Abstract
The DexAide right ventricular assist device (RVAD) is a magnetically and hydrodynamically levitated implantable centrifugal pump. Recent progress includes (1) redesign of the inflow/outflow conduits, which yielded two successful 3-month experiments, (2) development of alternative journal bearing materials, and (3) completion of an 18-month duration of in vitro endurance testing. Verification testing of the RVAD electronics has been completed, and a prototype biventricular assist device (BVAD) system has been tested. Acute DexAide/CorAide BVAD implantations via median sternotomy in two calves documented BVAD control algorithms and anatomical fit. A drug-induced calf chronic heart failure model, currently under development in our laboratory, resulted in a successful BVAD implantation in a calf with heart failure. Our future plans are to complete in vitro and in vivo validation of alternative bearing materials, perform preclinical DexAide in vivo and in vitro reliability studies, and obtain FDA approval for an Investigational Device Exemption to conduct a clinical pilot study. Two successful 3-month in vivo experiments and an 18-month in vitro endurance test were completed. Following final bearing material selection, the DexAide design will be “frozen” so that preclinical systems can be manufactured. BVAD experiments using a chronic heart failure model are in progress.
Keywords: ventricular assist device, right ventricular failure, circulatory support
Introduction
Various types of implantable left ventricular assist devices (LVADs) have been successfully used for patients with end-stage heart failure as a bridge to transplantation, bridge to recovery, or destination therapy. The clinical results with LVADs are improving, especially with continuous-flow devices.1 However, a poor prognosis has been shown for the subset of LVAD patients who develop concomitant right ventricular (RV) failure and require prolonged inotropic support.2 Unfortunately, the prognosis does not improve even when they are supported with existing right ventricular assist devices (RVADs).3,4 Clinically available RVADs are mostly extracorporeal devices and have resulted in a high mortality rate due to several limitations, including poor blood compatibility, high infection rates, poor long-term durability, need for anticoagulation, need for a prolonged hospital stay, and reduced quality of life. The current status of RVAD support is similar to that of the early days of LVAD support, when prognosis was poor because of crude extracorporeal devices and the fact that the device was warranted only as a life-saving measure in morbidly ill heart failure patients. The development of a safe and effective continuous flow implantable RVAD, combined with a growing understanding of reliable predictors of right heart failure prior to or soon after LVAD implantation, will greatly improve the clinical results for these patients.
With funding from a Bioengineering Research Partnerships grant from the National Heart, Lung, and Blood Institute, we are developing an implantable RVAD (the DexAide), a magnetically and hydrodynamically levitated centrifugal pump.5–9 This article describes our progress over the last 2 years in the areas of RVAD (1) mechanical systems, (2) external electronics, (3) computational fluid dynamics (CFD), and (4) chronic in vivo testing. We also report on biventricular assist device (BVAD) in vivo testing and development of a chronic calf heart failure model to evaluate RVAD and BVAD control algorithms.
RVAD Mechanical System Progress
The detailed design features of the DexAide RVAD have been published previously.5 Briefly, the pump consists of three subassemblies: the volute housing, the rotating assembly, and the stator assembly. Progress with the mechanical system included (1) inflow/outflow conduit redesign and testing, (2) possible replacement of fluorinated ethylene propylene copolymer (FEP)-coated titanium stator housings with zirconia ceramic materials, (3) diamond-like carbon (DLC) coating development for the rotating assembly and stator assembly, and (4) in vitro pulsatile endurance testing.
Inflow cannula obstruction caused by endocardial tissue overgrowth and associated severe depositions was a major issue with the previous RVAD calf inflow cannulae that were inserted into the outflow portion of the RV via a left thoracotomy approach in our first 16 calves.7 This inflow cannula obstruction issue has been resolved primarily by inserting the cannula from the diaphragmatic surface of the RV through a right thoracotomy instead and by a new cannula design to accommodate this alternative RV access. Figure 1 shows the RV diaphragmatic access and in it the redesigned inflow cannula after a 3-month implantation, demonstrating no obstruction or deposition. We believe that the new cannula design successfully avoided the cannula deposition issue by improving blood flow direction into the cannula. Previously, the direction of the blood flow into the cannula was almost 90 degrees at the RV outflow tract. With the new cannula inserted from the diaphragmatic surface of the RV, the cannula tip is directed towards the tricuspid valve, and thus the blood flow from the RV into the cannula is almost straight.
Figure 1.
The DexAide calf inflow cannula after 3-month implantation through a right thoracotomy. The cannula’s end and both side holes were widely patent with no obstruction or deposition.
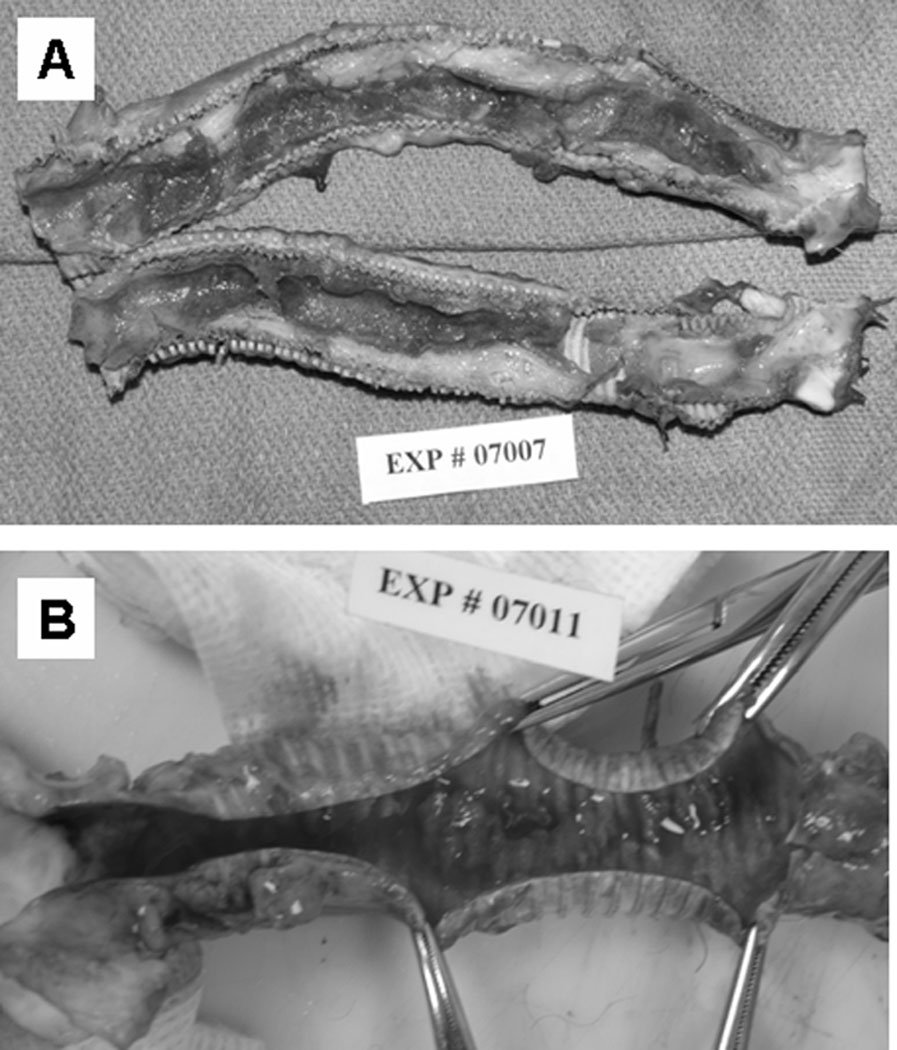
Excessive luminal deposition in the previous outflow conduit (Figure 2A), a 14-mm Hemashield® collagen-impregnated woven polyester graft (Boston Scientific, Natick, MA), has been eliminated by switching to a Gore-Tex® 14-mm Standard-walled, Ringed Vascular PTFE graft (W.L. Gore & Associates, Inc., Flagstaff, AZ; Figure 2B). It is interesting to note that this excessive luminal deposition was not observed in our CorAide™ LVAD animal studies using the same 14-mm Hemashield graft and was nonexistent in the CorAide LVAD clinical trial. It is postulated that the difference in response between the RVAD and LVAD systems may be related to the differences in pressure, flow, shear stress, or oxygen contents in the blood between the systemic and pulmonary circulations. A possible correlation can be drawn to the preference for use of PTFE grafts for vascular replacement in the pulmonary circulation in the clinical setting.
Figure 2.
(A) A Hemashield® outflow graft after a 43-day implantation. Severe neointimal tissue growth (range 4–9 mm in thickness) was observed on the entire surface of the outflow graft, obstructing the lumen by 25–75%. (B) A Gore-Tex® outflow graft after a 41-day implantation. Thin neointimal tissue (1–1.5 mm in thickness) was observed on the entire surface of the outflow graft, showing no obstruction.
We have extensively evaluated a medical grade zirconia ceramic material as a replacement for the previously used FEP-coated titanium stator in both in vitro and in vivo studies.10,11 The in vitro results showed that motor power consumption with a zirconia stator was 20% less than that with a titanium stator, and DexAide battery life was extended from 9 hours to over 12 hours on two fully charged batteries. In vivo data comparing average motor power consumption for 14 previous DexAide implants using the titanium stator (3.2 ± 0.2 watts at 6.0 ± 1.9 L/min and 2,539 ± 88 rpm), vs. 5 DexAide implants using the zirconia stator at similar average pump flows and pump speeds (2.6 ± 0.2 watts, 5.2 ± 0.4 L/min and 2,495 ± 71 rpm) demonstrates a 19% reduction in motor power consumption. When comparing in vivo motor power data recorded at maximum (3,200 rpm) and minimum (2,000 rpm) pump speeds and at average pump flows within 3 to 5 L/min, the titanium stator pumps required 5.77 and 2.17 watts, respectively, vs. 4.70 and 1.69 watts for the zirconia stator pumps, demonstrating a 19 and 22% reduction in motor power at maximum and minimum pump speeds.
The zirconia stator was also successfully evaluated in severe start/stop bearing tests, pre- and post-exposure of the zirconia stator to accelerated simulated biologic aging. Average in vivo pump flows and speeds using a zirconia stator in eight chronic calf implants showed no statistically significant difference to that for our previous 16 titanium stator pump calf implants, with the exception of a 19% reduction in power consumption, matching the in vitro results. Platelet aggregation tests, using adenosine diphosphate, arachidonic acid, collagen, and epinephrine, demonstrated no device-induced increase in platelet activation. Post-explant evaluation of the zirconia journal bearing surfaces showed no biologic deposition in any of the six implants (Figure 3). In vitro hemolysis test results using human blood and in vivo plasma free hemoglobin results were comparable for both stator types. These results showed that zirconia ceramic can be safely and more efficiently used as a blood-contacting journal bearing material in the DexAide RVAD with excellent blood compatibility.
Figure 3.
A zirconia stator after 3-month implantation. There was no deposition nor any signs of bearing wear or deformation on the bearing surfaces.
A new development program is under way to improve and evaluate DLC bearing coatings on the rotating assembly and titanium stator housing (a lower cost alternate to the zirconia). In vivo studies and in vitro start/stop bearing testing are being conducted using DLC/zirconia and DLC/DLC bearing pairs.
A pulsatile endurance test pump, submerged in 37 °C saline/glycerin, has achieved an 18-month duration without change in pump performance or the bearing condition.
RVAD External Electronics Development
The Cleveland Clinic’s Fixed Flow control algorithm for the RVAD system was implemented in the RVAD portable electronic module (RPEM) and the laptop serial interface (RSI) software. RVAD pumps have been successfully run in the Fixed Flow operating mode for 42 % of the total calf implant duration at requested fixed flow levels of 5.0 to 6.5 L/min This algorithm uses fixed speed increments at the completion of 10-second control cycles to match the RVAD pump flow to a clinician programmed target flow. The algorithm includes a method to detect ventricular unloading and prevent ventricular suction based on the pump flow response to the fixed speed increments.
Minnetronix, Inc. (Saint Paul, MN) has completed the verification testing of the RVAD electronics. This included the generation and review of the test protocols, unit testing of the RVAD RPEM and RSI software, and performing the verification tests. Minnetronix manufactured and delivered five RPEM controllers and five RSIs. A BVAD prototype system was designed and developed at Minnetronix. One complete system with BVAD controller and BVAD serial interface was delivered to the Cleveland Clinic.
Computational Fluid Dynamics Study
The previously validated whole pump computational fluid dynamics (CFD) model provided insight into the pump's hemodynamic operation and quantified the fluid shear and red blood cell (RBC) residence time throughout the pump. Recent CFD efforts used the CFD model to predict indices of hemolysis (shear stress × RBC residence time) and indices of potential thrombosis (transit time/shear stress) within the DexAide RVAD.12
RVAD Chronic In Vivo Testing
All animal studies follow NIH and institutional guidelines and are approved by our Institutional Animal Care and Use Committee. Since February 2007, the DexAide RVAD was implanted between the RV and the pulmonary artery in 11 healthy calves at an average pump flow of 5.1 ± 0.4 L/min. Either 2-week or 3-month biocompatibility studies were conducted to evaluate various combinations of the stator and rotator materials as alternate blood-contacting bearing surfaces. Eight implanted pumps incorporated a polished zirconia stator housing, and three used an FEP-coated titanium stator. Nine had either an FEP-coated or FEP-sleeved rotating assembly, and two used a DLC-coated rotating assembly. Five calf studies were completed at the scheduled durations: three at 2 weeks and two at 3 months. The other six calves were terminated prematurely: three on postoperative day (POD) 23, 36 and 43 because of either inflow or outflow conduit obstruction; two on POD 3 and 41 because of deposition on the secondary impellers associated with uncoated secondary impeller titanium surfaces; and one on POD 9 because of intestinal perforation and sepsis unrelated to pump performance. Eight of the 11 explanted pumps showed no biologic deposition at post-explant examination. In one case, nonadherent pump thrombus on the primary impeller was believed to have originated from deposition at the inflow cannula tip. In two cases, deposition on the secondary impellers was associated with uncoated titanium surfaces. The pump hydrodynamic bearing was clean in 10 of the 11 cases, including the bearing combinations of the FEP-coated, FEP-sleeved, or DLC-coated rotating assembly run on zirconia or DLC-coated stators. Bearing deposition in one case was associated with experimentation using an uncoated titanium rotating assembly as described above. In eight of the 11 RVAD autopsies conducted, there were no signs of ischemia, infarction, or embolus in the lungs with gross examination, 1.5-cm serial sectioning and dissection of the pulmonary artery to 2-mm branches. Two cases showed a small pulmonary embolus with no associated infarction, and another case showed large tubular pulmonary emboli originating from thrombosis in the pulmonary artery pressure monitoring line. The encouraging results of these biologic studies support the use of either DLC-coated, FEP-coated, or FEP-sleeved rotating assemblies on either a zirconia or DLC-coated titanium stator in the DexAide RVAD.
Development of a Bovine Chronic Heart Failure Model
We conducted five calf experiments to evaluate a drug-induced (oral monensin) large animal heart failure model for use in evaluating chronic BVAD performance. Monensin is a polyether ionophore antibiotic produced by fermentation of streptomyces cinnamonensis. It was approved by the FDA to treat ruminant coccidiosis and to improve milk production efficiency in dairy cows. However, it has a cardiac toxicity effect in high doses. We demonstrated the feasibility of inducing and maintaining stable yet severe heart failure for up to 3 weeks with decreases in cardiac output (from 11–13 L/min to 6–8 L/min) and mean systemic pressure (from 90–100 mm Hg to 80–90 mm Hg) and increases in left atrial pressure (from 3–5 mm Hg to 23–25 mm Hg) and central venous pressure (from 4–5 mm Hg to 10–13 mm Hg). However, it is very difficult to control monensin blood concentrations via oral administration due to variability in absorption from the alimentary tract; therefore, several animals died due to either fatal arrhythmia or severe heart failure. We are currently conducting a study using intravenous monensin administration to better control blood levels and increase the reliability and reproducibility of this chronic heart failure model.
BVAD In Vivo Testing
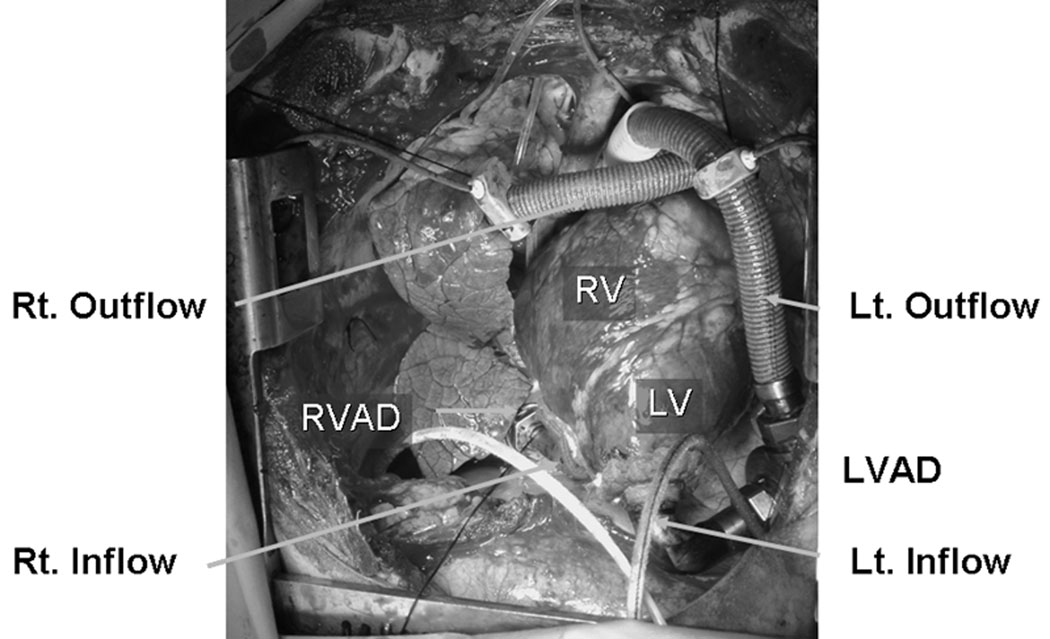
Since February 2007, two calves have been implanted with a BVAD configuration – both the CorAide LVAD and the DexAide RVAD through a median sternotomy – under an acute, open-chest biventricular support condition (Figure 4). Although a healthy calf was used for the first experiment, the second experiment was performed in a calf with stable, chronic heart failure induced by oral monensin, as described above. Both pumps were operated with balanced biventricular pump flows, demonstrating the capability to capture the total circulation at up to 6.0 L/min.
Figure 4.
The CorAide LVAD and the DexAide RVAD implantation through a median sternotomy. LVAD, left ventricular assist device; RVAD, right ventricular assist device; LV, left ventricle; RV, right ventricle; Lt., left; Rt., right.
Discussion
Our program has demonstrated steady progress in the development of the DexAide RVAD. The inflow/outflow conduits were redesigned, and several better blood-compatible materials have been identified to replace the previously used FEP-coated titanium journal bearing. We have completed two successful 3-month in vivo experiments and an18-month duration of in vitro pulsatile endurance testing. The verification testing of the RVAD electronics has been completed, and a BVAD prototype system has been designed and developed.
The severe inflow cannula obstruction that was a major issue with the previous calf inflow cannulae arose primarily because we used a calf model with a left thoracotomy approach,7 which would not be used in the RVAD or BVAD implantation in humans. With the left thoracotomy approach, the infundibular portion of the RV was chosen for inflow cannulation, but it was possible that the blood flow pattern at the infundibular portion of the RV may have caused significant stagnation of blood flow at the downstream side of the cannula. This inflow cannula obstruction issue has been resolved by inserting the redesigned cannula from the diaphragmatic surface of the RV through a right thoracotomy or a median sternotomy. Both our human intraoperative fitting studies at the time of LVAD implantation13 and human cadaver fitting studies9 demonstrated that the DexAide RVAD fit best with its inflow cannula inserted from the diaphragmatic surface of the RV through a median sternotomy.
The recent acute BVAD experiments were performed in two calves through a median sternotomy to evaluate BVAD control algorithms and anatomical fit. Contrary to the significant concerns of veterinary and experienced calf-device-implant surgeons, our experience using median sternotomy in our drug-induced chronic heart failure model calf studies demonstrated no observable difference to a standard lateral thoracotomy in terms of calf recovery time, incidence of infection, need for pain management, postoperative bleeding, stability of the chest wall or the ability to switch from sitting to standing.14 Better surgical access to all cardiac chambers and great vessels and more room for device placement were achieved using a median sternotomy. In all cases, at the time of autopsy, the sternum was well healed without any sign of infection.
Our future plans are to select the final alternative bearing materials, perform preclinical in vivo and in vitro reliability studies, and obtain FDA approval for an Investigational Device Exemption to conduct a clinical pilot study at our institution.
Conclusion
We are nearing completion of the design and development of the DexAide RVAD system with initiating technology transfer to industry to support preclinical testing. Two successful 3-month in vivo experiments and an 18-month in vitro endurance test were completed. In addition, BVAD control experiments using a unique heart failure model are in progress.
Footnotes
Presented at the 54th Annual Conference of the ASAIO, San Francisco, CA, June 19–21, 2008
Disclaimer: None
Disclosures: With the exception of the human fitting studies, the work reported here was funded by Bioengineering Research Partnerships grant 5R01HL074896 (to K.F.) from the National Heart, Lung, and Blood Institute/NIH.
References
- 1.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Schenk S, McCarthy PM, Blackstone EH, et al. Duration of inotropic support after left ventricular assist device implantation: risk factors and impact on outcome. J Thorac Cardiovasc Surg. 2006;131:447–454. doi: 10.1016/j.jtcvs.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Fukamachi K, McCarthy PM, Smedira NG, Vargo RL, Starling RC, Young JB: Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999;68:2181–2184. doi: 10.1016/s0003-4975(99)00753-5. [DOI] [PubMed] [Google Scholar]
- 4.Ochiai Y, McCarthy PM, Smedira NG, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002;106(12 Suppl 1):I198–I202. [PubMed] [Google Scholar]
- 5.Fukamachi K, Horvath DJ, Massiello AL, et al. Development of a small implantable right ventricular assist device. ASAIO J. 2005;51:730–735. doi: 10.1097/01.mat.0000181031.66900.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukamachi K, Ootaki Y, Horvath DJ, et al. Progress in the development of the DexAide right ventricular assist device. ASAIO J. 2006;52:630–633. doi: 10.1097/01.mat.0000240700.03478.d0. [DOI] [PubMed] [Google Scholar]
- 7.Ootaki Y, Saeed D, Ootaki C, et al. Development of the DexAide right ventricular assist device inflow cannula. ASAIO J. 2008;54:31–36. doi: 10.1097/MAT.0b013e31815b3ea4. [DOI] [PubMed] [Google Scholar]
- 8.Saeed D, Ootaki Y, Ootaki C, et al. Acute in vivo evaluation of an implantable continuous flow biventricular assist system. ASAIO J. 2008;54:20–24. doi: 10.1097/MAT.0b013e31815b2d1e. [DOI] [PubMed] [Google Scholar]
- 9.Ootaki Y, Ootaki C, Kamohara K, et al. Cadaver fitting study of the DexAide right ventricular assist device. Artif Organs. 2007;31:646–648. doi: 10.1111/j.1525-1594.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 10.Saeed D, Horvath DJ, Massiello AL, et al. In vitro evaluation of zirconia ceramic in the DexAide right ventricular assist device journal bearing. ASAIO J. 2008;54:23A. doi: 10.1111/j.1525-1594.2009.00757.x. (abstract). [DOI] [PubMed] [Google Scholar]
- 11.Saeed D, Shalli S, Fumoto H, et al. In vivo evaluation of zirconia ceramic in the DexAide right ventricular assist device journal bearing. ASAIO J. 2008;54:22A. doi: 10.1111/j.1525-1594.2009.00918.x. (abstract). [DOI] [PubMed] [Google Scholar]
- 12.Goodin MS, Horvath DJ, Massiello AL, Golding LA, Harrington SA, Fukamachi K. Validation of a computational fluid dynamics model for the Cleveland Clinic DexAide right ventricular assist device. ASAIO J. 2008;54:41A. (abstract). [Google Scholar]
- 13.Ootaki Y, Ootaki C, Massiello A, et al. Human clinical fitting study of the DexAide right ventricular assist device. Artif Organs. doi: 10.1111/j.1525-1594.2009.00741.x. in press. [DOI] [PubMed] [Google Scholar]
- 14.Saeed D, Zahr R, Shalli S, et al. Median sternotomy approach for chronic bovine experiments. ASAIO J. in press doi: 10.1097/MAT.0b013e31818a30d9. [DOI] [PMC free article] [PubMed] [Google Scholar]