Abstract
A recently developed method of synthesis of π-extended porphyrins made it possible to prepare a series of tetrabenzoporphyrins (TBP) with different numbers of meso-aryl substituents. The photophysical parameters of free-bases and Pd complexes of meso-unsubstituted TBP’s, 5,15-diaryl-TBP’s (Ar2TBP’s) and 5,10,15,20-tetraaryl-TBP’s (Ar4TBP’s) were measured. For comparison, similarly meso-arylsubstituted porphyrins fused with nonaromatic cyclohexeno-rings, i.e. Arn-tetracyclohexenoporphyrins (ArnTCHP’s, n = 0, 2, 4), were also synthesized and studied. Structural information was obtained by ab initio (DFT) calculations and X-ray crystallography. It was found that: 1) Free-base Ar4TBP’s are strongly distorted out-of-plane (saddled), possess broadened, red-shifted spectra, short excited-state lifetimes and low fluorescence quantum yields (τfl = 2–3 ns, ϕfl = 0.02–0.03). These features are characteristic of other nonplanar free-base porphyrins, including Ar4TCHP’s. 2) Ar2TBP free-bases possess completely planar geometries, although with significant in-plane deformations. These deformations have practically no effect on the singlet excited-state properties of Ar2TBP’s as compared to planar meso-unsubstituted TBP’s. Both types of porphyrins retain strong fluorescence (τfl = 10–12 ns, ϕfl = 0.3–0.4), and their radiative rate constants (kr) are 3–4 times higher than those of planar H2TCHP’s. 3) Nonplanar deformations dramatically enhance nonradiative decay of triplet states of regular Pd porphyrins. For example, planar PdTCHP phosphoresces with high quantum yield (ϕphos = 0.45, τphos = 1118 µs), while saddled PdPh4TCHP is practically nonemissive. In contrast, both ruffled and saddled PdArnTBP’s retain strong phosphorescence at ambient temperatures (PdPh2TBP: τphos = 496 µs, ϕphos = 0.15; PdPh4TBP: τphos = 258 µs, ϕphos = 0.08). It appears that π-extension is capable of counterbalancing deleterious effects of nonplanar deformations on triplet emissivity of Pd porphyrins.
Introduction
Porphyrins extended by way of annealing the pyrrole residues with external aromatic rings are commonly referred to as π-extended porphyrins. Symmetrically π-extended porphyrins, e.g., tetrabenzoporphyrins and tetranaphthoporphyrins, possess high absorption in the near-infrared region of the spectrum, and consequently draw attention in photomedicine,1 biomedical sensing and imaging.2 In addition, π-extended porphyrins have shown promise in up-conversion of noncoherent infrared light,3 optical power limiting and electrooptical applications4 as well as in material science.5 Such broad range of applicability warrants in-depth studies of structure–property relationships in the family of π-extended porphyrins, especially in the area of their photophysics, which is central to many of their applications.
Starting 1960s, influence of the π-extension on optical properties of porphyrins has been addressed by a number of researchers.6–8 As early as in 1961, Gouterman applied his four-orbital model to qualitatively explain differences between the electronic absorption spectra of regular porphyrins and tetrabenzoporphyrins.6a Soon after that his group6 and the group from Belarus7 published first reports on the luminescence of tetrabenzoporphyrin complexes. In more recent years, several in-depth theoretical studies9 provided insights into the electronic structure of π-extended tetrapyrroles, focusing mainly on their ground-state absorption spectra. For example, combined influence of π-extension and meso-tetraarylation on the spectra of tetrabenzo- and tetranaphthoporphyrins has been examined.10
In spite of these advances, effects of π-extension on the excited-state properties of porphyrins have been studied only scarcely, notwithstanding the fact that many potential uses of π-extended porphyrins rely on their singlet and triplet excited states. One particular question, much discussed in relation to regular porphyrins, is the interplay between the macrocycle structural deformations and its photophysics.11–13 Although fluorescence and phosphorescence of tetrabenzoporphyrins2,1a–c,7,8,10,14 and tetranaphthoporphyrins15 has been mentioned in a number of publications, relationships between structural features and excited-state properties of these porphyrins have never been studied systematically. Such structure/property analysis could not be done because structural information on π-extended porphyrins was extremely limited,16,17 in part due to their poor synthetic availability.
Over the past several years, a general method of synthesis of tetrabenzoporphyrins has been developed, based on the oxidative aromatization of porphyrins, annealed with nonaromatic hydrocarbon rings.18 This method provides an access to meso-tetraaryl-tetrabenzo-18a,b and tetranaphthoporphyrins (Ar4TBP’s and Ar4TNP’s)18c,d as well as to meso-unsubstituted tetrabenzo- and tetranaphthoporphyrins (TBP’s and TNP’s).18e A recent modification of the method, based on the use of 4,7-dihydroisoindole,18f also opened a pathway to 5,15-diaryl-tetrabenzoporphyrins (Ar2TBP).18g In all these cases, various functional groups can be added to the TBP macrocycles, increasing their solubility and facilitating crystal growth for X-ray structure determination. As a result, originally scarce structural information became more complete, enabling structure/property studies in the family of π-extended porphyrins.
In this work we analyzed photophysical properties of tetrabenzoporphyrins substituted with different numbers of meso-aryl groups. meso-Arylation is known to induce structural deformations in β-substituted porphyrins,19 and, therefore, it can be used to study effects of macrocycle distortions on tetrabenzoporphyrin photophysics. In particular, we focused on fluorescent free-base TBP’s, Ar2TBP’s and Ar4TBP’s and the corresponding phosphorescent Pd complexes, which to us present a special interest as near-infrared optical probes for biological imaging of oxygen.2
Effects of nonplanarity on the photophysics of regular porphyrins were systematically studied by Holten and co-workers,13 who elucidated deactivation pathways of singlet excited 1(π, π*) states (S1) of nonplanar free-bases,13a,b,d,f,h Zn porphyrins,13d Ni porphyrins13c,e and porphyrin dications.13g Formation and deactivation of triplet 3(π, π*) states (T1) in nonplanar porphyrins has also been addressed,20 including recent studies of nonplanar Pt and Pd porphyrins.21 In the present paper we compared tetrabenzoporphyrins ArnTBP (n = 0, 2, 4) with porphyrins annealed with nonaromatic cyclohexeno-rings, i.e. tetracyclohexenoporphyrins (ArnTCHP’s, (n = 0, 2, 4). These were chosen among other β-alkyl-substituted porphyrins because of their close structural similarity to ArnTBP’s. This comparison enabled us to directly evaluate π-extension as a factor influencing photophysics of nonplanar porphyrins.
Results and Discussion
In the beginning we briefly summarize relevant facts about the photophysics and structures of conformationally distorted porphyrins. For detailed discussion see refs.11, 13, 19.
The most common way to induce structural deformations in synthetic porphyrins19 is to position several bulky substituents at the macrocycle periphery. Steric repulsion between the β-pyrrole groups and meso-substituents leads to deviations of the macrocycle from its highly symmetrical D2h (free-base porphyrins) or D4h (metalloporphyrin) form. Macrocycle deformations fall into two main categories: in-plane (ip) and out-of-plain distortions (oop). There are several ways to quantify these distortions; however, the most general method is to decompose the macrocycle structure in the basis of distortions corresponding to the normal vibrational modes. The Normal mode Structural Decomposition (NSD) analysis, developed by Shelnutt and co-workers,22 quantifies not only the total in-plane and out-of-plane displacements (Dip and Doop), but distinguishes between individual contributions of different distortion modes,22,19 which are known to affect the photophysics of nonplanar porphyrins differently.13a,b
Distorted porphyrins typically exhibit mixtures of distortion modes; however, each structure is usually dominated by a single prevalent type of distortion. For example, out-of-plane distorted porphyrins are usually either mostly saddled (B2u-deformation, e.g., octaethyltetraphenylporphyrin (OETPP) or dodecaphenylporphyrin (DPP)) or ruffled (B1u-deformation, e.g., meso-tetra-tert-butylporphyrin (T(t-Bu)P)).23,19
Compared to planar porphyrins, such as octaethylporphyrin (OEP) or tetraphenylporphyrin (TPP), out-of-plane distorted porphyrins exhibit red-shifted and broadened optical spectra. The origin of red shifts in the spectra of nonplanar porphyrins has been extensively discussed in the literature. 11,12f-h In meso-tetraarylporphyrins the red shifts at least in part are caused by an increase in the conjugation between the macrocycle and meso-aryl substituents.10,24 In nonplanar porphyrins, the meso-aryl groups are rotated to a less than 90° angle with respect to the macrocycle average plane.
Nonplanar deformations of porphyrins also strongly affect properties of excited states. Both saddling and ruffling broaden the emission spectra and induce progressively large Stokes shifts with increasing degree of nonplanarity.13 Saddle-shaped freebase porphyrins (e.g., H2OETPP and H2DPP) typically exhibit S1 lifetimes (τfl) of about 0.5–1.0 ns and fluorescence quantum yields (ϕfl) in the order of 0.005 at room temperature.11,13a Ruffled free-base porphyrins have even shorter singlet lifetimes (e.g., τfl = 50 ps for H2T(t-Bu)P) and negligible emission yields (ϕfl < 0.0001).13b For comparison, singlet lifetimes and fluorescence quantum yields of the planar analogues (e.g., H2OEP or H2TPP) are 10–15 ns and 0.1–0.2, respectively, while their internal conversion quantum yields are in the range ϕic ≈ 0.1.13,25,26 Short S1 lifetimes of nonplanar porphyrins were shown to be affiliated with enhancements of both S1 → S0 internal conversion and S1 → T1 intersystem crossing.12c Polar solvents further increase the Stokes shifts, shorten the lifetimes and decrease the fluorescence quantum yields of nonplanar porphyrins,13h while lowering the temperature has the opposite effect, especially in the case of ruffled porphyrins.13d
Holten and co-workers rationalized these findings by considering increased conformational flexibility of nonplanar porphyrins in their S0 and S1 states.13 Broadening of the absorption and fluorescence spectra and large fluorescence Stokes shifts were associated with the existence of multiple excited-state conformations, easily accessible via low-energy out-of-plane vibrations. Some of these conformations can form so-called funnels27 on the S1 potential energy surface, separated from the global energy minimum by small kinetic barriers. Rapid internal conversion occurring in the funnels is due to the small S1–S0 energy gaps and favorable Franck–Condon factors, corresponding to out-of-plane vibrations. In addition, intersystem crossing near the funnels also can be enhanced. On the contrary, fluorescence occurs away from the funnels (e.g., near the global S1 minimum), where the S1–S0 gaps are quite large.
The photophysical properties of tetrabenzoporphyrins are discussed below within the framework of this model.
Free-Base Arn TBP’s and Arn TCHP’s (n = 0, 2, 4)
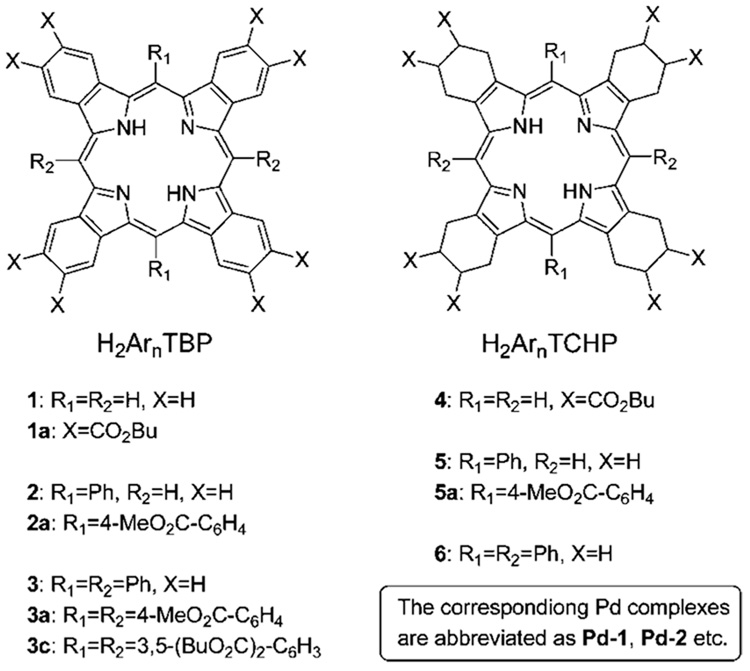
The structures of porphyrins studied in this work are summarized in Chart 1 (see Supporting Information, II for details of synthesis).
CHART 1.
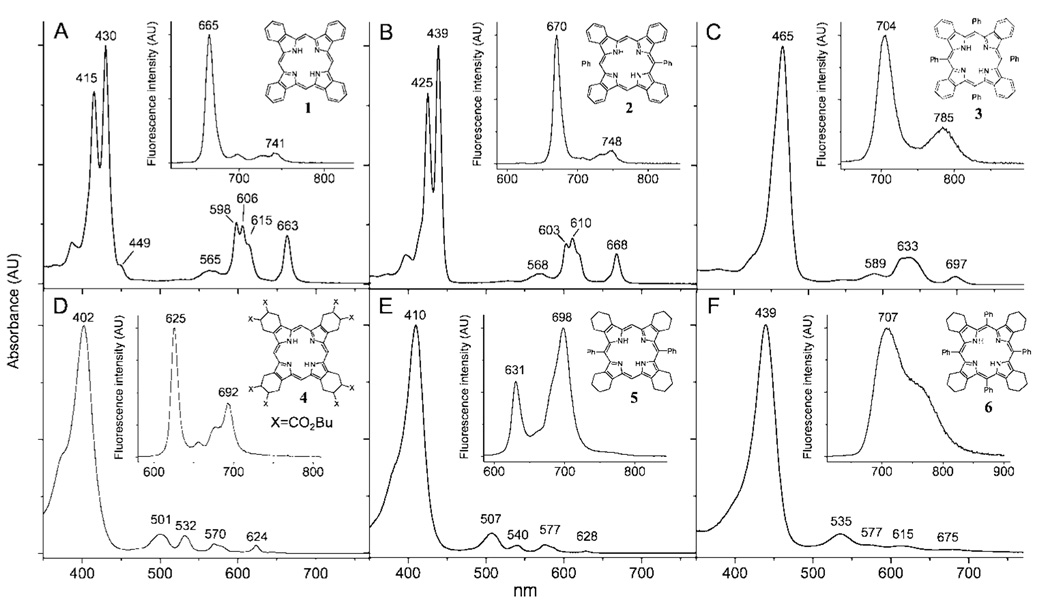
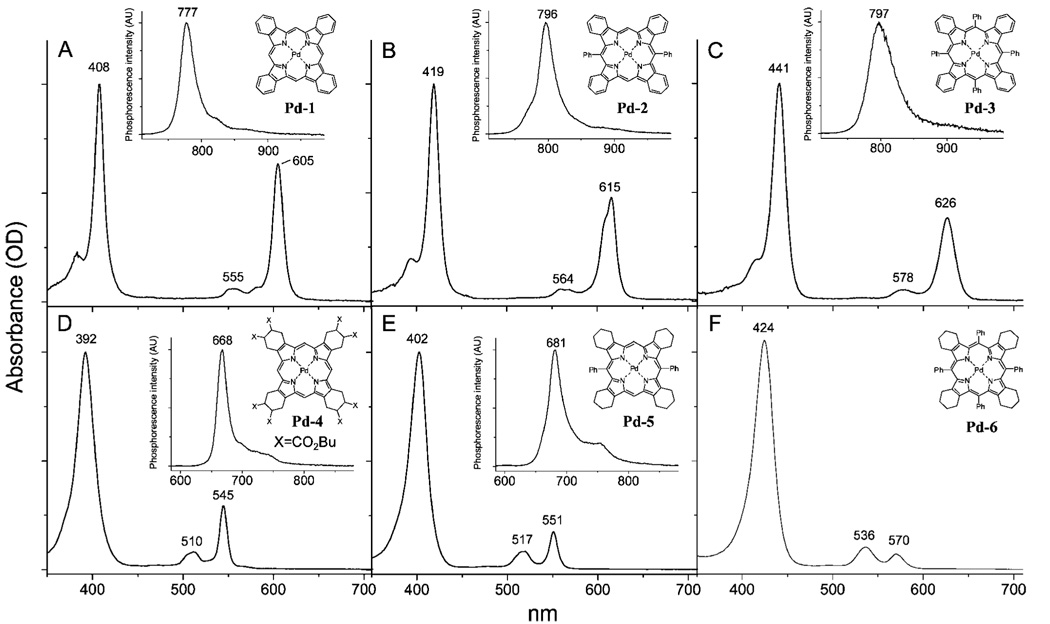
The optical absorption and fluorescence spectra of free-base ArnTBP’s (n = 0, 2, 4) are shown in Figure 1 (upper row). The spectra of the similarly substituted meso-aryl tetracyclohex-enoporphyrins (ArnTCHP’s, n = 0, 2, 4) are shown in the bottom row, and all the photophysical data are summarized in Table 1. All the measurements were performed in air-equilibrated toluene or dimethylacetamide (DMA) (noted) solutions at 295 K, except that: 1) to overcome solubility problems, H2TBP (1) was first dissolved in pyridine, and a drop of this solution was added to about 5 mL of toluene; 2) a small amount of TMEDA (tetramethylethylenediamine) was added to the solution of H2Ph4TCHP (6) to avoid protonation, since H2Ar4TCHP’s are known to possess very high basicities.28 Due to its improved solubility, H2TCHP (4), substituted with eight butoxycarbonyl groups, was used in our experiments instead of unsubstituted H2TCHP. The effect of the butoxycarbonyl groups on the macrocycle π-system in H2TCHP (5) is anticipated to be insignificant, since these substituents are separated from the macrocycle by several σ-bonds. In addition to free-bases shown in Figure 1, porphyrins with peripheral substituents (Chart 1) were used in some of the experiments (vide infra).
Figure 1.
Absorption and fluorescence (insets) spectra of H2TBP (1, A) (H2TBP was first dissolved in pyridine, and a drop of this solution was added to toluene.), H2Ph2TBP (2, B), H2Ph4TBP (3, C) and H2TCHP(CO2Bu)8 (4, D), H2Ph2TCHP (5, E) and H2Ph4TCHP (6, F) (Small amount of TMEDA was added to prevent dication formation.) in toluene at 295 K.
TABLE 1.
Photophysical Properties of Free-Base Porphyrins H2ArnTBP and H2ArnTCHP (n) = 0, 2, 4) in Toluene at 295 K and in Dimethylacetamide (DMA, Noted)a
| free-base porphyrin | fluorescence λmax (λex), nm | Stokes shift (cm−1) | ϕflb | τfl (ns)c |
|---|---|---|---|---|
| TBP | ||||
| 1 | 665, 741 (571) | 45.4 | 0.35 | 9.8 |
| 1 (in DMA) | 663, 740 (571) | 45.6 | 0.47 | – |
| 2 | 670, 746 (571) | 44.7 | 0.34 | 10.3 |
| 2 (in DMA) | 668, 744 (517) | 45.0 | 0.41 | – |
| 2a | 668, 744 (517) | 45.0 | – | 9.6 |
| 3 | 705, 786 (635) | 162.8 | 0.03 | 2.8c |
| 3 (in DMA) | 707, 791 (635) | 223.5 | 0.02 | – |
| 3a | 712, 792 (594) | 220.4 | 0.03 | 2.9c |
| TCHP | ||||
| 4 | 625, 692 (531) | 25.6 | 0.14 | 14.1 |
| 5 | 631, 698 (507) | 75.7 | 0.08 | 11.8 |
| 6 | 707 (535) | 670 | 0.01 | 2.6c |
| 6 (in DMA) | 720 (635) | 882 | <0.01 | – |
A drop of H2TBP solution in pyridine was added to toluene.
Quantum yields were measured relative to the fluorescence of H2TPP in deoxygenated C6H6 (ϕfl = 0.11 in deoxygenated benzene).25 Error in determination of yields is no more than 10%. Purging samples with Ar was shown to increase the fluorescence quantum yields by no more than 5% both in toluene and DMA.
Inhomogeneous multiexponential decays with broad lifetime distributions were detected. Average lifetime value is reported in the Table 1.
The optical absorption spectrum of planar H2TBP (1) (Figure 1A) shows a well-resolved vibronic structure in the Q-band region and a large splitting, 841 cm−1, of the Soret band. This splitting is caused by the strong mixing of the B and Q states and relatively high intensity of the Q-bands.6a,9b,c The lowest energy Q-band (663 nm) is red-shifted by 39 nm (943 cm−1) relative to the corresponding transition of the also planar (vide infra)29 H2TCHP (4) (Figure 1D) due to the effect of extended π-conjugation.9,10
Both H2TBP (1) and H2TCHP (4) exhibit small fluorescence Stokes shifts (45 cm−1 and 25.6 cm−1), indicating rigid planar structures. In good agreement with the previously published data,1b,8c H2TBP (1) shows a very high for porphyrins fluorescence quantum yield (ϕfl = 0.35 in toluene), while the yield of H2TCHP (4) is significantly lower (ϕfl = 0.14), nearly matching that of H2OEP (ϕfl = 0.16).26 The S1 lifetime of H2TBP (1)(9.8 ns) is shorter than that of H2TCHP (4) (14.1 ns). The calculated values of the corresponding rate constants are presented in Table 2. An almost 3.6 times higher value of the radiate rate constant (kr) for H2TBP (1) agrees well with much higher oscillator strength of its Q-band transition (log ε(663 nm) = 4.22 for H2TBP (1) vs log ε(624 nm) = 3.70 for H2TCHP (4)).18e
TABLE 2.
Deactivation Rate Constants for S1 State of Free-Base Porphyrins H2ArnTBP and H2ArnTCHP (n = 0, 2, 4) in Toluene at 295 K
| compound | k = 1/τfl (s−1) | kr (s−1)a | knr (s−1)b |
|---|---|---|---|
| TBP | |||
| H2TBP (1) | 1.02 × 108 | 3.57 × 107 | 6.63 × 107 |
| H2Ph2TBP (2) | 9.71 × 107 | 3.30 × 107 | 6.41 × 107 |
| H2Ph4TBP (3) | 3.57 × 108 | 1.07 × 107 | 3.46 × 108 |
| TCHP | |||
| H2TCHP (4) | 7.09 × 107 | 9.93 × 106 | 6.10 × 107 |
| H2Ph2TCHP (5) | 8.47 × 107 | 6.78 × 106 | 7.80 × 107 |
| H2Ph4TCHP (6) | 3.85 × 108 | 3.85 × 106 | 3.81 × 108 |
kr radiative decay rate constant, calculated as kr = ϕflk, where ϕfl is the fluorescece quantum yield.
knr, nonradiative decay rate constant (knr = kic + kisc), calculated as kr = k − knr.
The quantum yield of the nonradiative decay (ϕnr = ϕic + ϕisc; ϕic, internal conversion; ϕisc, intersystem crossing) for H2TCHP (4) (ϕnr = 0.86) is higher than that for H2TBP (1) (ϕnr = 0.65), but the corresponding nonradiative rate constant (knr = kic + kisc) for H2TCHP (4) is slightly lower (Table 2). The faster nonradiative decay of H2TBP (1) might be due to the higher rate of the internal conversion alone, since the S1–S0 gap for H2TBP (1) is almost 1000 cm−1 narrower than that in H2TCHP (4); however, it may also reflect the altered rate of intersystem crossing.
The optical properties of H2Ph4TBP (3) (Figure 1C) (strongly saddled structure, vide infra) in general follow the pattern predicted by the Holten’s model.13 Upon going from H2TBP (1) to H2Ph4TBP (3), the fine vibronic structure of the Q-band disappears, and both bands shift to the red: Q-band by 736 cm−1, Soret band by 2163 cm−1. Similar trends are seen in the absorption spectrum of also saddled (vide infra) H2Ph4TCHP (6) (Figure 1F) vs planar H2TCHP (4) (Figure 1D). The two latter spectra closely resemble the published spectra of H2OETPP and H2OEP, respectively.13a,26
The changes in the fluorescence of H2Ph4TBP (3) vs that of H2TBP (1) in general also conform to the model (see Table 1). For example, the fluorescence quantum yield of H2Ph4TBP (3) is lower than that of H2TBP (1) by about 10 times, almost matching the change in the pair H2Ph4TCHP (6)/H2TCHP (4). The Stokes shift of H2Ph4TBP (3) (162.8 cm−1) is somewhat lower than that of H2Ph4TCHP (6) (670 cm−1), and upon changing the solvent to more polar DMA it increases by 36% (to 223 cm−1). The Stokes shift for H2Ph4TCHP (6) increases in DMA by almost the same value (to 882 cm−1).
Typical of nonplanar porphyrins,13h the fluorescence quantum yield of H2Ph4TBP (3) decreases in more polar solvent and its emission spectrum broadens (see Supporting Information, III). Interestingly, the fluorescence quantum yields of planar H2TBP (1) increases in DMA to 0.47–a change not normally observed for planar porphyrins.
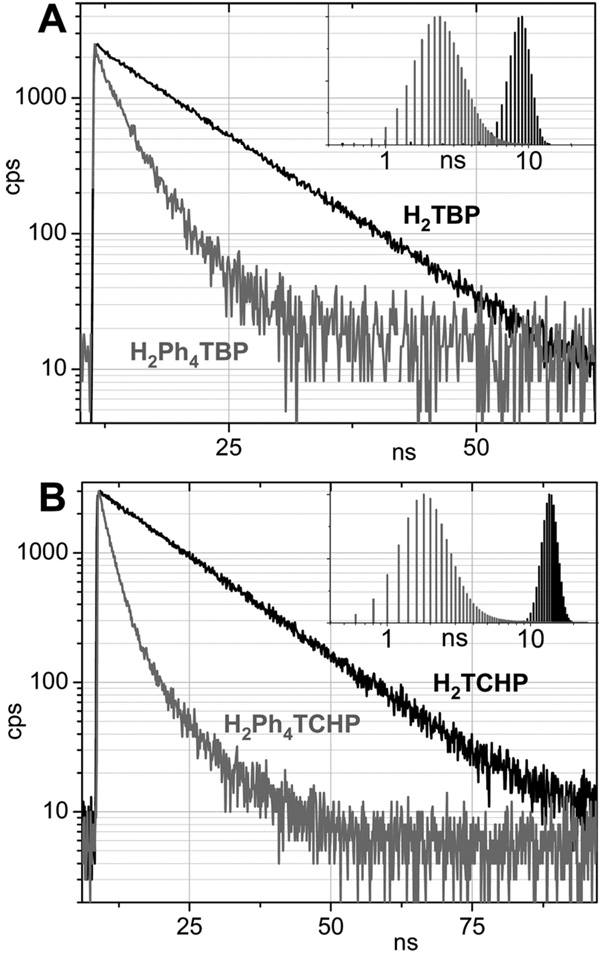
The dynamic fluorescence data for the most part are consistent with the steady-state measurements. The fluorescence decays of both H2Ph4TBP (3) and H2Ph4TCHP (6) reveal broad underlying distributions of lifetimes (recovered by the Maximum Entropy Method (MEM),30 Figure 2), which are indicative of multiple excited-state conformations in agreement with the model. The radiative rate constants (kr) (Table 2) decrease by about 3.3 times on going from H2TBP (1) to H2Ph4TBP (3) and by about 2.6 times on going from H2TCHP (4) to for H2Ph4TCHP (6). Therefore, low S1 lifetimes (see Table 1) of both meso-tetraarylated porphyrins are due to the strong enhancement of the nonradiative processes. Indeed, the corresponding rate constants (knr) increase by 6.2 times from H2TCHP (4) to H2Ph4TCHP (6) and by only slightly less, i.e. 5.2 times, from H2TBP (1) to H2Ph4TBP (3). Notably, the nonradiative rate for H2OETPP is significantly higher, i.e., knr ) 1.5 × 109 s−1,13a than for H2Ph4TCHP.
Figure 2.
Fluorescence decays for H2TBP (1), H2Ph4TBP (3) (A) and H2TBP (4) and H2Ph4TBP (6) (B) in toluene at 296 K and the underlying lifetime distributions, recovered by the Maximum Entropy Method.
The main conclusion drawn from these comparisons is that S1 states of nonplanar porphyrins are significantly less prone to nonradiative deactivation when these porphyrins are annealed with cyclic motifs (as in H2Ph4TCHP (6)) as opposed to β-substituted with pendant alkyl groups (as in H2OETPP). In addition, if the cyclic motifs are in conjugation with the macrocycle (as in H2Ph4TBP (3)), the nonradiative decay appears to weaken even more, although within the measurement error. The differences between nonradiative rates of these three types porphyrins can be explained by taking into account that their S0–S1 gaps change in parallel with their rate constants knr, i.e., H2Ph4TBP ≈ H2Ph4TCHP < H2OETPP. However, the observed effect may also be related to higher rigidity of macrocycles annealed with exocyclic fragments, especially with benzo- rings.
It has been shown that photophysical properties of free-base porphyrins change gradually with increasing degree of the macrocycle nonplanarity.12a,13h Therefore, one could expect that tetrabenzoporphyrins substituted with two, as opposed to four, meso-aryl groups would exhibit optical properties somewhat average to those of H2TBP’s and H2Ar4TBP’s. In contrast to this expectation, the absorption spectrum of H2Ph2TBP (2) (Figure 1B) appears to be nearly identical to that of H2TBP (1), with the Q and Soret bands only slightly shifted to the red, by 5 and 10 nm, respectively.31
The vibronic structure of the Q-band of H2Ph2TBP (2) and the shape of its Soret band are identical to those of H2TBP (1), except for a small extra peak at 449 nm in the spectrum of the latter. This peak is likely to be caused by an impurity. A similar feature was observed in the spectrum of H2TBP8d synthesized by the template condensation method.32 The method is known to yield complex mixtures of products. On the other hand, the spectrum of well-soluble octabutoxy-derivative H2TBP(CO2Bu)8 did not show this extra feature (see Supporting Information, III).
The fluorescence properties of H2TBP (1) and H2Ph2TBP (2) are also very close: small Stokes shifts (~45 cm−1), long fluorescence lifetimes (9.8 and 10.3 ns) and high emission yields (0.35 and 0.34), increasing to 0.47 and 0.41, respectively, in DMA.
Similarities in the optical properties of H2Ph2TBP (2) and H2TBP (1) suggest that the structures of these porphyrins should also be similar, i.e. that H2Ph2TBP (2) should be planar. On the other hand, based on the published data,16,18a,b,28 H2Ph4TBP (3) is expected to be strongly nonplanar, presumably saddled. The question arises as to why the interactions between meso-aryl rings and benzo-rings in H2Ph2TBP (2) do not affect its planarity, whereas the same interactions in H2Ph4TBP cause it to become strongly nonplanar?
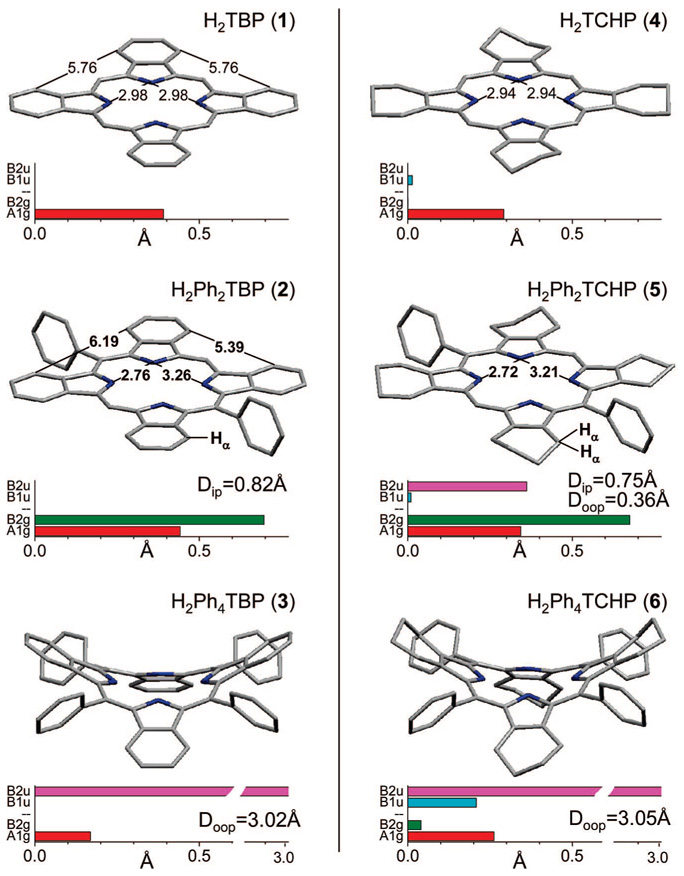
In order to perform structural comparisons we carried out DFT (B3LYP/6-31G(d)) calculations of porphyrins depicted in Figure 1. The calculated structures are shown in Figure 3 together with their normal distortion modes, as revealed by the NSD analysis.22 It should be mentioned that the X-ray structures of H2TBP (1) cations (planar),17 unsubstituted H2TCHP (anomalously planar dication)29 and H2Ph4TBP(CO2Me)8 (saddled)28 were published in the literature, providing the required reference points. However, for the purpose of the comparative analysis, computed structures may in fact present an advantage, since X-ray structures are potentially affected by crystal packing forces, which may or may not affect the macrocycle’s planarity.
Figure 3.
Computed structures (DFT B3LYP/6–31G(d)) of H2PhnTBP’s (left column) and PhnTCHP’s (right column) (n = 0, 2, 4). Distances are in Å. Main distortion modes, recovered by the NSD analysis,22 are shown by the bar graphs. In-plane distortions: A1g, red; B2g, green. Out-of-plane distortions: B1u (ruffling), cyan; B2u (saddling), magenta. (For complete list of modes see Supporting Information, V). Doop and Dip designate total mean-square out-of-plane and in-plane displacements, respectively.
It is seen from the figure that the macrocycle of H2Ph2TBP (2) is as planar as that of H2TBP (1). The total out-of-plain displacements (Doop) are practically zero for both porphyrins. The phenyl rings in H2Ph2TBP (1) are strictly orthogonal to the macrocycle plane and, as a result, their effect on the macrocycle electronic system is minor. Indeed, the absorption bands of H2Ph2TBP (2) are only slightly red-shifted vs those of H2TBP (1), and the substituents in the meso-phenyl rings, e.g., para-methoxycarbonyl groups in porphyrin 2a (Chart 1), have negligible effect on the peak positions (see Supporting Information, III).
As expected, the structure of H2Ph4TBP (3) is strongly nonplanar (Doop = 3.02 Å), with B2u-saddling mode being the dominant deformation. H2Ph4TCHP (6) is also saddled, but with a contribution of ruffling (B1u). B1u-mode is known to especially strongly enhance nonradiative decay of the S1 state,13b suggesting that the steeper increase in knr (vide supra) upon going form H2TCHP (4) to H2Ph4TCHP (6) vs the pair H2TBP (2)/H2Ph4TBP (3), may be associated with this particular distortion. The meso-phenyl rings in H2Ph4TBP (3) are tilted with respect to the macrocycle, and, therefore, are partially drawn into conjugation. As a result, para-methoxycarbonyl groups in 3a shift its spectrum by 4–5 nm to the red relative to H2Ph4TBP (3) (see Supporting Information, III).
The intrinsic asymmetry of free-base porphyrins manifests itself in all the calculated structures by the A1g-distortion, i.e., the elongation in the direction of the axis connecting the two opposite NH nitrogen atoms. In addition to that, H2Ph2TBP (2) and H2Ph2TCHP (5) exhibit strong B2g mode, which is associated with the in-plane rotation of the isoindole fragments, leading to the change in the distances between the opposite pairs of pyrrole N-atoms and yielding more space for the meso-aryl substituents. This rotation appears to lift the steric hindrance between the meso-aryl groups and the α-hydrogen atoms (Hα) of the benzo (H2Ph2TBP (2)) or cyclohexeno rings (H2Ph2TCHP (5)). For example, the edges of the rectangular, drawn through the corresponding α-carbon atoms of benzo-rings in H2Ph2TBP (2), are 5.41 and 6.12 Å (Dip = 0.82Å), while in symmetrical H2TBP (1) the corresponding distances are equal (5.76 Å).
Relief of the steric strain by way of in-plane as opposed to out-of-plane deformation appears to be more energetically favorable, suggesting that it might be a more effective way to preserve the macrocycle aromaticity. Interestingly, the structure of H2Ph2TCHP (5) in addition to being distorted in-plane (Doop = 0.75 Å) also shows a small contribution of saddling (B2u) (Doop = 0.36 Å). This distortion is completely absent in the structure of H2Ph2TBP (3), even though the distance between the HR-atom of the benzo-ring and the plane of the meso-phenyl ring (Figure 3) is actually shorter than the average distance between the meso-phenyl ring and the Hα-atoms of the cyclohexeno-ring in H2Ph2TCHP (5). Higher resistance of H2Ph2TBP (2) to nonplanar distortion may be another manifestation of the increased rigidity of TBP macrocycle. In this regard, in a recent theoretical study24 the propensity of meso-aryl-substituted porphyrins to undergo nonplanar deformations was considered to be an effect of two counterbalancing forces: one acting to preserve planarity and aromaticity and another striving to bring the meso-aryl group into conjugation with the tetrapyrrole system. From this point of view, the former force in H2Ph2TBP (2) appears to be stronger than in H2Ph2TCHP (5).
Remarkably, even a small out-of-plane deformation of H2Ph2TCHP (5) immediately reveals itself in its photophysical behavior. The absorption bands of H2Ph2TCHP (5) (Figure 2E) are broadened and red-shifted relative to H2TCHP (4), the fluorescence spectrum of H2Ph2TCHP (5) has a somewhat different shape, and its fluorescence yield (0.08) and lifetime (11.8 ns) are considerably lower than those of H2TCHP (4). The nonradiative rate constant for H2Ph2TCHP (5) (Table 2) increases by about 30% relative to knr of H2TCHP (4), and the radiative constant decreases by about 50% relative to kr of H2TCHP (4). In contrast to these changes, the in-plane distortion in H2Ph2TBP (2) has practically no influence on the S1 decay parameters optical properties as compared to H2TBP (1). These results strongly suggest that out-of-plane deformations play the dominant role in the excited-state properties of distorted porphyrins, whereas the role of in-plane deformations is insignificant.
To the best of our knowledge, the X-ray structure of H2Ph2TCHP (2) is not known. To verify that the computations adequately represented its structural features, we compared the calculated (B3LYP/6-31G(d)) structure of another 5,15-diphenyl-octalkylporphyrin with its published X-ray structure33 (see Supporting Information, V). The results confirmed that all the main features of the experimental structure were adequately reproduced by the calculations. It also should be mentioned, that most of the published X-ray structures of free-base β-alkyl-5,15-diarylporphyrins (obtained from Cambridge Structural Database) (see ref 33 and Supporting Information, IV) reveal certain degree of out-of-plane deformations, although in some cases these could be caused by structural elements (e.g., strapping substituents) capable of inducing out-of-plane distortions independent of meso-aryl/β-alkyl interactions.
The experimental evidence of virtually ideal planarity of H2Ar2TBP as well as of its strong in-plane distortion was obtained by X-ray crystallography (Figure 4).
Figure 4.
X-ray crystal structure of H2Ar2TBP (Ar = 3,5-tBu2C6H3) and its NSD analysis. Distances are in Å. Hydrogen atoms are omitted for clarity.
The crystal structure of H2Ar2TBP (Ar = 3,5-tBu2-C6H3) shows the essentially flat tetrapyrrole skeleton, practically orthogonal meso-aryl rings and significant A1g and B2g deformations (Dip = 0.67 Å). The N4-core is asymmetrical with adjacent edges of 3.18 and 2.76 Å. Overall, the structure is in excellent agreement with the calculations.
It follows from the above results that the spectroscopic signature of meso-unsubstituted TBP is as a sensitive indicator of planarity of TBP-based systems. For example, it is likely that the structures of H2ArTBP and 5,10-H2Ar2TBP, reported by Senge and Bischoff,34 as well as the structure of H2ArTBP (Ar = 4-MeO2C–C6H4), reported by Berova et al.,35 are planar. Similarly, meso-alkenyl-TBP’s, synthesized by Ono et al.,36 also are expected to be planar.
In summary of this section, planar free-base tetrabenzoporphyrins are strongly fluorescent and their radiative rate constants and quantum yields are 3–4 times higher that those of regular planar porphyrins. Nonplanar distortions affect the excited-state properties of free-base tetrabenzoporphyrins in a manner consistent with the model proposed for regular nonplanar porphyrins.13 In contrast to out-of-plane deformations, in-plane deformations, induced in tetrabenzoporphyrins by 5,15-diaryl-substitution, have practically no effect on their excited-state properties of tetrabenzoporphyrins.
PdArnTBP’s and PdArnTCHP (n = 0, 2, 4)
In Pd porphyrins, S1(π, π*) → T1(π, π*) intersystem crossing occurs with almost unity quantum yield.37 Because (d, d) transitions of Pd(II) ion are higher in energy than T1(π, π*) states, planar Pd porphyrins (e.g., PdOEP, PdTPP) strongly phosphoresce in solutions at room temperature.38
Recently, Zenkevich and co-workers21 showed that saddling (B2u) deformations in regular Pd porphyrins, such as PdOETPP,39,40 lead to a drastic decrease in their phosphorescence, whereas at low temperatures the phosphorescence is considerably regained (ϕphos=0.25 for PdOETPP at 77 K).21b Having that in mind, we set out to examine how meso-aryl substituents in PdArnTBP’s (n = 0, 2, 4) affect properties of their T1 states. As in the case of free-base porphyrins, Pd complexes of structurally related ArnTCHP’s (n = 0, 2, 4) (Chart 1) were used for comparison.
Structural information on Pd porphyrins is not as easily obtainable by ab initio methods as for free-bases. Nevertheless, previously published structural data on various types of metallated planar and nonplanar porphyrins allowed us to make the following conjectures. Similar to PdOEP,41 PdTCHP (Pd-4) is expected to have unperturbed planar geometry. Literature data on Cu, Co and Zn tetrabenzoporphyrins4b-d suggest that PdTBP (Pd-1) also has planar geometry. Similar to Cu, Zn and Ni OETPP’s,40 PdPh4TCHP (Pd-6) is expected to be saddled; while based on the existing X-ray data on metallated 5,15-diaryl β-octaalkylporphyrins,42 PdPh2TCHP is expected to be predominantly ruffled.
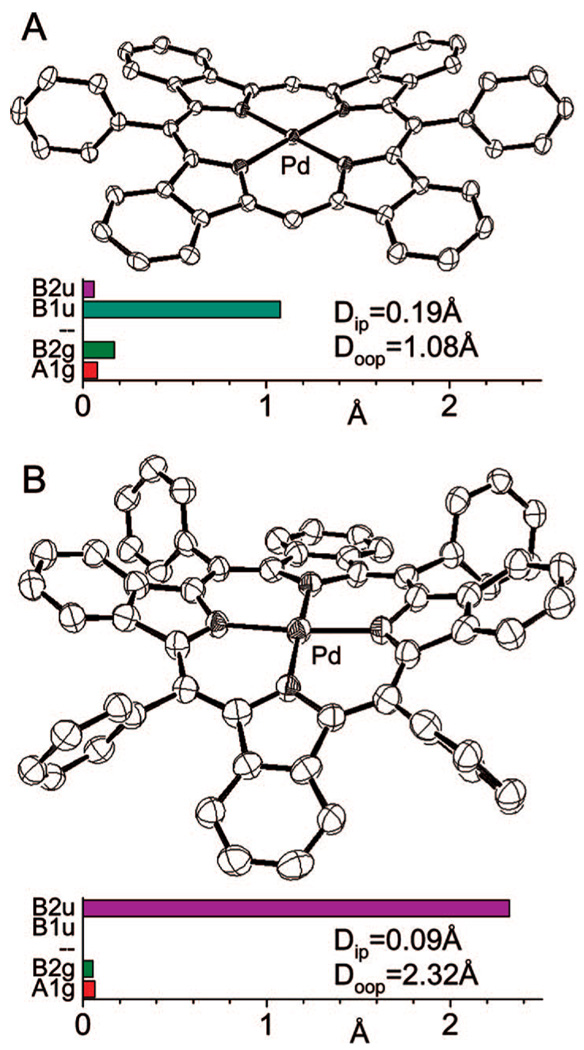
By analogy with regular 5,15-diarylporphyrins, we expected that metalation of Ar2TBP’s would change the geometry of the N4-core and, as a result, would lead to a closer contact between benzo and meso-aryl rings. Metallated β-alkyl 5,15-diarylporphyrins typically undergo nonplanar B1u-type ruffling deformation,42 and the same was expected for PdPh2TBP (Pd-2). The X-ray structure of PdPh2TBP (Pd-2) (Figure 5 A) confirmed this expectation. The predominant mode of its out-of-plane distortion (Doop = 1.08 Å) is indeed ruffling (B1u) with a small contribution of saddling (B2u), while the in-plane distortion is much smaller (Dip = 0.19 Å) than in the corresponding free-base H2Ph2TBP (2) (Figure 1 and Figure 4).
Figure 5.
X-ray crystal structures of PdPh2TBP (Pd-2) (A) and PdAr4TBP (Ar = 3,5-(BuO2C)2C6H3) (B) and their NSD analyses. Hydrogen atoms and substituents in the meso-aryl rings in PdAr4TBP (B) are not shown for clarity (see Supporting Information, IV for complete structure).
PdAr4TBP (Ar = 3,5-(BuO2C)2C6H3) (Figure 5B) is, on the contrary, strongly saddled. The value of the total out-of-plane deformation in PdAr4TBP (Doop = 2.32 Å) is similar to that determined for the earlier published structure of ZnPh4TBP·THF (Doop = 2.35 Å),16 but somewhat lower than in PtPh4TBP (Doop = 2.83 Å)4e and especially in NiPh4TBP(CO2Me)8 (Doop = 3.43 Å).18b These differences might be caused by the differences in ionic radia of the metals or by the crystal packing forces or by both.
The optical spectra of PdPhnTBP’s and PdPhnTCHP’s (n = 0, 2, 4) are shown in Figure 6, and the photophysical data are summarized in Table 3. The phosphorescence quantum yields and decays were measured in Ar-purged DMA solutions at 295 K, and the phosphorescence decays were found to be purely single-exponential. Porphyrin triplet states are extremely sensitive to various quenching processes, and that makes it difficult to compare the data on the phosphorescence of Pd porphyrins measured in different laboratories using different solvents and different deoxygenation methods. Therefore, in our experiments we attempted to maintain identical conditions (solvent, Ar purity, control of air displacement) for all the samples measured; however, the reported lifetimes and quantum yields should not be considered absolute.
Figure 6.
Absorption and phosphorescence (insets) spectra of PdTBP (Pd-1, A), PdPh2TBP (Pd-2, B), PdPh4TBP (Pd-3, C) and PdTCHP(CO2Bu)8 (Pd-4, D), PdPh2TCHP (Pd-5a, E) and PdPh4TCHP (6, F) in DMA at 295 K.
TABLE 3.
Photophysical Properties of PdArnTBP and PdArnTCHP (n = 0, 2, 4) in Deoxygenated DMA at 295 K
| Pd porphyrin | phosphorescence λmax (λex), nm | ϕphosa | τphos (µs)b |
|---|---|---|---|
| TBP | |||
| Pd-1 | 777 (605) | 0.13 | 399 |
| Pd-1a | 770 (615) | 0.35 | 496 |
| Pd-2 | 796 (615) | 0.15 | 423 |
| Pd-3 | 797 (626) | 0.08 | 258 |
| TCHP | |||
| Pd-4 | 668 (545) | 0.45 | 1118 |
| Pd-5 | 681 (550) | 0.02 | 97 |
| Pd-6 | – | –c | –c |
Measured relative to the fluorescence of H2TPP in deoxygenated C6H6 (ϕfl = 0.11).25 Error in determination of yields is no more than 10%.
Phosphorescence decays were nearly ideally single-exponential, as revealed by the Maximum Entropy analysis.
No signal could be detected.
Unsubstituted PdTBP (Pd-1) has very low solubility and shows tendency to aggregation. To account for possible effects of aggregation, a well-soluble PdTBP derivative substituted with eight butoxycarbonyl groups, PdTBP(CO2Bu)8 (Pd-1a), was also studied. It should be mentioned that insertion of Pd into ArnTCHP’s often leads to the appearance of extra bands above 600 nm in the absorption spectra. These bands are most likely associated with partially oxidized products, i.e. mono-, di- and tribenzoporphyrins. Special care needs to be taken to avoid contamination by these side products, and only freshly prepared and purified samples of PdPh2TCHP (Pd-5) must be used in photophysical measurements.
The changes in the ground-state absorption spectra of PdArnTBP’s and PdArnTCHP’s upon increase in n from 0 to 4 resemble the trends observed for the corresponding free-bases: red-shifting, broadening and, in the case of PdArnTCHP’s, gradual change in the relative intensity of Qx vs Qy band. As expected, the spectrum of PdAr4TCHP (Pd-6) was found to be similar to the published spectrum of PdOETPP.21 Noteworthy, there is a slight splitting of the Q-band (λmax = 615 nm) of PdAr2TBP (Pd-2), a feature absent in the spectrum of PdTBP (Pd-1).
The phosphorescence spectra in general follow the changes in the absorption. One exception is that the main peak of the phosphorescence of ruffled PdPh2TBP (Pd-2) (λmax = 796 nm) is located almost at the same position as that of saddled PdPh4TBP (Pd-3) (λmax = 797 nm), while the Q-bands shift gradually in the order PdTBP (Pd-1) → PdPh2TBP (Pd-2) → PdPh4TBP (Pd-3). As a result, the phosphorescence Stokes shift of PdPh4TBP (Pd-3) is less by more than 200 cm−1 compared to the two less substituted porphyrins.
The major difference between PdArnTBP’s and PdArnTCHP’s is in how their phosphorescence quantum yields and lifetimes respond to the changes in the macrocycle nonplanarity. Just like in the case of PdOETPP,21 saddling deformation in PdAr4TCHP (Pd-6) leads to the complete loss of its emissivity. In contrast, saddled PdAr4TBP (Pd-3) phosphoresces with quite high quantum yield (ϕphos = 0.08), which is only 1.6 lower than that of planar PdTBP (Pd-1) (ϕphos = 0.13). It is possible that the phosphorescence yield and lifetime of PdTBP (Pd-1) are affected (lowered) by aggregation. Indeed, the phosphorescence of much better soluble octabutoxycarbonyl-derivative Pd-1a was found to be significantly stronger (ϕphos = 0.35); but still, the difference between the quantum yields of Pd-1a and PdAr4TBP (Pd-3) is not nearly as drastic as for PdPh4TCHP (Pd-6) vs PdTCHP (Pd-4).
Both radiative and nonradiative rate constants for PdTBP (Pd-1a) are somewhat higher than those of PdTCHP (Pd-4) (Table 4). In response to nonplanar deformation, the radiative rate in PdPh4TBP (Pd-3) decreases slightly relative to that of PdTBP (Pd-1a), resembling the trend in the behavior of the corre sponding free-bases. Remarkably, upon going from PdTBP (Pd-1a) to PdPh4TBP (Pd-3) the nonradiative rate rises also by only about 2.7 times, whereas in PdPh4TCHP (Pd-6) knr is apparently so large, that this porphyrin completely looses its phosphorescence.
TABLE 4.
Deactivation Rate Constants for T1 state of PdArnTBP and PdArnTCHP (n = 0, 2, 4) in Deoxygenated DMA at 295 K
| compound | k = 1/τphos (s−1) | kr (s−1)a | knr (s−1)b |
|---|---|---|---|
| TBP | |||
| PdTBP (Pd-1) | 2.51 × 103 | 3.26 × 102 | 2.18 × 103 |
| Pd-1a | 2.02 × 103 | 7.06 × 102 | 1.31 × 103 |
| PdPh2TBP (Pd-2) | 2.36 × 103 | 3.55 × 102 | 2.00 × 103 |
| PdPh4TBP (Pd-3) | 3.88 × 103 | 3.10 × 102 | 3.57 × 103 |
| TCHP | |||
| PdTCHP (4) | 8.94 × 102 | 4.02 × 102 | 4.92 × 102 |
| PdPh2TCHP (Pd-5) | 1.03 × 104 | 2.06 × 102 | 1.01 × 104 |
kr, radiative decay rate constant, calculated as kr = ϕphosk, where ϕphos is the phosphorescence quantum yield.
knr, nonradiative decay rate constant (knr = kic + kisc), calculated as kr = k − knr.
For PdPh2TBP (Pd-2) and PhPh2TCHP (Pd-5) the differences are also quite striking. Upon going from planar PdTCHP (Pd-4) to presumably ruffled PdPh2TCHP (Pd-5), the phosphores cence quantum yield decreases by as much as 22.5 times, while in the case of PdTBP (Pd-1) vs PdPh2TBP (Pd-2) it seems to even slightly increase. Again, this can be related to the partial aggregation of PdTBP (Pd-1); but even in comparison with octabutoxycarbonyl-derivative Pd-1a, the quantum yield of PdPh2TBP (Pd-2) is only 4 times lower. Notably, this decrease in the quantum yield is largely due to the decrease in the radiative rate constant, while the increase in the nonradiative rate is only 1.5 times.
Overall, the photophysical data clearly show that π-extension strongly affects triplet state properties of Pd porphyrins. Nonplanarity, especially saddling, greatly promotes nonradiative decay of the T1 states of regular nonplanar Pd porphyrins, rendering them completely nonemissive. On the contrary, either saddling (B2u) or ruffling (B1u) deformations affect the T1 states of tetrabenzoporphyrins only slightly; both PdAr2TBP’s and PdAr4TBP’s remain strongly phosphorescent at ambient temperatures.
Disappearance of the phosphorescence in regular nonplanar porphyrins in principle can be caused by strong enhancement of S1 → S0 internal conversion, leading to the reduction in the yield of the T1 state. However, literature data suggest that the enhancement of internal conversion in nonplanar porphyrins occurs in parallel with the enhancement of the intersystem crossing.12c In the presence of Pd, S1 → T1 decay should become even faster, and, as a result, it is likely to reach the rate at least as high as that of the internal conversion. Therefore, the key factor influencing phosphorescence of nonplanar Pd porphyrins is most likely the rate of T1 → S0 decay.
The rates of radiative T1 → S0 transitions are somewhat higher for planar PdTBP’s than for planar PdTCHP’s, and upon nonplanar deformations they slightly decrease (vide supra). The nonradiative T1 → S0 rates increase sharply in response to non-planar deformations in PdTCHP’s, but only slightly in PdTBP’s. The fact that the phosphorescence of nonplanar Pd porphyrins, e.g. of PdOETPP, is regained at 77 K21 suggests that the enhancement of the nonradiative decay is associated with vibrational activity. Thus, vibrations greatly enhance T1 → S0 intersystem crossing in PdAr4TCHP’s, but have only small effect on the intersystem crossing in PdAr4TBP’s.
The rate of T1 → S0 intersystem crossing is determined by the combination of three factors: spin–orbit coupling due to the heavy atom effect; interactions of Pd d orbitals with T1 and S0 states, resulting in their partial mixing; and Franck–Condon factors associated with the participating vibrational levels of the states involved in the transition.21,43 It is likely that some of the low-energy out-of-plane vibrations near the equilibrium point on the T1 potential energy surface facilitate spin–orbit coupling and enhance the nonradiative decay.43 Even if favorable for intersystem crossing conditions are not met near the equilibrium, there are likely to be conformers (local minima) on the T1 surface characterized by higher magnitudes of spin–orbit coupling. High conformational flexibility of nonplanar Pd porphyrins is likely to be retained in their T1 states,20 and the conformers with increased magnitude of spin–orbit-coupling can be viewed as analogs of “funnels” proposed for nonradiative deactivation of S1 states.13 Considering low T1 → S0 radiative rates, the molecule has enough time to visit all these local minima, including the funnels, and undergo efficient nonradiative T1 → S0 decay.
The described above experiments suggest that the conformations characterized by high rates of intersystem crossing are not as easily attainable by PdAr4TBP’s as by PdAr4TCHP’s. On the one hand, this can be a result of lower flexibility of the PdAr4TBP system and, therefore, its limited ability to traverse the T1 energy surface. However, the data on free-base Ar4TBP’s (vide supra) suggest that even if there is an increase in the rigidity of the TBP macrocycle compared to that of TCHP, the difference is not large.
Alternatively, it is possible that for all possible conformers of PdAr4TBP’s the magnitude of spin–orbit coupling is never as high as for some selected conformers of PdAr4TCHP’s. Should this indeed be true, the reason for much weaker effects of nonplanar deformations on the phosphorescence of PdAr4TBP’s vs PdAr4TCHP’s could be explained by the difference between the spatial distributions of b1 (HOMO) and e (LUMO) orbitals of tetrabenzoporphyrins vs regular porphyrins.10 Indeed, b1 HOMO and eg LUMO orbitals in Zn and Pd Ph4TBP’s were shown to be partially moved out from the pyrroles into benzo-rings,10 away from the metal center. As a result, interactions of these orbitals with d-orbitals of Pd even in out-of-plane distorted macrocycle may be much weaker than in PdAr4TCHP’s.
An interesting observation was made that the triplet lifetimes of Pd porphyrins (both TBP’s and TCHP’s) are affected by ruffling much less than by saddling. Noteworthy, the structures of highly phosphorescent PdTPP44 and Pd meso-tetracarboxyphenylporphyrin45 apparently are also quite ruffled. In the case of S1 states of free-base porphyrins, however, the situation is exactly the opposite, i.e. nonradiative decay in ruffled porphyrins is much faster than in saddled porphyrins (vide supra). In fact, the very fast internal conversion of ruffled free-base porphyrins13b is perhaps one of strongest indications of the existence of funnels on their potential energy surfaces. Either analogous minima do not exist on T1 surfaces of ruffled Pd porphyrins, or spin–orbit coupling in these minima is inefficient. A separate study will be required to understand this phenomenon.
Conclusion
The presented data provide initial experimental framework for understanding mechanisms of photophysical processes occurring in planar and nonplanar π-extended porphyrins. The performed phenomenological analysis can be useful for building empirical correlations between structures and photophysical properties of these molecules and forming the initial picture; however, comprehensive computational work, such as recently performed on free-base porphin and its Mg and Zn complexes,43will be required to fully delineate the effects of the π-extension on the excited properties of nonplanar tetrapyrroles.
From practical point of view, the described photophysical measurements allowed identification of a subset of π-extended porphyrins, namely 5,15-diaryltetrabenzoporphyrins, which combine powerful near-infrared absorption and strong emission from singlet and triplet states with ease of derivatization via substitution in the meso-aryl rings. Convenient functionalization not leading to alterations in photophysical properties is the key to many potential applications of these chromophores, such as biomedical imaging and PDT.
Experimental Section
All porphyrins used in this study were synthesized and purified as described previously,18 except for complexes Pd-4, Pd-5, Pd-5a and Pd-6. The latter were obtained by refluxing the corresponding free-base porphyrins with Pd(OAc)2 in acetic acid and purified by column chromatography (see Supporting Information, II for details).
Absorption and emission spectra, fluorescence and phosphorescence quantum yields and lifetimes were measured using standard methods (see Supporting Information, I). Complete X-ray crystal structures are available in the Cambridge Crystallographic Data Centre (CCDC): 665410, 681173, 687685. For details of DFT calculations see Supporting Information, V. NSD analysis22 was performed using an online Java applet available at the Web site of Prof. John A. Shelnutt: http://jasheln.unm.edu/jasheln/content/nsd/NSDengine/start.htm (see Supporting Information, V for details).
Acknowledgment
Support of the grants EB007279 and HL081273 from the NIH USA and RFBR-04-03-32650 and RFBR-07-03-01121 from the Russian Foundation of Basic Research is gratefully acknowledged. We thank Dr. Thomas Troxler (Penn RLBL, NIH P41-RR001348) for assistance with fluorescence lifetime measurements, Drs. K. Lutsenko (INEOS RAN) and Patrick Carroll (Penn) for X-ray structure determination and Prof. Craig Medforth (University of New Mexico) for providing structural data on 5,15-diaryl-β-octaalkylporphyrins. SAV thanks Prof. Robin M. Hochstrasser for invaluable discussions.
Footnotes
Supporting Information Available: Description of synthesis, details of photophysical experiments, computations and X-ray structure determination, as well as characterization data for the new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.a Ehrenberg B, Malik Z, Nitzan Y, Ladan H, Johnson FM, Hemmi G, Sessler JL. Lasers Med. Sci. 1993;8:197. [Google Scholar]; b Gross E, Ehrenberg B, Johnson FM. Photochem. Photobiol. 1993;57:808. doi: 10.1111/j.1751-1097.1993.tb09215.x. [DOI] [PubMed] [Google Scholar]; c Lavi A, Johnson FM, Ehrenberg B. Chem. Phys. Lett. 1994;231:144. [Google Scholar]; d Yasuike M, Yamaoka T, Ohno O, Sakuragi M, Ichimura K. Inorg. Chim. Acta. 1991;184:191. [Google Scholar]; e Friedberg JS, Skema C, Baum ED, Burdick J, Vinogradov SA, Wilson DF, Horan AD, Nachamkin I. J. Antimicrob. Chemother. 2001;48:105. doi: 10.1093/jac/48.1.105. [DOI] [PubMed] [Google Scholar]; f Kepczynski M, Pandian RP, Smith KM, Ehrenberg B. Photochem. Photobiol. 2002;76:127. doi: 10.1562/0031-8655(2002)076<0127:dlbcop>2.0.co;2. [DOI] [PubMed] [Google Scholar]; g Ongayi O, Gottumukkala V, Fronczek FR, Vicente MGH. Bioorg. Med. Chem. Lett. 2005;15:1665. doi: 10.1016/j.bmcl.2005.01.043. [DOI] [PubMed] [Google Scholar]; h Gottumukkala V, Ongayi O, Baker DG, Lomax LG, Vicente MGH. Bioorg. Med. Chem. 2006;14:1871. doi: 10.1016/j.bmc.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 2.a Vinogradov SA, Wilson DF. J. Chem. Soc. Perkin Trans. 1995;2:103–111. [Google Scholar]; b Vinogradov SA, Lo L-W, Jenkins WT, Evans SM, Koch C, Wilson DF. Biophys. J. 1996;70:1609. doi: 10.1016/S0006-3495(96)79764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Finikova OS, Galkin A, Rozhkov VV, Cordero M, Hägerhäll C, Vinogradov SA. J. Am. Chem. Soc. 2003;125:4882. doi: 10.1021/ja0341687. [DOI] [PubMed] [Google Scholar]; d Rietveld IB, Kim E, Vinogradov SA. Tetrahedron. 2003;59:3821. [Google Scholar]; e Apreleva SV, Wilson DF, Vinogradov SA. Appl. Opt. 2006;45:8547. doi: 10.1364/ao.45.008547. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Wilson DF, Lee WMF, Makonnen S, Finikova O, Apreleva S, Vinogradov SA. J. Appl. Physiol. 2006;101:1648. doi: 10.1152/japplphysiol.00394.2006. [DOI] [PubMed] [Google Scholar]; g Finikova OS, Troxler T, Senes A, DeGrado WF, Hochstrasser RM, Vinogradov SA. J. Phys. Chem. A. 2007;111:6977. doi: 10.1021/jp071586f. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Mik EG, Johannes T, Ince C. Amer J. Physiol. Renal Physiol. 2008;294:F676. doi: 10.1152/ajprenal.00569.2007. [DOI] [PubMed] [Google Scholar]
- 3.a Baluschev S, Yakutkin V, Miteva T, Avlasevich Y, Chernov S, Aleshchenkov S, Nelles G, Cheprakov A, Yasuda A, Mullen K, Wegner G. Angew. Chem., Int. Ed. 2007;46:7693. doi: 10.1002/anie.200700414. [DOI] [PubMed] [Google Scholar]; b Baluschev S, Yakutkin V, Wegner G, Miteva T, Nelles G, Yasuda A, Chernov S, Aleshchenkov S, Cheprakov A. App. Phys. Lett. 2007;90:181103. [Google Scholar]; c Baluschev S, Yakutkin V, Miteva T, Wegner G, Roberts T, Nelles G, Yasuda A, Chernov S, Aleshchenkov S, Cheprakov A. New J. Phys. 2008;10:1. [Google Scholar]
- 4.a Hanack M, Zipplies T. J. Am. Chem. Soc. 1985;107:6127. [Google Scholar]; b Guha S, Kang K, Porter P, Roach JF, Remy DE, Aranda FJ, Rao DVGLN. Opt. Lett. 1992;17:264. doi: 10.1364/ol.17.000264. [DOI] [PubMed] [Google Scholar]; c Chen PL, Tomov IV, Dvornikov AS, Nakashima M, Roach JF, Alabran DM, Rentzepis PM. J. Phys. Chem. 1996;100:17507. [Google Scholar]; d Brunel M, Chaput F, Vinogradov SA, Campagne B, Canva M, Boilot JP, Brun A. Chem. Phys. 1997;218:301. [Google Scholar]; e Plagemann B, Renge I, Renn A, Wild UP. J. Phys. Chem. A. 1998;102:1725. [Google Scholar]; f Borek C, Hanson K, Djurovich PI, Thompson ME, Aznavour K, Bau R, Sun YR, Forrest SR, Brooks J, Michalski L, Brown J. Angew. Chem., Int. Ed. 2007;46:1109. doi: 10.1002/anie.200604240. [DOI] [PubMed] [Google Scholar]
- 5.a Phillips TE, Hoffman BM. J. Am. Chem. Soc. 1977;99:7734. [Google Scholar]; b Martinsen J, Pace LJ, Phillips TE, Hoffman BM, Ibers JA. J. Am. Chem. Soc. 1982;104:83. [Google Scholar]; c Liou K, Ogawa MY, Newcomb TP, Quirion G, Lee MH, Poirier M, Halperin WP, Hoffman BM, Ibers JA. Inorg. Chem. 1989;28:3889. [Google Scholar]; d Liou KY, Newcomb TP, Heagy MD, Thompson JA, Heuer WB, Musselman RL, Jacobsen CS, Hoffman BM, Ibers JA. Inorg. Chem. 1992;31:4517. [Google Scholar]; e Kobayashi N, Nevin WA, Mizunuma S, Awaji H, Yamaguchi M. Chem. Phys. Lett. 1993;205:51. [Google Scholar]; f Murata K, Liou KK, Thompson JA, McGhee EM, Rende DE, Ellis DE, Musselman RL, Hoffman BM, Ibers JA. Inorg. Chem. 1997;36:3363. doi: 10.1021/ic961490s. [DOI] [PubMed] [Google Scholar]; g Aramaki S, Sakai Y, Ono N. Appl. Phys. Lett. 2004;84:2085–2087. [Google Scholar]; h Shea PB, Johnson AR, Ono N, Kanicki J. IEEE Trans. Electron Devices. 2005;52:1497. [Google Scholar]
- 6.a Gouterman M. J. Mol. Spectrosc. 1961;6:138. [Google Scholar]; b Bajema L, Gouterman M, Rose C. J. Mol. Spectrosc. 1971;39:421. [Google Scholar]; c Edwards L, Gouterman M, Rose CB. J. Am. Chem. Soc. 1976;98:7638. doi: 10.1021/ja00440a031. [DOI] [PubMed] [Google Scholar]; d Aartsma TJ, Gouterman M, Jochum C, Kwiram AL, Pepich BV, Williams LD. J. Am. Chem. Soc. 1982;104:6278. [Google Scholar]
- 7.a Sevchenko AN, Solovev KN, Shkirman SF, Kachura TF. Doklady Akademii Nauk SSSR (Russ) 1965;161:1313. [Google Scholar]; b Tsvirko MP, Sapunov VV, Soloviyev KN. Optics Spectrosc.(Russ) 1973;34:1094. [Google Scholar]
- 8.a Vogler A, Kunkely H. Inorg. Chim. Acta. 1980;44:L211. [Google Scholar]; b Vogler A, Kunkely H, Rethwisch B. Inorg. Chim. Acta. 1980;46:101. [Google Scholar]; c Koehorst RBM, Kleibeuker JF, Tjeerd JS, de Bie DA, Geursten B, Henrie RN, van der Plas HC. J. Chem. Soc. 1981:1005. [Google Scholar]; d Ehrenberg B, Johnson FM. Spectrochim. Acta. 1990;46a:1521. [Google Scholar]; e Madoka Y, Tsiguo Y, Osami O, Kunihiro I, Hisayuki M, Masako S. Inorg. Chim. Acta. 1991;185:39. [Google Scholar]
- 9.a Kobayashi N, Konami H. J. Porphyrins Phthalocyanines. 2001;5:233. [Google Scholar]; b Rosa A, Ricciardi G, Baerends EJ, van Gisbergen SJA. J. Phys. Chem. A. 2001;105:3311. [Google Scholar]; c Nguyen KA, Pachter R. J. Chem. Phys. 2001;114:10757. [Google Scholar]; d Zamyatin AV, Soldatova AV, Rodgers MAJ. Inorg. Chim. Acta. 2007;360:857. [Google Scholar]
- 10.Rogers JE, Nguyen KA, Hufnagle DC, McLean DG, Su WJ, Gossett KM, Burke AR, Vinogradov SA, Pachter R, Fleitz PA. J. Phys. Chem. A. 2003;107:11331. [Google Scholar]
- 11.Shelnutt JA, Song XZ, Ma JG, Jia SL, Jentzen W, Medforth CJ. Chem. Soc. Rev. 1998;27:31. [Google Scholar]
- 12.a Medforth CJ, Dolores Berber M, Smith KM, Shelnutt JA. Tetrahedron Lett. 1990;31:3719. [Google Scholar]; b Shelnutt JA, Medforth CJ, Berber MD, Barkigia KM, Smith KM. J. Am. Chem. Soc. 1991;113:4077. [Google Scholar]; c Charlesworth P, Truscott TG, Kessel D, Medforth CJ, Smith KM. J. Chem. Soc. Faraday Trans. 1994;90:1073. [Google Scholar]; d Ravikanth M, Reddy D, Chandrashekar TK. J. Photochem. Photobiol. 1993;72:61. [Google Scholar]; e Reddy D, Chandrashekar TK, Vanwilligen H. Chem. Phys. Lett. 1993;202:120. [Google Scholar]; f Wertsching AK, Koch AS, DiMagno SG. J. Am. Chem. Soc. 2001;123:3932. doi: 10.1021/ja003137y. [DOI] [PubMed] [Google Scholar]; g Ryeng H, Ghosh A. J. Am. Chem. Soc. 2002;124:8099. doi: 10.1021/ja0202659. [DOI] [PubMed] [Google Scholar]; h Haddad RE, Gazeau S, Pecaut J, Marchon JC, Medforth CJ, Shelnutt JA. J. Am. Chem. Soc. 2003;125:1253. doi: 10.1021/ja0280933. [DOI] [PubMed] [Google Scholar]
- 13.a Gentemann S, Medforth CJ, Forsyth TP, Nurco DJ, Smith KM, Fajer J, Holten D. J. Am. Chem. Soc. 1994;116:7363. [Google Scholar]; b Gentemann S, Medforth CJ, Ema T, Nelson NY, Smith KM, Fajer J, Holten D. Chem. Phys. Lett. 1995;245:441. [Google Scholar]; c Drain CM, Kirmaier C, Medforth CJ, Nurco J, Smith KM, Holten D. J. Phys. Chem. 1996;100:11984. [Google Scholar]; d Gentemann S, Nelson NY, Jaquinod L, Nurco DJ, Leung SH, Medforth CJ, Smith KM, Fajer J, Holten D. J. Phys. Chem. B. 1997;101:1247. [Google Scholar]; e Drain CM, Gentemann S, Roberts JA, Nelson NY, Medforth CJ, Jia SL, Simpson MC, Smith KM, Fajer J, Shelnutt JA, Holten D. J. Am. Chem. Soc. 1998;120:3781. [Google Scholar]; f Retsek JL, Gentemann S, Medforth CJ, Smith KM, Chirvony VS, Fajer J, Holten D. J. Phys. Chem. B. 2000;104:6690. [Google Scholar]; g Chirvony VS, van Hoek A, Galievsky VA, Sazanovich IV, Schaafsma TJ, Holten D. J. Phys. Chem. B. 2000;104:9909. [Google Scholar]; h Sazanovich IV, Galievsky VA, van Hoek A, Schaafsma TJ, Malinovskii VL, Holten D, Chirvony VS. J. Phys. Chem. B. 2001;105:7818. [Google Scholar]
- 14.a Aaviksoo J, Frieberg A, Savikhin S, Stelmakh GF, Tsvirko MP. Chem. Phys. Lett. 1984;111:275. [Google Scholar]; b Ehrenberg B, Johnson FM. Spectrochim. Acta. 1990;46a:1521. [Google Scholar]; c Yasuike M, Koseki K, Yamaoka T, Ichimura K, Sakuragi M, Ohno O. Inorg. Chim. Acta. 1991;183:9. [Google Scholar]
- 15.a Roitman L, Ehrenberg B, Kobayashi N. J. Photochem. Photobiol. A Chem. 1994;77:23. [Google Scholar]; b Vinogradov SA, Wilson DF. Adv. Exp. Med. Biol. 1997;411:597. [PubMed] [Google Scholar]; c Rozhkov VV, Khajehpour M, Vinogradov SA. Inorg. Chem. 2003;42:4253. doi: 10.1021/ic034257k. [DOI] [PubMed] [Google Scholar]
- 16.Cheng RJ, Chen YR, Wang SL, Cheng CY. Polyhedron. 1993;12:1353. [Google Scholar]
- 17.A number of X-ray structures of tetrabenzoporphyrin-cations was determined by Ibers et al.5b–d
- 18.a Finikova O, Cheprakov A, Beletskaya I, Vinogradov S. Chem. Commun. 2001:261. [Google Scholar]; b Finikova OS, Cheprakov AV, Beletskaya IP, Carroll PJ, Vinogradov SA. J. Org. Chem. 2004;69:522. doi: 10.1021/jo0350054. [DOI] [PubMed] [Google Scholar]; c Finikova OS, Cheprakov AV, Carroll PJ, Vinogradov SA. J. Org. Chem. 2003;68:7517. doi: 10.1021/jo0347819. [DOI] [PubMed] [Google Scholar]; d Finikova OS, Aleshchenkov SE, Brinas RP, Cheprakov AV, Carroll PJ, Vinogradov SA. J. Org. Chem. 2005;70:4617. doi: 10.1021/jo047741t. [DOI] [PubMed] [Google Scholar]; e Finikova OS, Cheprakov AV, Vinogradov SA. J. Org. Chem. 2005;70:9562. doi: 10.1021/jo051580r. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Filatov MA, Cheprakov AV, Beletskaya IP. Eur. J. Org. Chem. 2007:3468. [Google Scholar]; g Filatov MA, Lebedev AY, Vinogradov SA, Cheprakov AV. J. Org. Chem. 2008;73:4175. doi: 10.1021/jo800509k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a Senge MO. In: Ch. 6 in The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Academic Press; 2000,and references therein;. [Google Scholar]; b Senge MO. Chem. Commun. 2006:243. doi: 10.1039/b511389j. [DOI] [PubMed] [Google Scholar]
- 20.a Avilov IV, Zenkevich EI, Sagun EI, Filatov IV. J. Physi. Chem. A. 2004;108:5684. [Google Scholar]; b Chirvony VS, Avilov IV, Panarin AY, Malinovskii VL, Galievsky VA. Chem. Phys. Lett. 2007;434:116. [Google Scholar]
- 21.a Knyukshto VN, Sagun EI, Shul’ga AM, Bachilo SM, Starukhin DA, Zen’kevich EI. Opt. Spectrosc. 2001;90:67. [Google Scholar]; b Knyukshto VN, Shul’ga AM, Sagun EI, Zen’kevich EI. Opt. Spectrosc. 2002;92:53. [Google Scholar]; c Knyukshto VN, Shul’ga AM, Sagun EI, Zen’kevich EI. Opt. Spectrosc. 2006;100:590–601. [Google Scholar]
- 22.a Jentzen W, Song X-Z, Shelnutt JA. J. Phys. Chem. B. 1997;101:1684. [Google Scholar]; b Jentzen W, Ma JG, Shelnutt JA. Biophys. J. 1998;74:753. doi: 10.1016/S0006-3495(98)74000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medforth CJ, Senge MO, Smith KM, Sparks LD, Shelnutt JA. J. Am. Chem. Soc. 1992;114:9859. [Google Scholar]
- 24.Rosa A, Ricciardi G, Baerends EJ. J. Phys. Chem. A. 2006;110:5180. doi: 10.1021/jp060931i. [DOI] [PubMed] [Google Scholar]
- 25.Seybold PG, Gouterman M. J. Mol. Spectrosc. 1969;31:1. [Google Scholar]
- 26.Gradyushko AT, Tsvirko MP. Optics Spectrosc. (Russ) 1971;31:291. [Google Scholar]
- 27.In photochemical literature, funnels or conical intersections (as opposed to avoided crossings) usually refer to the points in which the ground and excited state potential energy surfaces touch, resulting in sub-picosecond non-radiative relaxation (for review see Garavelli M. Theor. Chem. Acc. 2006;116:87.). In the aforementioned model,13 funnels more broadly refer to local minima on the S1 potential energy surface, in which the S1–S0 gaps are small and internal conversion is enhanced in accordance with the energy gap law.
- 28.Finikova OS, Cheprakov AV, Carroll PJ, Dalosto S, Vinogradov SA. Inorg. Chem. 2002;41:6944. doi: 10.1021/ic0260522. [DOI] [PubMed] [Google Scholar]
- 29.Senge MO, Forsyth T, Nguyen LT, Smith KM. Angew. Chem., Int. Ed. 1994;33:2485. [Google Scholar]
- 30.a Livesey AK, Brochon JC. Biophys. J. 1987;52:693. doi: 10.1016/S0006-3495(87)83264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Vinogradov SA, Wilson DF. Appl. Spectrosc. 2000;54:849. [Google Scholar]
- 31.Yasuike M, Yamaoka T, Ohno O, Kunihiro I, Morii H, Sakuragi M. Inorg. Chim. Acta. 1991;185:39.The reported absorption spectrum and the fluorescent properties, although in general consistent with our data, indicate that their samples contained other meso-aryl TBP’s.
- 32.Barret PA, Linstead RP, Rundall FG, Tuey GAP. J. Chem. Soc. 1940:1079. [Google Scholar]
- 33.Senge MO, Medforth CJ, Forsyth TP, Lee DA, Olmstead MM, Jentzen W, Pandey RK, Shelnutt JA, Smith KM. Inorg. Chem. 1997;36:1149. doi: 10.1021/ic961156w. [DOI] [PubMed] [Google Scholar]
- 34.Senge MO, Bischoff I. Heterocycles. 2005;65:879. [Google Scholar]
- 35.Giraud-Roux M, Proni G, Nakanishi K, Berova N. Heterocycles. 2003;61:417. [Google Scholar]
- 36.Yamada H, Kushibe K, Okujima T, Uno H, Ono N. Chem. Commun. 2006:383. doi: 10.1039/b513848e. [DOI] [PubMed] [Google Scholar]
- 37.Eastwood D, Gouterman M. J. Mol. Spectrosc. 1970;35:359. [Google Scholar]
- 38.Antipas A, Gouterman M. J. Am. Chem. Soc. 1983;105:4896. [Google Scholar]
- 39.Based on the experimental and calculated structures of other metal complexes of O ETPP (e.g. Zn, Ni, Cu),40 PdOETPP is considered to possess saddled geometry.
- 40.a Barkigia KM, Berber MD, Fajer J, Medforth CJ, Renner MW, Smith KM. J. Am. Chem. Soc. 1990;112:8851. [Google Scholar]; b Barkigia KM, Renner MW, Furenlid LR, Medforth CJ, Smith KM, Fajer J. J. Am. Chem. Soc. 1993;115:3627. [Google Scholar]; c Renner MW, Barkigia KM, Zhang Y, Medforth CJ, Smith KM, Fajer J. J. Am. Chem. Soc. 1994;116:8582. [Google Scholar]
- 41.Stolzenberg AM, Schussel LJ, Summers JS, Foxman BM, Petersen JL. Inorg. Chem. 1992;31:1678. [Google Scholar]
- 42.a Sessler JL, Johnson MR, Creager SE, Fettinger JC, Ibers JA. J. Am. Chem. Soc. 1990;112:9310. [Google Scholar]; b Stulz E, Scott SM, Ng YF, Bond AD, Teat SJ, Darling SL, Feeder N, Sanders JKM. Inorg. Chem. 2003;42:6564. doi: 10.1021/ic034699w. [DOI] [PubMed] [Google Scholar]
- 43.Minaev B, Ågren H. Chem. Phys. 2005;315:215. [Google Scholar]
- 44.Fleischer EB, Webb LE, Miller CK. J. Am. Chem. Soc. 1964;86:2342. [Google Scholar]
- 45.Lipstman S, Goldberg I. Acta Crystallogr. C: Cryst. Struct. Commun. 2008;64:M53–M57. doi: 10.1107/S0108270107063524. [DOI] [PubMed] [Google Scholar]