Abstract
Plasma gelsolin (pGSN) binds actin and bioactive mediators to localize inflammation. Low pGSN correlates with adverse outcomes in acute injury, whereas administration of recombinant pGSN reduces mortality in experimental sepsis. We found that mean pGSN levels of 150 patients randomly selected from 10,044 starting chronic hemodialysis were 140 ± 42 mg/L, 30 to 50% lower than levels reported for healthy individuals. In a larger sample, we performed a case-control analysis to evaluate the relationship of pGSN and circulating actin with mortality; pGSN levels were significantly lower in 114 patients who died within 1 yr of dialysis initiation than in 109 survivors (117 ± 38 mg/L versus 147 ± 42 mg/L, P < 0.001). pGSN levels had a graded, inverse relationship with 1-yr mortality, such that patients with pGSN <130 mg/L experienced a >3-fold risk for mortality compared with those with pGSN ≥150 mg/L. The 69% of patients with detectable circulating actin had lower pGSN levels than those without (127 ± 45 mg/L versus 141 ± 36 mg/L, P = 0.026). Compared with patients who had elevated pGSN and no detectable actin, those with low pGSN levels and detectable actin had markedly increased mortality (odds ratio 9.8, 95% confidence interval 2.9 to 33.5). Worsening renal function correlated with pGSN decline in 53 subjects with CKD not on dialysis. In summary, low pGSN and detectable circulating actin identify chronic hemodialysis patients at highest risk for 1-yr mortality.
Although chronic hemodialysis has markedly reduced the acute mortality of ESRD patients, these patients still die at a markedly accelerated rate, principally from cardiovascular events and recurrent infections.1–3 Patients with ESRD exhibit muscle depletion, malnutrition, hypoalbuminemia, and manifestations of diffuse tissue injury, and these parameters strongly correlate with early mortality.4–10 However, the specific factors linking wasting and inflammation with accelerated mortality remain under investigation.11
The extracellular actin binding proteins include vitamin D binding protein and plasma gelsolin (pGSN).12 pGSN also inactivates bioactive lipid mediators including lysophosphatidic acid,13 lipopolysaccharide endotoxin,14 and platelet-activating factor.15 pGSN is measurable in healthy individuals (approximately 190 to 300 mg/L, Supplementary Table 2) as a secreted variant of a cellular protein involved in the regulation of changes in cell shape.16,17 Many cell types secrete pGSN, but striated muscle accounts for most pGSN production.18 pGSN contributes to removing actin when injected into the circulation of experimental animals, and levels of pGSN fall after acute injuries, consistent with the release of actin in circulation after tissue damage.12,19–22
A highly evolutionarily conserved actin- and inflammatory mediator-binding protein present in diverse species from Drosophila to humans suggests that it functions to localize inflammatory and immune reactions to sites of injury.23 Local depletion of pGSN due to tissue damage and exposure of cytoplasmic actin, one of the most abundant body proteins, enables inflammatory mediators to locally exert adaptive defense and repair functions, whereas a reservoir of circulating pGSN prevents these mediators from injuring organs away from the primary site of insult.
Extensive injuries, however, may compromise the inflammation-localizing function of pGSN by depleting it because of excessive actin exposure. Consistent with this hypothesis, critical extents of pGSN level reductions predict adverse clinical outcomes, including death, in patients subjected to acute injuries such as major trauma or surgery, burns, or hematopoietic stem cell transplantation.20–21 Here we report that a critical level of pGSN depletion, measured at the initiation of chronic hemodialysis, correlates with the highest risk for 1-yr mortality in patients initiating chronic hemodialysis and that the presence of circulating actin markedly increases this risk. In addition, pGSN concentrations decline with deteriorating renal function in chronic kidney disease. These findings potentially link the pathophysiologies of protein-energy wasting, inflammation, and tissue injury and mortality in ESRD via low circulating muscle-derived pGSN. Because partial or complete repletion of depleted pGSN stores alleviates inflammatory manifestations and reduces mortality in experimental tissue injury,24–26 pGSN depletion is a potentially modifiable risk factor in this setting.
RESULTS
Baseline Characteristics
The initial random sample of 150 incident chronic kidney disease patients represented 148 separate dialysis centers across the United States and its baseline characteristics (Supplementary Table 1) resemble larger populations at hemodialysis initiation.27 Figure 1A shows the distribution of baseline pGSN levels, the means of which were 140 ± 42 mg/L; only 2 (1%) patients’ levels were at or above approximately 250 mg/L, the mean level reported in healthy volunteers (Supplementary Table 2). pGSN levels correlated inversely with age (r = −0.18, P = 0.01) and directly with baseline measures of muscle mass and nutrition, such as serum creatinine (r = 0.27, P < 0.001) and albumin levels (r = 0.34, P < 0.001). The correlation between pGSN and body mass index was 0.02 (P = 0.81). The levels did not differ by sex: men, 140 ± 48 mg/L; women, 140 ± 31 mg/L (P = 0.90). When baseline high-sensitivity C-reactive protein (hsCRP) levels were examined in tertiles, those with the lowest levels of hsCRP demonstrated the highest levels of pGSN: tertile 1, hsCRP <12 mg/L, pGSN 145 ± 39 mg/L; tertiles 2 and 3, hsCRP ≥12 mg/L, pGSN 131 ± 53 mg/L, P = 0.048). Linear regression analyses confirmed that only serum albumin independently correlated with pGSN levels (P < 0.001).
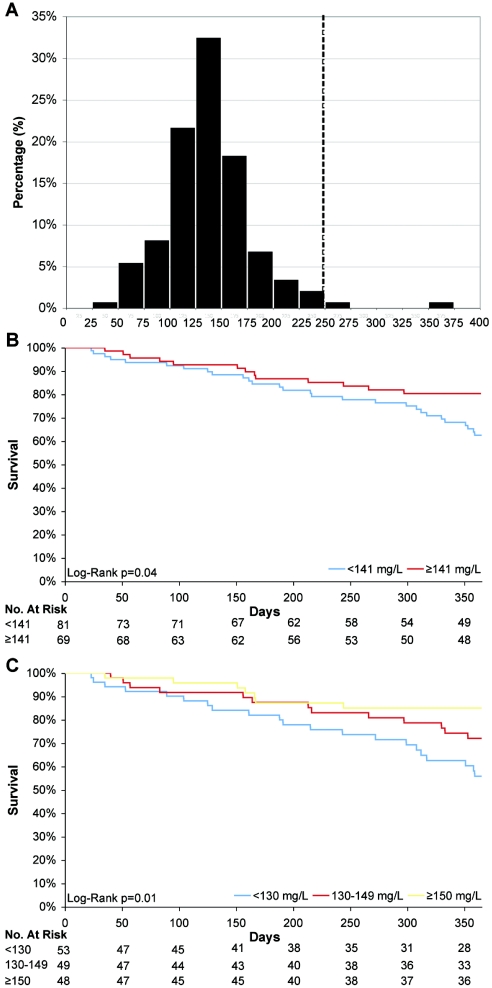
Figure 1.
(A) Distribution of plasma gelsolin levels in the random sample of 150 incident ESRD patients living throughout the United States. Dashed line represents the mean pGSN level of approximately 250 mg/L in healthy controls (also see Supplementary Table 2). (B and C) Kaplan-Meier analysis of 1-yr survival according to baseline pGSN levels categorized by (B) the median level and (C) tertiles.
pGSN and Time to Death
The median pGSN level among the 150 random patients was 141 mg/L [range, 28 to 357 mg/L, interquartile range (IQR) 116 to 161 mg/L]. Kaplan-Meier analyses of these 150 patients demonstrated a significant survival difference when baseline pGSN levels were dichotomized at the median level (P = 0.04, Figure 1B) or examined in tertiles (P = 0.01, Figure 1C). Among those that died within 1 yr, the median day of death was 154 d (IQR 68 to 233 d).
Patient Characteristics and 1-Yr Mortality
We analyzed the case-control sample of 223 patients (baseline characteristics in Table 1) to examine the risk factors for 1-yr survival. Those who died were slightly older, more likely to have a venovenous catheter as initial vascular access (compared with arteriovenous fistula or graft), had lower serum albumin and creatinine levels, and higher white blood cell counts, consistent with previous reports of hemodialysis mortality.27 Mean pGSN levels were significantly lower in patients who died (117 ± 38 mg/L) compared with survivors (147 ± 42 mg/L, P < 0.001). Baseline pGSN levels did not differ between cardiovascular (n = 59, 116 ± 41 mg/L) and infectious (n = 55, 117 ± 34 mg/L, P = 0.91) deaths.
Table 1.
Baseline characteristics of the case-control samplea
| Characteristic | Cases (n = 114) | Controls (n = 109) | P Value |
|---|---|---|---|
| Age (yr) | 67 ± 13 | 63 ± 15 | 0.02 |
| Female (%) | 45 | 45 | 0.99 |
| Race (%) | 0.22 | ||
| white | 59 | 47 | |
| black | 36 | 47 | |
| other | 5 | 6 | |
| Body mass index (kg/m2) | 26 ± 3 | 27 ± 7 | 0.20 |
| Etiology of renal failure (% diabetes) | 50 | 36 | 0.03 |
| Vascular access (% catheter) | 70 | 46 | <0.01 |
| Systolic blood pressure (mmHg) | 140 ± 25 | 145 ± 20 | 0.02 |
| Diastolic blood pressure (mmHg) | 71 ± 14 | 74 ± 12 | 0.05 |
| Hospitalization 14 ± 5 d from initiation of dialysis (%) | 17 | 10 | 0.17 |
| Albumin (g/dl) | 3.2 ± 0.6 | 3.5 ± 0.5 | <0.01 |
| Calcium (mg/dl) | 8.3 ± 0.7 | 8.4 ± 0.8 | 0.20 |
| Phosphorus (mg/dl) | 4.4 ± 1.4 | 4.6 ± 1.3 | 0.05 |
| Creatinine (mg/dl) | 5.5 ± 2.6 | 6.5 ± 2.6 | 0.01 |
| Estimated Kt/V | 1.3 ± 0.3 | 1.3 ± 0.4 | 0.56 |
| Hemoglobin (g/dl) | 10.1 ± 1.3 | 10.0 ± 1.4 | 0.40 |
| hsCRP (mg/L)b | 20 (7 to 47) | 13 (3 to 24) | 0.20 |
| White blood cell count (cells/μl) | 8.7 ± 4.1 | 7.5 ± 2.6 | 0.01 |
| Platelets (cells/dl) | 210 ± 84 | 236 ± 95 | 0.08 |
Values are frequencies or means ± SD.
hsCRP reported as median values and IQR (25 to 75%).
Multivariable Analysis of Mortality
In the model examining all-cause mortality, for every 10-mg/L reduction in baseline pGSN, the risk for subsequent mortality was increased by 15% [95% confidence interval (CI), 7 to 23%]. The lowest baseline levels (tertile 3, pGSN <130 mg/L) were associated with the highest risk for 1-yr all-cause and infection-related mortality (Table 2). The results for cardiovascular causes of death were less significant. In these analyses, hsCRP did not significantly associate with 1-yr mortality. In addition, serum creatinine, which was significant with univariate analysis, was no longer significant once the model was adjusted for pGSN. We then examined the effect of serum albumin on the multivariable models and noted that although the point estimates for each tertile of pGSN were modestly larger without serum albumin, the level and direction of significance did not change by adding serum albumin. Alternatively, including or excluding pGSN gave the following results with serum albumin:
- Excluding pGSN:
- tertile 1 (serum albumin <3.2 mg/dl), odds ratio (OR) 3.0, 95% CI 1.1 to 6.4
- tertile 2 (3.2 to 3.6 mg/dl), OR 1.1, 0.5 to 2.4
- tertile 3 (> 3.6 mg/dl), OR 1.0 (reference).
- Including pGSN:
- tertile 1, OR 2.0, 95% CI 0.8 to 4.9
- tertile 2, OR 1.0, 0.5 to 2.4; tertile 3, OR 1.0 (ref).
Table 2.
{P}Multivariable risk (OR) of 1-yr mortality according to tertiles of pGSN and all cause, cardiovascular disease (CVD), and infectious causes of death at 1 yra
| Tertiles of pGSN | Cases | Controls | OR | 95% CI | P Value |
|---|---|---|---|---|---|
| Risk for all-cause death | |||||
| tertile 1: ≥150 mg/L | 16 | 41 | 1.0 | Reference | |
| tertile 2: 130 to 149 mg/L | 24 | 33 | 2.1 | 0.7 to 6.7 | 0.19 |
| tertile 3: <130 mg/L | 74 | 35 | 3.4 | 1.2 to 9.4 | 0.01 |
| P for trend = 0.006 | |||||
| Risk for CVD deaths | |||||
| tertile 1: ≥150 mg/L | 8 | 41 | 1.0 | Reference | |
| tertile 2: 130 to 149 mg/L | 10 | 33 | 1.4 | 0.3 to 5.2 | 0.65 |
| tertile 3: <130 mg/L | 41 | 35 | 2.4 | 0.6 to 8.2 | 0.10 |
| P for trend = 0.052 | |||||
| Risk for infectious deaths | |||||
| tertile 1: ≥150 mg/L | 8 | 41 | 1.0 | Reference | |
| tertile 2: 130 to 149 mg/L | 14 | 33 | 3.2 | 0.7 to 15.5 | 0.13 |
| tertile 3: <130 mg/L | 33 | 35 | 5.4 | 1.3 to 22.5 | 0.03 |
| P for trend = 0.008 |
Model adjusted for baseline age, gender, race, body mass index, cause of ESRD, blood pressure, vascular access, and baseline serum albumin, calcium, phosphorus, creatinine, white blood cell count, platelet count, and hsCRP.
When serum albumin was modeled as a continuous variable, it remained significant even after adjustment for pGSN (excluding pGSN, OR 0.32 for each 1 mg/dl increase of serum albumin, 95% CI 0.15 to 0.67; including pGSN, OR 0.39, 95% CI 0.18 to 0.83). Therefore, addition of pGSN to the models attenuated but did not extinguish, the association between serum albumin and mortality.
pGSN, Circulating Actin, and Mortality
Polypeptides comigrating with purified actin were clearly visible as discrete bands by Western blot of uremic serum and verified as actin by mass spectrometry (data not shown). Sixty-nine percent of patients had circulating actin at baseline, and diabetic renal failure patients were more likely to have circulating actin (85%) than patients with other causes of renal failure (59%, P < 0.001). Compared with those with no actin, pGSN levels were lower in patients with actin (141 ± 36 mg/L versus 127 ± 45 mg/L, respectively, P = 0.02) (Figure 2A), consistent with previous results in sepsis samples.26
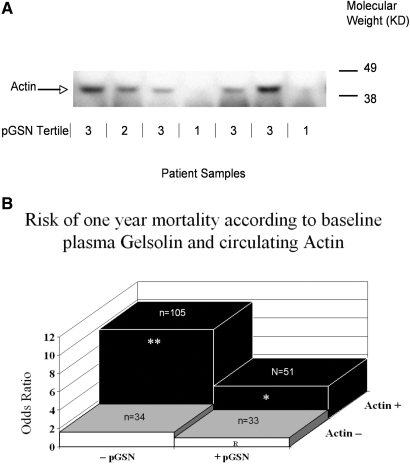
Figure 2.
(A) Western blot of plasma samples in relation to pGSN level at the initiation of chronic hemodialysis. Representative Western blot of plasma samples from sevean patients analyzed by E-PAGE 48 system as described in the Concise Methods section. The clear band migrating at 42 kD denotes the presence of actin in the sample analyzed. Corresponding pGSN levels stratified by tertiles are under each corresponding sample (tertile 1, ≥150 mg/L; tertile 2, 149 to 130 mg/L; tertile 3, <130 mg/L). Patients with lower pGSN levels tend to have more detectable actin compared with patients with higher pGSN levels. (B) Risk of 1-yr mortality according to baseline pGSN and presence or absence of actin. Elevated baseline pGSN (pGSN ≥141 mg/L, + pGSN), low baseline pGSN (pGSN <141 mg/L, −pGSN); no detectable actin (Actin−); detectable actin (Actin+): −pGSN, Actin+, OR 9.8, 95% CI 2.9 to 33.5; + pGSN, Actin+, OR 3.6, 95% CI 1.0 to 13.5; −pGSN, Actin−, OR 1.6, 95% CI 0.3 to 7.7; +pGSN, Actin−, OR 1.0 (reference). * P = 0.05, ** P = 0.003.
In univariate analysis, detectable circulating actin conferred 3.5-fold (95% CI 1.9 to 6.4) odds for 1-yr death. This relationship persisted with multivariable analyses (OR 4.6, 95% CI 2.0 to 10.5). The presence of diabetic renal failure, which significantly associated with early mortality upon univariate analyses (OR 1.8, 95% CI 1.1 to 3.0), became nonsignificant after adjusting for circulating actin (OR 1.3, 95% CI 0.7 to 2.3). Given that pGSN binds actin released by tissue damage and may abrogate actin-induced injury,12,28–30 we hypothesized that low pGSN and elevated actin would increase risk of adverse outcomes. We dichotomized pGSN (by median levels), and the formal test for effect modification with the interaction term (pGSN × detectable actin) in a multivariable model was significant (P = 0.03) (Figure 2B). The combined parameters, low pGSN and detectable actin, associated synergistically rather than additively with risk of death.
Venovenous Catheter and Mortality
We sought additional effect modifications by including interaction terms (e.g., pGSN × covariate) in multivariable models with covariates of interest. The only additional interaction suggested was vascular access type (P = 0.04). Although venovenous catheter vascular access associates with an increased risk for early mortality,31 deciphering those most susceptible to death has been challenging. Patients initiating hemodialysis with a catheter (129 ± 49 mg/L) or with an arteriovenous fistula or graft (136 ± 32 mg/L, P = 0.24) did not differ at baseline by pGSN levels, nor by the frequency of circulating actin (71 versus 68%, respectively, P = 0.67). Nevertheless, a venovenous catheter appeared to influence 1-yr mortality risk (Table 3). Among patients with a venovenous catheter, those with low pGSN and detectable circulating actin had a marked increase in overall mortality compared with those with high pGSN and no detectable actin (OR 25.9, 95% CI 4.3 to 157.0).
Table 3.
Multivariable risk (OR) of 1-yr mortality according to venovenous catheter status at baseline and pGSN and actin statusa
| Statusb | Cases | Controls | OR | 95% CI |
|---|---|---|---|---|
| No venovenous catheter (n = 93) | ||||
| +pGSN, Actin− | 4 | 14 | 1.0 | Reference |
| −pGSN, Actin− | 3 | 8 | 0.3 | 0.1 to 7.2 |
| +pGSN, Actin+ | 5 | 16 | 1.0 | 0.2 to 7.9 |
| −pGSN, Actin+ | 22 | 21 | 2.4 | 0.5 to 12.1 |
| Venovenous Catheter (n = 130) | ||||
| +pGSN, Actin− | 4 | 12 | 1.0 | Reference |
| −pGSN, Actin− | 11 | 12 | 3.9 | 0.6 to 26.4 |
| +pGSN, Actin+ | 15 | 14 | 11.1 | 1.8 to 69.5 |
| −pGSN, Actin+ | 50 | 12 | 25.9 | 4.3 to 157.0 |
Model adjusted for baseline age, gender, race, body mass index, cause of ESRD, blood pressure, vascular access, and baseline serum albumin, calcium, phosphorus, creatinine, white blood cell count, platelet count, and hsCRP.
Elevated baseline pGSN (pGSN ≥141 mg/L, +pGSN), low baseline pGSN (pGSN <141 mg/L, pGSN−); no detectable actin (Actin−); detectable actin (Actin+).
pGSN, Circulating Actin, and Chronic Kidney Disease
pGSN levels correlated directly with estimated GFR (r = 0.39, P = 0.003) in subjects with chronic kidney disease not on dialysis (Supplementary Figure 1). Men (153 ± 43 mg/L) tended to have higher levels of pGSN compared with women (136 ± 52 mg/L, P = 0.09). Levels in late stages of kidney disease (e.g., stages 3 and 4) were comparable to those found at the initiation of chronic hemodialysis. However, these levels were significantly lower than in samples obtained from stages 1 and 2 (P = 0.002) (Supplementary Figure 2). The frequency of circulating actin was 11% in this predialysis cohort, in contrast to 69% in the dialysis cohort (P < 0.001).
DISCUSSION
Patients initiating hemodialysis have pGSN levels reduced to an average 30 to 50% lower than those found in healthy controls. pGSN declines with progressive renal disease, suggesting mechanisms upstream of chronic dialysis initiation account for pGSN reduction. After the initiation of chronic hemodialysis, pGSN demonstrated a graded, inverse relationship with adverse outcomes—the lower the level, the higher the risk for 1-yr mortality. The findings represent the first chronic condition in which low pGSN levels relate to adverse outcomes, a finding previously limited to acute illnesses.
pGSN sequestration at sites of injury or clearance with circulating actin are the principal causes of decreased pGSN concentrations after acute insults. These factors presumably also contribute to diminished pGSN in ESRD; however, impairment of synthesis may be important. For example, uremia is characterized by increased activity of the ubiquitin-proteasome pathway,6 and recently increased activity of this pathway has been linked to increased degradation of cGSN, the intracellular isoform of pGSN.32 Moreover, because the molecular weight of pGSN is approximately 93 kDA, pGSN is unlikely to be cleared by hemodialysis. As highlighted in Figure 3, the combination of decreased production and increased consumption due to ongoing tissue injury in dialysis patients is most likely the etiology of the decreased circulating levels of pGSN in end-stage renal failure subjects. pGSN synthesis is constitutive and does not increase like acute-phase reactants in inflammation.25 Because muscle is a major source of pGSN, correlations with serum albumin and creatinine suggest that protein-energy wasting characteristic of ESRD contributes to pGSN reduction.4,6,8,33–37 pGSN attenuates the otherwise strong relationship between serum creatinine and albumin and hemodialysis mortality,4 suggesting at least a partial overlap between these parameters in explaining mortality.
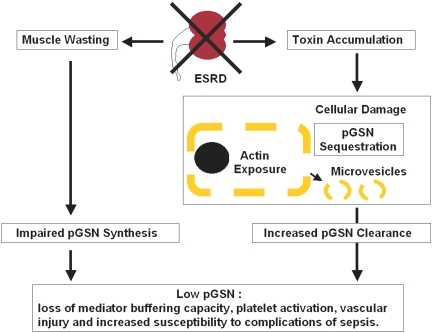
Figure 3.
Possible mechanisms for pGSN depletion and its consequences in chronic renal failure. Chronic renal failure inhibits pGSN synthesis and accelerates clearance. Muscle is the major source of pGSN biosynthesis, and the reduction of muscle mass associated with chronic renal failure predictably would reduce net pGSN production. The failure to eliminate toxins in renal failure causes widespread tissue destruction (especially endothelial), leading to exposure of cytoplasmic actin into the plasma and pGSN sequestration in broken cells. In addition, release of inside-out membrane vesicles with attached actin filaments from damaged cells would result in detectable circulating actin, and circulating actin accelerates pGSN clearance. Low pGSN results in impaired buffering of inflammatory mediators such as platelet-activating factor, promoting vascular complications and rendering patients susceptible to the lethal effects of sepsis.
Patients at greatest risk for death with the lowest pGSN levels were those with detectable circulating actin. Actin has been detectable in plasma of patients with acute lung or liver injury,12 patients with severe trauma, and even in healthy blood donors.38 Circulating actin in over two-thirds of hemodialysis patients is consistent with widespread tissue injury and excess muscle protein catabolism reported in patients with ESRD.6,7,39,40 Most (85%) patients with diabetic renal failure, a group with widespread endothelial cell injury and markedly elevated mortality rates,41–43 had circulating actin, and it was interesting to find that adjustment for circulating actin eliminated the relationship between diabetes status and mortality that has been previously reported.44 Circulating actin has been documented in patients with acute respiratory distress syndrome28 and in animal models of sepsis (Supplemental Table 2).26 In contrast to pGSN depletion, detectable circulating actin was far less prevalent in advanced renal disease before dialysis, suggesting that dialysis itself, possibly resulting from acute hemodynamic fluxes or dialysis membrane bioincompatibilies,45 may contribute to tissue damage, thereby releasing actin into the circulation.
pGSN depletion may link muscle wasting, tissue injury, inflammation, and death due to cardiovascular events and sepsis in ESRD. pGSN depletion may indeed characterize other chronic wasting states. pGSN avidly binds inflammatory mediators including platelet-activating factor, lysophosphatidic acid, lipoteichoic acid, aß peptide, and lipopolysaccharide endotoxin and decreases the effects of these agonists on target cells.13,15,46,47 Loss of buffering of these mediators due to pGSN depletion could exacerbate vascular disease and its contribution to mortality. Toxic effects of circulating actin on the vasculature might also be important.28,48 No evidence exists that pGSN depletion predisposes to infection per se; rather, pGSN deficiency may worsen the outcome of superimposed infection.21,22,26 Low pGSN and circulating actin conferred a markedly increased risk for early mortality in catheter compared with graft- or fistula-managed patients. Attenuation of pGSN's ability to disrupt actin-containing biofilms may be one mechanism by which low pGSN and elevated actin predispose to adverse outcomes in catheter-instrumented patients.49,50 Moreover, actin impairs the activity of the leukocyte-derived cationic antimicrobial polypeptides known as defensins.30
With the incidence of ESRD likely increasing,51 it is interesting to speculate that measuring pGSN levels and testing for circulating actin may help predict outcomes, assess which patients should not undergo catheter instrumentation, identify dialysis methods to reduce tissue injury or patients in need of more aggressive dialysis, or prioritize patients for transplantation. Our findings also suggest a novel therapeutic option: recombinant pGSN has been produced in Escherichia coli and administered by various routes to inflammation and infection-challenged test animals with the apparent absence of adverse effects and with the amelioration of adverse outcomes.24–26 If our results are confirmed and found to be generalizable, pGSN repletion to restore the “pGSN-actin imbalance” in chronic hemodialysis patients may attenuate the marked morbidity and mortality risk faced by such patients. Given that this is the first description of circulating pGSN depletion and actin detection in dialysis patients, further validation of the findings with high throughput pGSN and actin assays is necessary to justify pursuing the utility of pGSN measurement and replacement in this disease.
We acknowledge that residual and unmeasured confounders are limitations for all observational studies, and although we included subjects from over 100 U.S. dialysis centers, further validation is necessary in a larger population of comparable patients. These larger studies will better define traditional test characteristics for both pGSN and circulating actin. Errors in International Classification of Diseases (ICD)-9-based outcomes can misrepresent causes of death, although venovenous catheter exposure is accurately documentable, and our data strongly support the hypothesis that pGSN deficiency may pathophysiologically relate to infection-related deaths.
CONCISE METHODS
Study Population and Participants
We used a case-cohort design to examine hemodialysis patients. We first measured pGSN levels and circulating actin in plasma samples collected between 0 and 14 d after initiating hemodialysis from 150 randomly selected patients of the 10,044 enrolled prospectively between July 1, 2004 and June 30, 2005 in the Accelerated Mortality on Renal Replacement (ArMORR) cohort.52,53 Patients were from 1 of 1056 dialysis units by Fresenius Medical Care, North America (Waltham, MA) that compiles patient demographics, laboratory tests, intravenous therapies, and clinical outcomes and enters the data into a central database at the point of care with rigorous quality assurance/quality control auditing.54,55 Of these 150 random patients, 41 (27%) died within 365 d and 109 survived for at least 365 d. To increase power in mortality analysis, we supplemented the original 150 random patients with 75 additional incident dialysis patients who died within 365 d (n = 116 total patients) to create a case-control sample of an approximate 1:1 ratio with a total of 225 patients. We prespecified inclusion of a similar number of cardiovascular- and infection-related deaths (defined below) to examine in an unbiased fashion the relationship with each outcome. Subsequently, 2 patients had insufficient blood samples and were excluded, leaving 223 patients. With this case-control sample, we had over 80% power to detect an OR of at least 2 among patients with low (i.e., lowest tertile) compared with high levels.
We also measured pGSN and circulating actin in 53 outpatients at various stages of chronic kidney disease.56 We calculated GFRs using the Modification of Diet in Renal Disease equation and classified stages of kidney disease according to the National Kidney Foundation recommendations.57
Measurements
All samples (patients and controls) were collected, shipped, and stored in a similar manner, and each underwent one freeze-thaw cycle. pGSN levels remain stable even after long storage periods. We quantified pGSN with a previously described22,26 functional fluorimentric assay based on pGSN's acceleration of the initial rate of actin polymerization (actin nucleation assay) reported by increased fluorescence of pyrene actin,58 which is directly proportional to pGSN concentration and detects total pGSN irrespective of whether free or complexed to actin or other pGSN ligands. pGSN concentrations were estimated from a standard curve using purified recombinant human pGSN synthesized in E. coli. The assay coefficient of variation was 12%, and all measurements were performed with the supervising investigator and laboratory technician blinded to the outcomes.
For measurements of circulating actin, plasma samples were diluted 1:10-fold in PBS and electrophoresed using E-PAGE 48 8% gel system (Invitrogen, Carlsbad, CA). Western blotting for β-actin was performed using primary anti-β-actin antibodies (AC-15, Sigma, St. Louis, MO) and probed with horseradish peroxidase-linked anti-rabbit IgGs (Santa Cruz Biotechnology, Santa Cruz, CA). The presence of β-actin was defined as the appearance of discrete bands comigrating with purified rabbit skeletal muscle actin (Cytoskeleton, Denver, CO). The identity of the actin on the Western blots was confirmed by subjecting ten randomly selected samples to mass spectrometry (Beth Israel Deaconess Medical Center Mass Spectrometry Core Facility).
The primary exposure was baseline pGSN levels, and the primary outcome was 1-yr overall mortality. We examined pGSN as a continuous and dichotomized (on the basis of median levels in the random sample) variable and categorized by tertiles. In addition to overall mortality, we utilized ICD-9 codes to define outcomes with cardiovascular (e.g., died of diseases of the circulatory system, ICD-9 390 to 459.9; hypertensive diseases, 401 to 405; ischemic heart disease, 410 to 414; acute myocardial infarction, 410; and cerebrovascular disease, 430 to 438) and infectious causes of mortality (e.g., bacterial, fungal, and viral pneumonias, ICD-9 480.0 to 487.8; empyema, 510.0; lung abscess, 513.0; sepsis, severe sepsis, and septic shock, 038, 995 to 996, 785). We confirmed death by discharge diagnosis reports.
The primary covariate of interest was detectable circulating actin in plasma. Other covariates included age, race, sex, body mass index, assigned cause of renal failure (e.g., diabetes, hypertension, GN), blood pressure, vascular access at initiation (arteriovenous fistula or graft, or venovenous catheter), and dialysis dose (Kt/V) as in prior analyses.54,55 We analyzed baseline blood levels of albumin, creatinine, calcium, phosphorus, platelet and white blood cell counts, and hsCRP (Dade Behring).
Statistical Analysis
We summarized baseline characteristics of the initial 150 patient sample, expressing means (±SD) and medians as IQR (IQR 25 to 75%). We utilized Spearman correlation coefficients for the association between routine laboratory tests and pGSN levels and to examine the association between pGSN levels and estimated GFRs of chronic kidney disease patients not on dialysis. We used linear regression models to examine independent relationships between pGSN and other covariates, and univariate survival analysis using Kaplan-Meier curves with log-rank tests after dividing baseline pGSN values on the basis of median and tertile values. Patients censored for recovery of renal function (n = 4), voluntary discontinuation of dialysis (n = 3), or loss to follow up (n = 9) were <11%.
Case-Control Sample.
We used two-sample t test and Fisher's exact test to compare demographic and laboratory characteristics at dialysis initiation among patients and controls. We used multivariable logistic regression models to examine the independent association between baseline pGSN and all-cause, cardiovascular, and infectious causes of 1-yr mortality. We included covariates previously associated with mortality on dialysis from previous studies54,55 and those significantly different among patients and controls in the study presented here. We adjusted all models for C-reactive protein levels. Data points on individual covariates were missing in <5% of patients; for the multivariable logistic regression analyses, these covariates were treated as categorical variables with an additional category for missing values. Various imputations of missing data were also examined but these did not change the results. We examined the relationship between pGSN, circulating actin, and outcomes given the biologic relationship of these two measures. In exploratory analyses we examined first-order interactions between pGSN and covariates (pGSN × covariate) in univariate and multivariable models, and stratified models were presented when an interaction with P value <0.10 was detected. We also performed likelihood ratio χ2 testing, comparing models with and without the interaction terms as a formal test for interaction.
The Institutional Review Board of the Massachusetts General Hospital approved all of these protocols. All analyses were performed using SAS 9.1 (Cary, NC), and two-sided P values <0.05 were considered statistically significant.
DISCLOSURES
Four of the authors (P.S.L, S.A.K, T.S, and R.T) are named as co-inventors on a recently filed provisional patent filed by the Harvard Hospitals for the use of gelsolin in renal failure subjects. This patent has not been issued nor licensed to a third party. P.S.L and T.S. are equity holders in Critical Biological Corporation, a private company that is currently developing recombinant gelsolin for potential use in sepsis.
Supplementary Material
Acknowledgments
This work is supported by an American Heart Association Scientist Development Grant (P.S.L.), the Howard Hughes Medical Institute (S.A.K.), an American Cancer Society Clinical Research Professorship (T.P.S.), and grants (DK71674 and HL093954) from the National Institutes of Health (R.T.). We also thank Sze-Man Tsang for her technical support.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Naqvi SB, Collins AJ: Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis 13: 199–204, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM: Modality-specific nutrition support in ESRD: Weighing the evidence. Am J Kidney Dis 33: 193–197, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Mitch WE, Goldberg AL: Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Zoccali C, Mallamaci F, Tripepi G: Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int Suppl: S105–110, 2003 [DOI] [PubMed]
- 10.Johansen KL, Young B, Kaysen GA, Chertow GM: Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 80: 324–332, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Lee WM, Galbraith RM: The extracellular actin-scavenger system and actin toxicity. N Engl J Med 326: 1335–1341, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS: Gelsolin binding and cellular presentation of lysophosphatidic acid. J Biol Chem 275: 14573–14578, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bucki R, Georges PC, Espinassous Q, Funaki M, Pastore JJ, Chaby R, Janmey PA: Inactivation of endotoxin by human plasma gelsolin. Biochemistry 44: 9590–9597, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Osborn TM, Dahlgren C, Hartwig JH, Stossel TP: Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin. Am J Physiol Cell Physiol 292: C1323–C1330, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Yin HL, Stossel TP: Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature 281: 583–586, 1979 [DOI] [PubMed] [Google Scholar]
- 17.Kwiatkowski DJ, Stossel TP, Orkin SH, Mole JE, Colten HR, Yin HL: Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature 323: 455–458, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Kwiatkowski DJ, Mehl R, Izumo S, Nadal-Ginard B, Yin HL: Muscle is the major source of plasma gelsolin. J Biol Chem 263: 8239–8243, 1988 [PubMed] [Google Scholar]
- 19.Suhler E, Lin W, Yin HL, Lee WM: Decreased plasma gelsolin concentrations in acute liver failure, myocardial infarction, septic shock, and myonecrosis. Crit Care Med 25: 594–598, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Mounzer KC, Moncure M, Smith YR, Dinubile MJ: Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am J Respir Crit Care Med 160: 1673–1681, 1999 [DOI] [PubMed] [Google Scholar]
- 21.DiNubile MJ, Stossel TP, Ljunghusen OC, Ferrara JL, Antin JH: Prognostic implications of declining plasma gelsolin levels after allogeneic stem cell transplantation. Blood 100: 4367–4371, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lee PS, Drager LR, Stossel TP, Moore FD, Rogers SO: Relationship of plasma gelsolin levels to outcomes in critically ill surgical patients. Ann Surg 243: 399–403, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heintzelman MB, Frankel SA, Artavanis-Tsakonas S, Mooseker MS: Cloning of a secretory gelsolin from Drosophila melanogaster. J Mol Biol 230: 709–716, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Christofidou-Solomidou M, Scherpereel A, Solomides CC, Christie JD, Stossel TP, Goelz S, DiNubile MJ: Recombinant plasma gelsolin diminishes the acute inflammatory response to hyperoxia in mice. J Investig Med 50: 54–60, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Rothenbach PA, Dahl B, Schwartz JJ, O'Keefe GE, Yamamoto M, Lee WM, Horton JW, Yin HL, Turnage RH: Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J Appl Physiol 96: 25–31, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Lee PS, Waxman AB, Cotich KL, Chung SW, Perrella MA, Stossel TP: Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit Care Med 35: 849–855, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Erukhimov JA, Tang ZL, Johnson BA, Donahoe MP, Razzack JA, Gibson KF, Lee WM, Wasserloos KJ, Watkins SA, Pitt BR: Actin-containing sera from patients with adult respiratory distress syndrome are toxic to sheep pulmonary endothelial cells. Am J Respir Crit Care Med 162: 288–294, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Janmey PA, Lind SE: Capacity of human serum to depolymerize actin filaments. Blood 70: 524–530, 1987 [PubMed] [Google Scholar]
- 30.Weiner DJ, Bucki R, Janmey PA: The antimicrobial activity of the cathelicidin LL37 is inhibited by F-actin bundles and restored by gelsolin. Am J Respir Cell Mol Biol 28: 738–745, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W: Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int 55: 1081–1090, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Ni XG, Zhou L, Wang GQ, Liu SM, Bai XF, Liu F, Peppelenbosch MP, Zhao P: The ubiquitin-proteasome pathway mediates gelsolin protein downregulation in pancreatic cancer. Mol Med 14: 582–589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIntyre CW, Selby NM, Sigrist M, Pearce LE, Mercer TH, Naish PF: Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant 21: 2210–2216, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kaysen GA, Greene T, Daugirdas JT, Kimmel PL, Schulman GW, Toto RD, Levin NW, Yan G: Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am J Kidney Dis 42: 1200–1211, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Owen WF, Jr., Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Beddhu S, Cheung AK, Larive B, Greene T, Kaysen GA, Levey AS, Rocco M, Sarnak M, Toto R, Eknoyan G: Inflammation and inverse associations of body mass index and serum creatinine with mortality in hemodialysis patients. J Ren Nutr 17: 372–380, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semba RD, Ricks MO, Ferrucci L, Xue QL, Guralnik JM, Fried LP: Low serum selenium is associated with anemia among older adults in the United States. Eur J Clin Nutr 63: 93–99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mejean C, Roustan C, Benyamin Y: Anti-actin antibodies. Detection and quantitation of total and skeletal muscle actin in human plasma using a competitive ELISA. J Immunol Methods 99: 129–135, 1987 [DOI] [PubMed] [Google Scholar]
- 39.Mezzano D, Pais EO, Aranda E, Panes O, Downey P, Ortiz M, Tagle R, Gonzalez F, Quiroga T, Caceres MS, Leighton F, Pereira J: Inflammation, not hyperhomocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uremia. Kidney Int 60: 1844–1850, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Mezzano D, Tagle R, Pais E, Panes O, Perez M, Downey P, Munoz B, Aranda E, Barja P, Thambo S, Gonzalez F, Mezzano S, Pereira J: Endothelial cell markers in chronic uremia: Relationship with hemostatic defects and severity of renal failure. Thromb Res 88: 465–472, 1997 [DOI] [PubMed] [Google Scholar]
- 41. U.S. Renal Data System 2006 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006
- 42.Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T: Features of endothelial dysfunction in early diabetic nephropathy. Lancet 1: 461–463, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Hsueh WA, Anderson PW: Hypertension, the endothelial cell, and the vascular complications of diabetes mellitus. Hypertension 20: 253–263, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Lazarus JM, Owen WF: Role of bioincompatibility in dialysis morbidity and mortality. Am J Kidney Dis 24: 1019–1032, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Ito H, Nakamura H, Hayashi E, Kishimoto S, Hashimoto T, Tagawa K: Human plasma gelsolin binds adenosine triphosphate. J Biochem 108: 505–506, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Lind SE, Janmey PA: Human plasma gelsolin binds to fibronectin. J Biol Chem 259: 13262–13266, 1984 [PubMed] [Google Scholar]
- 48.Haddad JG, Harper KD, Guoth M, Pietra GG, Sanger JW: Angiopathic consequences of saturating the plasma scavenger system for actin. Proc Natl Acad Sci U S A 87: 1381–1385, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker TS, Tomlin KL, Worthen GS, Poch KR, Lieber JG, Saavedra MT, Fessler MB, Malcolm KC, Vasil ML, Nick JA: Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun 73: 3693–3701, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trautner BW, Darouiche RO: Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control 32: 177–183, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu CY, Vittinghoff E, Lin F, Shlipak MG: The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med 141: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, Camargo CA Jr, Melamed M, Norris K, Stampfer M, Powe NR, Thadhani R: Impact of activated vitamin D and race on hemodialysis survival. J Am Soc Nephrol 19: 1379–1388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr., Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Kouyama T, Mihashi K: Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem 114: 33–38, 1981 [PubMed] [Google Scholar]
- 59.Dahl B, Schiodt FV, Ott P, Gvozdenovic R, Yin HL, Lee WM: Plasma gelsolin is reduced in trauma patients. Shock 12: 102–104, 1999 [DOI] [PubMed] [Google Scholar]
- 60.DiNubile M, Antin J, Bressler S, Stossel T, Ferrara J: Decreased gelsolin levels are associated with interstitial pneumonia after allogeneic BMT. Blood 92: 683a, 1998. 9657771 [Google Scholar]
- 61.Ito H, Kambe H, Kimura Y, Nakamura H, Hayashi E, Kishimoto T, Kishimoto S, Yamamoto H: Depression of plasma gelsolin level during acute liver injury. Gastroenterology 102: 1686–1692, 1992 [DOI] [PubMed] [Google Scholar]
- 62.Smith DB, Janmey PA, Herbert TJ, Lind SE: Quantitative measurement of plasma gelsolin and its incorporation into fibrin clots. J Lab Clin Med 110: 189–195, 1987 [PubMed] [Google Scholar]
- 63.Smith DB, Janmey PA, Sherwood JA, Howard RJ, Lind SE: Decreased plasma gelsolin levels in patients with Plasmodium falciparum malaria: A consequence of hemolysis? Blood 72: 214–218, 1988 [PubMed] [Google Scholar]
- 64.Lind SE, Smith DB, Janmey PA, Stossel TP: Depression of gelsolin levels and detection of gelsolin-actin complexes in plasma of patients with acute lung injury. Am Rev Respir Dis 138: 429–434, 1988 [DOI] [PubMed] [Google Scholar]
- 65.Lofberg M, Paunio T, Tahtela R, Kiuru S, Somer H: Serum gelsolin and rhabdomyolysis. J Neurol Sci 157: 187–190, 1998a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.