Abstract
The 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors (statins) interfere with the mevalonate pathway. While initially developed for their lipid-lowering properties, statins have been extensively investigated with respect to their impact on autoantigen and alloantigen driven immune responses. Mechanistically it was shown that statins modify immune responses on several levels, including effects on dendritic cells, endothelial cells, macrophages, B cells and T cells. Several lines of evidence suggest that statins act in a disease-specific manner and are not effective in each immune disorder. This review discusses possible modes of action of statins in modulating immunity towards autoantigens and alloantigens.
Keywords: autoimmunity, graft-versus-host disease, mevalonate pathway, statins, T-cell responses
Introduction
Statins are widely used hypocholesterolaemic drugs that inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of the mevalonate pathway. Initially this class of drugs was established for their cholesterol-lowering effects in the treatment of cardiovascular diseases.1 Recently, the pleiotropic immunomodulatory effects of these drugs have attracted increasing interest. Initial evidence demonstrated their effect in preventing and reversing relapsing paralysis in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis (MS) – first demonstrated in 20022 and confirmed by other studies.3–5 Statins affect multiple cell populations relevant to the immune response, including B cells,6 T cells,4,7–9 regulatory T cells,10 macrophages,11 dendritic cells7,12 and endothelial cells.13
Mechanistically it was shown that atorvastatin decreased the expression of major histocompatibility complex (MHC) class II, CD80 and CD86 on microglial cells,2 an effect that may modulate antigen presentation and T-cell costimulation. Similar observations were made later in dendritic cells under conditions of graft-versus-host-disease (GVHD)7 and B cells in lupus-prone mice.6 Statin-mediated modification of T-cell proliferation and cytokine production acted via the mevalonate pathway because the effects could be reversed by the addition of l-mevalonate, the product of HMG-CoA reductase. That observation indicated that molecules that are downstream of the l-mevalonate pathway function as essential regulators of T helper type 1 (Th1)/Th2 fate4 and suggests that inhibition of HMG-CoA reductase is a potential mechanism to modulate pathogenic T-cell responses. Increased production of Th2 cytokines can be protective in disease states characterized by predominantly Th1 polarized inflammatory conditions. Other mechanisms of a subgroup of statins may include the modification of lymphocyte function-associated antigen 1 and interferon-γ-induced MHC class II expression.14
In light of the reports on statin-mediated effects in preclinical models of autoimmunity,2,15,16Table 1 clinical studies have been performed in patients with MS,17,18 rheumatoid arthritis19 and chronic GVHD.20
Table 1.
Preclinical studies on the effects of statins on different immune responses
| Human/rodent | In vitro/in vivo | Disease model/experimental design | Reduction in: 1. Clinical disease parameters 2. Cytokines/factors | Statin(s) | Reference |
|---|---|---|---|---|---|
| Murine | In vitro | Dendritic cells, endothelial cells | 1. Modification of LFA-1 2. Inhibition of induction of MHC-II expression by IFN-γ | Atorvastatin Lovastatin Pravastatin | 14 |
| Murine | b | EAE | 1. Paralysis reversed 2. Reduced Th1 cytokines 3. APC maturation | Atorvastatin | 2 |
| Murine | In vivo | Collagen-induced arthritis | 1. Disease score reduced 2. Th1 humoral and cellular immune responses reduced | Simvastatin | 16 |
| Murine | In vivo | Allergic asthma | 1. OVA-induced IL-4, IL-5, IL-6, and IFN-γ secretion reduced | Simvastatin | 65 |
| Murine | In vivo + in vitro | Lupus-prone NZB/W F1 mice | 1. Lupus development blocked 2. Reduced expression of CD80 and CD86 on B cells | Atorvastatin | 6 |
| Human | In vitro | Statin-incubation of DC | DC maturation blocked | Simvastatin or atorvastatin | 12 |
| Murine | b | EAE | 1. EAE severity 2. Th1 cytokines | Atorvastatin, FTI/GGTIs | 4 |
| Murine | In vivo | Major mismatch GVHD model | Reduced GVHD, Th2 cytokine production Costimulatory molecule expression on APC reduced | Atorvastatin and Fluvastatin | 7 |
| b | b | PBMC exposure in vitro or oral treatment | 1. Treg induction in vivo in humans (not in C57BL/6 mice) 2. FoxP3 expression in CD4 T cells after in vitro exposure | Simvastatin and pravastatin Atorvastatin (mevastatin and pravastatin did not work) | 10 |
| Murine | In vivo | SLE | No effect | Atorvastatin | 21 |
| Murine | In vitro | Macrophage phagocytotic activity, THP-1 cells | Reduced phagocytosis | Pravastatin | 11 |
APC, antigen-presenting cells; b, both; DC, dendritic cell; EAE, experimental allergic encephalitis; FTI, farnesyl transferase inhibitor; GGTI, geranygeranyl transferase inhibitor; GVHD, graft-versus-host disease; IFN-γ, interferon-γ; IL-4, interleukin-4; LFA-1, lymphocyte function-associated antigen 1; MHC-II, major histocompatibility complex class II; OVA, ovalbumin; PBMC, peripheral blood mononuclear cells; SLE, systemic lupus erythematosis; Th1, T helper type 1; Treg, CD4+ CD25+ T regulatory cells.
Possible modes of action of statins in modulating autoimmunity
Murine studies demonstrated the effects of statins in different autoimmune diseases. Interestingly in mouse models of systemic lupus erythematosus (SLE), atorvastatin was ineffective.21 In humans it was recently reported that the severity of SLE could be reduced by atorvastatin.22 A similar effect was also seen when simvastatin was used.23 Interestingly, the reduction of the disease score was paralleled by prominent suppression of tumour necrosis factor (TNF) concentration in the serum after 4 weeks of treatment with simvastatin at a dose of 20 mg.23 A major candidate for the endothelial damage seen in patients with SLE is TNF, so it may be speculated that suppression of TNF levels after statin therapy might be one mechanism by which restoration of endothelial functions occurs. Another mechanism of action of statins in vasculitis is that inhibition of protein isoprenylation can act directly on endothelial function by increasing endothelial nitric oxide synthase expression.24
Besides their anti-inflammatory and antithrombotic actions, statins have also been reported as immunomodulating agents, which act by inhibiting the transcription of various genes induced by nuclear factor-κB (NF-κB) and inhibiting interferon-δ-induced human leucocyte antigen (HLA) class II expression on endothelial cells.25 The suppression of HLA class II expression contributes to preventing local T-cell activation and this can minimize Th1-driven autoimmunity in an SLE model.16
Further clinical studies on the therapeutic potential of statins in patients with different inflammatory rheumatic diseases refractory to conventional therapy demonstrated that simvastatin (80 mg once daily for 8 days) induced a rapid and significant reduction in proteinuria levels in three patients with SLE.26 Also, simvastatin had a marked beneficial effect in a patient with Wegener's granulomatosis and a patient with erythema nodosum.26 Five patients with rheumatoid arthritis who received atorvastatin for 8 days (20 mg/day) showed a reduction in C-reactive protein (CRP) levels and a clinical improvement that was classified as an American College of Rheumatology (ACR) 20 response.26 Importantly, before the administration of statins, all these patients had received aggressive conventional therapy with no satisfactory response. A significant reduction in spontaneous apoptosis of peripheral blood lymphocytes and expression of CD69 and HLA-DR was observed in SLE patients after simvastatin therapy.26
In a pilot short-term comparative (simvastatin versus chloroquine) open clinical trial in 15 patients with rheumatoid arthritis 90% of the patients who received simvastatin (40 mg/day) showed an ACR50 or better response after 8 weeks, whereas such a response was not observed in any patient (0/5) treated with chloroquine,26 suggesting that statins may be an important therapeutic tool for the treatment of inflammatory rheumatic diseases. Clinical studies on the anti-inflammatory and immunomodulatory effects of low-dosage simvastatin on rheumatoid arthritis demonstrated that the Th1/Th2 and CD4/CD8 ratios in peripheral blood were significantly reduced by simvastatin.27 Additional evidence that statins may have an anti-inflammatory effect is provided by a randomized trial that found that patients with rheumatoid arthritis experienced clinical improvement, reduced CRP levels and lower erythrocyte sedimentation rates when treated with atorvastatin compared with placebo (Table 2).19
Table 2.
Clinical trials on the anti-inflammatory effects of statins
| Disease entity | Experimental design (number of patients) | Observed effects | Statin | Reference |
|---|---|---|---|---|
| Coronary artery disease | Prospective pretreatment (n = 5742) | Reduction in CRP serum levels by almost 15% | Lovastatin | 62 |
| Coronary artery disease | Prospective pretreatment prior percutaneous coronary interventions (n = 1552) | Statins improved survival in patients in the highest CRP levels | Multiple statins | 61 |
| Multiple sclerosis | Prospective study, statins given daily over 6 months (n = 30) | Significant reduction of contrast-enhancing brain lesions by MRI | Simvastatin | 17 |
| Multiple sclerosis | Prospective phase II open-label study statin ± IFN-β (n = 41) | MRI analysis indicates a possible beneficial effect of atorvastatin no major toxicity | Atorvastatin | 18 |
| Rheumatoid arthritis | Double-blind, randomized placebo-controlled trial (n = 116) | CRP and ESR declined, Swollen joint count reduced, clinical response in 31% | Atorvastatin | 19 |
| SLE | Prospective analysis, non-randomized (n = 64 treated, 24 untreated) | Significant increase in flow-mediated dilation | Atorvastatin | 22 |
| Chronic GVHD | Prospective open trial, phase I, non-randomized (n = 18) | Clinical response in 30%, trend towards Th2 cytokines in responder group | Pravastatin | 20 |
| Acute GVHD | Retrospective analysis, acute GVHD (n = 49) | Significantly reduced GVHD incidence in statin group, no increase in leukaemia relapse | Multiple statins | 45 |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GVHD, graft-versus-host disease; MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus; Th2, T helper type 2.
Regarding the mechanism of action, it was shown that simvastatin inhibits cytokine production and NF-κB activation in interleukin-1β (IL-1β)-stimulated synoviocytes from rheumatoid arthritis patients.28 Simvastatin significantly inhibited the production of IL-6 and IL-8 in IL-1-stimulated synoviocytes also, although to a lesser extent than in unstimulated cells, via an HMG-CoA reductase block with an interference in prenylation process and NF-κB activation.28 These data support the rationale for the use of statins in the treatment of rheumatoid synovitis.
Multiple sclerosis is a chronic progressive inflammatory disease with an immune response directed against myelin-derived proteins.29 Different clinically applied therapies for relapsing–remitting MS include the targeting of autoreactive T-cell activation with glatiramer acetate, inhibition of immunocompetent cell migration into the central nervous system (interferon-β), and the suppression of the inflammatory response (e.g. by glatiramer acetate, mitoxantrone).30 Based on murine studies of experimental autoimmune encephalomyelitis, an animal model of MS,2,31 statins are promising candidates for the treatment of MS, possibly in combination with other treatments such as glatiramer acetate.32 The murine studies demonstrated that atorvastatin induces a shift from a Th1 to a Th2 cytokine profile (Table 1).2 The immunomodulatory effects of statins resulted in the inhibition of central nervous system lesion formation, and the delayed onset and ameliorated severity of experimental autoimmune encephalomyelitis.2–4,31 Beside the impact on antigen-presenting cells and the Th1/Th2 cytokine profile, (Fig. 1) recent data suggest that simvastatin induces the expression of suppressor of cytokine secretion (SOCS) 3 and 7 in monocytes, which inhibit transcription of IL-6 and IL-23, cytokines that play an important role in the development of the autoimmune response in MS.33 Induction of SOCS3 by statins may have an inhibitory effect on multiple inflammatory cytokine signal transduction pathways that mediate the autoimmune response. The messenger RNA of IL-17 is elevated in active MS brain lesions,34 which may provide an additional explanation for the effectiveness of simvastatin in MS as the drug inhibits IL-17 secretion by targeting several IL-17-regulatory cytokines and by inhibiting the expression of the IL-17 transcription factor RORC in CD4+ T cells.33
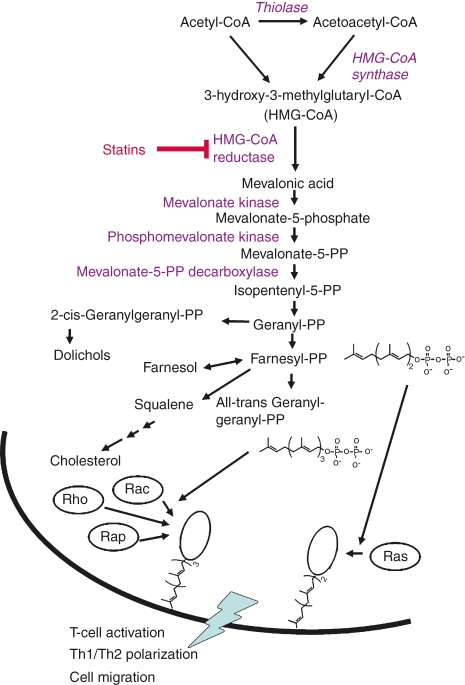
Figure 1.
Inhibition of the l-mevalonate pathway for immunomodulation. Statins interfere with 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of the l-mevalonate pathway which causes reduction of farnesylated and geranylgeranylated downstream proteins. Besides others, the GTPases Ras, Rho-B and Rap-1 that play a role in the process of activation and proliferation of T cells are not prenylated, which affects their binding to the T-cell membrane. This shift in active GTPase signalling is connected to a blockade of T helper type 1 (Th1) cytokine production.
In the light of these results from rodent models, clinical trials on statins for MS were initiated. An initial pilot study with oral simvastatin given daily over 6 months showed a significant reduction of contrast-enhancing lesions on brain magnetic resonance images from 30 patients with remitting–relapsing MS compared with a 3-month baseline period.17 A more recent study showed that treatment with high-dose atorvastatin (80 mg daily) over a period of 9 months is safe and is well tolerated in the majority of patients.18 Interestingly, a pronounced reduction in number and volume of contrast-enhancing lesions was observed under treatment when compared with baseline. In this clinical study, neither a disturbed proliferative response nor an inhibition of proinflammatory cytokines was observed in the treated MS patients and high-dose atorvastatin did not exhibit overall peripheral immunosuppressive effects.18 However, an upregulation of IL-10 production was observed in patients treated with atorvastatin, indicating an atorvastatin-mediated involvement of regulatory mechanisms in vivo.18 Based on these clinical studies as well as the data from the rodent models, statins hold promise for the effective treatment of MS, most likely in combination with other immunomodulatory medications.
Possible modes of action of statins in modulating alloantigen-driven immunity
Cardiac transplant recipients have a high incidence of hyperlipidaemia, which was the initial indication for statin administration in these patients. Interestingly, statin therapy improved patient survival and reduced the incidence and severity of transplant vasculopathy and allograft rejection.35 The effect of pravastatin therapy on the incidence of transplant vasculopathy was evaluated in a randomized, prospective open-label trial of 97 transplant recipients.36 Independent from its effects on the lipid profile, pravastatin was associated at 1 year with a significant reduction in the incidence of transplant vasculopathy as determined by angiography or autopsy, a lower maximal intimal thickness and a significant increase in patient survival,36 which was still seen at the 10-year follow-up study.37 Therefore, it was hypothesized that the benefits of statins may be related to a number of other factors including the attenuation of endothelial dysfunction,38 and immunosuppressive activity.39
In kidney transplantation, chronic allograft nephropathy still remains one of the leading causes of renal allograft loss. In a rat model atorvastatin showed favourable effects on blocking renal inflammation and fibrosis, and consequently it efficiently inhibited the development and progression of chronic allograft nephropathy, which improved the long-term survival rate of renal allografts.40 In an experimental model of cyclosporin A nephrotoxicity, Li et al.41 reported the inhibitory effects of pravastatin on macrophage infiltration and interstitial fibrosis. Atorvastatin downmodulated lymphocyte cellular infiltration and the expression of osteopontin in renal allografts in the early stages after kidney transplantation.42 In addition, statin therapy resulted in the downregulation of transforming growth factor-β-inducible gene h3, which is associated with reduced endothelial nitric oxide synthesis.43
We have previously shown that statins effectively reduce acute GVHD in a murine model. The effect of statins was through Th2 induction as increased levels of intracellular IL-4 and reduced TNF and interferon-γ production were found.7 Also, when T cells from animals deficient for signal transducer and activator of transcription 6, which almost completely lack Th2 responses,44 were used as donors for GVHD induction the protective effect of statins was partially antagonized.7
So far the effect of statins on GVHD has been investigated in two clinical studies. The first study was a prospective non-randomized study including 18 patients with refractory chronic GVHD. In this trial, pravastatin was given orally at a dose of 10 mg/day, with an increase up to 40 mg/day in 4 weeks.20 While there were no severe adverse events observed in the pravastatin group, the overall response rate was 28%. Th1 cells were found in 94% of the patients before treatment and the Th1/Th2 ratio tended to be lower in the responders than in the non-responders. In the second study, 67 consecutive patients with acute leukaemia underwent T-cell–replete allogeneic haematopoietic cell transplantation.45 Patients taking any type of statins at 40 mg/day or more for at least 1 month before and 3 months after allogeneic stem cell transplantation (n = 10) were compared with those without a history of statin use (n = 57). Acute GVHD was scored according to modified Glucksberg criteria.46
No difference in the incidence of chronic GVHD was seen in patients using statins (55%) compared with those in the no-statin group (57%; P = 0·9). No patient in either group experienced primary or secondary engraftment failure. On subgroup analysis of patients with acute myeloid leukaemia only (n = 49), a significantly reduced incidence of grades II–IV acute GVHD was seen in the statin group (0%) compared with 43% (n = 18) in the no-statin group (P = 0·02). Rates of chronic GVHD were 43% and 58% in the statin and no-statin groups, respectively (P = 0·68). We further investigated whether statin use, while reducing acute GVHD, mitigated the graft versus leukaemia effect in patients with acute myeloid leukaemia.45 Kaplan–Meier estimates of progression-free survival at 3 years in acute myeloid leukaemia patients with or without statin use were 54% and 28%, respectively (P = 0·17). This non-significant trend of improved progression-free survival indicates that the GVL is preserved in patients using statins at the time of allografting.45
Possible modes of action of statins in modulating immunity towards inflammatory mitogens in the arterial vessel wall
While initially contributed solely to lipid accumulation,47 dysregulated vascular smooth muscle cell homeostasis48 and calcification, recent evidence has revealed that inflammation contributes significantly to atherosclerosis and plaque rupture.49 Elevated serum markers of inflammation, and promoter gene variants for IL-1b and IL-18 are associated with progression of atherosclerosis and are predictive for a first myocardial infarction.49 Consecutively, these markers of inflammation are associated with a worse prognosis among patients with stable and unstable angina and those who undergo coronary stenting.49 Recent in vitro studies indicate that statins interfere with the interactions between neutrophils and endothelial cells. In an experimental in vitro setting, statins reduced histamine and TNF-mediated proinflammatory effects including neutrophil tethering and rolling which was accompanied by lower P/E-selectin expression on endothelial cells.50 Importantly, mevalonate pretreatment abrogated the beneficial effects of statins on endothelial cells. The results from another group demonstrate that the mevalonate pathway downstream products are critical for monocyte adhesion to endothelial cells induced by TNF or angiotensin II and that this process can be inhibited by simvastatin.51 Recent in vitro studies demonstrated that CRP-induced CD32 expression and NF-κB activation in endothelial cells were blocked by interferences with the mevalonate pathway, which is of clinical relevance because the finding provides a rationale for using statins on patients with high serum CRP levels.52 Clinical evidence for the anti-inflammatory effects of statins originates from the observation that statin therapy, given as primary or secondary prevention, reduces the concentration of CRP in the serum, an effect that is mostly unrelated to lipid levels at baseline or during therapy.53–55 Statin-mediated anti-inflammatory effects in atherosclerosis could contribute to the benefit from the early institution of statin therapy in patients with an acute coronary syndrome.55 Results from different clinical trials raise the possibility that the anti-inflammatory effects may differ among statins.56 While the first trials showed early benefit with atorvastatin,57 the following trial on simvastatin revealed no evidence of clinical benefit and no reduction in CRP.58 The potential importance of statin-induced reduction in serum markers of inflammation was illustrated by an analysis from the secondary prevention CARE trial.59 Patients with baseline serum concentrations of CRP and the acute-phase protein amyloid A in the highest quintile had a relative risk for a recurrent event 75% higher than those with levels in the lowest quintile.59 This risk reduction was not observed with all statins. For example, when using pravastatin, the association between inflammation and risk was attenuated and was no longer statistically significant.59 These data were compatible with a prospective study of patients with angiographically severe coronary disease showing that the improvement in survival with statin therapy occurred primarily in those with elevated serum CRP.60 Furthermore, a prospective observational study of patients who underwent percutaneous coronary interventions demonstrated that pretreatment with statins was associated with a marked improvement in survival in those patients in the highest CRP levels.61 Another prospective trial on lovastatin demonstrated that this drug reduced serum CRP by almost 15%.62 Interestingly, lovastatin was ineffective in patients with a low CRP, which is consistent with the hypothesis that statins are more effective when an inflammatory condition is present. Mechanistically it was suggested that statin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cyclooxygenase-2.63 Another protective effect may arise from the impact of statins on tissue factor. Tissue factor plays a pivotal role in thrombus formation in acute coronary syndromes. Endothelial tissue factor induction by thrombin is regulated by Rho/Rho-kinase, Akt, and p38 mitogen-activated protein kinase. Recently it was shown that simvastatin prevented tissue factor induction through inhibition of Rho/Rho-kinase and activation of Akt.64 In summary the protective action of statins in acute coronary syndromes is most likely to occur on several levels of the local inflammatory response in the vessel wall.
Conclusions and perspectives
The effects of statins on alloresponses and autoimmunity2,36 by pleiotropic mechanisms indicate a central role for the mevalonate downstream products in immunity. Mechanistically it was shown that isoprenoids that are downstream of the mevalonate pathway function as essential regulators of Th1/Th2 fate in T cells and are most likely relevant for the fate of other immune cells such as B cells, dendritic cells, macrophages and endothelial cells towards a tolerogenic versus proinflammatory phenotype.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH; RO1 CA0800065 to R.S.N.) and in part by the Deutsche Krebshilfe, Germany, grant no. 108034 to R.Z.
Conflict of interest
The authors have conflict of interest to declare.
References
- 1.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–13. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 2.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 3.Aktas O, Waiczies S, Smorodchenko A, et al. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–33. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn SE, Youssef S, Goldstein MJ, Prod’homme T, Weber MS, Zamvil SS, Steinman L. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med. 2006;203:401–12. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zipp F, Waiczies S, Aktas O, et al. Impact of HMG-CoA reductase inhibition on brain pathology. Trends Pharmacol Sci. 2007;28:342–9. doi: 10.1016/j.tips.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Lawman S, Mauri C, Jury EC, Cook HT, Ehrenstein MR. Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand Black/White F1 mice. J Immunol. 2004;173:7641–6. doi: 10.4049/jimmunol.173.12.7641. [DOI] [PubMed] [Google Scholar]
- 7.Zeiser R, Youssef S, Baker J, Kambhan N, Steinman L, Negrin RS. HMG-CoA reductase inhibitors (statins) provide acute-graft-versus-host disease protection by Th2 cytokine induction while sparing graft-versus-leukemia activity. Blood. 2007;110:4588–98. doi: 10.1182/blood-2007-08-106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raki M, Molberg O, Tollefsen S, Lundin KE, Sollid LM. The effects of atorvastatin on gluten-induced intestinal T cell responses in coeliac disease. Clin Exp Immunol. 2005;142:333–40. doi: 10.1111/j.1365-2249.2005.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waiczies S, Prozorovski T, Infante-Duarte C, et al. Atorvastatin induces T cell anergy via phosphorylation of ERK1. J Immunol. 2005;74:5630–5. doi: 10.4049/jimmunol.174.9.5630. [DOI] [PubMed] [Google Scholar]
- 10.Mausner-Fainberg K, Luboshits G, Mor A, Maysel-Auslender S, Rubinstein A, Keren G, George J. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+ CD25+ T cells. Atherosclerosis. 2008;197:829–39. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Broz P, Ben-Haim N, Grzelakowski M, Marsch S, Meier W, Hunziker P. Inhibition of macrophage phagocytotic activity by a receptor-targeted polymer vesicle-based drug delivery formulation of pravastatin. J Cardiovasc Pharmacol. 2008;51:246–52. doi: 10.1097/FJC.0b013e3181624aed. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz A, Reiss C, Tantawi O, et al. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis. 2004;172:85–93. doi: 10.1016/j.atherosclerosis.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Xu SZ, Zhong W, Watson NM, Dickerson E, Wake JD, Lindow SW, Newton CJ, Atkin SL. Fluvastatin reduces oxidative damage in human vascular endothelial cells by upregulating Bcl-2. J Thromb Haemost. 2008;4:692–700. doi: 10.1111/j.1538-7836.2008.02913.x. [DOI] [PubMed] [Google Scholar]
- 14.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood J, Walters CE, Pryce G, Kanuga N, Beraud E, Baker D, Adamson P. Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J. 2003;17:905–7. doi: 10.1096/fj.02-1014fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung BP, Sattar N, Crilly A, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer T, Key L, Durkalski V, Tyor W, Corboy J. Oral simvastatin treatment in relapsing–remitting multiple sclerosis. Lancet. 2004;363:1607–8. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 18.Paul F, Waiczies S, Wuerfel J, et al. Oral high-dose atorvastatin treatment in relapsing–remitting multiple sclerosis. PLoS ONE. 2008;3:1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 20.Hori A, Kanda Y, Goyama S, et al. A prospective trial to evaluate the safety and efficacy of pravastatin for the treatment of refractory chronic graft-versus-host disease. Transplantation. 2005;79:372–4. doi: 10.1097/01.tp.0000151001.64189.1d. [DOI] [PubMed] [Google Scholar]
- 21.Graham KL, Lee LY, Higgins JP, Steinman L, Utz PJ, Ho PP. Failure of oral atorvastatin to modulate a murine model of systemic lupus erythematosus. Arthritis Rheum. 2008;58:2098–104. doi: 10.1002/art.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira GA, Navarro TP, Telles RW, Andrade LEC, Sato I. Atorvastatin therapy improves endothelial-dependent vasodilatation in patients with systemic lupus erythematosus: an 8 weeks controlled trial. Rheumatology. 2007;46:1560–5. doi: 10.1093/rheumatology/kem186. [DOI] [PubMed] [Google Scholar]
- 23.Kotyla PJ. Comment on: atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: an 8 week controlled trial. Rheumatology. 2008;47:381–2. doi: 10.1093/rheumatology/kem381. [DOI] [PubMed] [Google Scholar]
- 24.Mason JC. Statins and their role in vascular protection. Clin Sci. 2003;105:251–66. doi: 10.1042/CS20030148. [DOI] [PubMed] [Google Scholar]
- 25.Meroni PL, Luzzana C, Ventura D. Anti-inflammatory and immunomodulating properties of statins. An additional tool for the therapeutic approach of systemic autoimmune disease? Clin Rev Allerg Immunol. 2002;23:263–77. doi: 10.1385/CRIAI:23:3:263. [DOI] [PubMed] [Google Scholar]
- 26.Abud-Mendoza C, de la Fuente H, Cuevas-Orta E, Baranda L, Cruz-Rizo J, González-Amaro R. Therapy with statins in patients with refractory rheumatic diseases: a preliminary study. Lupus. 2003;12:607–11. doi: 10.1191/0961203303lu429oa. [DOI] [PubMed] [Google Scholar]
- 27.Kanda H, Yokota K, Kohno C, et al. Effects of low-dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Mod Rheumatol. 2007;17:364–8. doi: 10.1007/s10165-007-0589-4. [DOI] [PubMed] [Google Scholar]
- 28.Lazzerini PE, Lorenzini S, Selvi E, et al. Simvastatin inhibits cytokine production and NF-κB activation in IL-1β-stimulated synoviocytes from rheumatoid arthritis patients. Clin Exp Rheumatol. 2007;25:696–700. [PubMed] [Google Scholar]
- 29.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–75. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 30.Hafler DA. Multiple sclerosis. J Clin Invest. 2004;113:788–94. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172:1273–86. doi: 10.4049/jimmunol.172.2.1273. [DOI] [PubMed] [Google Scholar]
- 32.Stüve O, Youssef S, Weber MS, et al. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J Clin Invest. 2006;116:1037–44. doi: 10.1172/JCI25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–96. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 34.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 35.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:39–42. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 36.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 37.Kobashigawa JA, Moriguchi JD, Laks H, et al. Ten-year follow-up of a randomized trial of pravastatin in heart transplant patients. J Heart Lung Transplant. 2005;24:1736–43. doi: 10.1016/j.healun.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Weis M, Pehlivanli S, Meiser BM, von Scheidt W. Simvastatin treatment is associated with improvement in coronary endothelial function and decreased cytokine activation in patients after heart transplantation. J Am Coll Cardiol. 2001;38:814–21. doi: 10.1016/s0735-1097(01)01430-9. [DOI] [PubMed] [Google Scholar]
- 39.Holschermann H, Hilgendorff A, Kemkes-Matthes B, et al. Simvastatin attenuates vascular hypercoagulability in cardiac transplant recipients. Transplantation. 2000;69:1830–9. doi: 10.1097/00007890-200005150-00017. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Liu M, Wu Y, Zhu P, Yin C, Zhang W, Gu M. Protective effects of atorvastatin on chronic allograft nephropathy in rats. J Surg Res. 2007;143:428–36. doi: 10.1016/j.jss.2006.12.557. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Yang CW, Park JH, et al. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol. 2004;286:46–53. doi: 10.1152/ajprenal.00428.2002. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Wu YC, Zhang W, et al. Effect of atorvastatin on intrarenal osteopontin expression in rat allogeneic kidney transplantation. Chin J Clin Rehab. 2006;62:1651–8. [Google Scholar]
- 43.Langham RG, Egan MK, Dowling JP, et al. Transforming growth factor-beta1 and TGF-β-inducible gene-H3 in nonrenal transplant cyclosporine nephropathy. Transplantation. 2001;72:1826–32. doi: 10.1097/00007890-200112150-00019. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 45.Hamadani M, Awan FT, Devine SM. The impact of HMG-CoA reductase inhibition on the incidence and severity of graft-versus-host disease in patients with acute leukemia undergoing allogeneic transplantation. Blood. 2008;111:3901–2. doi: 10.1182/blood-2008-01-132050. [DOI] [PubMed] [Google Scholar]
- 46.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 47.Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14–23. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]
- 48.Zeiser R, Albrecht-Bellingrath W, Schaefer HE. Age-dependent leiomuscular atrophy in vertebral arteries of individuals under low fat diet. In Vivo. 2000;14:631–4. [PubMed] [Google Scholar]
- 49.Bis JC, Heckbert SR, Smith NL, et al. Variation in inflammation-related genes and risk of incident nonfatal myocardial infarction or ischemic stroke. Atherosclerosis. 2008;198:166–73. doi: 10.1016/j.atherosclerosis.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eccles KA, Sowden H, Porter KE, Parkin SM, Homer-Vanniasinkam S, Graham AM. Simvastatin alters human endothelial cell adhesion molecule expression and inhibits leukocyte adhesion under flow. Atherosclerosis. 2008;200:69–79. doi: 10.1016/j.atherosclerosis.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Park SY, Lee JS, Ko YJ, et al. Inhibitory effect of simvastatin on the TNF-alpha- and angiotensin II-induced monocyte adhesion to endothelial cells is mediated through the suppression of geranylgeranyl isoprenoid-dependent ROS generation. Arch Pharm Res. 2008;31:195–204. doi: 10.1007/s12272-001-1141-2. [DOI] [PubMed] [Google Scholar]
- 52.Liang YJ, Shyu KG, Wang BW, Lai LP. Simvastatin inhibits C-reactive protein-induced pro-inflammatory changes in endothelial cells by decreasing mevalonate pathway products. Cardiology. 2008;110:182–90. doi: 10.1159/000111928. [DOI] [PubMed] [Google Scholar]
- 53.Plenge JK, Hernandez TL, Weil KM, et al. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;106:1447–52. doi: 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 54.Rosenson R, Brown AS. Statin use in acute coronary syndromes: cellular mechanisms and clinical evidence. Curr Opin Lipidol. 2002;13:625–31. doi: 10.1097/00041433-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Nissen SE. High-dose statins in acute coronary syndromes: not just lipid levels. JAMA. 2004;292:1365–71. doi: 10.1001/jama.292.11.1365. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes The MIRACL Study: a randomized controlled trial. JAMA. 2001;285:1711–8. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 57.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–503. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 58.de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. JAMA. 2004;292:1307–12. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 59.Ridker PM, Rifai N, Pfeffer MA, et al. For the cholesterol and recurrent events (CARE) investigators. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98:839–46. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 60.Horne BD, Muhlestein JB, Carlquist JF, et al. Statin therapy, lipid levels, C-reactive protein and the survival of patients with angiographically severe coronary artery disease. J Am Coll Cardiol. 2000;36:1774–86. doi: 10.1016/s0735-1097(00)00950-5. [DOI] [PubMed] [Google Scholar]
- 61.Chan AW, Bhatt DL, Chew DP, et al. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation. 2003;107:1750–62. doi: 10.1161/01.CIR.0000060541.18923.E9. [DOI] [PubMed] [Google Scholar]
- 62.Ridker PM, Rifai N, Clearfield M, et al. For the air force/texas coronary atherosclerosis prevention study investigators measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–67. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 63.Atar S, Ye Y, Lin Y, et al. Atorvastatin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cyclooxygenase-2. Am J Physiol Heart Circ Physiol. 2006;290:1960–8. doi: 10.1152/ajpheart.01137.2005. [DOI] [PubMed] [Google Scholar]
- 64.Masato E, Kozai T, Cosentino F, et al. Statin prevents tissue factor expression in human endothelial cells. Role of Rho/Rho-kinase and AKT pathways. Circulation. 2002;105:1756–9. doi: 10.1161/01.cir.0000015465.73933.3b. [DOI] [PubMed] [Google Scholar]
- 65.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–8. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]