells purified from and IL-6−/−TNF-α−/− recipients manifest significantly reduced responses versus the other groups in all of the assays (P < 0.05).
Defective Polyclonal T Cell Responses in IL-6−/−TNF-α−/− Transplant Recipients Treated with Co-stimulatory Blockade
Evidence of synergy between IL-6 and TNF-α was also demonstrated when T cell responses were measured ex vivo from the experimental groups. Without co-stimulatory blockade, we did not measure defects in T cell alloimmune responses between the experimental groups after transplantation (data not shown). In contrast, when recipients received co-stimulatory blockade, the polyclonal T cell alloimmune responses were diminished in the IL-6−/− skin allograft recipients as compared with the WT and TNF-α−/− groups (Figure 1, C and D); however, T cells purified from IL-6−/−TNF-α−/− recipients exhibited responses lower than the IL-6−/− group (Figure 1, C and D), providing further evidence of synergy between IL-6 and TNF-α in promoting T cell alloimmune responses in vivo, even in the presence of co-stimulatory blockade.
Synergy between IL-6 and TNF-α Promotes T Cell Alloimmunity
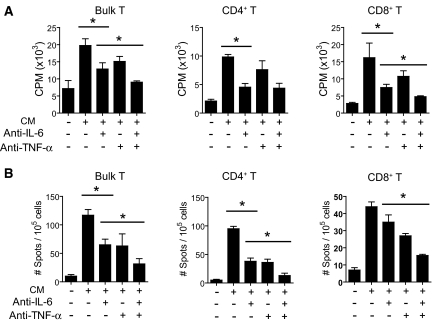
We established an in vitro culture system to examine the roles of IL-6 and TNF-α in the inflammatory milieu, extrinsic to the T cell, during alloimmune T cell activation. Hence, we harvested conditioned medium from LPS-treated and, therefore, TLR-activated bone marrow–derived DCs (BMDCs) after confirming that the conditioned medium contained elevated levels of proinflammatory cytokines (data not shown). The use of LPS in this system was an experimental tool to produce TLR signaling–dependent cytokines by DCs. The conditioned medium was then used to culture polyclonal T cells that were stimulated with allogeneic antigen-presenting cells (APCs). Compared with T cells that were activated by allogeneic APCs in control nonconditioned medium, which did not contain inflammatory cytokines (data not shown), T cells that were stimulated in the medium harvested from TLR-activated BMDCs exhibited augmented T cell responses (Figure 2, A and B). Thus, the presence of TLR-dependent cytokines in the inflammatory milieu promotes T cell alloimmunity.
Figure 2.
Inhibition of IL-6 and TNF-α impairs the ability of TLR-dependent cytokines to augment T cell alloimmunity. Conditioned media (CM) were harvested from LPS-activated BMDCs and used to perform an MLR. Inhibition of either IL-6 or TNF-α reduced T cell proliferation (A) or T cell IFN-γ (B) production. Similar results were noted for CD4+ and CD8+ T cells. Isotype control antibodies did not inhibit T cell responses in this assay (data not shown). *P < 0.05.
We next performed experiments to examine the role of either IL-6 or TNF-α on the T cell alloimmune-promoting effects of the conditioned medium in our in vitro culture system. Treatment of T cells with either an inhibitory anti–IL-6 mAb or an anti–TNF-α mAb reduced T cell responses during allostimulation (Figure 2, A and B); however, the greatest reduction in T cell alloimmune responses were noted when we treated with both IL-6 and TNF-α inhibitory mAbs (Figure 2, A and B). In this case, the T cell responses were similar to the T cell responses obtained in nonconditioned control medium. These data indicate that IL-6 and TNF-α cooperate to promote T cell alloimmunity in this experimental system.
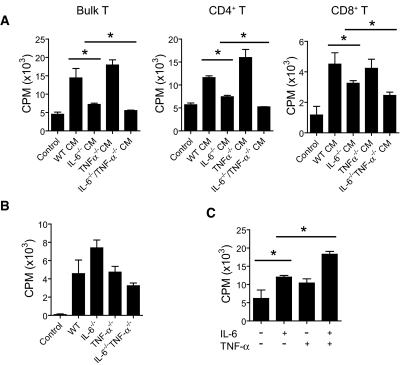
To determine the role of each cytokine in the promotion of T cell alloimmunity, we repeated the in vitro culture assay, but this time we harvested conditioned medium from WT, IL-6−/−, TNF-α−/−, or IL-6−/−TNF-α−/− TLR-activated BMDCs. These media were used to culture T cells during stimulation with allogeneic APCs. We found that T cells that were stimulated in conditioned medium harvested from IL-6−/−TLR-activated DCs but not from TNF-α−/− DCs had reduced T cell responses as compared with T cells that were allostimulated in conditioned medium from TLR-activated WT DCs (Figure 3A). Finally, T cells that were allostimulated in culture medium harvested from TLR-activated IL-6−/−TNF-α−/− DCs had defective responses, which were slightly but significantly reduced compared with T cells stimulated in the IL-6−/− conditioned medium (Figure 3A).
Figure 3.
Deletion of IL-6 and TNF-α reduces the ability of TLR-dependent cytokines to augment T cell antigen–specific, alloimmune responses. Addition of IL-6 and TNF-α together augments T cell alloimmunity versus either cytokine alone. (A) CM were harvested from WT, IL-6−/−, TNF-α−/−, and IL-6−/−TNF-α−/− TLR-activated BMDCs and used to culture T cells in an MLR. The absence of IL-6 but not TNF-α reduced T cell responses in this assay (*P < 0.05). Absence of both cytokines further reduced T cell responses to a small degree (*P < 0.05). (B) TLR4−/− T cells were cultured in standard media or in CM harvested from WT, IL-6−/−, TNF-α−/−, and IL-6−/−TNF-α−/− TLR-activated BMDCs without the addition of allogeneic APCs, and T cell proliferation was measured. The use of TLR4−/− T cells (i.e., hyporesponsive to LPS) was to assess the ability of the CM (which contained LPS) to promote T cell proliferation independent of the effect of LPS on T cells. (C) Addition of both IL-6 and TNF-α to the MLR augmented T cell alloimmune responses as compared with T cells stimulated in standard media, devoid of cytokines (*P < 0.05). Addition of either cytokine augmented T cell alloimmune proliferation to a small degree, which was less than that induced by both cytokines (*P < 0.05).
We noted that without allogeneic APCs, the conditioned medium harvested from TLR-activated BMDCs promoted T cell proliferation (Figure 3B) but to a lesser degree compared with when T cells were stimulated by allogeneic APCs in conditioned medium (compare Figure 3, A and B). However, the ability of conditioned medium to promote T cell proliferation without APCs was not dependent on either IL-6 or TNF-α (Figure 3B), demonstrating that IL-6 and TNF-α cooperate to promote Ag-specific but not nonspecific T cell proliferation.
We next performed experiments to test whether either IL-6 or TNF-α was sufficient to promote T cell alloimmunity. Hence, we stimulated T cells with allogeneic APCs in standard medium, which was devoid of cytokines, and either supplemented the culture with recombinant IL-6, TNF-α, or both cytokines. Our results indicate that the presence of either IL-6 or TNF-α slightly increased T cell alloimmune responses (Figure 3C). However, we noted the greatest augmentation of T cell alloimmune proliferation, compared with T cells stimulated in standard medium, occurred when both cytokines were added to the culture medium (Figure 3C).
Endogenous Expression of IL-6 and TNF-α in T Cells Is Dispensable for T Cell Alloimmunity
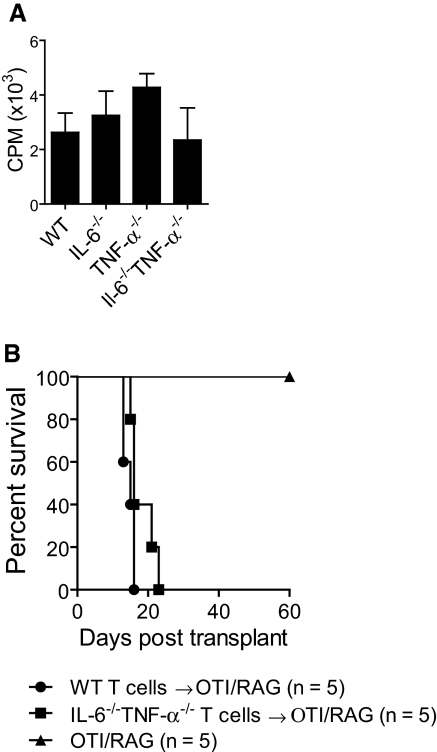
We next conducted experiments to examine the importance of endogenously expressed IL-6 or TNF-α in T cells for the induction of alloimmune responses. We stimulated WT, IL-6−/−, TNF-α−/−, or IL-6−/−TNF-α−/− polyclonal T cells with allogeneic APCs in standard medium that completely lacked cytokines. We found that the absence of endogenous IL-6 and/or TNF-α within T cells did not impair the ability of T cells to respond to allostimulation (Figure 4A).
Figure 4.
T cell intrinsic IL-6 and TNF-α are dispensable for T cell alloimmunity. (A) Polyclonal T cells were purified from C57BL/6 WT, IL-6−/−, TNF-α−/−, and IL-6−/−TNF-α−/− mice and stimulated by allogeneic APCs in standard media (lacking cytokines). No differences in T cell proliferation were noted between the groups. (B) Polyclonal T cells (1 × 106) were purified from either C57BL/6 WT or IL-6−/−TNF-α−/− mice and adoptively transferred via intravenous tail vein to syngeneic RAG−/−OTI TCR transgenic mice, which received a BALB/c skin allograft. The rate of graft rejection between the groups was similar. Nonadoptively transferred mice did not reject their allografts.
We next performed an in vivo adoptive transfer experiment to examine the ability of WT or IL-6−/−TNF-α−/− T cells to induce acute allograft rejection. We transferred polyclonal WT or IL-6−/−TNF-α−/− T cells into RAG−/− mice, which contained T cells that were transgenic for an irrelevant peptide (OVA257 to 264). The use of this host, as opposed to a RAG−/− host, was to limit the effects of lymphopenia-induced proliferation after adoptive transfer of T cells (data not shown). We found that when these recipients of allogeneic skin transplants received the adoptive transfer of either WT or IL-6−/−TNF-α−/− polyclonal T cells, allograft rejection occurred at similar rates (Figure 4B). Overall, these in vivo and in vitro data indicate that the ability of T cells to endogenously express IL-6 or TNF-α was dispensable for T cell alloimmune responses in this experimental system.
IL-6 and TNF-α Act Together to Impair the Ability of Tregs to Suppress T Effector Cell Alloimmunity
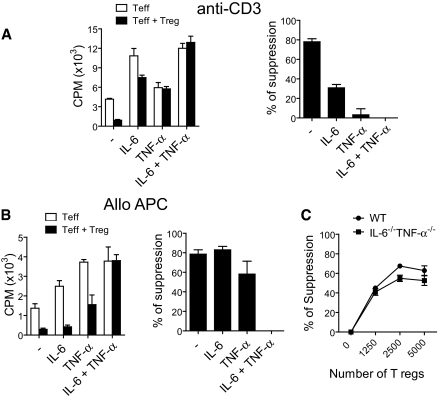
Previous work indicated that co-stimulatory blockade may mediate its allograft prolonging effects via Tregs.1,14 Furthermore, reports in nonalloimmune models indicate that IL-6 and TNF-α may prevent Treg-mediated suppression of effector T cell proliferation,6,15 although the interaction of both IL-6 and TNF-α on the suppressive functions of Tregs has not been examined. Hence, we examined whether IL-6, TNF-α, or both altered the ability of Tregs to suppress effector T cell alloimmunity. First, we used an in vitro system in which Tregs (CD4+CD25+) are added to CD3 activated effector (CD4+CD25−) T cells. Without addition of either IL-6 or TNF-α, Tregs were highly effective at suppressing T cell proliferation (Figure 5A). When recombinant IL-6 was added to the culture, effector T cell proliferation increased and the degree of Treg-mediated suppression reduced by approximately 50% (Figure 5A). When TNF-α was added to the culture, T cell proliferation was not appreciably increased, but the degree of Treg-mediated suppression was nearly entirely abrogated (Figure 5A). When both cytokines were added to the culture, the ability of Tregs to suppress effector T cell proliferation was completely repressed (Figure 5A).
Figure 5.
IL-6 and TNF-α synergized to impair the suppressive function of Tregs. (A) CD4+CD25− T cells were stimulated with soluble anti-CD3 with or without the co-culture of CD4+CD25+ Tregs. Recombinant IL-6, TNF-α, or both were added to the cultures. Results are presented as counts per minute or percentage suppression of T effector proliferation mediated by Tregs. (B) C57BL/6 CD4+CD25− T cells were stimulated with BALB/c APCs with or without co-culture of CD4+CD25+ Tregs. Recombinant IL-6, TNF-α, or both were added to the cultures. Results are presented as counts per minute or percentage suppression of T effector proliferation mediated by Tregs. (C) CD4+CD25− T cells were stimulated with soluble anti-CD3 and cultured alone or co-cultured with increasing numbers of CD4+CD25+ Tregs from C57BL/6 WT and IL-6−/−TNF-α−/− mice, and proliferation was measured. IL-6−/−TNF-α−/− Tregs exhibited a similar ability to suppress T effector proliferation compared with WT Tregs.
We next used an in vitro alloimmune T cell proliferation assay to examine the impact of IL-6, TNF-α, or the combination of both on Treg-mediated suppression of effector T cells. In this assay, Tregs were added to cultures of effector T cells, which were stimulated with allogeneic APCs. Without the addition of either IL-6 or TNF-α, Tregs were highly effective at reducing T effector cell alloimmune proliferation (Figure 5B). Tregs were able to induce suppression of effector T cells despite the addition of either IL-6 or TNF-α (Figure 5B); however, when both cytokines were added to the culture, the ability of Tregs to induce effector T cell suppression was prevented completely (Figure 5B). These results indicate that IL-6 and TNF-α act synergistically to impair the suppressive functions of Tregs in this alloimmune assay.
These experiments in two different T cell proliferation assays demonstrate that non–T cell–derived IL-6 and TNF-α impair the ability of Tregs to mediate suppression of effector T cell proliferation. We next examined the importance of Treg-derived IL-6 and TNF-α in the suppressive function of Tregs. Hence, we compared the ability WT and IL-6−/−TNF-α−/− Tregs to suppress effector T cell proliferation. We found that IL-6−/−TNF-α−/− Tregs exhibited a similar ability to suppress effector T cell proliferation in comparison with WT Tregs (Figure 5C). These data demonstrate that Treg-derived IL-6 and TNF-α are dispensable for the suppressive functions of Tregs.
IRAK-M−/− Macrophages Produce Augmented IL-6 and TNF-α upon TLR Activation, which Promotes T Cell Alloimmunity
The previous data indicate that both IL-6 and TNF-α synergize to promote T cell alloimmune responses, and also their absence synergizes with the administration of co-stimulatory blockade to promote skin allograft acceptance; therefore, one would predict that conditioned media derived from cells that produce excessive amounts of IL-6 and TNF-α would enhance T cell alloimmune responses. Furthermore, one would predict that hosts that have higher IL-6 and TNF-α responses would resist the effects of co-stimulatory blockade to extend allograft survival. To examine this, we first harvested conditioned media from LPS-activated IRAK-M−/− macrophages. Previous work indicated that these macrophages release elevated levels of IL-6 and TNF-α upon TLR activation,16 which we confirmed (data not shown). Furthermore, other work has demonstrated that IRAK-M−/− mice mount an exaggerated inflammatory responses during sepsis.17
We found that T cells that were cultured in media harvested from TLR-activated IRAK-M−/− macrophages had a greater T cell alloimmune proliferation compared with allostimulated T cells cultured in conditioned media harvested from TLR-activated WT macrophages (Figure 6A). This difference was abrogated by inhibition of either IL-6 or TNF-α using cytokine-specific antibodies (Figure 6A), indicating that the augmented T cell alloimmune response mediated by the IRAK-M−/− conditioned media was IL-6 or TNF-α dependent.
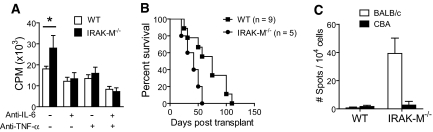
Figure 6.
Inflammatory cytokines augment T cell proliferation in an IRAK-M–dependent manner. IRAK-M−/− transplant recipients resist the graft-prolonging effects of anti-CD45RB and anti-CD154 to extend allograft survival. (A) WT and IRAK-M−/− macrophages were stimulated with LPS, and CM were harvested. These media were used to culture T cells that were stimulated with allogeneic APCs. IRAK-M−/− CM augmented T cell alloimmune proliferation versus WT CM (*P < 0.05). This difference was blocked by either IL-6 or TNF-α inhibition. (B) C57BL/6 IRAK-M−/− recipients of BALB/c skin allografts that were treated perioperatively with anti-CD45RB and anti-CD154 rejected their allografts at a faster rate than similarly treated WT recipients (P = 0.03, log rank). Both groups rejected their allografts at a similar rate when co-stimulatory blockade was not administered (data not shown). (C) Bulk purified T cells from recipient mice in B were harvested 21 d after transplantation and were re-stimulated with irradiated BALB/c or third-party CBA (H2K) APCs. IFN-γ response was then measured via ELISPOT. T cells from IRAK-M−/− recipients exhibited an enhanced donor-specific IFN-γ versus WT recipients (P = 0.03; n = 3 per group, two independent experiments).
IRAK-M−/− Recipients Resist the Allograft-Prolonging Effects of Co-stimulatory Blockade
We next examined whether IRAK-M−/− recipients would resist the effects of co-stimulatory blockade to extend allograft survival. IRAK-M−/− recipients treated with CTLA-4 Ig and anti-CD154 rejected allografts at a faster rate compared with WT recipients that were similarly treated (Supplemental Figure 1); however, as a result of the rapid rate of graft rejection observed in this model, we were unable to measure a significant difference between IRAK-M−/− recipients and WT recipients (Supplemental Figure 1). Thus, we used a different co-stimulatory blockade formulation, consisting of anti-CD45RB and anti-CD154, which has been shown to provide a longer allograft survival than the combination of CTLA-4 Ig and anti-CD154.13,18 The use of the alternative co-stimulatory blockade formulation extended the survival of the allograft on IRAK-M−/− recipients and provided us with a longer window to determine whether IRAK-M−/− recipients resist the allograft-prolonging effects of co-stimulatory blockade. As expected, we observed that IRAK-M−/− recipients exhibited an accelerated rate of allograft rejection compared with similarly treated WT recipients (Figure 6B). Furthermore, T cells harvested from IRAK-M−/− recipients had greater donor-specific IFN-γ alloimmune responses compared with T cells harvested from WT recipients (Figure 6C); therefore, recipients that are prone to heightened inflammatory responses resist the pro-allograft survival effects of co-stimulatory blockade.
DISCUSSION
Previous work indicated that increased levels of IL-6 and TNF-α are associated with acute allograft rejection19,20; however, it is not known whether IL-6 and TNF-α cooperate in preventing the induction of transplant tolerance. In this study, we found that IL-6 and TNF-α released into the inflammatory milieu, extrinsic to the effector T cell, enhanced T cell alloimmunity and allograft rejection. Importantly, we demonstrated that absence of both IL-6 and TNF-α in the recipient promoted allograft acceptance mediated by co-stimulatory blockade in the majority of transplant recipients. Thus, we provide evidence that these cytokines cooperate to impair the allograft-prolonging effects of co-stimulatory blockade.
Our work and that of others have demonstrated that the ability of co-stimulatory blockade to induce indefinite allograft acceptance is dependent on the absence of MyD88, a key TLR signal adaptor protein.1,3 In particular, we demonstrated that the absence of MyD88 impaired the ability of DCs to produce both IL-6 and TNF-α during acute allograft rejection.1 MyD88-dependent cytokines augmented T cell alloimmunity and impaired the ability of Tregs to suppress T effector alloimmune proliferation.1 Furthermore, co-stimulatory blockade mediated its effects in inducing enhanced allograft survival via Tregs.14 In this study, we identified IL-6 and TNF-α as the MyD88-dependent inflammatory cytokines that cooperate to increase T effector alloimmunity and impair the suppressive function of Tregs. Overall, our work indicates that MyD88 signaling within recipient DCs induces the production of both IL-6 and TNF-α during allotransplantation, which impair the immune regulatory properties of co-stimulatory blockade. The importance of inflammatory-activated recipient DCs to impede transplantation tolerance should be examined in future studies.
Recent studies have demonstrated that administration of TLR ligands prevent the induction of transplantation tolerance.3,4,21 In one study using CpG, a TLR9 agonist, the ability of CpG to impair co-stimulatory blockade–induced allograft acceptance was independent of IL-6.21 This is in general agreement with our study that found that IL-6 played a minor role in impairing the effects of co-stimulatory blockade. It is not yet clear what the relevant TLR ligands are that are activated in the setting of organ transplantation.22 It is possible that multiple ligands that use several inflammatory pathways are involved. This may be reflected in the model used in our study, which is dependent on MyD88, an adaptor downstream of multiple TLRs.
Anti–IL-6 and anti–TNF-α therapies have been used in clinical medicine, particularly for the treatment of autoimmune diseases.23,24 Hence, therapeutic tools that inhibit these cytokines are available and could be used in future clinical trials examining immunologic tolerance induction in organ transplantation; however, the use of such agents in immunosuppressed patients will require caution, because they may also alter host defense to pathogens.25 Clearly, future clinical studies are warranted to address these important issues.
IL-6 in combination with TGF-β, a cytokine, which can also be produced by Tregs, is sufficient to induce the generation of Th17-producing effector T cells.26,27 The role of IL-17 and Th17 T cell effectors in solid organ transplantation is not fully appreciated, although there is emerging evidence for a role of IL-17 inflammatory responses in acute and chronic lung rejection.28–30 Future studies are needed to examine the impact of IL-17 in transplantation tolerance induction.
Our study also found that IRAK-M−/− recipient animals, which produce elevated levels of IL-6 and TNF-α, resisted the allograft-prolonging effects of two different co-stimulatory blockage formulations. Although the anti-CD45RB and anti-CD154 formulation may include a certain degree of T cell deletion,31 it is possible that regulation also occurs with this approach, similar to the CTLA-4 Ig and anti-CD154 formulation. Because IRAK-M−/− recipients resisted the allograft-prolonging effects of both regimens, this indicates that the absence of the IRAK-M negative regulatory inflammatory pathway may apply to several therapies that have the potential to induce transplantation tolerance. IRAK-M is a negative regulator of TLR activation,16 and a recent study indicated that an inactivating polymorphism of IRAK-M is associated with the pathogenesis of human asthma.32 Our work suggests that such a polymorphism may predict transplant tolerance resistance in humans, which should be evaluated in a future clinical study.
In conclusion, our study has demonstrated that IL-6 and TNF-α cooperate to impede co-stimulatory blockade–induced allograft acceptance. This information may be useful for future clinical trials aimed at inducing immunologic tolerance of transplanted organs.
CONCISE METHODS
Mice and Reagents
C57BL/6 (H2b) WT, C57BL/6 IL-6−/−, and BALB/c (H2d) mice were purchased from Jackson Laboratory (Bar Harbor, ME). B6;129 TNF-α−/− mice (Jackson Laboratory) were backcrossed eight times onto the C57BL/6 background. We generated IL-6−/−TNF-α−/− by crossbreeding of IL-6−/− with TNF-α−/− mice. C57BL/6 IRAK-M−/− and RAG−/− OTI TCR transgenic mice were provided by Dr. Richard A. Flavell (Yale University, New Haven, CT). C57BL/6 TLR4−/− mice were provided by Dr. Akira (Osaka University, Osaka, Japan). All mice were kept in pathogen-free conditions. The Yale University Institutional Animal Care and Use Committee approved use of animals in this study.
LPS from Salmonella Minnesota R595 was obtained from Alexis (San Diego, CA). Recombinant mouse IL-6 (used in vitro at 50 ng/ml), TNF-α (used in vitro at 10 ng/ml), monoclonal rat anti-mouse IL-6 (used in vitro at 4 μg/ml antibody, clone MP520F3), and rat IgG1 isotype control were obtained from R&D Systems (Minneapolis, MN). The neutralizing antibody to TNF (2E2 mAb) was obtained from the NCI BRB Preclinical Repository (Rockville, MD) and used at 2 μg/ml, and hamster IgG isotype control was obtained from Biolegend (San Diego, CA). Anti-CD154, CTLA-4 Ig, and anti-CD45RB were purchased from BioXcell (West Lebanon, NH).
Skin Transplantation and Ab Treatment
Full-thickness BALB/c skin was transplanted to the dorsum of C57BL/6 recipients as described previously.1 Rejection was defined as complete loss of viable skin on visual inspection. Recipients received anti-CD154 (clone MR1, 500 μg/mouse intraperitoneally on days 2, 4, 6, and 8 relative to transplantation) and CTLA-4 Ig (500 μg/mouse intraperitoneally on day 2) or anti-CD45RB (clone HB220, 100 μg intraperitoneally on days −1, 0, 1, 2, 5, and 8) and anti-CD154 (250 μg intraperitoneally on days 0, 2, 6, and 8).
Cell Preparation and MLR
CD4+, CD8+, or bulk T cells were purified via negative selection (>95% purity) using EasySep T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada). Irradiated (28 Gy) allogeneic splenocytes were used as APCs. BMDCs were prepared in the presence of GM-CSF as per our previously published work.33 After 4 d of culture, the BMDCs were harvested and stimulated with 50 ng/ml LPS in complete Bruff's medium (Invitrogen, Carlsbad, CA). After 12 h, the supernatants were harvested and designated “conditioned medium.” Control medium consisted of standard complete Bruff's medium supplemented with 50 ng/ml LPS in the absence of BMDCs. Irradiated whole allogeneic splenocytes (1 × 105) were cultured with T cells (1 × 105) per well for 3 d in 96-well plates in the indicated conditioned medium or control medium. For all experiments used to generate the conditioned medium, T cells were syngeneic to the BMDCs. At this point, [3H]thymidine was added to the wells, and DNA was harvested and analyzed by a scintillation counter as an indicator of cell proliferation (PerkinElmer Life Science, Boston, MA). Other control wells included T effector cells without APCs, medium only, and APCs only. Thioglycollate-elicited peritoneal cells were prepared as previously published.34 In certain experiments, supernatant from LPS-stimulated thioglycollate-elicited peritoneal cells were used as conditioned medium. To perform the in vitro Treg suppression assay, we purified 2 × 104 C57BL/6 CD4+CD25− T effectors cells via FACS, as previously reported,1 and were subsequently stimulated by either soluble anti-CD3 (1 μg/ml) or 2 × 104 irradiated allogeneic (BALB/c) APCs with or without co-culture of FACS-purified 2 × 104 (or indicated numbers) CD4+CD25+ T cells for 96 h. Recombinant cytokines were added at the concentrations stated already. Cellular proliferation was measured as described already. Results are expressed as absolute counts per minute or the percentage suppression of T effector cell proliferation mediated by the presence of Tregs.
ELISPOT
ELISPOT was performed as described previously.1 To assess the T cell recall response after transplantation, we harvested recipient spleen cells at day 21 after transplantation, and bulk T, CD4+, and CD8+ T cells were purified. T cells (1 × 105/well) and irradiated donor stimulator spleen cells (1 × 105/well) were plated overnight in 96-well ELISPOT plates coated with the Ab of interest. Plates were read on a CTL automatic ELISPOT reader and analyzed using Immunospot 3.1 software (CTL, Cleveland, OH).
Statistical Analysis
Repeated measures were evaluated with a two-way ANOVA. Comparison of means was analyzed by t test. Significance was evaluated at P < 0.05. Statistical analysis was performed on GraphPad Prism Software (San Diego, CA).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant AI064660 to D.R.G.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Walker WE, Nasr IW, Camirand G, Tesar BM, Booth CJ, Goldstein DR: Absence of innate MyD88 signaling promotes inducible allograft acceptance. J Immunol 177: 5307–5316, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DR, Tesar BM, Akira S, Lakkis FG: Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 111: 1571–1578, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, Akira S, Wang CR, Fairchild RL, Alegre ML, Chong A: TLR engagement prevents transplantation tolerance. Am J Transplant 6: 2282–2291, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, Greiner DL: TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J Immunol 176: 1561–1570, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braudeau C, Ashton-Chess J, Giral M, Dugast E, LS, Pallier A, Braud C, Moreau A, Renaudin K, Soulillou JP, Brouard S: Contrasted blood and intragraft toll-like receptor 4 mRNA profiles in operational tolerance versus chronic rejection in kidney transplant recipients. Transplantation 86: 130–136, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Pasare C, Medzhitov R: Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299: 1033–1036, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Samoilova EB, Horton JL, Hilliard B, Liu, T-ST, Chen Y: IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: Roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol 161: 6480–6486, 1998 [PubMed] [Google Scholar]
- 8.Hata H, Sakaguchi N, Yoshitomi H, Iwakura Y, Sekikawa K, Azuma Y, Kanai C, Moriizumi E, Nomura T, Nakamura T, Sakaguchi S: Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest 114: 582–588, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown GR, Lee EL, Thiele DL: TNF enhances CD4+ T cell alloproliferation, IFN-gamma responses, and intestinal graft-versus-host disease by IL-12-independent mechanisms. J Immunol 170: 5082–5088, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Skak K, Guerder S, Picarella DE, Brenden N, Flavell RA, Michelson BK: TNF-alpha impairs peripheral tolerance towards beta-cells, and local costimulation by B7.1 enhances the effector function of diabetogenic T cells. Eur J Immunol 33: 1341–1350, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kelso A: The enigma of cytokine redundancy. Immunol Cell Biol 72: 97–101, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP: Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest 104: 1715–1722, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sho M, Kishimoto K, Harada H, Livak M, Sanchez-Fueyo A, Yamada A, Zheng XX, Strom TB, Basadonna GP, Sayegh MH, Rothstein DM: Requirements for induction and maintenance of peripheral tolerance in stringent allograft models. Proc Natl Acad Sci U S A 102: 13230–13235, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW: Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med 201: 1037–1044, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ: Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol 179: 154–161, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA: IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110: 191–202, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ: Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 116: 2532–2542, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothstein DM, Livak MFA, Kishimoto K, Ariyan C, Qian H-Y, Fecteau S, Sho M, Deng S, Zheng XX, Sayegh MH, Basadonna GP: Targeting signal 1 through CD45RB synergizes with CD40 ligand blockade and promotes long term engraftment and tolerance in stringent transplant models. J Immunol 166: 322–329, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hodge G, Hodge S, Chambers D, Reynolds PN, Holmes M: Acute lung transplant rejection is associated with localized increase in T-cell IFNgamma and TNFalpha proinflammatory cytokines in the airways. Transplantation 84: 1452–1458, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Christopher K, Finn PW, Colson Y, Perkins DL: Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation 84: 771–777, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W, Li P, Zhang J, Ansari JM, Hancock WW, Sayegh MH, Koulmanda M, Strom TB, Turka LA: Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol 181: 1692–1699, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein DR: The identity of innate immune activators in organ transplantation: Origins from within or exterior to the host? Am J Transplant 7: 1692–1694, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Beck JT, Hsu, S-M, Wijdenes J, Bataille R, Klein B, Vesole D, Hayden K, Jagannath S, Barlogie B: Alleviation of systemic manifestations of Castleman's disease by monoclonal anti-interleukin-6 antibody. N Engl J Med 330: 602–605, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Feldmann M, Maini R: Role of cytokines in rheumatoid arthritis: An education in pathophysiology and therapeutics. Immunol Rev 223: 7–19, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Saliu OY, Sofer C, Stein DS, Schwander SK, Wallis RS: Tumor-necrosis-factor blockers: Differential effects on mycobacterial immunity. J Infect Dis 194: 486–492, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM: Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity 24: 677–688, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Weaver CT, Hatton RD, Mangan PR, Harrington LE: IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25: 821–852, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Vanaudenaerde BM, Dupont LJ, Wuyts WA, Verbeken EK, Meyts I, Bullens DM, Dilissen E, Luyts L, Van Raemdonck DE, Verleden GM: The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J 27: 779–787, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, Blum JS, Brand DD, Wilkes DS: Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant 6: 724–735, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS: IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 11: 3498–3506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luke PP, Deng JP, O'Brien CA, Everest M, Hall AV, Chakrabarti S, O'Connell PJ, Zhong R, Jevnikar AM: Alteration in CD45RBhi/CD45RBlo T-cell ratio following CD45RB monoclonal-antibody therapy occurs by selective deletion of CD45RBhi effector cells. Transplantation 76: 400–409, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Balaci L, Spada MC, Olla N, Sole G, Loddo L, Anedda F, Naitza S, Zuncheddu MA, Maschio A, Altea D, Uda M, Pilia S, Sanna S, Masala M, Crisponi L, Fattori M, Devoto M, Doratiotto S, Rassu S, Mereu S, Giua E, Cadeddu NG, Atzeni R, Pelosi U, Corrias A, Perra R, Torrazza PL, Pirina P, Ginesu F, Marcias S, Schintu MG, Giacco GS, Manconi PE, Malerba G, Bisognin A, Trabetti E, Boner A, Pescollderungg L, Pignatti PF, Schlessinger D, Cao A, Pilia G: IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet 80: 1103–1114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR: The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant 6: 2622–2635, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Shen H, Kanoh M, Maruyama S, Matsumoto A, ZhangW, Asano Y: Listeria infection activates natural killer cell cytotoxicity to regress melanoma growth in vivo. Microbiol Immunol 52: 107–117, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.