Abstract
Impacting a significant portion of the world’s population with increasing incidence in minorities, the young, and the physically active, diabetes mellitus (DM) and its complications affect approximately 20 million individuals in the United States and over 100 million individuals worldwide. In particular, vascular disease from DM may lead to some of the most serious complications that can extend into both the cardiac and nervous systems. Unique strategies that can prevent endothelial cell (EC) demise and elucidate novel cellular mechanisms for vascular cytoprotection become vital for the prevention and treatment of vascular DM complications. Here, we demonstrate that erythropoietin (EPO), an agent that has recently been shown to extend cell viability in a number of systems extending beyond hematopoietic cells, prevents EC injury and apoptotic nuclear DNA degradation during elevated glucose exposure. More importantly, EPO employs Wnt1, a cysteine-rich glycosylated protein involved in gene expression, cell differentiation, and cell apoptosis, to confer EC cytoprotection and maintains the integrity of Wnt1 expression during elevated glucose exposure. In addition, application of anti-Wnt1 neutralizing antibody abrogates the protective capacity of both EPO and Wnt1, illustrating that Wnt1 is an important component in the cytoprotection of ECs during elevated glucose exposure. Intimately linked to this cytoprotection is the downstream Wnt1 pathway of glycogen synthase kinase (GSK-3β) that requires phosphorylation of GSK-3β and inhibition of its activity by EPO. Interestingly, inhibition of GSK-3β activity during elevated glucose leads to enhanced EC survival, but does not synergistically improve protection by EPO or Wnt1, suggesting that EPO and Wnt1 are closely tied to the blockade of GSK-3β activity. Our work exemplifies an exciting potential application for EPO in regards to the treatment of DM vascular disease complications and highlights a previously unrecognized role for Wnt1 and the modulation of the downstream pathway of GSK-3β to promote vascular cell viability during DM.
Keywords: apoptosis, diabetes, endothelial cells, erythropoietin, glucose, growth factors, GSK-3β, oxidative stress, SB21, vascular disease, wingless, Wnt, Wnt1 antibody
INTRODUCTION
Affecting close to 20 million individuals in the United States and over 100 million individuals worldwide, diabetes mellitus (DM) is recognized with increasing incidence in minorities, the young, and the physically active (Maiese, et al., 2007b, Quinn, 2001). Of these individuals, Type 1 DM accounts for approximately 10% of all diabetics that represents a highly significant health concern, since this disorder begins early in life and leads to cardiovascular, renal, and nervous system disease. Type 2 DM represents at least 80 percent of all diabetics and is rapidly increasing in incidence as a result of changes in human behavior and increased body mass index (Laakso, 2001). Both Type 1 and type 2 DM represent important health concerns whether they begin early or later in life (Maiese, et al., 2007a), since each can result in long-term complications throughout the body (Daneman, 2006). In regards to the vascular and nervous systems, patients with DM can develop severe neurological and vascular disease that can lead to an increased risk for cognitive decline especially from vascular disease (Chong, et al., 2005b, Li, et al., 2006a, Schnaider Beeri, et al., 2004).
Individuals with DM can suffer from either increased as well as depressed serum glucose levels as a result of poor control or unrecognized disease progression (Saydah, et al., 2004). These clinical observations are directly relevant to processes that occur at the cellular level in vascular cells during DM. Recent work has shown that cerebral endothelial cells (ECs) are susceptible to advanced glycation end products that can occur during DM (Niiya, et al., 2006). In experimental models of DM with elevated glucose levels, cerebral endothelial cell dysfunction may lead to blood-brain barrier permeability (Huber, et al., 2006).
Given that EC injury can occur during elevated glucose (Maiese, et al., 2007b), agents that reduce cellular loss may be highly successful for the treatment of complications arising from DM. Erythropoietin (EPO) may be an agent that closely fits this desired profile since protection by EPO can be quite robust for a number of disorders (Li, et al., 2004, Maiese, et al., 2004, Maiese, et al., 2005c, Mikati, et al., 2007, Santhanam and Katusic, 2006). EPO is approved by the Food and Drug Administration for the treatment of anemia, but a body of recent work has revealed that EPO is not only required for erythropoiesis, but also functions in other organs and tissues outside of the liver and the kidney, such as the brain, heart, and vascular system (Chong, et al., 2003b, Chong, et al., 2002, Chong and Maiese, 2007, Mikati, et al., 2007, Moon, et al., 2006, Um, et al., 2007). EPO can be detected in the breath of healthy individuals (Schumann, et al., 2006) suggesting its ubiquitous presence and has been correlated with increased Mental Development Index scores (Bierer, et al., 2006). In clinical studies with DM, plasma EPO is often low in diabetic patients with (Mojiminiyi, et al., 2006) or without anemia (Symeonidis, et al., 2006). Furthermore, the failure to produce erythropoietin in response to a declining hemoglobin level suggests an impaired EPO response in diabetic patients (Thomas, et al., 2005). Yet, increased EPO secretion during diabetic pregnancies may represent the body’s endogenous protection mechanisms against DM complications (Teramo, et al., 2004). Similar to the potential protective role of insulin (Duarte, et al., 2006), EPO has been shown in diabetics and non-diabetics with severe, resistant congestive heart failure have shown to decrease fatigue, increase left ventricular ejection fraction, and significantly decrease the number of hospitalization days (Silverberg, et al., 2006).
Unfortunately, agents such as EPO may not be tolerated by all individuals, especially those with co-morbid conditions such as congestive heart failure (van der Meer, et al., 2004), hypertension (Maiese, et al., 2005c), and neoplasms (Henry, et al., 2004, Maiese, et al., 2005b). For these reasons, it is vital to elucidate novel pathways that may influence cellular toxicity during DM (Berent-Spillson and Russell, 2007, Li, et al., 2006a, Maiese, et al., 2005a). In this instance, it is the cellular mechanisms controlled by EPO that can specifically target DM vascular complications that garner particular interest. One novel pathway for EPO to block cellular injury during elevated glucose involves Wnt1. Wnt proteins, derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes, are secreted cysteine-rich glycosylated proteins that play a role in a variety of cellular functions that involve gene expression, gene replication, cell differentiation, and cell apoptosis (Abe and Takeichi, 2007, Chong and Maiese, 2004, Cohen, et al., 2006, Jozwiak, et al., 2007, Li, et al., 2005, Li, et al., 2006c, Wang and MacNaughton, 2005). Variations in genes in the Wnt signaling pathway, such as transcription factor 7-like 2 gene, may impart increased risk for Type 2 DM in some populations (Grant, et al., 2006) as well as have increased association with obesity (Guo, et al., 2006). In addition, experimental work in mice suggest that some Wnt family members may offer glucose tolerance and insulin sensitivity (Wright, et al., 2007). Additional studies have described the expression of Wnt5b in adipose tissue, the pancreas, and the liver in diabetic patients with a suggested function for the regulation of adipose cell function (Kanazawa, et al., 2004) as well as the ability of Wnt4 or Wnt5a to protect glomerular mesangial cells from elevated glucose induced apoptosis (Lin, et al., 2006). Interestingly, Wnt1 which is considered to be one of the best characterized members of the Wnt family has been closely linked to the control of apoptotic cellular injury. Loss of Wnt1 expression (He, et al., 2005, You, et al., 2004) leads to apoptosis while the presence of Wnt1 can promote cell survival during insults such as serum deprivation (Bournat, et al., 2000) or amyloid toxicity (Chong, et al., 2007a).
A downstream pathway to consider that can involve both EPO and Wnt1 cytoprotection during DM is glycogen synthase kinase (GSK-3β). Wnt-1 can inhibit GSK-3β to prevent the phosphorylation and degradation of β-catenin to allow β-catenin to translocate to the nucleus to allow the Wnt pathway to block cell injury (Chong, et al., 2005c, Rhee, et al., 2002). Inhibition of GSK-3β activity can influence cell survival and inflammation (Chong, et al., 2007b) and, as a result, GSK-3β is considered to be a therapeutic target for a number of disorders (Balaraman, et al., 2006, Chong, et al., 2005b, Nurmi, et al., 2006). In regards to DM, inactivation of GSK-3β by small molecule inhibitors or RNA interference prevents toxicity from high concentrations of glucose and increases rat beta cell replication (Mussmann, et al., 2007) while cardioprotective agents during experimental DM have been linked to the inactivation of GSK-3β (Yue, et al., 2005). Furthermore, pharmacologic inhibition of GSK-3β by recombinant Wnt5a or other agents prevents high glucose-mediated apoptosis in glomerular mesangial cells (Lin, et al., 2006). In clinical studies, physical exercise is one of the important lifestyle interventions for DM to promote glycemic control (Maiorana, et al., 2002) and at the cellular level, physical exercise has been shown to phosphorylate and inhibit GSK-3β activity (Howlett, et al., 2006). We demonstrate that EC protection by EPO during elevated glucose relies upon the activation of the Wnt1 pathway, since EPO promotes the expression of Wnt1 and EC protection is abolished by co-treatment with an anti-Wnt1 neutralizing antibody. Additionally, inhibition of GSK-3β activity during elevated glucose leads to enhanced EC survival, but does not synergistically improve protection by EPO or Wnt1, illustrating that the pathways of EPO and Wnt1 are intimately linked to the blockade of GSK-3β activity.
Materials and Methods
Cerebral microvascular endothelial cell cultures
Per our prior protocols, vascular ECs were isolated from male Sprague-Dawley adult rat brain cerebra by using a modified collagenase/dispase-based digestion protocol (Chong, et al., 2003a, Chong, et al., 2002, Chong and Maiese, 2007). Briefly, ECs were cultured in endothelial growth media consisting of M199E (M199 with Eagle’s salt) with 20% heat-inactivated fetal bovine serum, 2 mmol/l L-glutamine, 90 μg/ml heparin, and 20 μg/ml EC growth supplement (ICN Biomedicals, Aurora, OH). Cells from the third passage were identified by positive direct immunocytochemistry for factor VIII-related antigen (Chong, et al., 2003a, Chong, et al., 2002, Chong and Maiese, 2007) and possessed characteristic spindle-shaped morphology with antigenic properties shown to resemble brain endothelium in vivo (Abbott, et al., 1992).
Experimental treatments
Elevated glucose concentrations in ECs was performed by replacing the media with serum-free M199E media with 2 mmol/l L-glutamine and 90 μg/ml heparin containing 25 mM D-glucose and then incubated at 37°C for 48 hours. In this injury paradigm for elevated glucose, hyperosmolarity did not play a significant role in cell toxicity. A mannitol concentration of 25 mM resulted in similar and not significantly different survival rates than untreated control ECs with survival equal to approximately 90% (data not shown), suggesting that hyperosmolarity was not a significant factor in cell injury. Furthermore, we performed additional studies with the biologically inactive agent L-glucose plus 5.6 mM D-glucose and have observed that L-glucose in concentrations of 25 mM did not significantly alter cell survival (data not shown).
For treatments applied 1 hour prior to elevated glucose concentrations, application of erythropoietin (EPO) (R&D Systems, Minneapolis, MN), human recombinant Wnt1 protein (R&D Systems, Minneapolis, MN), a mouse monoclonal anti body against Wnt1 (R&D Systems, 0olis, MN), or the glycogen synthase kinase (GSK)-3β inhibitor SB216763 [3-(2,4-Dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione] (SB21) (Tocris, Ellisville, MO) were continuous.
Cell survival and DNA fragmentation
EC injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 48 hours per our previous protocols (Chong and Maiese, 2007) following elevated glucose. Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (Chong, et al., 2003a, Chong, et al., 2003b, Chong, et al., 2002) with the 3′-hydroxy ends of cut DNA labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Expression of Wnt1 and phosphorylated glycogen synthase kinase
Cells were homogenized and following protein determination, each sample (50 μg/lane) was then subjected to 12.5% SDS-polyacrylamide gel electrophoresis. After transfer, the membranes were incubated with a rabbit polyclonal antibody against Wnt1 (1: 1000, R&D Systems, Minneapolis, MN) or a rabbit antibody against phosphorylated GSK-3β (p-GSK-3β, Ser9) (Cell Signaling, Beverly, MA). Following washing, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody (goat anti-mouse IgG, 1:1000 (Wnt1) or goat anti-rabbit IgG, 1:10000 (p-GSK-3β). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Statistical analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Student’s t-test. Statistical significance was considered at p<0.05.
Results
Elevated glucose leads to EC injury
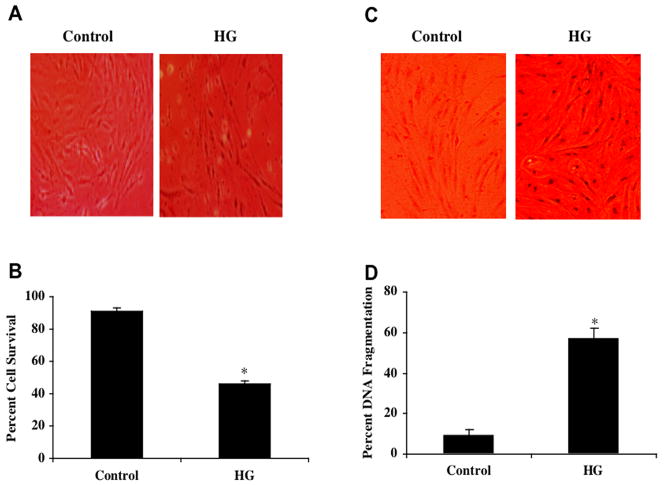
Primary cultures of microvascular ECs were exposed to elevated D-glucose (25 mM) and cell survival was determined 48 hours later by the trypan blue exclusion method. As shown in Fig. 1A, representative pictures demonstrate that elevated glucose treatment results in a loss of membrane integrity and staining in a significant number of ECs cells with trypan blue. The quantitative results demonstrate that EC survival was significantly decreased from 90 ± 2% to 46 ± 2% 48 hours following administration of D-glucose (Fig. 1B).
Fig. (1). Elevated glucose decreases primary EC survival and increases apoptotic demise.
(A)Primary ECs were exposed to elevated D-glucose (HG) at the concentration of 25 mM and EC survival was determined 48 hours later. Representative images illustrate increased trypan blue staining during elevated glucose. (B) EC survival was significantly decreased from 90 ± 2% to 46 ± 2% 48 hours following administration of D-glucose (*p< 0.01 vs. Control). (C) Primary ECs were exposed to elevated D-glucose (HG) (25 mM) and EC nuclear DNA degradation was determined 48 hours later. Representative images illustrate increased TUNEL staining during elevated glucose. (D) EC apoptotic nuclear DNA degradation was significantly increased from 9 ± 3% to 57 ± 5% 48 hours following administration of D-glucose (*p< 0.01 vs. Control). In all cases, each data point represents the mean and SEM and control = untreated EC cultures.
We next assessed genomic DNA fragmentation in primary cultures of microvascular ECs 48 hours following administration of elevated D-glucose (25 mM) with TUNEL. Representative pictures in Fig. 1C demonstrate significant apoptosis with chromatin condensation and nuclear fragmentation in ECs during elevated D- glucose. As shown in Fig. 1D, percent DNA fragmentation was significantly increased from 9 ± 3% of control cells to 57 ± 5% 48 hours following administration of D-glucose.
Wnt1 provides necessary cellular protection against high glucose in ECs
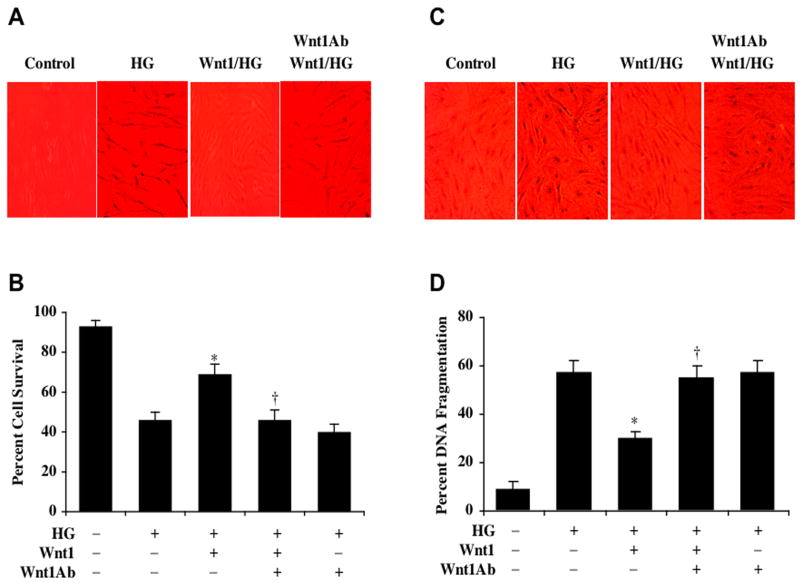
Application of recombinant human Wnt1 protein (100 ng/ml) in EC cultures 1 hour prior to elevated D-glucose (25 mM) exposure significantly reduced trypan blue uptake in ECs when assessed 48 hours later following elevated D-glucose application (Fig. 2A). In Figure 2B, EC survival was significantly reduced to 46 ± 4% following exposure to elevated D-glucose when compared with untreated control cultures (93 ± 2%, p<0.01). Yet, application of Wnt1 of 100 ng/ml 1 hour prior to elevated D-glucose significantly reduces trypan blue uptake in ECs resulting in an EC survival of 69 ± 5% (p<0.01).
Fig. (2). Wnt1 fosters necessary cellular protection against elevated glucose in primary ECs.
(A)Recombinant human Wnt1 protein (100 ng/ml) was applied to EC cultures 1 hour prior to the exposure of D-glucose (25 mM) (HG) and cell survival was determined 48 hours later. Representative images illustrate increased trypan blue staining during elevated glucose, but administration of Wnt1 significantly decreased trypan blue uptake by ECs. In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to the administration of Wnt1 protein antagonized the ability of Wnt1 to significantly reduce trypan blue uptake in ECs during elevated glucose exposure. (B) Wnt1 (100 ng/ml) administration significantly increased EC survival when compared with cultures exposed to elevated glucose alone (*p<0.01 vs. HG). In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to the administration of Wnt1 protein blocked Wnt1 cytoprotection in ECs during elevated glucose (†p<0.01 vs. Wnt1/HG). (C) Recombinant human Wnt1 protein (100 ng/ml) was administered to EC cultures 1 hour prior to the exposure of D-glucose (25 mM) (HG) and nuclear DNA fragmentation with TUNEL was determined 48 hours later. Representative images illustrate increased TUNEL staining during elevated glucose, but administration of Wnt1 significantly decreased TUNEL labeling in ECs. In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to the administration of Wnt1 protein antagonized the ability of Wnt1 to significantly reduce TUNEL labeling in ECs during elevated glucose exposure. (D) Wnt1 (100 ng/ml) administration significantly decreased EC nuclear DNA degradation when compared with cultures exposed to elevated glucose alone (*p<0.01 vs. HG). In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to the administration of Wnt1 protein prevented Wnt1 from reducing nuclear DNA degradation in ECs during elevated glucose (†p<0.01 vs. Wnt1/HG). In all cases, each data point represents the mean and SEM and control = untreated EC cultures.
Administration of an antibody to Wnt1 (Wnt1Ab, 1 μg/ml) alone did not significantly alter EC survival when compared to untreated control cultures (data not shown). In studies with exposure to elevated D-glucose (25 mM), application of the Wnt1Ab, 1 μg/ml slightly decreased EC survival when compared to cultures treated with elevated glucose alone, suggesting that endogenous Wnt1 may offer a minimum level of protection to ECs (Fig. 2B).
We next examined whether specific antagonism against exogenous Wnt1 application with the Wnt1Ab could neutralize the protective capacity of Wnt1 during elevated glucose exposure. In the presence of the Wnt1Ab (1 μg/ml), the protective capacity of Wnt1 was significantly reduced yielding EC survivals of 46 ± 5% (p<0.01) when compared to a survival of 69 ± 5% in ECs with Wnt1 only treatment 48 hours following administration of D-glucose (Fig. 2B).
Similarly, application of Wnt1 protein (100 ng/ml) to EC cultures 1 hour prior to administration of elevated D-glucose at the concentration of 25 mM significantly reduced apoptotic chromatin condensation and nuclear fragmentation determined 48 hours later by TUNEL (Figs. 2C, 2D). Yet, co-application of the Wnt1Ab (1 μg/ml) with Wnt1 resulted in an increase in percent DNA fragmentation in ECs during elevated glucose treatment, illustrating the necessity of the Wnt1 pathway for cytoprotection (Figs. 2C, 2D).
EPO prevents EC injury and apoptotic loss through Wnt1
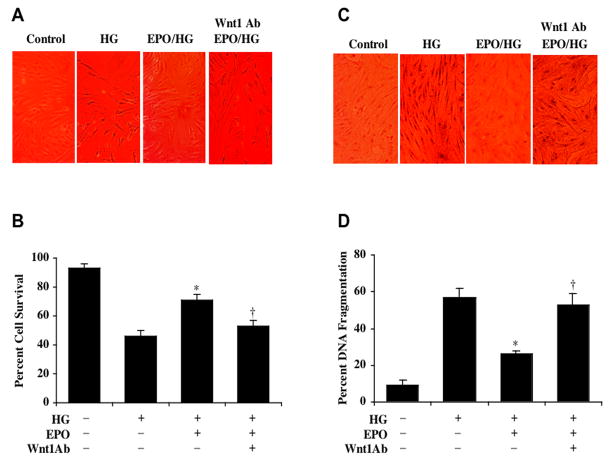
To investigate the ability of EPO to offer cytoprotection during elevated glucose treatment, EPO (10 ng/ml) was applied to ECs 1 hour prior to the administration of D-glucose at the concentration of 25 mM. Cell survival was determined by trypan blue dye exclusion 48 hours following administration of D-glucose. EPO significantly reduced trypan blue uptake in ECs during elevated glucose (Fig. 3A). Interestingly, application of the Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to EPO administration abolished the cytoprotective capacity of EPO resulting in a significant increase in trypan blue staining in ECs. Quantitation of these results in Figure 3B illustrates that EC survival was significantly increased with EPO administration from 46 ± 4% during elevated D-glucose to 71 ± 4% (p<0.01). Yet, co-application of Wnt1Ab (1 μg/ml) with EPO blocked cytoprotection by EPO to reduce cell survival to 53 ± 4% (Fig. 3B).
Fig. (3). Cytoprotection in ECs by EPO requires Wnt1 during elevated glucose exposure.
(A)EPO (10 ng/ml) was applied to EC cultures 1 hour prior to the exposure of D-glucose (25 mM) (HG) and cell survival was determined 48 hours later. Representative images illustrate increased trypan blue staining during elevated glucose, but administration of EPO significantly decreased trypan blue uptake by ECs. In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to the administration of EPO antagonized the ability of EPO to prevent trypan blue uptake in ECs during elevated glucose exposure. (B) EPO (10 ng/ml) application significantly increased EC survival when compared with cultures exposed to elevated glucose alone (*p<0.01 vs. HG). In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to the EPO treatment blocked Wnt1 cytoprotection in ECs during elevated glucose (†p<0.01 vs. EPO/HG). (C) EPO (10 ng/ml) was applied to EC cultures 1 hour prior to the exposure of D-glucose (25 mM) (HG) and nuclear DNA fragmentation with TUNEL was determined 48 hours later. Representative images illustrate increased TUNEL staining during elevated glucose, but administration of EPO significantly decreased nuclear DNA fragmentation as demonstrated by reduced TUNEL labeling in ECs. In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to the administration of EPO prevented EPO from significantly reducing TUNEL labeling in ECs during elevated glucose exposure. (D) EPO (10 ng/ml) administration significantly decreased EC nuclear DNA degradation when compared with cultures exposed to elevated glucose alone (*p<0.01 vs. HG). In contrast, application of Wnt1 antibody (Wnt1Ab, 1 μg/ml) 30 min prior to EPO application prevented EPO from reducing nuclear DNA degradation in ECs during elevated glucose (†p<0.01 vs. EPO/HG). In all cases, each data point represents the mean and SEM and control = untreated EC cultures.
Furthermore, application of EPO (10 ng/ml) to EC cultures 1 hour prior to administration of D-glucose at the concentration of 25 mM significantly reduced apoptotic chromatin condensation and nuclear DNA fragmentation when assesses 48 hours later by TUNEL (Figs. 3C, 3D). In contrast, co-application of Wnt1Ab (1 μg/ml) with EPO lead to a significant increase in percent DNA fragmentation in ECs during high glucose treatment from 26 ± 2% with EPO only administration to 53 ± 6% (p<0.01) during EPO and Wnt1Ab application.
EPO prevents the loss of Wnt1 expression and inhibits GSK-3β activity during elevated glucose exposure
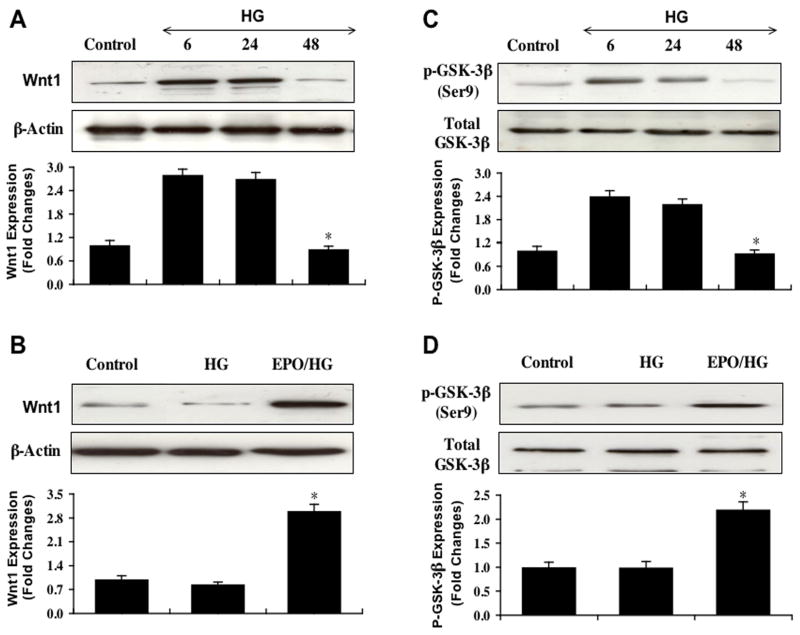
Western blot assay was performed for Wnt1 expression (Figure 4A) and phosphorylated GSK-3β (p-GSK-3β, Ser9) expression (Fig. 4C) at 6, 24, and 48 hours following exposure to elevated D-glucose (25 mM). In Fig. 4A, elevated D-glucose initially increased the expression of Wnt1 at 6 and 24 hours when compared to EC control cultures. After 24 hours post elevated glucose exposure, expression of Wnt1 was lost and approached control levels (Figs. 4A, 4B). In contrast, EPO (10 ng/ml) during elevated D-glucose exposure significantly maintained the expression of Wnt1 over a 48 hour course, suggesting that EPO can prevent the degradation of Wnt1 during elevated glucose exposure (Fig. 4B).
Fig (4). EPO modulates the expression of Wnt1 and phosphorylated glycogen synthase kinase-3 β during elevated glucose exposure.
EC protein extracts (50 μg/lane) were immunoblotted with anti-Wnt1 (A and B), anti-phosphorylated glycogen synthase kinase-3β (anti-p-GSK-3β) (C and D). Representative images of Western blot detection for Wnt1 (A) and p-GSK-3β (C) were performed at 6, 24, and 48 hour time intervals following administration of elevated D-glucose (25 mM) (HG). Wnt1 and p-GSK-3β expression increased at 6 and 24 hours following exposure to high glucose, but expression of these proteins was lost 48 hours following elevated glucose (*p< 0.01 vs. 6 hours or 24 hours HG). Application of EPO (10 ng/ml) 1 hour prior to the administration of elevated glucose significantly increased Wnt1 (B) (*p< 0.01 vs. HG) and p-GSK-3β (D) (*p< 0.01 vs. HG) expression 48 hours following elevated glucose treatment. In all cases, each data point represents the mean and SEM and control = untreated EC cultures.
EPO also modulated the expression of p-GSK-3β at the conserved regulatory residue of Ser9 (Eldar-Finkelman, et al., 1996). Elevated D-glucose also initially increased the expression of p-GSK-3β at 6 and 24 hours when compared to EC control cultures. After 24 hours post elevated glucose exposure, expression of p-GSK-3β was lost suggesting that p-GSK-3β activity was no longer inhibited (Figs. 4C, 4D). Yet, EPO (10 ng/ml) during elevated D-glucose exposure was able to maintain the inhibition of GSK-3β and significantly promote the expression of p-GSK-3β over a 48 hour course (Fig. 4D).
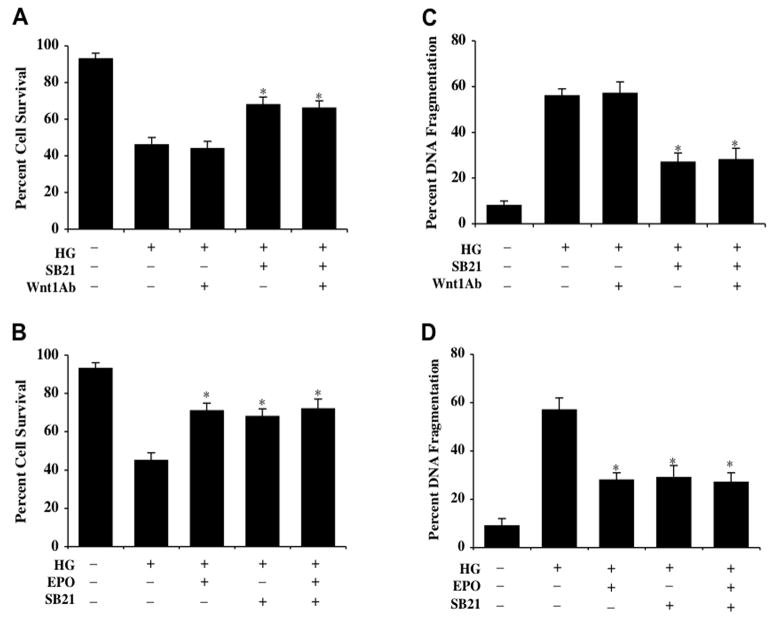
Glycogen synthase kinase-3β (GSK-3β) activity inhibition confers cytoprotection to ECs and parallels protection with EPO application
Exposure to elevated D-glucose (25 mM) leads to EC injury 48 hours late (Figs. 5A, 5B). In contrast, application of the GSK-3β inhibitor (SB216763, (SB21), 5 μM 1 hour prior to elevated D-glucose exposure (25 mM) in concentrations consistent with the current literature (Yoshimura, et al., 2005) increases EC survival. Furthermore, inhibition of GSK-3β activity alone or in combination with the application of EPO (10 ng/ml) administered 1 hour prior to elevated D-glucose significantly increases EC survival during application of EPO alone or during co-administration with EPO, suggesting that EPO relies upon the inhibition of GSK-3β activity to provide EC cytoprotection. Increased EC survival during inhibition of GSK-3β activity was not altered by co-application of the Wnt1Ab, indicating that modulation of the GSK-3β activity to preserve EC survival occurs downstream from the initial activation of Wnt1 (Fig. 5A).
Fig. (5). EPO protects ECs during elevated glucose exposure by inhibiting glycogen synthase kinase-3 β GSK-3β) activity.
Primary ECs were exposed to elevated D-glucose (25 mM) (HG) and EC survival or nuclear DNA fragmentation were determined 48 hours following elevated glucose exposure. (A and C) Elevated glucose resulted in a significant decrease EC survival and a significant increase in nuclear DNA fragmentation in ECs. Application of the GSK-3β inhibitor SB21 (5 μM) 1 hour prior to administration of elevated D-glucose significantly increased cell survival and decreased nuclear DNA fragmentation 48 hours following elevated glucose treatment (*p<0.01 vs. HG). Co-application of Wnt1 antibody (Wnt1Ab) did not alter the ability of SB21 to protect ECs during elevated glucose treatment (*P<0.01 vs. HG). (B and D) EPO (10 ng/ml) administered 1 hour prior to elevated D-glucose (25 mM) application significantly increased cell survival and decreased nuclear DNA fragmentation in ECs 48 hours following elevated glucose treatment (*p<0.01 vs. HG). Co-application of GSK-3β inhibitor SB21 with EPO significantly increased survival and decreased apoptotic nuclear DNA degradation during elevated glucose exposure, but lead to similar survival levels and DNA degradation during EPO administration alone with elevated glucose without a synergistic increase, suggesting that EPO requires the inhibition of GSK-3β activity for cytoprotection in ECs (*p<0.01 vs. HG alone). In all cases, each data point represents the mean and SEM and control = untreated EC cultures.
Similarly, application of the GSK-3β inhibitor (SB216763, (SB21), 5 μM 1 hour prior to elevated D-glucose exposure (25 mM) prevented EC apoptotic DNA fragmentation (Fig. 5C) assessed by TUNEL without evidence of a synergistic increase in protection against DNA fragmentation during co-administration of EPO (10 ng/ml) (Fig. 5D), further supporting the premise that EPO utilizes inhibition of GSK-3β activity to prevent EC injury and apoptotic demise. In addition, EC apoptotic injury during inhibition of GSK-3β activity was not altered by co-application of the Wnt1Ab (Fig. 5D).
Discussion
EPO has been identified as a possible candidate for a number of disease entities that involve cardiac, nervous, and vascular system diseases (Maiese, et al., 2004, Maiese, et al., 2005c). In particular, EPO may have significant relevance for the treatment of vascular complications in DM in light of prior clinical studies illustrating that EPO can decrease fatigue and enhance cardiac output in patients with DM (Silverberg, et al., 2003). Protection by EPO in vascular cells may directly prevent cell toxicity during elevated glucose or modulate inflammatory pathways that ultimately lead to cellular disposal. For example, EPO not only can preserve microglial integrity (Li, et al., 2006b), but it also can prevent microglial cell activation and proliferation to block phagocytosis of injured cells through pathways that involve early apoptotic cellular membrane phospahtidylserine exposure (Maiese, et al., 2004, Maiese, et al., 2005c).
Employing an elevated glucose model for ECs, we illustrate that a final glucose concentration of 25 mM over a 48 hour course leads to a significant loss in cell survival and correspondingly a significant increase in genomic DNA degradation when compared to control ECs. Our work also demonstrates that primary cerebral ECs are extremely sensitive to elevations in D-glucose that are similar to clinical glucose concentrations not only during poorly controlled diabetes (Pagano, et al., 1994), but also during early clinical onset diabetes (Ryan, et al., 2004) and during expected diurnal variations with diabetes (Troisi, et al., 2000) known to occur in a range from 15 mM–25 mM (270 mg/dl – 450 mg/dl). In addition, the application of the biologically inactive agent L-glucose (25 mM) as well as factors related to hyperosmolarity were not responsible for the observed neuronal injury (data not shown).
In regards to the ability of EPO to offer direct EC cell protection during elevated glucose of 25 mM, we demonstrate that EPO alone is not toxic to EC in a concentration of 10 ng/ml. Administration of EPO (10 ng/ml) with a 1 hour pre-treatment significantly enhanced EC survival during elevated glucose. EPO also blocked apoptotic DNA degradation in ECs during elevated glucose similar to alternate models of oxidative stress in cardiac and vascular cell models (Avasarala and Konduru, 2005, Chong, et al., 2003a, Chong, et al., 2002, Chong and Maiese, 2007, Moon, et al., 2006). The concentration of EPO of 10 ng/ml that achieved cytoprotection in ECs during elevated glucose is similar to serum levels of EPO in patients with cardiac or renal disease that have been associated with potential EPO cellular protection (Mason-Garcia, et al., 1990, Namiuchi, et al., 2005) and clinical protocols with EPO administration have been shown to significantly increase plasma EPO levels well above the 1.0 ng/ml range similar to experimental in vitro work and confer beneficial results (Bierer, et al., 2006, Sohmiya, et al., 1998).
EPO modulates a variety of signal transduction pathways for cytoprotection that can involve protein kinase B, signal transducer and activator of transcription pathways, forkhead transcription factors, caspases, and nuclear factor κB (Bahlmann, et al., 2004, Chong, et al., 2003a, Chong, et al., 2005a, Chong and Maiese, 2007, Menon, et al., 2006, Urao, et al., 2006), but pathways of EPO protection especially in the vascular system that rely upon Wnt signaling have not been previously described. Although clinical trials in patients with DM have suggested that EPO may improve cardiac function (Silverberg, et al., 2003) or offer protection against complications in woman with diabetic pregnancies suggests (Teramo, et al., 2004), the cellular pathways responsible for EPO cytoprotection during DM are unknown. Prior work has suggested that Wnt family members may regulate glucose tolerance (Wright, et al., 2007), adipose cell function (Kanazawa, et al., 2004), and glomerular mesangial cells protection during elevated glucose (Lin, et al., 2006). We show that endogenous activation of Wnt1 may offer a minimum level of protection during elevated glucose exposure, since application of the Wnt1Ab resulted in a slight increase in EC injury. Furthermore, administration of exogenous Wnt1 protein significantly increased EC survival and prevented apoptotic EC degeneration during elevated glucose exposure. More importantly, administration of the Wnt1Ab could neutralize the protective capacity of Wnt1, illustrating that Wnt1 is an important component in the cytoprotection of ECs during elevated glucose exposure. Interestingly, EPO cytoprotection in ECs during elevated glucose exposure also relies upon Wnt1. EPO maintains the expression of Wnt1 over a 48 hour course during elevated glucose exposure and prevents loss of Wnt1 expression that would occur in the absence of EPO during elevated glucose. In addition, loss of EC protection with EPO during the administration of the Wnt1Ab demonstrates that Wnt1 is critical for EPO to protect against EC injury and apoptosis during elevated glucose.
EPO recently has been shown to block the activation of GSK-3β and employ this pathway to maintain microglial cell integrity during oxidative stress (Li, et al., 2006b). Given that the GSK-3β pathway is a significant regulatory component during Wnt signaling (Chong, et al., 2007a, Chong, et al., 2005d, Maiese, et al., 2007a) and that GSK-3β may influence beta cell survival (Mussmann, et al., 2007) and cardioprotection (Yue, et al., 2005) during DM, we examined whether the GSK-3β pathway played a role in EC injury and EPO cytoprotection during elevated glucose exposure. We demonstrate that GSK-3β becomes phosphorylated over a 24 hour course elevated glucose exposure, but that EPO in the presence of elevated glucose significantly maintains the inhibitory phosphorylation of GSK-3β over a 48 hour period following the initial exposure of elevated glucose. This inhibition of GSK-3β activity is closely linked to EC survival, since inhibition of GSK-3β activity during administration of the GSK-3β antagonist SB216763 (SB21) prevents EC injury and apoptotic cell loss during elevated glucose. Increased EC survival during inhibition of GSK-3β activity was not altered by the co-application of Wnt1Ab, suggesting that prevention of the GSK-3β activity to preserve EC survival occurs downstream from the initial activation of Wnt1. Finally, inhibition of GSK-3β activity during co-administration of EPO results in similar survival levels without a synergistic increase, also illustrating that EPO relies upon blockade of GSK-3β activity to offer cytoprotection in ECs during elevated glucose.
Acknowledgments
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), and NIH NINDS/NIA.
References
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992;103 (Pt 1):23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–97. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Avasarala JR, Konduru SS. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-alpha-treated primary cultures of human microvascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25:183–9. doi: 10.1385/JMN:25:2:183. [DOI] [PubMed] [Google Scholar]
- Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, Kirsch T, de Groot K, Laudeley R, Niemczyk E, Guler F, Menne J, Haller H, Fliser D. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–12. doi: 10.1161/01.CIR.0000139335.04152.F3. [DOI] [PubMed] [Google Scholar]
- Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer’s disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63:1226–35. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent-Spillson A, Russell JW. Metabotropic glutamate receptor 3 protects neurons from glucose-induced oxidative injury by increasing intracellular glutathione concentration. J Neurochem. 2007;101:342–54. doi: 10.1111/j.1471-4159.2006.04373.x. [DOI] [PubMed] [Google Scholar]
- Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:e635–40. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- Bournat JC, Brown AM, Soler AP. Wnt-1 dependent activation of the survival factor NF-kappaB in PC12 cells. J Neurosci Res. 2000;61:21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003a;23:320–30. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003b;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007a;19:1150–62. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005a;2:387–99. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005b;75:207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005c;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007b;19:263–72. [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li FQ, Maiese K. Employing new cellular therapeutic targets for Alzheimer’s disease: A change for the better? Curr Neurovasc Res. 2005d;2:55–72. doi: 10.2174/1567202052773508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–50. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol. 2004;19:495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Furey TS, Doggett NA, Kaufman DG. Genome-wide sequence and functional analysis of early replicating DNA in normal human fibroblasts. BMC Genomics. 2006;7:301. doi: 10.1186/1471-2164-7-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman D. Type 1 diabetes. Lancet. 2006;367:847–58. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- Duarte AI, Proenca T, Oliveira CR, Santos MS, Rego AC. Insulin restores metabolic function in cultured cortical neurons subjected to oxidative stress. Diabetes. 2006;55:2863–70. doi: 10.2337/db06-0030. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Argast GM, Foord O, Fischer EH, Krebs EG. Expression and characterization of glycogen synthase kinase-3 mutants and their effect on glycogen synthase activity in intact cells. Proc Natl Acad Sci U S A. 1996;93:10228–33. doi: 10.1073/pnas.93.19.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006;43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–8. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- Henry DH, Bowers P, Romano MT, Provenzano R. Epoetin alfa. Clinical evolution of a pleiotropic cytokine. Arch Intern Med. 2004;164:262–76. doi: 10.1001/archinte.164.3.262. [DOI] [PubMed] [Google Scholar]
- Howlett KF, Sakamoto K, Yu H, Goodyear LJ, Hargreaves M. Insulin-stimulated insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity is enhanced in human skeletal muscle after exercise. Metabolism. 2006;55:1046–52. doi: 10.1016/j.metabol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Huber JD, VanGilder RL, Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. 2006;291:H2660–8. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- Jozwiak J, Kotulska K, Grajkowska W, Jozwiak S, Zalewski W, Oldak M, Lojek M, Rainko K, Maksym R, Lazarczyk M, Skopinski P, Wlodarski P. Upregulation of the WNT pathway in tuberous sclerosis-associated subependymal giant cell astrocytomas. Brain Dev. 2007;29:273–80. doi: 10.1016/j.braindev.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakamura Y, Maeda S. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet. 2004;75:832–43. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med. 2001;249:225–35. doi: 10.1046/j.1365-2796.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006a;13:883–95. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Erythropoietin on a Tightrope: Balancing Neuronal and Vascular Protection between Intrinsic and Extrinsic Pathways. Neurosignals. 2004;13:265–89. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006b;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–40. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006c;21:103–24. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006;17:2812–20. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005a;2:425–46. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Mechanisitic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007a;14:1689–1699. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin and cancer. JAMA. 2005b;293:1858–1859. [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005c;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007b;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorana A, O’riscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56:115–23. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- Mason-Garcia M, Beckman BS, Brookins JW, Powell JS, Lanham W, Blaisdell S, Keay L, Li SC, Fisher JW. Development of a new radioimmunoassay for erythropoietin using recombinant erythropoietin. Kidney Int. 1990;38:969–75. doi: 10.1038/ki.1990.299. [DOI] [PubMed] [Google Scholar]
- Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest. 2006;116:683–94. doi: 10.1172/JCI25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Hokayem JA, Sabban ME. Effects of a single dose of erythropoietin on subsequent seizure susceptibility in rats exposed to acute hypoxia at p10. Epilepsia. 2007;48:175–81. doi: 10.1111/j.1528-1167.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- Mojiminiyi OA, Abdella NA, Zaki MY, El Gebely SA, Mohamedi HM, Aldhahi WA. Prevalence and associations of low plasma erythropoietin in patients with Type 2 diabetes mellitus. Diabet Med. 2006;23:839–44. doi: 10.1111/j.1464-5491.2006.01893.x. [DOI] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C, Austen M. Inhibition of glycogen synthase kinase (GSK) 3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007 doi: 10.1074/jbc.M609637200. [DOI] [PubMed] [Google Scholar]
- Namiuchi S, Kagaya Y, Ohta J, Shiba N, Sugi M, Oikawa M, Kunii H, Yamao H, Komatsu N, Yui M, Tada H, Sakuma M, Watanabe J, Ichihara T, Shirato K. High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;45:1406–12. doi: 10.1016/j.jacc.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Niiya Y, Abumiya T, Shichinohe H, Kuroda S, Kikuchi S, Ieko M, Yamagishi S, Takeuchi M, Sato T, Iwasaki Y. Susceptibility of brain microvascular endothelial cells to advanced glycation end products-induced tissue factor upregulation is associated with intracellular reactive oxygen species. Brain Res. 2006;1108:179–87. doi: 10.1016/j.brainres.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Goldsteins G, Narvainen J, Pihlaja R, Ahtoniemi T, Grohn O, Koistinaho J. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40:1776–84. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Pagano G, Bargero G, Vuolo A, Bruno G. Prevalence and clinical features of known type 2 diabetes in the elderly: a population-based study. Diabet Med. 1994;11:475–9. doi: 10.1111/j.1464-5491.1994.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Quinn L. Type 2 diabetes: epidemiology, pathophysiology, and diagnosis. Nurs Clin North Am. 2001;36:175–92. v. [PubMed] [Google Scholar]
- Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–32. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, Katusic ZS. Erythropoietin and cerebral vascular protection: role of nitric oxide. Acta Pharmacol Sin. 2006;27:1389–94. doi: 10.1111/j.1745-7254.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. Jama. 2004;291:335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63:1902–7. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- Schumann C, Triantafilou K, Krueger S, Hombach V, Triantafilou M, Becher G, Lepper PM. Detection of erythropoietin in exhaled breath condensate of nonhypoxic subjects using a multiplex bead array. Mediators Inflamm. 2006;2006:18061. doi: 10.1155/MI/2006/18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Blum M, Tchebiner JZ, Sheps D, Keren G, Schwartz D, Baruch R, Yachnin T, Shaked M, Schwartz I, Steinbruch S, Iaina A. The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant. 2003;18:141–6. doi: 10.1093/ndt/18.1.141. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Iaina A, Steinbruch S, Wollman Y, Schwartz D. Anemia, chronic renal disease and congestive heart failure--the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. 2006;38:295–310. doi: 10.1007/s11255-006-0064-8. [DOI] [PubMed] [Google Scholar]
- Sohmiya M, Kakiba T, Kato Y. Therapeutic use of continuous subcutaneous infusion of recombinant human erythropoietin in malnourished predialysis anemic patients with diabetic nephropathy. Eur J Endocrinol. 1998;139:367–70. doi: 10.1530/eje.0.1390367. [DOI] [PubMed] [Google Scholar]
- Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, Leotsinidis M, Kyriazopoulou V, Vassilakos P, Vagenakis A, Zoumbos N. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol. 2006;85:79–85. doi: 10.1007/s00277-005-1102-9. [DOI] [PubMed] [Google Scholar]
- Teramo K, Kari MA, Eronen M, Markkanen H, Hiilesmaa V. High amniotic fluid erythropoietin levels are associated with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancies. Diabetologia. 2004;47:1695–703. doi: 10.1007/s00125-004-1515-3. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Cooper ME, Tsalamandris C, MacIsaac R, Jerums G. Anemia with impaired erythropoietin response in diabetic patients. Arch Intern Med. 2005;165:466–9. doi: 10.1001/archinte.165.4.466. [DOI] [PubMed] [Google Scholar]
- Troisi RJ, Cowie CC, Harris MI. Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. Jama. 2000;284:3157–9. doi: 10.1001/jama.284.24.3157. [DOI] [PubMed] [Google Scholar]
- Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–45. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–13. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Voors AA, Lipsic E, Smilde TD, van Gilst WH, van Veldhuisen DJ. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:63–7. doi: 10.1016/j.jacc.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wang H, MacNaughton WK. Overexpressed beta-catenin blocks nitric oxide-induced apoptosis in colonic cancer cells. Cancer Res. 2005;65:8604–7. doi: 10.1158/0008-5472.CAN-05-1169. [DOI] [PubMed] [Google Scholar]
- Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, Susulic VS, Macdougald OA. Wnt10b Inhibits Obesity in ob/ob and Agouti Mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–49. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- You L, He B, Uematsu K, Xu Z, Mazieres J, Lee A, McCormick F, Jablons DM. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res. 2004;64:3474–8. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- Yue TL, Bao W, Gu JL, Cui J, Tao L, Ma XL, Ohlstein EH, Jucker BM. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005;54:554–62. doi: 10.2337/diabetes.54.2.554. [DOI] [PubMed] [Google Scholar]