Abstract
Neurocysticercosis is caused by Taenia solium infection of the brain. Diagnosis is most often made by visualization of the parasitic scolex by magnetic resonance imaging of the brain or by characteristic neuroimaging findings with serologic test results positive for T. solium. A patient who presents with a solitary brain lesion usually poses a diagnostic dilemma, because the differential diagnosis often includes neurocysticercosis and other infections or neoplasm. Although the sensitivity of serologic testing for T. solium approaches 100% in patients with multiple intraparenchymal cysts, the sensitivity of testing for patients with solitary cysts is <50%, which makes serologic testing a less useful diagnostic tool for patients with solitary central nervous system (CNS) lesions. We describe 2 patients with solitary CNS lesions who received a neurocysticercosis diagnosis after identification of T. solium DNA in brain biopsy tissue with use of a global DNA screening platform. Global screening is a promising tool for the diagnosis of CNS infection, especially when traditional diagnostic tools are insensitive.
Neurocysticercosis (NCC) is the most common cause of acquired epilepsy in the world and occurs when the larval form of the tapeworm Taenia solium forms encapsulated cysts in the CNS [1]. Neuroimaging manifestations of NCC vary by geographic region. In Latin America, Africa, and Asia, NCC is typically associated with multiple cysts, whereas in India, NCC is usually associated with a solitary degenerating cyst [2].
The absolute criteria for diagnosis of NCC include (1) histologic demonstration of T. solium in brain or spinal cord tissue, (2) demonstration of the T. solium scolex within a cystic lesion by CT or MRI, or (3) fundoscopic visualization of subretinal parasites [3]. Definitive diagnosis of NCC can also be made in persons with characteristic neuroimaging findings, clinical symptoms, serologic test results positive for T. solium, or exposure to a person with T. solium infection or an area where T. solium infection is endemic. In clinical practice, a solitary CNS lesion presents a diagnostic dilemma and may be difficult to differentiate by neuroimaging alone. Serologic assays for T. solium have positive results in 14%–40% of patients with solitary lesions, and false-positive results occur in individuals with other parasitic infections [2, 4, 5]. Molecular approaches targeting genetic sequences of Taenia species have been used to detect NCC in CSF and other tissues and for geographic genotyping; however, these assays typically require an a priori suspicion of T. solium infection [6-11].
Global DNA screening platforms using ribosomal RNA (rRNA) gene sequencing can detect a broad range of bacterial or fungal DNA and, simultaneously, can differentiate among them through comparison of genetic variability of conserved regions. The 16s rRNA gene is a standard target that is used for identification of bacterial species, whereas the internal transcribed spacer (ITS) regions 1 and 2 and 28s rRNA gene are targets for identification of fungal species [12-14]. Although the ITS region could screen for parasitic DNA, as well, we are not aware of a study or report that has demonstrated this technique as a screening tool. We report 2 patients with single enhancing CNS lesions who had T. solium detected in their brain tissue samples by ITS rRNA gene sequencing in a global screening platform; the presence of T. solium was subsequently confirmed in a secondary analysis using Taenia species–specific primers.
Case report 1
A 23-year-old man presented with seizures that involved his left arm and leg. CT demonstrated a solitary enhancing polycystic lesion in the right superior parietal lobe, with surrounding vasogenic edema (figure 1). The patient had no other neurologic deficits, headaches, chills, night sweats, or weight loss. He reported a temperature of 37.8°C before onset of seizures and also reported a cough that had resolved several days before presentation. The patient was born in India and had immigrated to the United States at 18 years of age. He had traveled throughout India but was unaware of potential tuberculosis contacts. A PPD test performed in India was non-reactive. Serologic tests for T. solium, HIV types 1 and 2, and hepatitis B virus had negative results. CT of the chest, abdomen, and pelvis had normal findings. The patient initiated therapy with an anticonvulsant and a 12-day course of albendazole and dexamethasone as presumptive treatment for NCC. He experienced no additional seizures. An MRI, obtained 2 months after initiation of therapy, demonstrated decreased edema but no change in lesion size. Additional serologic tests for T. solium in serum and CSF samples had negative results. The patient’s CSF profile was normal, and cultures for bacteria, mycobacteria, and fungi showed no growth. Because of concern regarding tuberculosis, the patient elected to undergo surgical resection of the lesion; histopathologic examination revealed a mixed lymphoplasmacytic inflammatory infiltrate without evidence of parasite or Mycobacteria species (figure 2). Cultures and DNA analysis of brain tissue samples for bacteria, mycobacteria, and fungi had negative results, but universal primers targeting the ITS1 region detected T. solium DNA. Additional testing targeting the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene specific for Taenia species identified T. solium. Additional MRI performed 7 months after the surgical procedure revealed no recurrence of the lesion, and the patient remained free of seizures.
Figure 1.
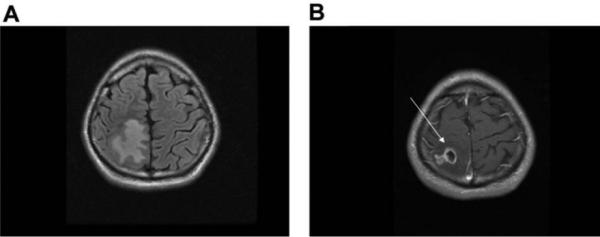
A, MRI of the brain revealing a large area of vasogenic edema in the right parietal lobe on fluid-attenuated inversion-recovery imaging. B, MRI after administration of gadolinium, revealing a single enhancing lesion (arrow).
Figure 2.
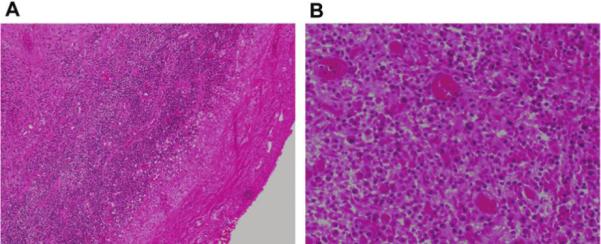
A, Histopathology sections revealing dense eosinophilic fibrous tissue with mixed inflammatory infiltrate of plasma cells, lymphocytes, and occasional neutrophils (hematoxylin and eosin stain; original magnification, ×100). B, Histopathology sections under high power oil immersion revealing no parasitic protoscolex (hematoxylin and eosin stain; original magnification, ×1000); stains for acid-fast organisms (Ziehl Nielson stain) and fungi (Gomori methenamine silver stain) had negative findings.
Case report 2
A 23-year-old Mexican man presented with incoordination of his left hand and a generalized tonic-clonic seizure. MRI revealed a single enhancing lesion in the right parietal lobe, with surrounding vasogenic edema (figure 3). The patient initiated treatment with dexamethasone but received no antihelminthics or antibiotics. The findings of detailed general and neurologic examinations were normal. The patient denied any known contacts with tuberculosis. A PPD test had negative results, and the findings of a chest radiograph were unremarkable. Serologic testing of blood and CSF samples had results that were negative for T. solium, HIV, cytomegalovirus, Epstein-Barr virus, Toxoplasma gondii, Cryptococcus neoformans, Echinococcus granulosus, Brucella species, Bartonella species, Mycobacterium tuberculosis, and Treponema pallidum. The patient remained clinically stable during a 4-month period. Additional MRI and brain perfusion imaging revealed no change; the CNS lesion was considered to represent a granulomatous infectious process. Steroid therapy was tapered, and subsequent MRI demonstrated an increase in the size of the lesion.
Figure 3.

A, MRI of the brain revealing a large area of vasogenic edema in the right parietal lobe on fluid-attenuated inversion-recovery imaging. B, Sagittal MRI after administration of gadolinium, revealing a single enhancing lesion (arrow).
Five months after presentation, the patient underwent surgical extirpation of the lesion. Gross macroscopic examination revealed small cystic pockets with extensive suppurative inflammation, consistent with an intraparenchymal abscess. Hemotoxylin-eosin staining demonstrated a mixed inflammatory cell infiltrate interspersed with bands of collagen (figure 4). Examination of multiple levels of the lesion did not reveal cysticerci. Culture and DNA analysis for bacteria, mycobacteria, and fungi had negative results, but T. solium DNA was detected by ITS1 regionand cox1 sequence analyses. Additional MRI performed 5 weeks after resection revealed only postsurgical changes, and a neurologic examination, performed 3 months after resection, had normal findings.
Figure 4.
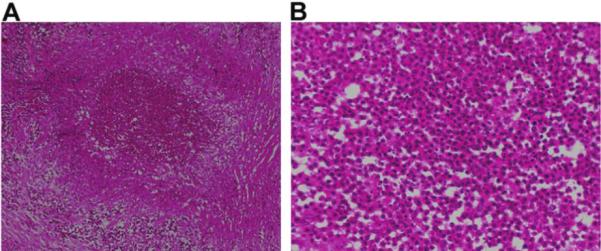
A, Hematoxylin-eosin stain of histopathology sections revealing a nodular, mixed inflammatory cell infiltrate consisting of neutrophils, plasma cells, lymphocytes, and histiocytes interspersed with bands of collagen and associated with brain parenchyma, which is characterized by dense reactive astrocytosis and extensive endothelial proliferation. No bacterial, fungal, or mycobacterial organisms were identified. B, Histopathology sections under high power oil immersion revealing no parasitic protoscolex; stains for acid-fast organisms (Ziehl Nielson stain) and fungi (Gomori methenamine silver stain) had negative findings.
Molecular diagnosis
Brain tissue was digested using proteinase K, and DNA was extracted using a QIAamp DNA Mini Kit (Qiagen). Template DNA was amplified by PCR assays using primers specific for ITS1 and ITS2 regions and 28s eukaryotic ribosomal subunit and was visualized using 1.0% agarose gels. For the ITS1 region, the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′) were used; for the ITS2 region, the primers ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS 4 (5′-TCCTCCGCTTATTGATATGC-3′) were used; and for 28s, the primers NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) were used. PCR amplification was performed with initial denaturation at 95°C for 10 min, 30 cycles of denaturation at 95°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 2 min, final extension at 72°C for 10 min, and holding at 4°C. All other primer pairs, including primers for the first ∼500 base pairs of the 16S rRNA gene, the heat shock protein 65, and RNA polymerase β subunit, were negative for amplification products. All reactions were positive for extraction and amplification controls. Samples were prepared for sequencing using the ABI PRISM BigDye Terminator Cycle Sequencing Kit, and DNA sequencing was performed on the ABI PRISM 3100 Analyzer (Applied Biosystems). Single-strand sequences derived from forward and reverse primers were assembled into double-stranded contig using Sequencher (Gene Codes Corporation) and were submitted to the GenBank BLAST database for sequence matches. Both sequence fragments were genetically related to T. solium (276 base pairs evaluated, with 99.73% sequence similarity for case 1; 478 base pairs evaluated, with 100% sequence similarity for case 2). Analysis of products from the 28s region and ITS2 region yielded nonanalyzable sequences. Extracted template DNA was reamplified using primers binding to the mitochondrial cox1 gene from Taenia species, as described elsewhere: JB3 (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and JB4.5 (5′-AAAGAAAGAACATAATGAAAATG-3′) [8]. Samples were prepared and sequenced as described. Sequence identification of the contig of the cox1 gene showed that both isolates were genetically identified as T. solium (100% and 99.5% sequence similarity for case 1 and case 2, respectively).
Discussion
We report, to our knowledge, the first use of a global screening platform to detect T. solium infection in 2 patients with solitary CNS lesions. In both patients, the differential diagnosis was broad and included NCC, tuberculosis, and neoplasm. Histologic examination of multiple levels of each lesion, stains, cultures, cytologic examination, and serologic testing for T. solium had negative results. A definitive diagnosis of NCC was made by detection of T. solium DNA. It is plausible that the cysticerci were not identified by histopathologic examination because of extensive damage from albendazole, dexamethasone, or the inflammatory response; if so, the global screening platform may be an even more useful tool for patients in whom histopathologic examination does not detect cysticerci.
Analysis of ribosomal ITS1 and ITS2 regions has been used for geographic genotyping and molecular phylogeny of Taenia species [13, 14]. ITS sequences are located in eukaryotic rRNA genes between 18S and 5.8S rRNA coding regions (ITS1 region) and between 5.8S and 25S rRNA coding regions (ITS2 region). The coding regions flanking the ITS regions are highly conserved, which allows for primer recognition. The evolution rate of ITS regions is slower than that of other genetic regions, permitting differentiation between parasitic species [15-18]. Mitochondrial DNA (mtDNA) is multicopied, relatively small (20,000 base pairs), and possesses a high degree of variability because of a more rapid evolution. Several mtDNA sequences, such as cox1, are excellent targets for interspecies differentiation for both helminths and filaria [19-21].
The global screening platform incorporates rRNA gene sequencing analysis using primers targeting the ITS regions and 28s for identification of fungal DNA, 16s for identification of bacterial DNA and rpoB, and hsp65 for detection of mycobacterial DNA. In our patients, identification of T. solium DNA with use of this universal screening approach was confirmed by a specific mtDNA target, cox1. Global screening is a valuable and efficient diagnostic tool for clinicians that allows identification of a diverse range of suspected or unsuspected infectious organisms. As this technology is used more frequently, the sensitivity and specificity of these assays for identifying infectious pathogens from human tissue samples will be better defined.
Acknowledgments
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Taenia solium cysticercosis. Lancet. 2003;362:547–56. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh G. Neurocysticercosos in South-Central America and the Indian subcontinent: a comparative evaluation. Arq Neuropsiquiatr. 1997;55:349–56. doi: 10.1590/s0004-282x1997000300001. [DOI] [PubMed] [Google Scholar]
- 3.Del Brutto OH, Rajshekhar V, White AC, Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson M, Bryan RT, Fried JA, et al. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164:1007–9. doi: 10.1093/infdis/164.5.1007. [DOI] [PubMed] [Google Scholar]
- 5.Carpio A. Neurocysticercosis: an update. Lancet Infect Dis. 2002;2:751–62. doi: 10.1016/s1473-3099(02)00454-1. [DOI] [PubMed] [Google Scholar]
- 6.Almeida CR, Ojopi EP, Nunes CM, et al. Taenia solium DNA is present in the cerebrospinal fluid of neurocysticercosis patients and can be used for diagnosis. Eur Arch Psychiatry Clin Neurosci. 2006;256:307–10. doi: 10.1007/s00406-006-0612-3. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa E, Komatsu Y, Kikuchi K, et al. Neurocysticercosis as solitary parenchymal lesion confirmed by mitochondrial deoxyribonucleic acid sequence analysis. Neurol Med Chir (Tokyo) 2007;47:40–4. doi: 10.2176/nmc.47.40. [DOI] [PubMed] [Google Scholar]
- 8.Varcasia A, Lightowlers MW, Cattoli G, et al. Genetic variation within Taenia multiceps in Sardinia, Western Mediterranean (Italy). Parasitol Res. 2006;99:622–6. doi: 10.1007/s00436-006-0179-y. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki H, Allan JC, Sato MO, et al. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–53. doi: 10.1128/JCM.42.2.548-553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki H, Nakao M, Sako Y, Nakaya K, Ito A. Molecular identification of Taenia solium cysticercus genotype in the histopathological specimens. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 4):131–4. [PubMed] [Google Scholar]
- 11.Yamasaki H, Nakao M, Sako Y, Nakaya K, Sato MO, Ito A. Mitochondrial DNA diagnosis for taeniasis and cysticercosis. Parasitol Int. 2006;55(Suppl):S81–5. doi: 10.1016/j.parint.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Clarridge JE., III Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17:840–62. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Eisner JD, Kattar MM, et al. Polymorphic internal transcribed spacer region 1 DNA sequences identify medically important yeasts. J Clin Microbiol. 2001;39:4042–51. doi: 10.1128/JCM.39.11.4042-4051.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakeman JL, Bui U, Lafe K, Chen YC, Honeycutt RJ, Cookson BT. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J Clin Microbiol. 2005;43:3324–33. doi: 10.1128/JCM.43.7.3324-3333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando K, Tsunemori M, Akahane H, Tesana S, Hasegawa H, Chinzei Y. Comparative study on DNA sequences of ribosomal DNA and cytochrome c oxidase subunit 1 of mitochondrial DNA among five species of gnathostomes. J Helminthol. 2006;80:7–13. doi: 10.1079/joh2005315. [DOI] [PubMed] [Google Scholar]
- 16.Cutillas C, de Rojas M, Ariza C, Ubeda JM, Guevara D. Molecular identification of Trichuris vulpis and Trichuris suis isolated from different hosts. Parasitol Res. 2007;100:383–9. doi: 10.1007/s00436-006-0275-z. [DOI] [PubMed] [Google Scholar]
- 17.Ishiwata K, Shinohara A, Yagi K, Horii Y, Tsuchiya K, Nawa Y. Identification of tissue-embedded ascarid larvae by ribosomal DNA sequencing. Parasitol Res. 2004;92:50–2. doi: 10.1007/s00436-003-1010-7. [DOI] [PubMed] [Google Scholar]
- 18.Nolan MJ, Cribb TH. The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv Parasitol. 2005;60:101–63. doi: 10.1016/S0065-308X(05)60002-4. [DOI] [PubMed] [Google Scholar]
- 19.McManus DP. Molecular discrimination of taeniid cestodes. Parasitol Int. 2006;55(Suppl):S31–7. doi: 10.1016/j.parint.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.McManus DP, Le TH, Blair D. Genomics of parasitic flatworms. Int J Parasitol. 2004;34:153–8. doi: 10.1016/j.ijpara.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki H, Matsunaga S, Yamamura K, et al. Solitary neurocysticercosis case caused by Asian genotype of Taenia solium confirmed by mitochondrial DNA analysis. J Clin Microbiol. 2004;42:3891–3. doi: 10.1128/JCM.42.8.3891-3893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


