Abstract
An improved synthesis of 3-hydroxy-4-pyridone via an Elbs oxidation of 4-pyridone and isolation of 4-pyridone-3-sulfate is described.
Findings
3-Hydroxy-4-pyridone is a constituent of mimosine (also called leucenol) where it occurs as a derivative of L-alanine (Fig. 1) [1]. It is also an intermediate in the bacterial metabolism of 4-pyridone [2] and affects thyroid function [3]. 3-Hydroxy-4-pyridone was first synthesized by Ost in 1873 [4] from pyrone precursors and since then by other related transformations of the pyrone ring system [5-8], by degradation of mimosine [2,9,10], and by the Elbs peroxydisulfate oxidation of both 3-hydroxypyridine and 4-pyridone [2,11,12]. None of these routes is entirely satisfactory: the required pyrones are easily synthesized from meconic (poppy) acid but this is no longer commercially available [13] (the closest available structures are maltol and kojic acid); mimosine currently sells at about $200/g; and the yields in the Elbs oxidation have been, at best, less than 10% and have involved difficult purifications. There is a promising route via lithiation of 4-methoxypyridine which has been carried as far as 3-hydroxy-4-methoxypyridine in connection with the synthesis of orelline, a mushroom toxin [14].
Figure 1.
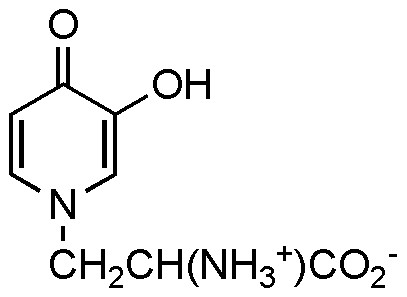
Mimosine.
I report improvements to the Elbs procedure although the yield has been increased only modestly. The oxidation of 4-pyridone by peroxydisulfate is an example of an Elbs oxidation in which the substitution occurs ortho to the existing phenolic group (Fig. 2). In a phenol with both ortho and para positions free, the ortho-para ratio is typically about 0.1. Yields of the ortho-substitution product when the para-position is blocked (as in 4-pyridone) are usually low [15]. However, considerable quantities of starting material are recoverable and increasing the peroxydisulfate-phenol ratio increases the yield of the ortho-product in these cases [16]. A speculation about the reason for this situation involves destruction of peroxydisulfate by reaction with a phenolic free-radical and regeneration of the phenol by decomposition of an aromatic hydroperoxide according to Scheme 1[17]. It is probably relevant that Huyser has found catalysis of the free-radical oxidation of secondary alcohols by 4-pyridone but not by the 2-isomer [18]. The kinetics of the reaction between 4-pyridone and peroxydisulfate have also been measured [19]. The reaction has the smallest rate constant known for any Elbs oxidation: k2(30°C) = 0.0026 L/mol-min, which corresponds to a half-time at 0.1 M of about three days.
Figure 2.
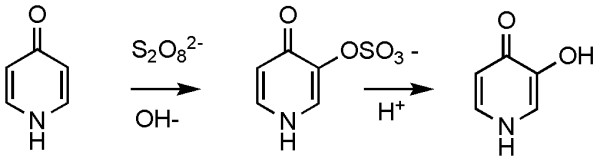
Synthesis of 3-hydroxy-4-pyridone via the 3-sulfate.
Scheme 1.
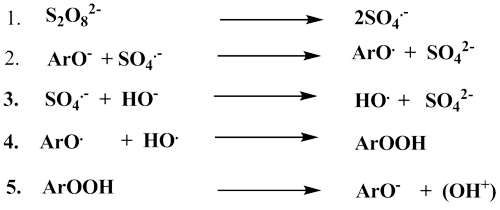
Destruction of peroxydisulfate by reaction with a phenolic free-radical.
Singh and Venkatarao [20] have shown that peroxydisulfate decomposes under alkaline conditions according to the two-term rate law: v = k1 [S2O82-] + k2 [S2O82-] [OH-] (eq. 1). Elbs oxidations are necessarily conducted in alkali but allowances for eq. 1 are usually not required because the rates of most Elbs oxidations are much greater than eq. 1. For example, the half-time for the decomposition of peroxydisulfate in 2 M NaOH at 40°C is about 80 hr (consistent with an extrapolation of the data given in [20]) whereas the Elbs oxidation of 2-pyridone (v = [S2O82-] [2-pyridone anion]) has a half-time of the order of 30 min. 4-Pyridone, however, reacts very slowly with peroxydisulfate so that eq. 1 cannot be ignored. The innovations here involve proper maintenance of the pH, removal of starting material before isolation of the product, easy separation of the product from by-products by crystallization as the monomethanolate, and isolation of the intermediate sulfate ester, 4-pyridone-3-sulfate. Unreacted 4-pyridone is removed from the dried, neutralized reaction mixture by Soxhlet extraction with methyl ethyl ketone. Then acid-catalyzed hydrolysis of the sulfate ester, neutralization, and extraction of the dried salt cake with 2-propanol gives the product 3-hydroxy-4-pyridone, following crystallization from methanol, in about 13% yield. This is about a 30% increase over previous procedures. The material has been crystallized in the past from water and from ethanol (1–2, 4–11). It crystallizes from ethanol well except that in the present synthesis there are impurities which make ethanol a poor choice for the first crystallization; sticky and hygroscopic materials co-precipitate. Methanol, however, solves these problems; nice crystals of the monomethanolate form readily and the impurities remain in solution. The NMR spectrum of the monomethanolate establishes the ratio of methanol to the pyridine as one. Contrary to expectations, reaction yields were not improved by lowering the temperature.
Examination of crude (and salty) reaction mixtures (which smell strongly of ammonia, indicating ring destruction) by proton NMR (D2O) shows the presence of starting material (δ 7.76 & 6.45, d, J = 7.3 Hz), the expected monosulfate ester (δ 7.99, s, 7.70 & 6.50, d, J = 6.9 Hz), and a downfield singlet (δ 8.3) attributable to the presence of a small amount (5% of product) of the disulfation product, 4-pyridone-3,5-disulfate (the resonance for 3,4,5-trihydroxypyridine itself is a singlet at δ 7.570) (lit[21], 7.50). In addition, under certain conditions, there appear two doublets at δ 6.41 and 8.18 with a coupling constant of 7.7 Hz. Formation of this molecule is suppressed at high concentration of alkali.
4-Pyridone-3-sulfate has been isolated from the urine of cattle but without details [3]. It was isolated in this work by first extracting the crude neutralized Elbs oxidation product with methyl ethyl ketone as described above to remove unreacted 4-pyridone. The extracted salt cake was dried and then re-extracted with 95% ethanol. Cooling the ethanolic extract gave crystals of the sodium salt of 4-pyridone-3-sulfate. 3-Hydroxy-4-pyridone can then be made from this ester by acid-catalyzed hydrolysis. This route gives the better overall yield.
Experimental
3-Hydroxy-4-Pyridone
4-Pyridone monohydrate (Alfa-Aesar, 5.5 g, 0.049 mol) was dissolved in 125 mL of water. NaOH (10 g, 0.25 mol) was added followed by 16.6 g sodium peroxydisulfate (0.07 mol) added in portions during 10 min. at 90 – 95°C. The solution was kept at this temperature for 15 min. more after which a test for peroxydisulfate with iodide was negative. Then 7 g additional NaOH was added followed by 16.6 g of sodium peroxydisulfate again added in portions during 10 min. Following another 15 min., the solution was cooled to RT and neutralized with concentrated sulfuric acid. The neutralized solution was taken to dryness on a rotary evaporator at 45°C using ethanol for final drying. The salt cake was removed from the flask and air-dried. The dry salt cake was powdered using a mortar and pestle and then extracted for seven hours in a Soxhlet apparatus with methyl ethyl ketone yielding about 1 g of 4-pyridone (20%). The extracted salt cake was dried and then dissolved in 125 mL water, 3 mL concentrated sulfuric acid added, and the solution hydrolyzed by boiling for 30 min. The hydrolysate was cooled on an ice bath and neutralized with a concentrated solution of sodium hydroxide. The neutral solution was taken to dryness by rotary evaporation again using ethanol for final drying. The salt cake was air-dried overnight and finally extracted for seven hours using the Soxhlet apparatus with 2-propanol. The light brown solution was dried by rotary evaporation to yield a sticky brown solid. This was dissolved in 5 mL of hot methanol. Crystals of product form slowly at RT. Cooling at 5°C gives a yield of 1 g of tan crystals of the monomethanolate (13%). It can be recrystallized from ethanol or freed of solvent by vacuum sublimation.
MP: sinters at 180–185, 242–243 (dec.)
MS: electrospray, time-of-flight: 223.0712 (calc. 223.0719, protonated dimer) and 334.1054 (calc. 334.1039, protonated trimer)
1H NMR: 600 MHz(D2O) [DMSO-d6] δ 7.576 [7.73], dd, H-6(J = 6.8, 1.3 Hz); 7.546 [7.67], d, H-2(J = 1.3 Hz); 6.496 [6.57], d, H-5(J = 6.8 Hz). (DMSO-d6)
13C NMR: 151 MHz (DMSO-d6) δ 169.0, C-4; 148.2, C-3; 135.8, C-6; 123.2, C-2; 113.6, C-5
IR (Nujol):
Methanolate: 1632, 1539, 1337, 1300, 1192, 1035, 882, 829, 788 cm-1
Ethanolate: 1633, 1549, 1510, 1324, 1295, 1241, 1187, 1145, 1050, 883, 821, 789, 755, 722 cm-1
Hydrate: 1622, 1562, 1503, 1288, 1193, 1138, 1052, 819, 781 cm-1
Anhydrous: 1639, 1543, 1486, 1287, 1250, 1145, 1049, 847, 824, 773 cm-1.
FeCl3 complex: λmax 535 nm
UV: λmax 273 nm, ε 12,000 M-1cm-1 (water)
4-Pyridone-3-Sulfate
Following removal of 4-pyridone by extraction with methyl ethyl ketone as described above, the dried salt cake was re-extracted with about 250 mL of 95% ethanol for seven hours. Cooling the solution gave 2 g of crude material as off-white crystals (17%). It was recrystallized from 95% ethanol. It gradually decomposes between 250–290°C without melting. Anal. calcd. for C5H4NO5SNa + 1.5H2O: C, 25.00%; H, 2.94%; N, 5.83%. Found: C, 25.17%, H, 2.70%; N, 5.87%. Hydrolysis of the ester in 0.2 M sulfuric acid for 30 min at 95°C followed by neutralization with NaOH and drying gives a residue from which 3-hydroxy-4-pyridone may be extracted quantitatively by trituration with boiling 95% ethanol and purification by crystallization from methanol as above.
IR (Nujol): 1640, 1586, 1556, 1542, 1283, 1242, 1193, 1177, 1078, 1052, 913, 799 cm-1
1H NMR: 600 MHz (D2O) δ 8.136, d, H-2(J = 1.55); 7.850, dd, H-6(J = 7.11, 1.50); 6.700, d, H-5(J = 7.11)
13C NMR: 151 MHz (D2O) δ 173.5(C-4), 140.3(C-3), 137.3(C-6), 132.2(C-2), 117.4(C-5)
UV: λmax 260 nm, ε 12,100 M-1cm-1 (water)
Competing interests
The author declares that he has no competing interests.
References
- Spenser ID, Notation AD. A synthesis of mimosine. Can J Chem. 1962;40:1374–1379. doi: 10.1139/v62-210. [DOI] [Google Scholar]
- Houghton C, Cain RB. Microbial metabolism of the pyridine ring. Biochem J. 1972;130:879–893. doi: 10.1042/bj1300879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie GS, Lee CP, Hegarty MP. Antithyroid properties of 3-hydroxy-4(1H)-pyridone: Antiperoxidase activity and effect on thyroid function. Endocrinology. 1979;105:342–347. doi: 10.1210/endo-105-2-342. [DOI] [PubMed] [Google Scholar]
- Ost H. Stickstoffhaltige derivate der mekonsäure und ihre umwandlung in pyridin. J Prakt Chem. 1883;24:257–294. doi: 10.1002/prac.18830270114. [DOI] [Google Scholar]
- Peratoner A, Tamburello A. Sopra alcuni piridoni dall'acido piromenico e dal maltolo. Gazz Chim Ital. 1906;36:50–57. [Google Scholar]
- Bickel AF. On the structure of leucaenine. J Am Chem Soc. 1947;69:1805–1806. doi: 10.1021/ja01199a068. [DOI] [PubMed] [Google Scholar]
- Belonosov IS. Synthesis of 3,4-dihydroxysulfapyridine. Zhur Priklad Khim. 1949;22:1103–1107. [CA, 1951, 45: 5650g]. [Google Scholar]
- Harris RLN. Potential wool growth inhibitors. Aust J Chem. 1976;29:1329–1334. doi: 10.1071/CH9761329. [DOI] [Google Scholar]
- Adams R, Cristol SJ, Anderson AA, Albert AA. The structure of leucenol. J Am Chem Soc. 1945;67:89–92. doi: 10.1021/ja01217a032. [DOI] [Google Scholar]
- Hart NK, Hofmann A, Lamberton J, Richards CM. Mimosine, mimosinamine, and 3,4-dihydroxypyridine. Heterocycles. 1977;7:265–272. doi: 10.3987/S-1977-01-0265. [DOI] [Google Scholar]
- Behrman EJ, Pitt BM. The Elbs peroxydisulfate oxidation in the pyridine series. J Am Chem Soc. 1958;80:3717–3718. doi: 10.1021/ja01547a063. [DOI] [Google Scholar]
- Behrman EJ, Goswami MND. Quantitative determination of o- and p-dihydric phenols. Anal Chem. 1964;36:2189–2191. doi: 10.1021/ac60217a046. [DOI] [Google Scholar]
- Lovell S, Subramony P, Kahr B. Poppy acid. J Am Chem Soc. 1999;121:7020–7025. doi: 10.1021/ja990402a. [DOI] [Google Scholar]
- Trécourt F, Mallet M, Mongin O, Gervais B, Queguiner G. New synthesis of orelline. Tetrahedron. 1993;49:8373–8380. doi: 10.1016/S0040-4020(01)81920-7. [DOI] [Google Scholar]
- Behrman EJ. The persulfate oxidation of phenols and arylamines. Organic Reactions. 1988;35:421–511. [Google Scholar]
- Rao KB, Rao NVS. Observations on the Elbs persulphate oxidation. J Sci Ind Res. 1955;14B:130–131. [Google Scholar]
- Behrman EJ. The Elbs and Boyland-Sims peroxydisulfate oxidations. Beilstein J Org Chem. 2006;2:#22. doi: 10.1186/1860-5397-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyser ES, Feng RHC. Catalytic effect of 4-pyridone on the free-radical oxidations of secondary alcohols with t-butyl peroxide. J Org Chem. 1969;34:1727–1729. doi: 10.1021/jo01258a043. [DOI] [Google Scholar]
- Behrman EJ. Studies on the mechanism of the Elbs peroxydisulfate oxidation. J Am Chem Soc. 1963;85:3478–3482. doi: 10.1021/ja00904a038. [DOI] [Google Scholar]
- Singh UC, Venkatarao K. Decomposition of peroxodisulphate in aqueous alkaline solution. J Inorg Nucl Chem. 1976;38:541–543. doi: 10.1016/0022-1902(76)80300-4. [DOI] [Google Scholar]
- Krowicki K. 3,4,5-Trisubstituted alkoxy and hydroxy derivatives of pyridine. Rocz Chem. 1976;50:337–340. [Google Scholar]


