Abstract
Background
Pancreatic cancer is a near uniformly lethal disease and a better understanding of the molecular basis of this malignancy may lead to improved therapeutics. The Axl receptor tyrosine kinase is implicated in cellular transformation and tumor progression, although its role in pancreatic cancer has not been previously documented.
Results
Axl labeling was present in 54 of 99 (55%), and was absent in 45 of 99 (45%) cases, respectively. Axl expression in pancreatic cancer was significantly associated with lymph node metastases (p < 0.01), and a shorter median survival (12 versus 18 months, p < 0.01), than in tumors with negative labeling. Stable knockdown of Axl resulted in significant reduction in cell viability (p < 0.001), anchorage independent growth (p = 0.0031), as well as attenuation of migratory (p < 0.001) and invasive properties (p < 0.005), compared to vector-transfected cells. Profound inhibition of p42/p44 MAP kinase and PI-3kinase/Akt effector pathways was observed in MIAPaCa-2 cells with loss of Axl function. The reduction in invasion and migration upon Axl knockdown was mirrored by a decrease in the amounts of activated (GTP-bound) GTPase proteins Rho and Rac, significant downregulation in transcript levels of the epithelial mesenchymal transition (EMT)-associated transcription factors slug, snail and twist, and significant decrease in matrix metalloproteinase MMP-9 mRNA levels.
Materials
The immunohistochemical expression of Axl protein was assessed in a panel of 99 archival pancreatic cancers. Endogenous Axl expression was stably downregulated by lentiviral short hairpin shRNA directed against AXL mRNA in MIAPaCa-2 cells, and the effects on cell viability, anchorage independent growth, invasion, migration and intracellular effector pathways was assessed, by comparing to lentiviral vector-transfected cells.
Conclusion
Expression of Axl tyrosine kinase in pancreatic cancers confers an adverse prognostic influence, and represents a new therapeutic target in this malignancy.
Keywords: Axl, tyrosine kinase, pancreatic cancer, migration, epithelial mesenchymal transition, Rho, Rac, snail, slug, twist
Introduction
Ductal adenocarcinoma of the pancreas (a.k.a. pancreatic cancer) is a near uniformly lethal malignancy that accounts for approximately 34,000 deaths in the United States every year.1 Worldwide pancreatic cancer causes an estimated 213,000 deaths each year and is the fourth most common cause of cancer-related mortality. For all stages combined, the 1-year survival is around 20%, and the overall 5-year survival rate is less than 5%, despite even the most aggressive therapies currently available.2 The overwhelming majority of patients present with locally advanced or distant metastatic disease, rendering their disease surgically unresectable. Currently available conventional chemo-radiation therapies are minimally effective in prolonging the median survival of patients with advanced pancreatic cancer.3 Thus, there is an urgent need for developing more potent therapies, particularly those targeted at aberrantly expressed molecules or pathways in this malignancy.4
Receptor tyrosine kinases (RTKs) play an important role in signal transduction in both normal and malignant cells.5,6 There are different families of RTKs which are mainly characterized by differences in the ligand-binding extracellular domains. The mammalian TAM RTK family includes three closely related members: Tyro-3, Axl, Mer.7 The extracellular domain of these three RTKs is comprised of two immunoglobulin-like domains followed by two fibronectin type 3-like domains. Axl, also known as Ark, UFO or Tyro7, was originally isolated as a transforming gene from human leukemia cells.8,9 Since then, aberrant expression of Axl has been reported in a variety of solid cancers, including gliomas, melanomas and lung and breast cancers, amongst others.10–16 In a subset of these studies, overexpression of Axl in the cancer cells was associated with adverse clinical prognosis, such as advanced stage of disease at presentation and shorter median survival compared to corresponding non-expressing cancers.11,14,17 Not surprisingly, in addition to promoting transformation and survival, the Axl RTK has been shown to play an important role in mediating cellular migration and invasion, properties that are critical to tumor progression and metastases.10,14,18
We undertook the current study in light of the surprising paucity of data on the putative role of Axl in pancreatic cancer, particularly given the pervasive importance of metastatic disease as a determinant of survival in this malignancy. Our studies demonstrate that the Axl protein is commonly overexpressed in pancreatic cancer tissues, and that this aberrant expression is associated with a higher propensity for lymphatic metastases and significantly shorter median survival compared to Axl-negative cases within the same cohort. Further, our studies identify an unequivocal role for Axl in enhancing pancreatic cancer growth, migration and invasion, and provide evidence that this RTK is a new therapeutic target in this malignancy.
Results
Axl expression in pancreatic adenocarcinoma correlates with an adverse prognosis
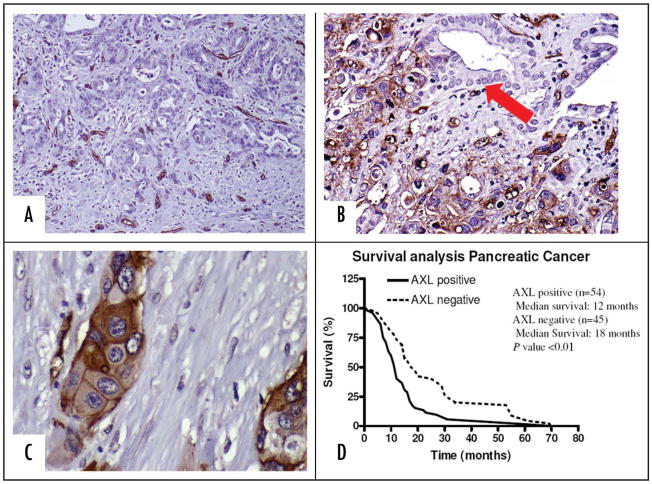
Immunohistochemical analysis for Axl expression was performed on a panel of 99 pancreatic cancers using an anti-Axl-specific antibody. The clinicopathologic features of the 99 patients stratified by their Axl expression are summarized in Table 1. Axl labeling was observed in 54 of 99 (55%) pancreatic cancers (Fig. 1B), and was absent in 45 of 99 (45%) cases (Fig. 1A), respectively. In the neoplastic cells, Axl protein was distributed in the cytoplasm with a pronounced membranous accentuation, as would be expected of a RTK (Fig. 1C). Normal pancreatic ductal epithelium, by contrast, had minimal to no expression of Axl protein (Fig. 1B, arrow). In Axl-negative cancers, expression of the protein was restricted to the tumor-associated vasculature (Fig. 1A), a not unexpected finding given the previously reported association between Axl and endothelial function in both normal and tumor tissues.18,28 In terms of clinicopathological correlation, Axl protein expression in pancreatic cancers was significantly associated with lymph node metastases (p < 0.01). Further, Kaplan Meier survival analysis demonstrated that patients harboring Axl-positive tumors had a significantly shorter median survival than patients with Axl-negative cancers (12 months versus 18 months, respectively; p < 0.01), underscoring the adverse prognostic influence conferred by Axl expression (Fig. 1D).
Table 1.
Clinicopathological correlates of Axl expression in archival pancreatic cancers
| Clinicopathologic features | AXL expression | |||
|---|---|---|---|---|
| Total | Negative (n = 45) | Positive (n = 54) | p value | |
| Gender | ||||
| Male | 43 | 19 | 24 | |
| Female | 56 | 26 | 30 | 0.84 |
| Tumorsize (cm) | ||||
| ≤3,0 | 64 | 28 | 36 | |
| >3,0 | 35 | 17 | 18 | 0.65 |
| Lymph Node Status | ||||
| <15% positive | 52 | 31 | 21 | |
| >15% positive | 47 | 14 | 33 | <0.01* |
| Margins | ||||
| Positive | 65 | 31 | 34 | |
| Negative | 34 | 14 | 20 | 0.54 |
| Differentiation | ||||
| Well or Moderated | 53 | 28 | 25 | |
| Poorly | 46 | 17 | 29 | 0.12 |
| Staging | ||||
| Localized | 16 | 5 | 11 | |
| Advanced | 83 | 40 | 43 | 0.22 |
Statistically Significant.
Figure 1.
The Axl receptor tyrosine kinase is overexpressed in pancreatic cancer. (A) A pancreatic cancer with absence of Axl labeling. Only the intra-tumoral blood vessels are positive for Axl expression. (B) A pancreatic cancer with diffuse overexpression of Axl in the neoplastic cells. Note absence of labelling in adjacent normal ductal epithelium (arrow). (C) Higher magnification of a pancreatic cancer with Axl expression. The infiltrating adenocarcinoma shows cytoplasmic staining with membranous accentuation for Axl, consistent with the pattern of expression for a receptor tyrosine kinase. (D) Axl expression is associated with adverse prognosis in pancreatic cancer. Kaplan Meier survival analysis confirms that patients with Axl-expressing tumors demonstrate a significantly shorter median survival than those with Axl-negative cancers (12 months versus 18 months, p < 0.05).
Axl protein is overexpressed in pancreatic cancer cell lines compared to HPNE cells
Axl expression was assessed in a panel of five pancreatic cancer cell lines (PANC1, CFPAC, MIAPaCa-2, PK-9 and SU.86.86), and in the hTERT-immortalized HPNE cells. Minimal Axl expression was observed in HPNE, while three cancer cell lines (MIAPaCa-2, CFPAC and PANC1) had variable Axl overexpression, when compared to the “baseline” level in HPNE cells (Fig. 2A). The MIAPaCa-2 cells were selected for subsequent functional studies comparing Axl-expressing cells to those with knockdown of endogenous protein.
Figure 2.
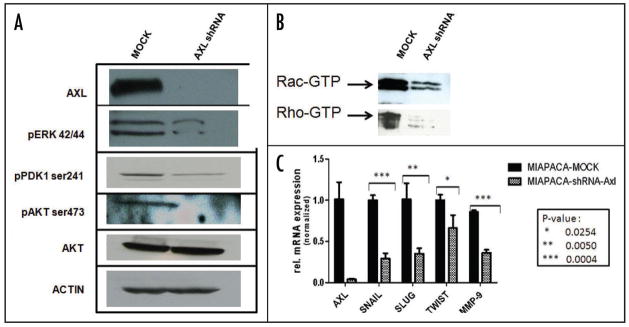
Axl expression in pancreatic cancer cell lines and stable knockdown of Axl in MIAPaCa-2 cells. (A) Axl protein is overexpressed in three of five pancreatic cancer cell lines (PANC-1, CFPAC1 and MIAPaCa-2), compared to the hTERT immortalized human pancreatic epithelial line HPNE. (B) Endogenous Axl was downregulated in the Axl-expressing MIAPaCa-2 cells using a lentiviral shRNA vector. Compared to vector-transfected (“mock”) clones, the shRNA-expressing cells demonstrate essentially complete loss of Axl protein. (C) Quantitative real-time PCR (qRT-PCR) confirms the downregulation of AXL transcripts in shRNA expressing MIAPaCa-2 clones. The assays were performed in triplicate and SDHA was used as housekeeping control.
Knockdown of endogenous Axl inhibits cell viability, anchorage independent growth, invasion and migration of MIAPaCa-2 cancer cells
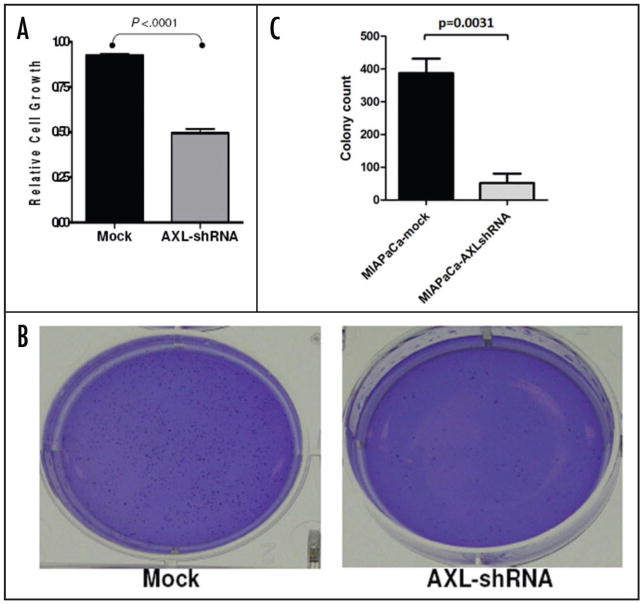
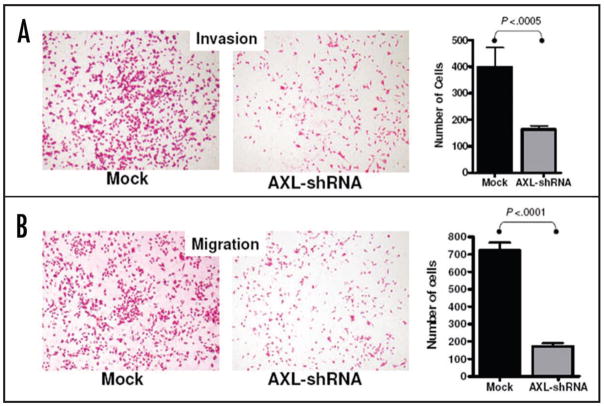
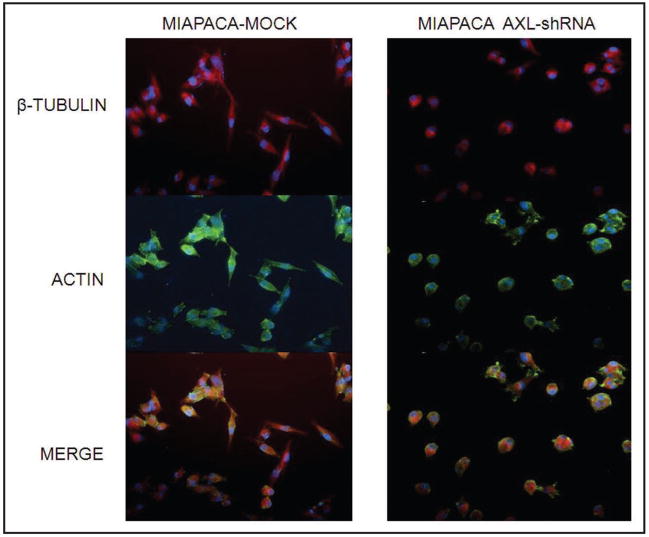
Parental MIAPaCa-2 cells were stably infected with either empty lentiviral vector or virus expressing AXL shRNA. Both Western blot analysis (Fig. 2B) and qRT-PCR (Fig. 2C) confirmed significant knockdown of the endogenous protein in AXL shRNA-expressing cells compared to the empty vector infected MIAPaCa-2 cells. Endogenous AXL knockdown led to significant reduction in viability of MIAPaCa-2 cells, compared to vector-transfected cell line, as assessed by in vitro MTS assay (Fig. 3A) (p < 0.001). Moreover, Axl knockdown inhibited the phenotype of anchorage-independent growth, with a significant reduction in colony formation in soft agar (Fig. 3B and C) (p = 0.0031). Multiple studies have reported that Axl plays an important role in promoting the migration of cancer cells, facilitating tumor progression.10,14,18 Therefore, we utilized modified Boyden chamber assays to assess the effects of Axl knockdown on in vitro invasion and migration, and found a significant reduction in both phenomena compared to MIAPaCa-2 cells with retained Axl function (Fig. 4A and B, p < 0.0005 and p < 0.0001, respectively). We also examined the morphology of MIAPaCa-2 cells following Axl knockdown, and these cells demonstrated a striking loss of polarity and absence of filopodia, compared to cells with retained Axl function, which displayed an organized polarity and well-formed filopodia formation (Fig. 5).
Figure 3.
Knockdown of endogenous Axl in MIAPaCa-2 inhibits in vitro cell viability and anchorage independent growth. (A) In vitro cell viability of Axl shRNA-expressing MIAPaCa-2 cells was significantly reduced compared to vector-transfected cells (p < 0.0001), as measured using MTS assay. The MTS assays were performed in triplicate, and mean and standard deviations are plotted. (B) Anchorage independent growth of Axl shRNA-expressing MIAPACA-2 cells, as assessed by colony formation in soft agar, was significantly reduced compared to vector-transfected cells (p = 0.0031). Colony assays were performed in triplicate, and the mean and standard deviations of colony counts were calculated for each condition. (C) Representative soft agar assay of Axl shRNA-expressing MIAPACA-2 compared to vector-transfected cells.
Figure 4.
Knockdown of endogenous Axl in MIAPaCa-2 cells inhibits in vitro invasion and migration. (A) Modified Boyden chamber assay (with Matrigel plug) was performed to assess in vitro invasion in MIAPaCa-2 cells. At 72 hours, loss of endogenous Axl function was associated with significant reduction in invasive capacity compared to vector-transfected cells (p < 0.0005), when normalized for cell viability. The histogram represents mean and standard deviation of invasion assay performed in triplicate. (B) Modified Boyden chamber assay (without Matrigel plug) was performed to assess in vitro migration in MIAPaCa-2 cells. At 72 hours, loss of endogenous Axl function was associated with significant reduction in migratory capacity compared to vector-transfected cells (p < 0.0001), when normalized for cell viability. The histogram represents mean and standard deviation of migration assay performed in triplicate.
Figure 5.
Knockdown of endogenous Axl is associated with reduction in filopdial extensions and loss of polarity in MIAPaCa-2 cells. Immunofluorescence studies demonstrate that vector-transfected MIAPaCa-2 cells have a spindled morphology, with well formed filopdial extensions as seen by β-tubulin/actin compound immunostaining. In contrast, loss of Axl is associated with loss of polarity and cell rounding, and reduction in the filopodial extensions. DAPI is used as a nuclear counterstain.
Knockdown of endogenous Axl is associated with inhibition of multiple effector pathways in pancreatic cancer
We then explored the status of multiple effector pathways that are implicated in pancreatic cancer growth and progression. Two major intracellular effectors downstream of RTKs in both normal and cancer cells are the p42/p44 MAP kinase and the PI-3-kinase/Akt signaling pathways.29 In MIAPaCa-2 shRNA expressing cells, we found inhibition of both effector arms, as evidenced by reduced phosphorylation of ERK1/ERK2, PDK1ser241 and Aktser473, compared to vector transfected cells (Fig. 6A). In contrast, total Akt levels were unaffected. In light of the profound inhibition of migration and invasion of MIAPaCa-2 cells with loss of endogenous Axl function, we looked at the activation status of the Rho family of GTPases, which control a number of cytoskeletal dynamics including cell migration,30,31 using immunoprecipitation for the active (GTP-bound) complement of Rho and Rac. In contrast to vector-transfected cells, loss of Axl function was associated with marked reduction in the levels of intracellular activated Rho/Rac GTPases (Fig. 6B). Finally, we examined the level of expression of the epithelial-mesenchymal-transition (EMT)-associated transcriptional repressors, snail, slug and twist,32 and mRNA levels of all three genes were significantly downregulated upon Axl knockdown (p = 0.0004, p = 0.005 and p = 0.0254, for differential expression of snail, slug and twist, respectively) (Fig. 6C). There was also significant downregulation in mRNA levels of the matrix metalloproteinase MMP-9 in MIAPaCa-2 shRNA-expressing cells compared to vector transfected controls.
Figure 6.
Multiple intracellular effector pathways are disrupted upon inhibition of endogenous Axl function in MIAPaCa-2 cells. (A) Lentiviral shRNA-mediated stable knockdown of Axl in MIAPaCa-2 cells blocks the activation of critical intracellular effector pathways known to be activated in pancreatic cancer, including the MAP kinase and PI-3-kinase/Akt signaling pathways. Western blot assay was performed for phospho-ERK1/2, phospho-Aktser473, and phospho-PDK1ser241, all of which demonstrated reduction in levels of specific phosphorylated protein, compared to vector-transfected cells. In contrast, no changes were seen in the levels of total Akt protein. Actin was used as loading control. (B) Knockdown of endogenous Axl is associated with reduction in GTP-bound (active) Rho and Rac in MIAPaCa-2 cells compared to vector-transfected cells. The immunoprecipitation assay specifically “pulls down” GTP-bound forms of both proteins. (C) qRT-PCR demonstrates that knockdown of endogenous Axl results in significant reduction in transcripts for snail (p = 0.0004), slug (p = 0.005), and twist (p = 0.0254), whose protein products are transcription factors implicated in epithelial-mesenchymal-transition (EMT), compared to vector-transfected MIAPaCa-2 cells. Loss of Axl function was also associated with significant reduction in transcripts for the matrix metalloproteinase MMP-9 (p = 0.0004). All qRT-PCR assays were performed in triplicate, and GUSB was used as housekeeping control. The Y-axis represents relative fold level in Axl shRNA-expressing cells normalized to vector-transfected cells, and mean and standard deviations are plotted.
Discussion
The Axl RTK belongs to the TAM family of protein kinases, which is comprised of Tyro-3, Axl and Mer proteins, and which has been implicated in a diverse array of cellular functions such as cell adhesion, migration, proliferation and survival, and regulation of inflammation, amongst others.7 Following its isolation as a transforming gene in hematological malignancies,8,9 an increasing array of human cancers have been identified with aberrant Axl experession.10–16 The ligand for the Axl receptor is the vitamin K-dependent protein growth arrest specific-6 (Gas6),33,34 and this molecule is also overexpressed concurrently with Axl in numerous cancers, thereby sustaining an aberrant Gas6-Axl axis.11,35–39 To the best of our knowledge, this is the first study to document aberrant expression of Axl in pancreatic adenocarcinomas. By immunohistochemical analysis, 55% of surgically resected pancreatic cancers demonstrated Axl overexpression, and this was correlated with significantly higher prevalence of lymph node metastases, as well as a significantly shorter median survival compared to Axl-negative tumors. The association between Axl overexpression in cancers and an adverse prognosis is not unprecedented, as similar findings have been reported in lung cancer and gliomas, amongst others.11,14 Since the cohort of cases included for immunohistochemistry was, by definition, restricted to surgically resectable pancreatic cancers without evidence of concurrent metastatic disease, we are unable to document whether Axl upregulation also correlates with distant metastases. Nevertheless, the higher prevalence of nodal positivity and shortened median survival of patients with Axl-expressing pancreatic cancers suggests that aberrant expression of this protein likely facilitates disease dissemination in vivo. This is reaffirmed by the in vitro phenotype observed in MIAPaCa-2 pancreatic cancer cells with genetic knockdown of endogenous Axl protein, which results in profound loss of invasive and migratory capabilities, accompanied by a near-complete loss of filopodial extensions. The decrease in invasive/migratory capacity occurs independent of the deleterious effects of Axl knockdown on cell viability, and the substance of these findings is essentially identical to those reported in glioma cell lines with blockade of endogenous Axl function using a dominant negative mutant protein.10 Conversely, ectopic expression of Axl in lung adenocarcinoma cell lines results in increased filopodia formation and enhanced migratory ability.40 Not unexpectedly, Axl has a well documented role in physiological migration of neuronal and vascular smooth muscle cells,41,42 underscoring the association between pathological Axl expression and dissemination of cancer cells from their primary site.
In addition to the observed deleterious phenotype on cell viability, anchorage independent growth, migration and invasion of MIAPaCa-2 cells upon Axl knockdown, we were also able to demonstrate blockade of multiple intracellular effectors in the Axl shRNA expressing cells. Axl promotes the growth and survival of neoplastic as well as non-neoplastic cell types through activation of the p42/44 MAP kinase and the PI-3-kinase/Akt signaling pathways,7,38,43–45 and both effector arms are inhibited in MIAPaCa-2 cells upon Axl knockdown, as evidenced by the decreased phosphorylation of Erk1/2, and PDK1ser241 and Aktser473, respectively. The p42/44 MAP kinase and the PI-3-kinase/Akt pathways are constitutively activated in the majority of pancreatic cancers as a result of upstream somatic KRAS2 gene mutations,46 and the blockade of these pathways by downregulation of Axl in MIAPaCa-2 cells suggests a role for this RTK in ras-dependent signaling. In light of the inhibitory effects of Axl knockdown on invasion/migration, we also examined the status of the Rho family of GTPases in AXL shRNA-expressing cells. The Rho GTPases are multi-functional proteins regulating a plethora of cellular activities like endocytosis, vesicle trafficking and gene transcription, but one of their most prominent effects is on cell migration through modulation of the actin cytoskeleton.30,31 The active state of Rho proteins is characterized by bound GTP, rather than GDP, which forms the basis for the immunoprecipitation assay described here. We demonstrate a marked reduction in GTP-bound Rho and Rac proteins, consistent with inactivation of these signaling molecules upon Axl knockdown. Previous studies in neuronal cells and NIH3T3 cells with ectopic Axl expression have reported a PI-3-kinase-dependent activation of the Rho GTPases by Axl,41,47 and our results suggest that such as axis is maintained in pancreatic cancer as well. We also demonstrate downregulation of numerous transcripts associated with the phenomenon of cancer cell invasion, including variably significant reduction in levels of snail, slug and twist mRNA, whose products regulate the process of “epithelial-mesenchymal-transition” or EMT.48 Acquisition of EMT is required for vascular intravasation and metastatic seeding by tumor cells, and the snail/slug/twist family of transcriptional repressors play an essential role in this phenotypic switch. To the best of our knowledge, this is the first description of an association between transcription factors facilitating EMT and Axl, and provides further mechanistic bases for the observed blockade in invasion/migration upon knockdown of this RTK in MIAPaCa-2 cells. Finally, we document significant reduction in transcripts for the matrix metalloproteinase MMP-9 in AXL shRNA-expressing MIAPaCa-2 cells. MMP-9 (also known as gelatinase-B) is a type IV collagenase involved in basement membrane proteolysis and in tumor angiogenesis, and its expression within the tumor microenvironment promotes invasion and metastases.49,50 MMP-9 is reported as overexpressed in pancreatic cancer across multiple prior studies.51–53 Further, a recent study demonstrates that ectopic Axl in cancer cells can upregulate MMP-9, and render the cells more invasive.54 Our results are in concert with these prior findings, and underscore the importance of sustained Axl expression towards promoting multiple facets of the invasion and metastatic cascade in pancreatic cancer.
Our study does not address the molecular basis for Axl overexpression in pancreatic cancer, although some conclusions can be drawn from existing literature. One common mechanism of RTK activation, with or without associated protein overexpression, is via somatic mutations, and this is best exemplified by the EGFR family of oncogenic receptors in solid tumors.55,56 Although rare somatic mutations of the AXL gene have been reported in lung, ovarian and gastric cancers (see the Catalog of Somatic Mutations in Cancer, or COSMIC database at http://www.sanger.ac.uk/genetics/CGP/cosmic),57 the recently completed pancreatic cancer genome sequencing project has failed to detect somatic mutations of this gene in a panel of 24 pancreatic cancers, upon analysis of all coding exons.58 Thus, mutational events are likely to be a rare to absent basis for Axl activation in pancreatic cancer. Another common mechanism for oncogene overexpression is amplification of the corresponding genomic region in cancer cells.59 The AXL gene is located at chromosome 19q13.1, a site of frequent amplification in solid tumors,60 including in pancreatic cancer.61,62 While Axl upregulation in a subset of cancers may be the result of copy number alteration, the frequency of 19q13.1 genomic amplification in pancreatic cancer is significantly lower than the prevalence of Axl overxpression in this malignancy (55%), as observed in the current study. This is a trend observed with other recurrent amplicons in pancreatic cancer such as MYC and GATA-6 as well, wherein the frequency of protein overexpression typically exceeds that of genomic copy number alterations.63–65 Thus, prima facie evidence points to transcriptional deregulation of AXL as the most frequent cause for overexpression of its protein product in pancreatic cancer, although the proximate transcriptional factor(s) driving this phenomenon remain to be elucidated. Most recently, Mudduluru and colleagues have elucidated the promoter sequence of the AXL gene, and have identified two potential sources of regulation: first, the existence of multiple specificity protein (Sp)-binding sites to which can bind the Sp1 family of transcription factors, and second, the presence of CpG islands within these Sp-binding motifs that can be reversibly methylated and thereby epigenetically silence AXL transcription.66 Multiple studies have documented overexpression of the Sp1 transcription factor in pancreatic cancer,67–69 and it is tempting to speculate that cancer-specific hypomethylation of the Sp-binding motifs permits unrestricted AXL transcription to occur in pancreatic cancers with Axl overexpression. We emphasize the speculative nature of this chain of events, and future studies to better elucidate the mechanism of Axl overexpression in pancreatic cancer are certainly warranted.
An encouraging aspect of our results is the possibility that Axl might become a relevant therapeutic target in pancreatic cancer, a disease where conventional chemo-radiation therapies have had minimal impact on ameliorating prognosis.1 RTKs have proven to be facile targets for the development of small molecule kinase inhibitors and monoclonal antibodies. Of note, potent small molecule Axl antagonists have recently been identified and are in active preclinical development.70–72 In addition to inhibiting tumor growth, these antagonists have also been shown to block angiogenesis in the tumor microenvironment, which is not an unexpected finding given that the Axl RTK plays a role in the survival of normal endothelial cells,73 as well as that of tumor-associated endothelium.10,18 In our own immunohistochemical analysis (see Fig. 1), robust Axl expression was seen within the endothelial cells within the tumor milieu, even in pancreatic cancers where the neoplastic cells per se did not express Axl. The in vivo application of these, or related, small molecule antagonists in preclinical models of pancreatic cancer will undoubtedly provide additional insights as to the validity of Axl as a therapeutic target in this malignancy.
Material and Methods
Immunohistochemistry for Axl protein expression
Immunohistochemistry for Axl was performed on formalin fixed paraffin embedded specimens of 99 surgically resected pancreatic adenocarcinomas, with the samples embedded in a tissue microarray (TMA) format, as previously described.19–21 Each case was represented by two 1.4 mm cores of neoplastic tissue, and two of the corresponding non-neoplastic pancreatic parenchyma. Four-micron sections were cut from the TMAs, and deparaffinized by routine techniques. Thereafter, the sections were quenched with 3% H2O2 for 10 minutes. The slides were steamed in 10 mM citrate buffer (ph 6.0) to unmask the epitopes for 20 minutes at 95°C, and then allowed to cool down for 20 minutes to room temperature. Prior to incubating with the primary antibody, the slides were blocked for 30 minutes with a 10% fetal bovine serum solution (Invitrogen, Carlsbad, CA). As primary antibody, we used a 1:100 dilution of hAxl (catalog # AF154, R&D systems, Minneapolis, MN) for 2 hours at room temperature. Labeling was detected with the Dako Envision system (Dako, Envision Plus Detection Kit, Carpinteria, CA) as per the manufacturer’s protocol. Sections were counterstained with Harris hematoxylin, a cover slip was applied. Immunohistochemical labeling was evaluated by two of the authors (J.-B.M.K. and A.M.) at a multi-headed microscope with consensus reached in all cases. A simple two-tier classification of “positive” and “negative” was used, as we have previously described for TMA-based evaluation of immunohistochemical labeling.19–21
Cell lines and culture conditions
Five pancreatic cancer cell lines (PANC1, CFPAC, MIAPaCa-2, PK-9, SU.86.86) were grown in either RPMI 1640 medium (Invitrogen) or in DMEM medium, supplemented with both 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Biofluids, Camarillo, CA). The sources of the pancreatic cancer cell lines used in this study have been described elsewhere.22 Human pancreatic epithelial cells (HPNE), immortalized by human telomerase reverse transcriptase (hTERT), were used as a control for non-neoplastic pancreas. The derivation and culture conditions for HPNE cells have been previously described.23,24
Lentiviral shRNA knockdown of AXL in MIAPaCa-2 cells
Axl-expressing MIAPaCa-2 cells were seeded into 6-well plates at 5 × 105 cells per well, and infected with either empty pLKO.1 lentiviral vector or with lentivirus expressing AXL shRNA (Open Biosystems, Huntsville, AL). Stably infected were selected by adding 3 μg/ml of puromycin to the cell culture media. Quantitative reverse transcription PCR (qRT-PCR) and Western blot analysis to confirm AXL mRNA and Axl protein knockdown, respectively, was performed as described below.
Western blot analysis for Axl and downstream effector pathways
Western Blot analysis was performed for detecting Axl expression in the five cancer cell lines, and compared to baseline levels in HPNE cells. Briefly, protein lysates were made from cell pellets, using the following lysis buffer [20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate, 1 mmol/L EDTA, 0.1% SDS]; protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and phenylmethylsulfonyl fluoride (PMSF) were added before lysing the cells. Cells were lysed on ice for 30 min with occasional gentle agitation of the tubes. Cell debris was separated by centrifugation at 14,000 rpm for 30 min at 4°C. Protein lysates were resolved by electrophoresis on 12% Tris-glycine gel (Invitrogen) and transferred on to nitrocellulose membranes (LC 2000, Invitrogen). Standard immunoblotting procedures were followed with slight modification: nitrocellulose membranes were blocked for one hour at room temperature and incubated with primary anti-hAxl antibody (dilution 1:1,000, catalog #4939S, Cell Signaling, Danvers, MA,) overnight at 4°C. Anti-actin antibody (catalog #sc-1615, Santa Cruz Biotechnology, Santa Cruz, CA) was used as a loading control. The identical protocol was used to confirm Axl protein knockdown in MIAPaCa-2 cells stably infected with lentiviral shRNA. Downstream effector pathways (p42/p44 MAP kinase and PI-3 kinase/Akt) were evaluated in empty vector infected versus AXL shRNA expressing cells, using the following primary antibodies and conditions (all antibodies were purchased from Cell Signaling and used at a 1:1000 dilution with incubation overnight at 4°C): total Akt (catalog #9272), phospho-Akt Ser473 (catalog #9271), phospho-ERK (catalog #9102) and phospho-PDK1 Ser241 (catalog #3061).
RNA extraction and quantitative reverse transcription PCR (qRT-PCR)
MIAPaca-2 cells with empty or AXL shRNA-expressing vectors were lysed and RNA was extracted using RNeasy Mini kit (Qiagen, Valencia, CA). RNA was reverse transcribed with oligo-d(T)12–18 primers at 42°C for 50 minutes using the SuperScript™ First Strand System (Invitrogen), according to manufacturer’s protocol. Primer sequences were designed using Primer3 online primer design software product, and are available upon request. qRT-PCR for AXL transcripts and candidate effector targets of Axl (Snail, Slug, Twist and matrix metalloproteinase MMP-9) was performed using the Quantitect™ SYBRGreen PCR kit on a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA). Relative fold levels were determined using the 2(−ΔΔCt) method, with GUSB used as housekeeping control.25
Rho/Rac immunoprecipitation assay
Rho and Rac activation was determined using the Rho/Rac Activation Assay Combo Kit (Cell Biolabs, Inc., San Diego, CA). Briefly, vector-transfected and shRNA-expressing MIAPaCa-2 clones were cultured in T-75 flask in RPMI with 10% FBS. When cells reached 80% confluent, cells were serum starved in RPMI with 0.5% FBS overnight, followed by stimulation with epidermal growth factor (EGF 10 ng/mL) for 30 minutes. Cells were washed in cold PBS twice, collected with a cell scraper and lysed on ice using the lysis buffer enclosed in the kit. After pre-cleaning of lysates with glutathione agarose, and shearing of DNA by passing 3 times through a 27-gauge needle, protein concentrations were determined by means of Bradford assays and equal amounts of total protein used for the pull-down step. Beads were loaded on a SDS polyacrylamide gel in laemmli buffer containing 5% beta-mercapto-ethanol (Sigma-Aldrich) and membranes developed with anti-Rho or anti-Rac antibodies at a dilution of 1:1,000, as recommended.
In vitro cell viability assays
Cell growth assays using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide reagent (CellTiter96™, Promega, Madison, WI) were performed on vector-transfected and shRNA-expressing MIAPaCa-2 cells, as described previously.26 Upon completion of the assay, 20 μl/well of the CellTiter96™ solution was added for 1 hour and plates were read on a Wallac-1420 Plate reader at OD = 490 nm (PerkinElmer, Boston, MA). All experiments were set up in triplicate to determine means and standard deviations.
Anchorage-independent growth in soft agar
Anchorage-independent growth was assessed by colony formation assays in soft agar, as previously described.26 Briefly, the soft agar assays were set up in 6-well plates, each well containing a bottom layer of 1% agarose (Invitrogen), a middle layer of 0.6% agarose including 10 × 104 cells, and a top layer of medium only. Subsequently, the plates were kept in a tissue culture incubator maintained at 37°C and 5% CO2 for 21 days to allow for colony growth, with top medium being changed on a weekly basis. The assay was terminated at day 21, when plates were stained with 0.5 ml of 0.005% crystal violet (Sigma-Aldrich) solution at 37°C for 2 hours, and colonies were counted in three independent wells for each condition, using an automated ChemiDoc XRS instrument (Bio-Rad, Hercules, CA).
Modified boyden chamber invasion and migration assays
Modified Boyden chamber assays to assess the in vitro invasion and migration of MIAPaCa-2 cells, with or without endogenous Axl expression, were performed as previously described.26,27 Briefly, 20 μg/well of Matrigel (BD Biosciences, San Jose, CA) was applied to 24-well trans-well plates with 8-μm pore size (BD Biosciences) and allowed to solidify overnight. Then, culture medium was added and 5 × 104 cells were seeded into each well. The plates were incubated at 37°C for 72 hours, and thereafter, cells on top of the membrane were removed using a cotton swab, and cells at the bottom were fixed in ethanol and stained with Harris hematoxylin solution. Cells in ten randomly selected microscopic fields were counted in three independent wells for each condition, and means and standard deviations calculated. Transmigrated cells were normalized for viable cell counts in each case, as previously described.26,27 For assessment of migration, the Boyden chamber assay was performed without addition of the Matrigel plug.
Fluorescence microscopy
The following antibodies were used for immunofluorescence studies: β-tubulin (catalog # 2116, AlexaFluor 555 conjugate, Cell Signaling) and actin (catalog # MAB1501x, Alexa fluor 488 conjugate, Millipore, Temecula, CA.). Briefly, 1.5 × 104 cells of both MIAPaca-2 vector-transfected and Axl shRNA-expressing clones were each cultured in RPMI with 10% FBS in tissue culture-slides (BD Falcon, Bedford, MA) overnight. Cells were briefly washed in PBS, and were fixed in 2 ml of 4% paraformaldehyde in PBS for 15 minutes at ambient temperature. Cells were then washed 3 times for 5 minutes each, and covered with ice-cold 100% methanol and incubated at −20°C for 10 minutes. Following that, cells were rinsed in PBS for 5 minutes. Cells were then blocked in 5% normal rabbit serum in PBS with 0.3% Triton X-100 for 1 hr, and incubated in a cocktail of β-tubulin and actin antibodies at a dilution to 1:200 in PBS/0.3% Triton X-100 overnight at 4°C. Cells were then rinsed 3 times for 5 minutes each with PBS. Slides were covered using coverslip with Prolong® Gold Antifade reagent with DAPI (Invitrogen). The edges of the slides were sealed with nail polish. Slides were examined and pictures taken using the appropriate filters.
Statistical analysis
Statistical analyses were performed using SPSS version 11 (SPSS Inc., Chicago, IL). Associations between categorical variables were examined using the Pearson’s chi-square tests, and using the two-tailed t-tests for continuous variables, with a p-value <0.05 being considered statistically significant. Kaplan-Meier survival analysis was performed in GraphPad Prism for Windows version 4.0 (GraphPad Software Inc., San Diego, CA).
Acknowledgments
A.M. was supported by the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Foundation, NIH SPORE (Specialized Programs of Research Excellence) in Gastrointestinal Cancer P50CA62924 and NIH R01CA113669. J-B.K. was supported by the Dutch Cancer Research Foundation (KWF) and the International Exchange Program grant provided by the University Medical Center Utrecht. G.F. was supported by a fellowship grant within the postdoctoral program of the German Academic Exchange Service (DAAD).
References
- 1.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly EM, Abou-Alfa GK. Cytotoxic therapy for advanced pancreatic adenocarcinoma. Seminars in oncology. 2007;34:347–53. doi: 10.1053/j.seminoncol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Strimpakos A, Saif MW, Syrigos KN. Pancreatic cancer: from molecular pathogenesis to targeted therapy. Cancer metastasis reviews. 2008;27:495–522. doi: 10.1007/s10555-008-9134-y. [DOI] [PubMed] [Google Scholar]
- 5.Bennasroune A, Gardin A, Aunis D, Cremel G, Hubert P. Tyrosine kinase receptors as attractive targets of cancer therapy. Critical reviews in oncology/hematology. 2004;50:23–38. doi: 10.1016/j.critrevonc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Robertson SC, Tynan JA, Donoghue DJ. RTK mutations and human syndromes: when good receptors turn bad. Trends Genet. 2000;16:265–71. doi: 10.1016/s0168-9525(00)02077-1. [DOI] [PubMed] [Google Scholar]
- 7.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling and potential therapeutic targeting in human cancer. Advances in cancer research. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, 3rd, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Molecular and cellular biology. 1991;11:5016–31. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neubauer A, O’Bryan JP, Fiebeler A, Schmidt C, Huhn D, Liu ET. Axl, a novel receptor tyrosine kinase isolated from chronic myelogenous leukemia. Seminars in hematology. 1993;30:34. [PubMed] [Google Scholar]
- 10.Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H, Kiessling F, Eichelsbacher U, Essig M, Read TA, Erber R, Ullrich A. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5799–804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, Kostron H, Stockham mer G, Ullrich A. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–8. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 12.van Ginkel PR, Gee RL, Shearer RL, Subramanian L, Walker TM, Albert DM, Meisner LF, Varnum BC, Polans AS. Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer research. 2004;64:128–34. doi: 10.1158/0008-5472.can-03-0245. [DOI] [PubMed] [Google Scholar]
- 13.Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001;37:2264–74. doi: 10.1016/s0959-8049(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 14.Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, Wu CW. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia (New York, NY) 2005;7:1058–64. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berclaz G, Altermatt HJ, Rohrbach V, Kieffer I, Dreher E, Andres AC. Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Ann Oncol. 2001;12:819–24. doi: 10.1023/a:1011126330233. [DOI] [PubMed] [Google Scholar]
- 16.Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, Szabadkai I, Daub H, Keri G, Ullrich A. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer research. 2008;68:1905–15. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 17.Rochlitz C, Lohri A, Bacchi M, Schmidt M, Nagel S, Fopp M, Fey MF, Herrmann R, Neubauer A. Axl expression is associated with adverse prognosis and with expression of Bcl-2 and CD34 in de novo acute myeloid leukemia (AML): results from a multicenter trial of the Swiss Group for Clinical Cancer Research (SAKK) Leukemia. 1999;13:1352–8. doi: 10.1038/sj.leu.2401484. [DOI] [PubMed] [Google Scholar]
- 18.Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE, McLaughlin J, Swift SE, Pali ES, Yam G, Wong S, Lasaga J, Shen MR, Yu S, Xu W, Hitoshi Y, Bogenberger J, Nor JE, Payan DG, Lorens JB. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer research. 2005;65:9294–303. doi: 10.1158/0008-5472.CAN-05-0993. [DOI] [PubMed] [Google Scholar]
- 19.Lee KM, Cao D, Itami A, Pour PM, Hruban RH, Maitra A, Ouellette MM. Class III beta-tubulin, a marker of resistance to paclitaxel, is overexpressed in pancreatic ductal adenocarcinoma and intraepithelial neoplasia. Histopathology. 2007 doi: 10.1111/j.1365-2559.2007.02792.x. [DOI] [PubMed] [Google Scholar]
- 20.Cao D, Zhang Q, Wu LS, Salaria SN, Winter JW, Hruban RH, Goggins MS, Abbruzzese JL, Maitra A, Ho L. Prognostic significance of maspin in pancreatic ductal adenocarcinoma: tissue microarray analysis of 223 surgically resected cases. Mod Pathol. 2007;20:570–8. doi: 10.1038/modpathol.3800772. [DOI] [PubMed] [Google Scholar]
- 21.Swierczynski SL, Maitra A, Abraham SC, Iacobuzio-Donahue CA, Ashfaq R, Cameron JL, Schulick RD, Yeo CJ, Rahman A, Hinkle DA, Hruban RH, Argani P. Analysis of novel tumor markers in pancreatic and biliary carcinomas using tissue microarrays. Hum Pathol. 2004;35:357–66. doi: 10.1016/j.humpath.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Calhoun ES, Hucl T, Gallmeier E, West KM, Arking DE, Maitra A, Iacobuzio-Donahue CA, Chakravarti A, Hruban RH, Kern SE. Identifying Allelic Loss and Homozygous Deletions in Pancreatic Cancer without Matched Normals Using High-Density Single-Nucleotide Polymorphism Arrays. Cancer research. 2006;66:7920–8. doi: 10.1158/0008-5472.CAN-06-0721. [DOI] [PubMed] [Google Scholar]
- 23.Lawson T, Ouellette M, Kolar C, Hollingsworth M. Culture and immortalization of pancreatic ductal epithelial cells. Methods Mol Med. 2005;103:113–22. doi: 10.1385/1-59259-780-7:113. [DOI] [PubMed] [Google Scholar]
- 24.Campbell PM, Groehler AL, Lee KM, Ouellette MM, Khazak V, Der CJ. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer research. 2007;67:2098–106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, Gabrielson KL, Matsui W, Maitra A. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer research. 2007;67:2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 28.D’Arcangelo D, Ambrosino V, Giannuzzo M, Gaetano C, Capogrossi MC. Axl receptor activation mediates laminar shear stress anti-apoptotic effects in human endothelial cells. Cardiovascular research. 2006;71:754–63. doi: 10.1016/j.cardiores.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinases as targets for anticancer drugs. Trends in molecular medicine. 2002;8:17–23. doi: 10.1016/s1471-4914(01)02217-1. [DOI] [PubMed] [Google Scholar]
- 30.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 31.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–70. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–70. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 34.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–6. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- 35.Sun WS, Fujimoto J, Tamaya T. Coexpression of growth arrest-specific gene 6 and receptor tyrosine kinases Axl and Sky in human uterine endometrial cancers. Ann Oncol. 2003;14:898–906. doi: 10.1093/annonc/mdg257. [DOI] [PubMed] [Google Scholar]
- 36.Sun W, Fujimoto J, Tamaya T. Coexpression of Gas6/Axl in human ovarian cancers. Oncology. 2004;66:450–7. doi: 10.1159/000079499. [DOI] [PubMed] [Google Scholar]
- 37.Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. Journal of cellular physiology. 2005;204:36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- 38.Sawabu T, Seno H, Kawashima T, Fukuda A, Uenoyama Y, Kawada M, Kanda N, Sekikawa A, Fukui H, Yanagita M, Yoshibayashi H, Satoh S, Sakai Y, Nakano T, Chiba T. Growth arrest-specific gene 6 and Axl signaling enhances gastric cancer cell survival via Akt pathway. Molecular carcinogenesis. 2007;46:155–64. doi: 10.1002/mc.20211. [DOI] [PubMed] [Google Scholar]
- 39.Mc Cormack O, Chung WY, Fitzpatrick P, Cooke F, Flynn B, Harrison M, Fox E, Gallagher E, Goldrick AM, Dervan PA, Mc Cann A, Kerin MJ. Growth arrest-specific gene 6 expression in human breast cancer. British journal of cancer. 2008;98:1141–6. doi: 10.1038/sj.bjc.6604260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lay JD, Hong CC, Huang JS, Yang YY, Pao CY, Liu CH, Lai YP, Lai GM, Cheng AL, Su IJ, Chuang SE. Sulfasalazine suppresses drug resistance and invasiveness of lung adenocarcinoma cells expressing AXL. Cancer research. 2007;67:3878–87. doi: 10.1158/0008-5472.CAN-06-3191. [DOI] [PubMed] [Google Scholar]
- 41.Allen MP, Linseman DA, Udo H, Xu M, Schaack JB, Varnum B, Kandel ER, Heidenreich KA, Wierman ME. Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase. Molecular and cellular biology. 2002;22:599–613. doi: 10.1128/MCB.22.2.599-613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridell YW, Villa J, Jr, Attar EC, Liu ET. GAS6 induces Axl-mediated chemotaxis of vascular smooth muscle cells. The Journal of biological chemistry. 1998;273:7123–6. doi: 10.1074/jbc.273.12.7123. [DOI] [PubMed] [Google Scholar]
- 43.Fridell YW, Jin Y, Quilliam LA, Burchert A, McCloskey P, Spizz G, Varnum B, Der C, Liu ET. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Molecular and cellular biology. 1996;16:135–45. doi: 10.1128/mcb.16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Molecular and cellular biology. 1997;17:4442–53. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen MP, Zeng C, Schneider K, Xiong X, Meintzer MK, Bellosta P, Basilico C, Varnum B, Heidenreich KA, Wierman ME. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Molecular endocrinology (Baltimore, Md) 1999;13:191–201. doi: 10.1210/mend.13.2.0230. [DOI] [PubMed] [Google Scholar]
- 46.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & development. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 47.Goruppi S, Ruaro E, Varnum B, Schneider C. Gas6-mediated survival in NIH3T3 cells activates stress signalling cascade and is independent of Ras. Oncogene. 1999;18:4224–36. doi: 10.1038/sj.onc.1202788. [DOI] [PubMed] [Google Scholar]
- 48.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–23. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 49.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer metastasis reviews. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 50.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bloomston M, Zervos EE, Rosemurgy AS., 2nd Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9:668–74. doi: 10.1007/BF02574483. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Staren ED, Howard JM, Iwamura T, Bartsch JE, Appert HE. Invasiveness and MMP expression in pancreatic carcinoma. J Surg Res. 2001;98:33–9. doi: 10.1006/jsre.2001.6150. [DOI] [PubMed] [Google Scholar]
- 53.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer research. 2003;63:8614–22. [PubMed] [Google Scholar]
- 54.Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW. Axl promotes cell invasion by inducing MMP-9 activity through activation of NFkappaB and Brg-1. Oncogene. 2008;27:4044–55. doi: 10.1038/onc.2008.57. [DOI] [PubMed] [Google Scholar]
- 55.Arteaga CL. EGF receptor mutations in lung cancer: from humans to mice and maybe back to humans. Cancer cell. 2006;9:421–3. doi: 10.1016/j.ccr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. The New England journal of medicine. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 57.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, Edkins S, O’Meara S, Vastrik I, Schmidt EE, Avis T, Barthorpe S, Bhamra G, Buck G, Choudhury B, Clements J, Cole J, Dicks E, Forbes S, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jenkinson A, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, Webb T, West S, Widaa S, Yates A, Cahill DP, Louis DN, Goldstraw P, Nicholson AG, Brasseur F, Looijenga L, Weber BL, Chiew YE, DeFazio A, Greaves MF, Green AR, Campbell P, Birney E, Easton DF, Chenevix-Trench G, Tan MH, Khoo SK, Teh BT, Yuen ST, Leung SY, Wooster R, Futreal PA, Stratton MR. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science (New York, NY) 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nature genetics. 2003;34:369–76. doi: 10.1038/ng1215. [DOI] [PubMed] [Google Scholar]
- 60.Myllykangas S, Himberg J, Bohling T, Nagy B, Hollmen J, Knuutila S. DNA copy number amplification profiling of human neoplasms. Oncogene. 2006;25:7324–32. doi: 10.1038/sj.onc.1209717. [DOI] [PubMed] [Google Scholar]
- 61.Nowak NJ, Gaile D, Conroy JM, McQuaid D, Cowell J, Carter R, Goggins MG, Hruban RH, Maitra A. Genome-wide aberrations in pancreatic adenocarcinoma. Cancer Genet Cytogenet. 2005;161:36–50. doi: 10.1016/j.cancergencyto.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Aguirre AJ, Brennan C, Bailey G, Sinha R, Feng B, Leo C, Zhang Y, Zhang J, Gans JD, Bardeesy N, Cauwels C, Cordon-Cardo C, Redston MS, DePinho RA, Chin L. High-resolution characterization of the pancreatic adenocarcinoma genome. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9067–72. doi: 10.1073/pnas.0402932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer research. 2002;62:2890–6. [PubMed] [Google Scholar]
- 64.Kwei KA, Bashyam MD, Kao J, Ratheesh R, Reddy EC, Kim YH, Montgomery K, Giacomini CP, Choi YL, Chatterjee S, Karikari CA, Salari K, Wang P, Hernandez-Boussard T, Swarnalata G, van de Rijn M, Maitra A, Pollack JR. Genomic profiling identifies GATA6 as a candidate oncogene amplified in pancreatobiliary cancer. PLoS genetics. 2008;4:1000081. doi: 10.1371/journal.pgen.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu B, Luo M, Lakkur S, Lucito R, Iacobuzio-Donahue CA. Frequent genomic copy number gain and overexpression of GATA-6 in pancreatic carcinoma. Cancer biology & therapy. 2008;7:1593–601. doi: 10.4161/cbt.7.10.6565. [DOI] [PubMed] [Google Scholar]
- 66.Mudduluru G, Allgayer H. The human receptor tyrosine kinase Axl gene—promoter characterization and regulation of constitutive expression by Sp1, Sp3 and CpG methylation. Bioscience reports. 2008;28:161–76. doi: 10.1042/BSR20080046. [DOI] [PubMed] [Google Scholar]
- 67.Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1648–52. doi: 10.1158/1055-9965.EPI-07-2791. [DOI] [PubMed] [Google Scholar]
- 68.Jungert K, Buck A, von Wichert G, Adler G, Konig A, Buchholz M, Gress TM, Ellenrieder V. Sp1 is required for transforming growth factor-beta-induced mesenchymal transition and migration in pancreatic cancer cells. Cancer research. 2007;67:1563–70. doi: 10.1158/0008-5472.CAN-06-1670. [DOI] [PubMed] [Google Scholar]
- 69.Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, Xiong Q, Wang B, Li XC, Xie K. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer research. 2001;61:4143–54. [PubMed] [Google Scholar]
- 70.Holland S, Hu Y, Chang B, Pan A, Franci C, Li W, Duan M, Bagos A, Torneros A, McLaughlin J, Zhang J, Yu J, Ding P, Heckrodt T, Litvak J, Stauffer E, Apatira A, Lin D-L, Morgan A, Clemens G, Daniel-Issakani S, Pine P, Goff D, Singh R, Payan D, Hitoshi Y. Suppression of tumor growth and angiogenesis by novel small molecule inhibitors of the axl receptor tyrosine kinase. AACR Meeting Abstracts. 2008;2008:4866. [Google Scholar]
- 71.Holland S, Hu Y, Chang B, Pan A, Franci C, Li W, Duan M, Bagos A, Torneros A, McLaughlin J, Zhang J, Yu J, Ding P, Heckrodt T, Litvak J, Stauffer E, Clemens G, Daniel-Issakani S, Pine P, Goff D, Singh R, Payan D, Hitoshi Y. Novel small molecule inhibitors of the Axl receptor tyrosine kinase * block tumor growth. AACR Meeting Abstracts. 2007;2007:230. [Google Scholar]
- 72.Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, Wisner L, Iorio M, Shakalya K, Garewal H, Nagle R, Bearss D. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–19. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 73.Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. American journal of physiology. 2004;287:1207–13. doi: 10.1152/ajpheart.00020.2004. [DOI] [PubMed] [Google Scholar]