Abstract
Undesired immunological responses to products of therapeutic gene replacement have been obstacles to successful gene therapy. Understanding such responses of the host immune system to achieve immunological tolerance to a transferred gene product is therefore crucial. In this article, we review relevant studies of immunological responses to gene replacement therapy, the role of immunological tolerance mediated by regulatory T cells in down-regulating the unwanted immune responses, and the interrelationship of the two topics.
Keywords: gene therapy, muscle immunology/disease, regulatory T cells, tolerance
Introduction
The host's immune system is a major obstacle to successful replacement gene therapy. Similar to the case of allogeneic organ or cell transplantation, where the recipient's immune system responds to the transplanted organ or cells as it would to a foreign organism, the replacement of an absent or defective gene by gene therapy leads to an immune response directed towards protein(s) encoded by the transferred gene. Among different approaches that have been taken, induction of immune suppression mediated by the self-regulators of the immune system known as regulatory T (Treg) cells has the potential to address the problem of immune activation by gene replacement therapy. Treg cells function to prevent activation of self-reactive immune cells and to limit ongoing responses to pathogens. Such Treg cells can be specific for self as well as foreign antigens so expansion of these cells in the context of gene therapy is considered to be a secure way of down-regulating the unwanted immune reactions to a transferred gene. Many techniques have been tested to expand Treg cell populations with desired antigen-specificity; and gene transfer itself can be the means by which the desired Tregs are obtained. The approach to use these modified cells for regulation of the immune response to transferred genes holds promise. However, there is much to discover with regard to the effects of Treg cells in different gene delivery settings for various genetic diseases. Although many comprehensive reviews have previously dealt with either the progress in gene transfer or the therapeutic use of Treg cells in various disease settings, to date the interrelationship between gene delivery and Treg cells has not been fully addressed. Here we review some of the important findings in these fields and discuss some of the links between therapeutic gene transfer and Treg cells, ending with a focus on pre- and post-gene transfer immunity associated with the genetic disorder Duchenne muscular dystrophy (DMD) and the related recent studies.
Immunity and gene transfer
Strategies of gene transfer must contend with the risk of a host immune reaction against the products encoded by the transferred gene. Studies on gene therapy for genetic diseases such as haemophilia B or DMD, where a gene is either defective or missing, have revealed that expression of the therapeutic gene product may be hampered by the host immune system. This occurs because the normal mechanisms of self-tolerance, such as autoreactive T-cell deletion in the thymus during T-cell development,1 cannot occur if the self protein is absent or mutated. As a result, helper CD4+ T cells may develop that recognize the transferred gene product when it is presented by professional antigen-presenting cells known as dendritic cells (DC), and become activated. In some cases, particularly when the therapeutic gene product is a soluble protein, these activated T cells can then induce B-cell activation and as a consequence neutralizing antibodies against the introduced protein are formed, as reviewed by Tarlinton et al.,2 leading to inactivation of its therapeutic role. In addition, the helper T cells can provide signals for activation of cytotoxic CD8+ T cells that in turn can induce killing of the cells expressing the transferred gene product. Robust activation of the adaptive immune response requires the presentation of the foreign protein in the context of an inflammatory response usually mediated by the innate immune system. The choice of vector for gene therapy becomes very important because DNA and RNA motifs from viral vectors can activate the cells of the innate immune system, leading to more effective presentation of the gene product to T and B cells.
With gene therapy, the induction of a host immune response significantly depends on factors such as the type of vector used for gene delivery,3–6 the target tissue,7 and the route of gene transfer.8–10 Therefore, in a variety of different gene replacement strategies, variable types and degrees of immunological response to vector particles and transgene product are observed that primarily lead to the loss of transgene expression. In response, one approach has been to use less immunogenic vectors or to employ specific routes of delivery that lead to more effective gene replacement, at least in preclinical models. An example of such an approach is the use of the adeno-associated viral (AAV) vector for administration of the factor IX gene to hepatocytes in animal models of haemophilia B, where sustained gene expression, as well as immune tolerance to the transferred gene, was observed.11,12 More often, however, high levels of target tissue inflammation are observed after gene transfer, with the consequent elimination of the gene product.
Furthermore, even if a preclinical study was successful, the results of subsequent clinical trials do not necessarily mirror what was found in animal studies. A recent example comes from the clinical trials on haemophilia B, where an AAV vector carrying the factor IX gene was introduced by hepatic gene transfer to participants enrolled in a human study. Following gene transfer, elevated liver enzymes were observed in these patients, indicating rejection of their transduced hepatocytes, probably as a result of re-activation of pre-existing anti-AAV-capsid CD8+ T cells in the recipients.13 Such an outcome highlights the importance of generating animal models that better resemble human subjects.
To achieve the therapeutic goals of gene delivery into a host with a genetic disorder, modification of the host immune system to completely accept the transgene product is necessary. Various manipulations of the immune system have been attempted to minimize the immune recognition of transferred gene products to allow for prolonged gene product expression.14,15 One promising approach is to encourage the host immune system to suppress undesirable gene product-specific immune responses by stimulating the generation of Treg cells. Similar strategies have been used in studies on autoimmune diseases, including type 1 diabetes, where Treg cells have been either generated or stimulated to prevent the undesired anti-self immunological responses.16–18 In fact, considering the link between the unwanted anti-self responses in autoimmunity, the responses against a graft in organ transplantation, and the responses against a gene product in the case of therapeutic gene transfer, one could compare studies in each of these fields to better understand the role of Tregs in down-regulating undesirable immune reactions.
Immune system self-regulators
Functional aspects
The Treg cells are a remarkable and not fully characterized T-cell subset in the mammalian immune system with a crucial role in controlling specific immune responses, to self as well as foreign antigens. The adaptive immune system in higher vertebrates is capable of reacting to countless non-self antigens. In addition, it is known that autoreactive T cells survive thymic negative selection; the process by which T cells that recognize self-antigens with high avidity are eliminated. Such autoreactive T cells have the potential to cause autoimmune disease. Moreover, a very strong and prolonged reaction to a pathogen may be dangerous to the host19 because this can lead to massive inflammation that can seriously damage the involved tissue. Treg cells play an essential role in controlling desired immune reactions and preventing unwanted autoimmune responses. In fact, the critical role for Treg cells in the prevention of autoimmunity is best illustrated by the elimination of Treg cells in various animal models.20,21 In Scurfy mice, which have a defective foxp3/scurfin gene and therefore lack Treg cells,22 for instance, a massive lymphoproliferative syndrome is observed, where autoreactive T cells proliferate indefinitely leading to overwhelming autoimmunity.21–23
Subpopulations of Treg cells, including natural and adaptive Treg cells, are important in maintaining peripheral immunological tolerance, as reviewed by Del.24 Natural Treg cells primarily utilize direct or cell-to-cell25 interactions with other cells of the immune system, while adaptive Treg cells utilize indirect or cytokine-mediated25,26 interactions. Such interactions are mainly with DC and activated conventional (helper or cytotoxic) T cells reviewed by Vignali et al.27 Interactions of Treg cells with DC through a surface molecule known as lymphocyte activated gene-3 (LAG-3),28 as well as the effects of the secreted products of Treg cells such as transforming growth factor-β (TGF-β), interleukin10 (IL-10) and IL-3529 on responder T cells, are largely responsible for the immunosuppressive role of Treg cells. These direct and indirect contacts lead to the activation and proliferation of Treg cells and consequently to the inactivation of responder T cells. The ability of Treg cells to down-regulate immunity, which is solely based on the molecular characteristics associated with these cells, renders them promising candidates for the treatment of autoimmune diseases,17,30 and also for the inhibition of inflammatory reactions against a transplanted organ,31–33 or a transferred therapeutic gene.
Molecular features
The two Treg subpopulations mentioned above, natural and adaptive, arise either in the thymus as a consequence of T-cell development or in the periphery in response to self as well as foreign antigens, respectively. Natural Treg cells are thought to arise as a result of partial negative selection in which self-reactive T cells are not deleted but rather differentiate into Treg cells. Adaptive Treg cells can be induced in the periphery by the cytokines TGF-β and IL-10, or by interaction with peripheral immature DC expressing low levels of self proteins.34 As a result of this diversity there is no single cell surface marker that can be used to define Treg cells. In addition, many of the markers associated with Treg cells can also be found on activated responder CD4+ T cells. To date, a standard approach of most laboratories has been to use a combination of different characteristic markers that together are able to specifically identify Treg cells efficiently. In addition to the CD4 molecule, these markers include the α subunit of IL-2 receptor (CD25) in higher levels than on the activated responder T cells, cytotoxic T lymphocyte antigen-4 (CTLA-4), membrane-bound TGF-β, l-selectin (CD62L), glucocorticoid-induced tumour necrosis factor-related receptor, LAG-3, neuropilin-1 (Nrp1),35–37 Galectin-10 (Gal-10),38 and the transcription factor forkhead box p3 (Foxp3). Foxp3 seems to be mostly specific to the Treg population. Although recent studies have reported transient expression of Foxp3 in activated human T cells,39 this has not been seen in mouse studies. Recently it has also been suggested that high expression levels of the cell surface molecule folate receptor 4 (FR-4) on T cells could be used to separate Treg cells from activated effector T cells in certain conditions after T-cell stimulation.40
Each of the molecules mentioned is important in the function of Treg cells in down-regulating an immune reaction. For instance, the CTLA-4 molecule interacts with CD80 and CD86 molecules on DC and inhibits the ability of these important costimulatory molecules to bind to CD28 on CD4 T cells, which is crucial for T-cell activation. Likewise, TGF-β is an example of an immunosuppressive molecule and its signalling along with the help of IL-2 leads to Foxp3 expression in Treg cells.41–43 CD62L is important for the homing of Treg cells to the lymph nodes;44 LAG-3 is a CD4-related molecule with the potential to bind the major histocompatiblity complex class II molecule on DC;45,46 and Nrp1 is a TGF-β receptor on the cell surface of Treg cells and is suggested to enhance Treg–DC interaction.35,37 Galectin-10 is a lectin family member and has been shown to be constitutively expressed in Treg cells, at least in humans, and to be essential for the suppressive activity of human-derived Treg cells.38 Foxp3 is a transcription factor that is crucial for the suppression of IL-2 expression, as well as for up-regulation of Treg-associated proteins such as CD25 and CTLA-4.47 In addition to the marker molecules that are used to track and identify Treg cells, there are other markers, such as secreted TGF-β, IL-10 and IL-35,29,48 in the cell culture media, that have been used in Treg functional assays, in which generally Treg-mediated suppression of responder T-cell activity is tested. Together, these different molecules make the ongoing research on Treg cells in such clinical cases as autoimmunity, transplantation, cancer and therapeutic gene replacement possible.
The interface of gene therapy and regulatory T cells
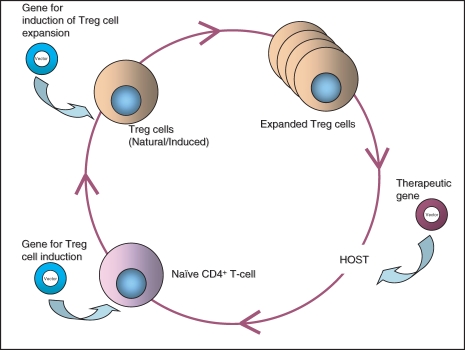
Significant advances in therapeutic gene transfer are reflected in many developments in gene and vector design. In gene replacement settings, where an immune response to the transferred gene product is not desirable, various techniques have been used to create a vector that delivers the gene of interest to its target tissue efficiently in a way that is non-toxic and non-immunogenic to the host. Such techniques include engineering the viral vector to be non-replicating49 and to remove some or all of the viral genes to eliminate viral protein expression,50,51 hence limiting anti-vector immune reactions. Furthermore, some laboratories have worked on increasing the period of time that the gene of interest is expressed in the target tissue by adding a gene with an immunosuppressive product to the vector expression cassette.14 The additional gene product usually mimics some natural regulatory function of the immune system itself, such as that of the Treg cells. Gene transfer applications, in which the vector transgene causes Treg cells to be induced and expanded and Treg-induced immune suppression have the potential to work synergistically and to lead to successful gene transfer (Fig. 1). Therefore, although Treg cells can be used to down-regulate responses to a transferred gene, gene transfer can be used to improve immune regulation and Treg cell function to a great extent as well.
Figure 1.
The synergistic inter-relation between immunosuppressive regulatory T (Treg) cells and therapeutic gene replacement. Just as gene transfer may induce and expand the Treg cell population, gene replacement therapy may benefit the immune suppression induced by Treg cells in a host system.
The ideal way to enlist the help of Treg cells for a successful therapeutic gene replacement would be to induce adaptive Treg cells specific to the introduced gene product at the same time as gene transfer. Approaches could include the addition of vectors encoding cytokines known to induce Treg cell differentiation such as TGF-β, which has been shown to be crucial for Treg cell development.52 Another approach would be to expand the existing Treg population in vivo using genes or proteins that are associated with Treg cells and their function under normal conditions. For instance, Elpek et al.53 have shown that stimulating the 4-IBB protein (CD137), a membrane co-stimulatory receptor on the surface of the CD4+ T cells, using a vector carrying the 4-IBB ligand (4-IBBL) gene, induces the proliferation and survival of Treg cells in mice, through increasing the production of IL-2, which is vital for Treg survival and expansion. In another study, Jiang et al.54 showed that the addition of a CTLA-4–immunoglobulin complementary DNA (cDNA), coding for a soluble CTLA-4 molecule that mimics some Treg function, to a vector carrying the enhanced green fluorescent protein (EGFP) cDNA induces a longer and stronger EGFP expression in murine muscle, compared with that when the muscle fibres are transduced with an EGFP vector alone.
Furthermore, different techniques have been applied to generate specific Treg cells and to induce the expansion of a certain Treg population. For example, Chai et al.31 have demonstrated that retroviral induction of T-cell receptor transgenic CD4+ CD25− naïve T cells to express the Foxp3 transcription factor generates immunosuppressive Treg cells that are capable of preventing allograft rejection when adoptively transferred into recipient transgenic mice with the same T-cell receptor specificity. Also, Skapenko et al.55 have successfully transformed CD4+ CD25− T cells into anergic and suppressive Foxp3+ T cells using the cytokines IL-4 and IL-13. Other factors, including TGF-β, have also been shown to have direct impact on Treg expansion by regulating Foxp3 expression, for instance.56,57 In addition, an example for expanding specific Treg populations used in a therapeutic gene transfer setting comes from the work of Gross et al.58 In these experiments cotransfer of an AAV vector carrying an influenza haemagglutinin (HA) gene along with in vitro expanded HA-specific Treg cells into a naïve mouse induced the suppression of the naïve conventional T cells and successful expression of HA in the recipient mouse.58 Consequently, many approaches for the manipulation of the immune system of the gene recipient can be considered to allow effective and long-lasting gene transfer. However, there is much more to be done to actually translate these techniques and findings into efficient clinical treatments for all possible genetic diseases. Depending on factors such as the onset of a disease and the ease of transfer of the desired gene into a specific target tissue, the application of such treatments for a wide variety of disorders remains distant from direct clinical application. For instance, similar studies are yet to be carried out for genetic disorders such as DMD, in which inflammation is a prominent feature of the muscle pathology associated with the disease.59 This disease-related inflammation further complicates both the immunological responses to the transferred therapeutic gene product in gene transfer therapeutic approaches and the manipulations required to prevent those unwanted responses.
Future goals for regulatory T cells in gene therapy
A focus on gene therapy for DMD
Gene transfer has considerable potential to provide a cure for the genetic disease DMD, which is an X-linked disorder affecting 1 in 3500 live male births per year, worldwide. This lethal disease, which usually presents at 3–5 years of age, is the result of the absence of a functional dystrophin protein leading to loss of muscle fibre and its replacement with fibrous tissue in young patients. Affected boys generally become wheelchair-dependent for mobility at 10–12 years of age and often die in their third decade.60 To date, there is no known cure for this disease.
Various studies have transferred either the 14-kilobase full-length dystrophin cDNA15,50,51,61 or the truncated ‘mini-dystrophin’62,63 into skeletal muscle in experimental animal models. The most commonly used animal model of DMD is the mdx mouse.64 Depending on the vector system used, varying degrees of host immunity are induced against viral capsid proteins62,63 and more importantly, the dystrophin protein that is expressed as a result of gene transfer.61,63 Such an immune response may cause the rejection of the transferred gene product, and may also preclude vector re-administration unless immune suppression is used with the first administration. However, the short-term approach of immune suppression to address the transient problem of vector antigens is not appropriate for the long-term regulation required to address the immunity to the transferred gene product that must be expressed for the lifespan of the recipient.
When considering an effective treatment for a genetic myopathy like DMD, it is important to note that the dystrophic muscle phenotype associated with DMD is not entirely the result of dystrophin deficiency, in which the sarcolemmal membrane of the muscle fibres becomes fragile and leads to muscle necrosis. This muscle necrosis leads to massive inflammation in the muscle fibres and infiltrating, potentially autoreactive, helper (CD4+) and cytotoxic (CD8+) T cells accumulate, which leads to further damage.59,65 Moreover, Farini et al.66 have shown that the infiltrating T cells, as well as activated B cells, in dystrophic mdx muscle significantly affect the amount of fibrosis that occurs in the irreversible process of muscle replacement with fibrotic tissue. It is therefore hypothesized that in DMD, Treg cells not only have the potential to prevent undesired post-gene-transfer reactions of the host immune system against dystrophin protein but, depending on the age of the host at vector administration, Treg cells may also help to slow the progression of the disease by avoiding much of the autoimmune-mediated damage occurring in the muscles of the patients. In fact, it is important to avoid such immunity in a potential gene-transfer host because the existing inflammation in a dystrophic muscle may actually function as a driving force for an even stronger and faster-acting immune response against the vector as well as the transgene product.
Surprisingly, despite the clear contribution of immune cells to the pathology of DMD there are no reports on the role of Treg cells in untreated or treated dystrophic muscle. Among the investigations on the induction of tolerance in a gene therapy setting for DMD are the examples of in utero gene transfer to dystrophic animal models, with the purpose of inducing tolerance to the delivered dystrophin protein.67 The idea behind performing gene transfer to the fetus of an mdx mouse is that the murine fetal immune system is functionally immature at the age of gene delivery and therefore does not react against the transgene product. In related studies, it has been shown that expression of a transferred gene product before the introduction of Treg cells to the host system can lead to induction of tolerance to that protein in the recipient. A major question for similar immunological in utero studies is how these findings will translate into a cure for the human disease, knowing that the immune system in humans matures before birth.
This important fact further highlights the need to better understand the role of immune system regulators in myopathies such as DMD. At the same time, the previous findings on the phenotype associated with DMD, as an example of a genetic disorder with immunological and non-immunological complications, provides fertile ground for an immense variety of studies dealing with reducing both the immunological symptoms associated with the disease, as well as the post-gene-delivery immune problems that are the main obstacle for successful gene replacement therapy. Many questions arising from the interface of therapeutic gene transfer and immunological tolerance should be further studied if successful gene therapy for genetic disorders is to be achieved.
Acknowledgments
S.E. is supported by National Institutes of Health grant 1-F31-NS056780-01A2 and P.R.C. is supported by VA resources (VA Pittsburgh Healthcare System, Pittsburgh, PA).
Disclosures
None of the authors have any conflict of interest with any of the content of this manuscript.
References
- 1.Carl JW, Jr, Liu JQ, Joshi PS, et al. Autoreactive T cells escape clonal deletion in the thymus by a CD24-dependent pathway. J Immunol. 2008;181:320–8. doi: 10.4049/jimmunol.181.1.320. [DOI] [PubMed] [Google Scholar]
- 2.Tarlinton DM, Batista F, Smith KG. The B-cell response to protein antigens in immunity and transplantation. Transplantation. 2008;85:1698–704. doi: 10.1097/TP.0b013e3181777a39. [DOI] [PubMed] [Google Scholar]
- 3.Chu D, Sullivan CC, Weitzman MD, et al. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J Thorac Cardiovasc Surg. 2003;126:671–9. doi: 10.1016/s0022-5223(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 4.Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–23. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10:955–63. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 6.Yuasa K, Yoshimura M, Urasawa N, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–60. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- 7.Harvey BG, Hackett NR, El-Sawy T, et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol. 1999;73:6729–42. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrzynski E, Herzog RW. Tolerance induction by viral in vivo gene transfer. Clin Med Res. 2005;3:234–40. doi: 10.3121/cmr.3.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jerebtsova M, Batshaw ML, Ye X. Humoral immune response to recombinant adenovirus and adeno-associated virus after in utero administration of viral vectors in mice. Pediatr Res. 2002;52:95–104. doi: 10.1203/00006450-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Xiao W, Chirmule N, Schnell MA, Tazelaar J, Hughes JV, Wilson JM. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol Ther. 2000;1:323–9. doi: 10.1006/mthe.2000.0045. [DOI] [PubMed] [Google Scholar]
- 11.Dobrzynski E, Mingozzi F, Liu YL, et al. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–77. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 12.Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–6. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 13.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–24. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z, Schiedner G, Gilchrist SC, Kochanek S, Clemens PR. CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Ther. 2004;11:1453–61. doi: 10.1038/sj.gt.3302315. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z, Schiedner G, van Rooijen N, Liu CC, Kochanek S, Clemens PR. Sustained muscle expression of dystrophin from a high-capacity adenoviral vector with systemic gene transfer of T cell costimulatory blockade. Mol Ther. 2004;10:688–96. doi: 10.1016/j.ymthe.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Song L, Wang J, Wang R, et al. Retroviral delivery of GAD-IgG fusion construct induces tolerance and modulates diabetes: a role for CD4+ regulatory T cells and TGF-beta? Gene Ther. 2004;11:1487–96. doi: 10.1038/sj.gt.3302327. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Han G, Wang J, et al. Foxp3-expressing CD4(+)T cells under the control of INF-gamma promoter prevent diabetes in NOD mice. Mol Ther. 2007;15:1551–7. doi: 10.1038/sj.mt.6300208. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Song L, Han G, et al. Mechanisms of regulatory T-cell induction by antigen-IgG-transduced splenocytes. Scand J Immunol. 2007;66:515–22. doi: 10.1111/j.1365-3083.2007.02004.x. [DOI] [PubMed] [Google Scholar]
- 19.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 23.Appleby MW, Ramsdell F. Scurfy, the Foxp3 locus, and the molecular basis of peripheral tolerance. Curr Top Microbiol Immunol. 2008;321:151–68. doi: 10.1007/978-3-540-75203-5_7. [DOI] [PubMed] [Google Scholar]
- 24.Del PG. The complexity of the CD4 T-cell responses: old and new T-cell subsets. Parassitologia. 2008;50:9–16. [PubMed] [Google Scholar]
- 25.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–60. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 30.Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci USA. 2005;102:17418–23. doi: 10.1073/pnas.0507454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai JG, Xue SA, Coe D, et al. Regulatory T cells, derived from naive CD4+ Transplantation. 2005;79:1310–6. doi: 10.1097/01.tp.0000159147.56408.9c. [DOI] [PubMed] [Google Scholar]
- 32.Raimondi G, Turner MS, Thomson AW, Morel PA. Naturally occurring regulatory T cells: recent insights in health and disease. Crit Rev Immunol. 2007;27:61–95. doi: 10.1615/critrevimmunol.v27.i1.50. [DOI] [PubMed] [Google Scholar]
- 33.Seveno C, Coulon F, Haspot F, et al. Induction of regulatory cells and control of cellular but not vascular rejection by costimulation blockade in hamster-to-rat heart xenotransplantation. Xenotransplantation. 2007;14:25–33. doi: 10.1111/j.1399-3089.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 34.Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol. 2004;16:1769–80. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 35.Bruder D, Probst-Kepper M, Westendorf AM, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–30. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 36.Glinka Y, Prud’homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–10. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–13. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubach J, Lutter P, Bopp T, et al. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–8. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Ioan-Facsinay A, van der Voort EIH, Huizinga TWJ, Toes REM. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–59. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur J Immunol. 2008;38:912–5. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 42.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–21. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shevach EM, Davidson TS, Huter EN, Dipaolo RA, Andersson J. Role of tgf-Beta in the induction of foxp3 expression and T regulatory cell function. J Clin Immunol. 2008;28:640–6. doi: 10.1007/s10875-008-9240-1. [DOI] [PubMed] [Google Scholar]
- 44.Ochando JC, Yopp AC, Yang Y, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Workman CJ, Martin SM, Vignali DA. Biochemical analysis of the regulatory T cell protein lymphocyte activation gene-3 (LAG-3; CD223) J Immunol. 2004;173:6806–12. doi: 10.4049/jimmunol.173.11.6806. [DOI] [PubMed] [Google Scholar]
- 46.Liang B, Workman C, Lee J, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–26. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 47.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 48.Niedbala W, Wei XQ, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–9. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 49.Ragot T, Opolon P, Perricaudet M. Adenoviral gene delivery. Methods Cell Biol. 1997;52:229–60. [PubMed] [Google Scholar]
- 50.Alba R, Bosch A, Chillon M. Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Ther. 2005;12(Suppl. 1):S18–27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- 51.Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–6. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–40. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 53.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol. 2007;179:7295–304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Z, Feingold E, Kochanek S, Clemens PR. Systemic delivery of a high-capacity adenoviral vector expressing mouse CTLA4Ig improves skeletal muscle gene therapy. Mol Ther. 2002;6:369–76. doi: 10.1006/mthe.2002.0676. [DOI] [PubMed] [Google Scholar]
- 55.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175:6107–16. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 56.Pyzik M, Piccirillo CA. The TGF-beta1/Foxp3 regulatory axis in immune self-tolerance: implications for health and disease. Inflamm Allergy Drug Targets. 2006;5:167–77. doi: 10.2174/187152806778256089. [DOI] [PubMed] [Google Scholar]
- 57.Pyzik M, Piccirillo CA. TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leukoc Biol. 2007;82:335–46. doi: 10.1189/jlb.1006644. [DOI] [PubMed] [Google Scholar]
- 58.Gross DA, Leboeuf M, Gjata B, Danos O, Davoust J. CD4+CD25+ regulatory T cells inhibit immune-mediated transgene rejection. Blood. 2003;102:4326–8. doi: 10.1182/blood-2003-05-1454. [DOI] [PubMed] [Google Scholar]
- 59.Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–43. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- 60.Appel SH. The muscular dystrophies. Clinical update on two major types. Postgrad Med. 1978;64:93–102. doi: 10.1080/00325481.1978.11714902. [DOI] [PubMed] [Google Scholar]
- 61.Gilchrist SC, Ontell MP, Kochanek S, Clemens PR. Immune response to full-length dystrophin delivered to Dmd muscle by a high-capacity adenoviral vector. Mol Ther. 2002;6:359–68. doi: 10.1006/mthe.2002.0675. [DOI] [PubMed] [Google Scholar]
- 62.Bilbao R, Reay DP, Wu E, et al. Comparison of high-capacity and first-generation adenoviral vector gene delivery to murine muscle in utero. Gene Ther. 2005;12:39–47. doi: 10.1038/sj.gt.3302392. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y, Haecker SE, Su Q, Wilson JM. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–12. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 64.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–92. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinman L. State of the art. Four easy pieces: interconnections between tissue injury, intermediary metabolism, autoimmunity, and chronic degeneration. Proc Am Thorac Soc. 2006;3:484–6. doi: 10.1513/pats.200603-061MS. [DOI] [PubMed] [Google Scholar]
- 66.Farini A, Meregalli M, Belicchi M, et al. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol. 2007;213:229–38. doi: 10.1002/path.2213. [DOI] [PubMed] [Google Scholar]
- 67.David AL, Peebles D. Gene therapy for the fetus: is there a future? Best Pract Res Clin Obstet Gynaecol. 2008;22:203–18. doi: 10.1016/j.bpobgyn.2007.08.008. [DOI] [PubMed] [Google Scholar]