Abstract
Human trophoblast cells express an unusual repertoire of human leucocyte antigen (HLA) molecules which has been difficult to define. Close homology between and extreme polymorphism at the classical HLA class-I (HLA-I) loci has made it difficult to generate locus-specific monoclonal antibodies (mAbs). The problem of defining an antibody's reactivity against the thousands of existing HLA-I allotypes has often made it impossible to determine the HLA bound by a mAb in biological samples from a normal outbred population. Here we have used commercially available beads coated with individual HLA-I to characterize experimentally the reactivity of nine mAb against 96 common HLA-I allotypes. In conjunction with donor HLA-I genotyping, we could then define the specific HLA molecules bound by these antibodies in normal individuals. We used this approach to analyse the HLA expression of primary trophoblast cells from normal pregnancies; the choriocarcinoma cells JEG-3 and JAR; and the placental cell lines HTR-8/SVneo, Swan-71 and TEV-1. We confirm that primary villous trophoblast cells are HLA null whereas extravillous trophoblast cells express HLA-C, HLA-G and HLA-E, but not HLA-A, HLA-B or HLA-DR molecules in normal pregnancy. Tumour-derived JEG-3 and JAR cells reflect extravillous and villous trophoblast HLA phenotypes, respectively, but the HLA repertoire of the in vitro derived placental cell lines is not representative of either in vivo trophoblast phenotype. This study raises questions regarding the validity of using the placental cell lines that are currently available as model systems for immunological interactions between fetal trophoblast and maternal leucocytes bearing receptors for HLA molecules.
Keywords: human leucocyte antigen, major histocompatibility complex, placenta, reproductive immunology, trophoblast
Introduction
Eutherian mammals depend on a placenta for growth and development in utero. Placental trophoblast cells form the interface between a mother and her conceptus throughout gestation. Trophoblast cells are, therefore, the primary fetal cells directly exposed to the maternal immune system.1 As a normal fetus is allogeneic, it has seemed paradoxical that trophoblast can avoid maternal alloreactivity. The main molecules involved in allorecognition by both T cells and natural killer cells belong to the highly polymorphic major histocompatibility complex, and are known in humans as human leucocyte antigens (HLA). There are two classes of HLA molecules, class-I (HLA-I) and class-II (HLA-II), that are recognized by CD8 and CD4 T cells, respectively. The HLA-I molecules are also ligands for killer immunoglobulin-like receptors (KIR) and leucocyte immunoglobulin-like receptors (LILR) expressed by natural killer cells and myelomonocytic cells of the innate immune system.2
In humans, trophoblast cells differentiate from the trophoblast shell that surrounds the post-implantation embryo into two main lineages, villous trophoblast and extravillous trophoblast (EVT). Villous trophoblast cells cover the villous tree with an inner cytotrophoblast layer and an overlying continuous syncytium in contact with maternal blood of the intervillous space. In contrast, EVT (which include endovascular trophoblast, interstitial trophoblast and placental bed giant cells) migrate from the cytotrophoblast shell through the decidual stroma and transform the maternal arteries, altering blood flow to the implantation site. Endovascular trophoblast cells invade arteries, displacing endothelium and becoming incorporated into the arterial wall. Interstitial trophoblast cells invade through the decidual stroma before fusing to form placental bed giant cells in the inner myometrium.3 As they infiltrate through the uterine mucosa, EVT mingle closely with maternal leucocytes, composed predominantly of the distinctive CD56bright uterine natural killer cells as well as HLA-DR+ macrophages and dendritic cells and a few CD3+ T cells. Contact with the maternal immune system therefore differs for the two main trophoblast lineages: villous trophoblast is in contact with the systemic immune system, whereas EVT interact with the local mucosal immune cells.4
There are six HLA-I loci that have expressed protein products: three classical molecules – HLA-A, HLA-B, HLA-C – and three non-classical HLA-I molecules – HLA-E, HLA-F, HLA-G. Classical HLA-A and HLA-B are ubiquitously expressed, with the exception of neurons, germ cells and trophoblast. Furthermore, the levels of classical HLA-I expression can vary significantly among cell types, and can be upregulated by cytokines such as interferon-γ (IFN-γ). In contrast, cell surface HLA-G expression is restricted to EVT.5 The HLA-II loci consist of HLA-DR, HLA-DQ and HLA-DP. These genes are coordinately regulated at the level of transcription, with HLA-DR tending to be the most highly expressed.6 Constitutive expression of HLA-II genes is restricted to a limited number of cells that function as antigen-presenting cells to T cells. These include dendritic cells, B cells, macrophages and thymic epithelium. Fibroblasts and epithelial and endothelial cells can also express HLA-II following exposure to IFN-γ. Both constitutive and IFN-γ-inducible HLA-II transcription are mediated by the class II transactivator (CIITA), the master regulator of HLA-II transcription.7 Studies of HLA expression of trophoblast subpopulations in vivo show that villous trophoblast cells do not express messenger RNA (mRNA) or protein for any of the HLA-I molecules or HLA-DR and are therefore considered immunologically inert.1 In contrast, although EVT do not express HLA-II proteins, they do display an unusual array of HLA-I molecules: HLA-G, HLA-C and HLA-E.8–14 This is a unique combination that has not been found on any other normal somatic or extraembryonic cell.
A major difficulty in studying the biological role of these trophoblast HLA-I molecules, in particular their interaction with receptors on maternal leucocytes, has been the availability of trophoblast cells for use in in vitro experiments. Primary trophoblast cells can be isolated from first-trimester placentae but this is ethically and technically problematic and a degree of contamination from fetal mesenchymal and Hofbauer cells (placental macrophages) always occurs. Because of these difficulties, a number of cell lines have been generated from both first-trimester and term placentae using a variety of methods.15 These cell lines would have obvious advantages over primary cells in the study of trophoblast behaviour but their relevance as models for the immunology of placentation depends on whether their HLA expression is the same as normal villous or extravillous trophoblast.
Since the early days of monoclonal antibody (mAb) technology, there have been many reagents generated to HLA-I and HLA-II molecules including the widely-used mAbs W6/32 and BBM.1, that react with all HLA-I molecules.16,17 It has been difficult, though, to generate locus-specific reagents because of both the close homology between classical HLA-I molecules and their extreme polymorphism. Furthermore, the difficulty of defining the reactivity of a mAb against the thousands of HLA-I allotypes has often made it impossible to define the HLA bound by a mAb in normal biological samples. Here we demonstrate a method to characterize experimentally the reactivity of mAbs against 100 of the common classical HLA-I allotypes. We then use these mAbs in conjunction with HLA-I genotyping to define the trophoblast repertoire of HLA expression and show that, in this respect, three placental cell lines are not representative of either of the main trophoblast cell lineages in vivo.
Materials and methods
Monoclonal antibodies
Purified mAbs W6/3216 and BBM.117 were purchased from Serotec (Oxford, UK) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Hybridoma supernatants of B1.23.2,18 Tu149,19 Tu155,20 PA2.1,21 MA2.122 and 22E-123,24 mAbs, all known to bind HLA-I molecules, were kindly supplied by Drs P. LeBouteiller, B. Uchanska-Ziegler, N. Holmes and I. Smith. Three purified mAbs recognizing HLA-G molecules were used: MEM-G/1125 from Abcam (Cambridge, UK), fluorescein isothiocyanate (FITC)-conjugated MEM-G/925 from Serotec and G2339 made in our own laboratory. Hybridoma supernatant of the well-established anti-HLA-DR mAb L24326 was supplied by Dr N. Lapaque. Purified isotype control immunoglobulin G2a (IgG2a) and IgG2b mouse mAbs were obtained from Sigma-Aldrich (Poole, UK). To label 2 × 105 cells in a total volume of 100 μl, 0·5 μg of purified mAbs and 20 μl of each hybridoma supernatant was used. Binding of these unlabelled mAbs was detected by polyclonal FITC-conjugated or phycoerythrin (PE)-conjugated secondary antibody to murine IgG or IgM (all Sigma-Aldrich). Conjugated mAbs also used were, LILRB3-PE-Cy5 (clone ZM3.8) from Beckman Coulter Immunotech (Marseilles, France), epidermal growth factor receptor (EGFR)-FITC (clone EGFR1) from Insight Biotechnology (Wembley, UK), HLA-DR-PE (clone L243) from Biolegend (San Diego, CA) and CD3-FITC (clone SK7), control IgG1-FITC or IgG2a-FITC (all Becton Dickinson, Oxford, UK).
Characterization of HLA-I antibodies
Binding of mAbs recognizing HLA-I molecules was characterized for 96 common classical HLA-I allotypes using commercially available beads each coated with individual HLA purified from transfected 721 221 cells.27 These LABScreen Single Antigen beads (One Lambda, Canoga Park, CA) were incubated with unlabelled primary or isotype control mAb for 30 min at room temperature, washed five times and labelled with polyclonal PE-conjugated secondary antibody to murine IgG or IgM (both Sigma-Aldrich). Fluorescence intensities of infra-red dyes identifying each bead and the PE-conjugated mAb labelling were analysed using a Luminex 100 reader (Luminex, Austin, TX). Results shown are median fluorescence intensities of a minimum of 100 events for each HLA-I-coated bead.
Primary tissue and cell lines
JAR, JEG-3 and HeLa cells were purchased from the American Type Culture Collection (Rockville, MD; reference numbers HTB-144, HTB-36 and CCL-2, respectively) and cultured as previously described.28,29 Placenta-derived cell lines HTR-8/SVneo,30 Swan-7131 and Tev-132 were obtained from Drs C. Graham, G. Mor and H. Feng, respectively. They were cultured in RPMI-1640/5% fetal calf serum (FCS) (HTR-8/SVneo), Hams F12/Dulbecco's modified Eagle's medium (DMEM)/5% FCS/1·75 mm HEPES (TEV-1), or DMEM/10% FCS (Swan-71) on plastic, fibronectin or laminin (Becton Dickinson).
Placental tissue was obtained from elective terminations of normal pregnancies between 6 and 12 weeks gestation. Ethical approval for the use of these tissues was obtained from the Cambridge Local Research Ethics Committee. Trophoblast cells were isolated as previously described.33 Briefly, trophoblast was released from chorionic villi by trypsin digestion and macrophages were depleted by adherence to plastic. Freshly isolated cells are predominantly villous trophoblast, identified as the only cell in these preparations expressing EGFR.34 After culture overnight on fibronectin 50–80% of the cells express HLA-G, a marker unique to EVT cells. Each cell type was also cultured in the presence of IFN-γ (from both Miltenyi Biotech, Bisley, UK and PBL, Piscataway, NJ) at 100 or 500 U/ml for 24 and 48 hr.
Flow cytometry
Adherent cultured primary trophoblast cells and cell lines were dissociated using 0·2% trypsin. An initial incubation with human IgG (Sigma-Aldrich) was performed to block Fcγ receptor-mediated mAb binding. Unlabelled primary antibodies were then added and detected with fluorochrome-conjugated anti-mouse IgG or IgM (Sigma-Aldrich). Free secondary antibody-binding sites were blocked with murine immunoglobulin, before staining with directly conjugated mAb to identify leucocyte or trophoblast cell populations. Cells were analysed using a FACscan flow cytometer and cellquest software (Becton Dickinson).
Reverse transcription–polymerase chain reaction
RNA isolation and reverse transcription–polymerase chain reaction (RT-PCR) analysis were performed as previously described.28,29 Briefly, RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) as specified by the manufacturer. Reverse transcriptase reactions were performed on 2 μg total RNA using Superscript II reverse transcriptase (Invitrogen). The primers and annealing temperatures used for RT-PCR analysis were described previously as follows: CIITA, HLA-DRα and invariant chain (Ii),35 HLA-DPα, IRF-136 and GAPDH.37 The PCR analysis was performed for 30 cycles for CIITA, HLA-DRα, HLA-DPα and Ii, 24 for IRF-1 and 20 cycles for GAPDH.
Results
Characterization of mAbs
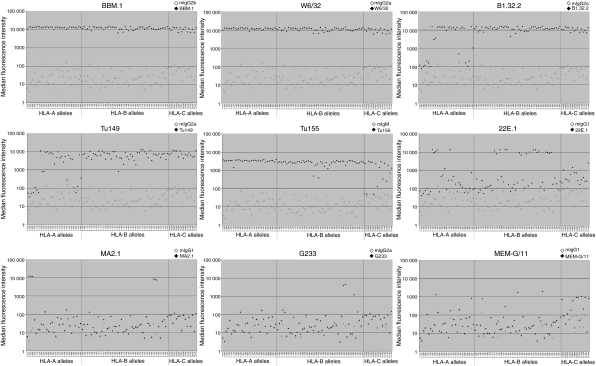
Initially we characterized the specificities of anti-HLA-I mAbs used in this study using a panel of commercially available beads coated with individual HLA-I molecules. Thirty-one HLA-A, 49 HLA-B and 16 HLA-C molecules were included, representing the common allotypes in Caucasian populations. Results are shown in Fig. 1 and summarized in Table 1. The mAbs BBM.1 and W6/32 bound strongly to all beads in the panel. BBM.1 binds to β2-microglobulin (β2m) and therefore will have the same affinity for each HLA-I allotype. W6/32 only binds to properly conformed HLA-I heterotrimers and confirms that there is no degradation of the HLA-I molecules. Binding of BBM.1 and W6/32 was almost identical to all HLA-I beads and therefore we can conclude that each bead was coated with a similar level of the conformed HLA-I complex. The mAbs MA2.1 and PA2.1, established to bind HLA-A2/B57/58 and HLA-A2/A28, respectively, only bound HLA-I allotypes consistent with these known specificities (Fig. 1 and data not shown).38 These results do therefore confirm the validity of this method in characterizing specificities of mAbs to HLA. Using single antigen beads to define antibody specificity was previously validated by comparing with results from antibody-mediated cytotoxicity assays.39
Figure 1.
Characterization of antibody reactivity against human leucocyte antigen class-I (HLA-I) molecules. The reactivity of antibodies used in this study was tested experimentally against 96 of the common HLA-I allotypes, using commercially available beads coated with individual HLA-I molecules. Binding of isotype control (open points) or the indicated murine monoclonal antibodies (filled points) is shown for each of the HLA-I allotypes tested. Plotted results are the median fluorescence intensity of at least 100 normally-distributed beads, and representative of at least three independent experiments for each antibody.
Table 1.
Summary of common human leucocyte antigen class I (HLA-I) allotype recognition of antibodies used in this study
| BBM.1 | W6/32 | B1.23.2 | Tu149 | Tu155 | 22E-1 | MA2.1 | G233 | MEM-G/11 | |
|---|---|---|---|---|---|---|---|---|---|
| A*0101 | + | + | − | − | + | − | − | − | − |
| A*0201 | + | + | − | − | + | − | + | − | − |
| A*0203 | + | + | − | − | + | − | + | − | − |
| A*0206 | + | + | − | − | + | − | + | − | − |
| A*0301 | + | + | − | − | + | − | − | − | − |
| A*1101 | + | + | − | − | + | − | − | − | − |
| A*1102 | + | + | − | − | + | − | − | − | − |
| A*2301 | + | + | + | + | + | + | − | − | − |
| A*2402 | + | + | ± | ± | + | + | − | − | − |
| A*2403 | + | + | ± | ± | + | + | − | − | − |
| A*2501 | + | + | + | + | + | + | − | − | − |
| A*2601 | + | + | + | + | + | − | − | − | − |
| A*2901 | + | + | + | + | + | − | − | − | − |
| A*2902 | + | + | + | + | + | − | − | − | − |
| A*3001 | + | + | + | + | + | ± | − | − | − |
| A*3002 | + | + | + | + | + | − | − | − | − |
| A*3101 | + | + | + | + | + | − | − | − | − |
| A*3201 | + | + | + | + | + | + | − | − | − |
| A*3301 | + | + | + | + | + | − | − | − | − |
| A*3303 | + | + | + | + | + | − | − | − | − |
| A*3401 | + | + | + | + | + | − | − | − | − |
| A*3402 | + | + | + | + | + | − | − | − | − |
| A*3601 | + | + | − | − | + | − | − | − | − |
| A*4301 | + | + | + | + | + | − | − | − | − |
| A*6601 | + | + | + | + | + | − | − | − | − |
| A*6602 | + | + | + | + | + | − | − | − | − |
| A*6801 | + | + | − | − | + | − | − | − | − |
| A*6802 | + | + | − | − | + | − | − | − | − |
| A*6901 | + | + | − | − | + | − | − | − | − |
| A*7401 | + | + | + | + | + | − | − | − | ± |
| A*8001 | + | + | ± | ± | + | − | − | − | − |
| B*0702 | + | + | + | + | + | − | − | − | − |
| B*0801 | + | + | + | + | + | − | − | − | − |
| B*1301 | + | + | + | + | + | − | − | − | − |
| B*1302 | + | + | + | + | + | − | − | − | − |
| B*1401 | + | + | + | + | + | − | − | − | ± |
| B*1402 | + | + | + | + | + | − | − | − | − |
| B*1501 | + | + | + | + | + | − | − | − | − |
| B*1502 | + | + | + | + | + | − | − | − | − |
| B*1503 | + | + | + | + | + | − | − | − | − |
| B*1510 | + | + | + | + | + | − | − | − | − |
| B*1512 | + | + | + | + | + | − | − | − | − |
| B*1513 | + | + | + | + | + | + | − | − | − |
| B*1516 | + | + | + | + | + | + | − | − | − |
| B*1801 | + | + | + | + | + | − | − | − | − |
| B*2705 | + | + | + | + | + | + | − | − | − |
| B*2708 | + | + | + | + | + | − | − | − | − |
| B*3501 | + | + | + | + | + | − | − | − | − |
| B*3701 | + | + | + | + | + | + | − | − | − |
| B*3801 | + | + | + | + | + | + | − | − | − |
| B*3901 | + | + | + | + | + | − | − | − | − |
| B*4001 | + | + | ± | ± | ± | − | − | − | − |
| B*4002 | + | + | + | + | + | − | − | − | − |
| B*4006 | + | + | + | + | + | − | − | − | − |
| B*4101 | + | + | + | + | ± | − | − | − | − |
| B*4201 | + | + | + | + | + | − | − | − | ± |
| B*4402 | + | + | + | + | + | + | − | − | − |
| B*4403 | + | + | + | + | + | + | − | − | − |
| B*4501 | + | + | + | + | + | − | − | − | − |
| B*4601 | + | + | + | + | + | − | − | − | − |
| B*4701 | + | + | + | + | + | + | − | − | − |
| B*4801 | + | + | + | + | + | − | − | − | − |
| B*4901 | + | + | + | + | + | + | − | − | − |
| B*5001 | + | + | + | + | + | − | − | − | − |
| B*5101 | + | + | + | + | + | + | − | − | − |
| B*5102 | + | + | + | + | + | + | − | − | − |
| B*5201 | + | + | + | + | + | + | − | − | − |
| B*5301 | + | + | + | + | + | + | − | − | − |
| B*5401 | + | + | + | + | + | − | − | ± | − |
| B*5501 | + | + | + | + | + | − | − | ± | ± |
| B*5601 | + | + | + | + | + | − | − | − | − |
| B*5701 | + | + | + | + | + | + | + | − | − |
| B*5703 | + | + | + | + | + | + | + | − | − |
| B*5801 | + | + | + | + | + | + | + | − | − |
| B*5901 | + | + | + | + | + | + | − | ± | − |
| B*6701 | + | + | + | + | + | − | − | − | − |
| B*7301 | + | + | + | + | + | − | − | − | − |
| B*7801 | + | + | + | + | + | − | − | − | − |
| B*8101 | + | + | + | + | + | − | − | − | − |
| B*8201 | + | + | + | + | + | − | − | − | − |
| Cw*0102 | + | + | + | + | + | − | − | − | ± |
| Cw*0202 | + | + | + | + | − | ± | − | − | − |
| Cw*0302 | + | + | + | + | + | − | − | − | − |
| Cw*0303 | + | + | + | + | + | − | − | − | − |
| Cw*0304 | + | + | + | + | + | − | − | − | − |
| Cw*0403 | + | + | + | + | − | − | − | − | − |
| Cw*0501 | + | + | + | + | + | ± | − | − | ± |
| Cw*0602 | + | + | + | + | ± | − | − | − | − |
| Cw*0702 | + | + | + | + | + | − | − | − | ± |
| Cw*0801 | + | + | + | + | + | − | − | − | ± |
| Cw*1203 | + | + | + | + | ± | − | − | − | − |
| Cw*1402 | + | + | + | + | + | − | − | − | ± |
| Cw*1502 | + | + | + | + | ± | − | − | − | − |
| Cw*1601 | + | + | + | + | + | − | − | − | ± |
| Cw*1701 | + | + | + | + | ± | − | − | − | − |
| Cw*1802 | + | + | + | + | + | ± | − | − | ± |
We next tested mAbs to various HLA-A, HLA-B and HLA-C allotypes. The mAbs B1.23.2 and Tu149 bound all HLA-B and HLA-C allotypes as well as several HLA-A molecules. The mAb 22E.1 bound certain HLA-A and HLA-B allotypes consistent with its known Bw4 supertype reactivity.23 The mAb Tu155 has been used as an antibody to HLA-A but in our assay shows much wider reactivity to almost all HLA-A and HLA-B as well as some HLA-C allotypes.
We also tested the anti-HLA-G mAbs, G233 and MEMG/11 and observed low levels of binding to some classical HLA-I molecules. These are shown as ± in Table 1. To establish whether these levels of binding to the beads result in detectable binding of mAbs to normal cells, we tested G233 and MEMG/11 against peripheral blood CD3+ cells from individuals positive for the HLA-I alleles shown as ± in Table 1. No binding of either mAb was detected in identical flow cytometry conditions to that used for trophoblast (data not shown).
Overall our results show that this is a useful method to define accurately the specificity of mAbs to multiple HLA-I allotypes. Our results agree with, but substantially extend, the previously described characterization of the mAbs used. With these results and in conjunction with HLA-I typing of selected individual donors, it is now possible to define the precise HLA-I bound by these mAbs in biological samples from a normal, outbred population.
HLA expression on normal first-trimester placental cells
Extravillous trophoblast
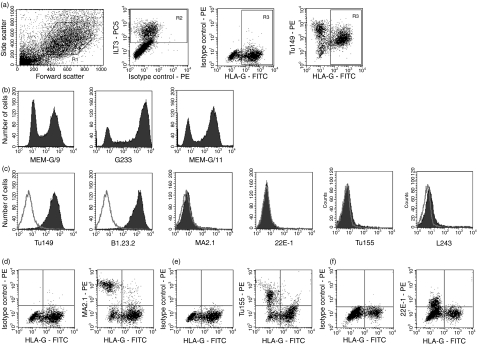
To confirm the HLA status of primary EVT we analysed first-trimester trophoblast cells plated overnight on fibronectin. Live cells were gated on the basis of forward and side scatter (Fig. 2a). Placental macrophages (Hofbauer cells) were stained with a mAb to ILT3 (LILRB3) allowing exclusion of these cells from the analysis.40 Staining with mAbs specific to HLA-G identified cells with an extravillous phenotype. All three antibodies to HLA-G (G233, MEMG/9 and MEMG/11) consistently bound to primary EVT cells (Fig. 2b).
Figure 2.
Human leucocyte antigen (HLA) expression of primary extravillous trophoblast cells (EVT). Trophoblast cells were isolated from normal human pregnancies in the first trimester and analysed by triple-colour flow cytometry. (a) EVT were identified using the following gates. Live cells selected by scatter (R1), placental macrophages labelled with monoclonal antibody (mAb) to ILT3 and excluded from subsequent analysis (R2), and the specific marker HLA-G identifies EVT (R3). (b) HLA-G expression by EVT was confirmed by three different mAbs: MEM-G/9, G233 and MEM-G/11, with histograms shown using the gates R1 AND NOT R2. (c) Expression of classical HLA-I (Tu149, B1.23.2, MA2.1, 22E-1, Tu155) and HLA-II (L243) was investigated using the mAbs indicated. Histograms shown use the gates R1 AND R3 AND NOT R2. Staining of the indicated mAb is shown as a filled trace with isotype controls as open plots. The results shown in (a–c) are representative of the majority of trophoblast samples from over 50 independent pregnancies. (d–f) Dot plots are shown for mAb MA2.1 (d), Tu155 (e) and 22E-1 (f) binding to placental cell preparations from selected donors. In these individuals the mAb can be seen binding to HLA-G–, but not EVT cells.
Labelling of the ILT3-, HLA-G+ cells with additional mAbs allows investigation of other HLA-I molecules expressed specifically by EVT (Fig. 2c). The EVT from all individuals stain strongly with mAbs Tu149 and B1.23.2. Since trophoblast cells have not been shown to transcribe other classical HLA-I, these mAbs are likely to be labelling HLA-C on trophoblast.12 The HLA-G+ trophoblast cells did not stain with mAbs MA2.1 or 22E-1, which react with some HLA-A or HLA-B allotypes. EVT from only occasional individuals bound Tu155, which does recognize a few HLA-C allotypes (see Table 1).
To definitively prove that EVT does not express HLA-A or HLA-B molecules we genotyped selected samples. This allowed us to study donors encoding particular HLA-A or HLA-B allotypes that we have shown were detected by the mAbs Tu155, MA2.1 or 22E-1 (Fig. 1). For each of the individuals shown mAbs Tu155, MA2.1 or 22E-1 bound to the contaminating HLA-G− but not to HLA-G+ EVT cells (Fig. 2d–f). For example, in Fig. 2(f) the trophoblast sample shown encodes the HLA-I genotype: A*0301, A*2402; B*0702, B*1801; Cw*0702, Cw*1203. These six allotypes are all included in our panel of HLA-I beads, but only HLA-A*2402 bound the mAb 22E-1. These results provide clear evidence that EVT does not express HLA-A or HLA-B molecules.
Of the remaining non-classical HLA-I molecules, trophoblast cells do not bind a well-characterized mAb to HLA-F,41 but do bind several mAbs to HLA-E.14,42 We also confirmed the absence of the HLA-II molecule HLA-DR on trophoblast cells because none of the purified primary trophoblast cells stained with the mAb L243 (Fig. 2c). All these findings extend and confirm previous studies from several groups, to definitively show that trophoblast cells with an extravillous phenotype express surface HLA-G, HLA-C and HLA-E but not HLA-A, HLA-B, HLA-F or HLA-DR molecules.8–14,41–45
Villous trophoblast
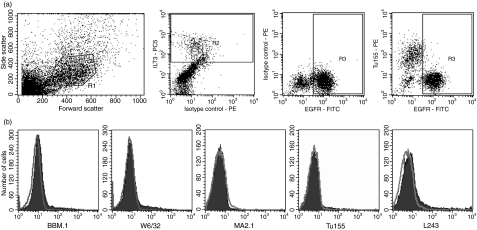
To analyse HLA expression on villous trophoblast, we obtained primary cell isolates after a brief enzyme digestion of first-trimester villi, which results in preparations rich in villous cytotrophoblast cells.3 Villous cytotrophoblasts are the only cells in these placental cell digests that are EGFR+.34 By gating on ILT3− EGFR+ cells it is apparent that villous trophoblast does not express β2m or any HLA-I or HLA-II molecules (Fig. 3).
Figure 3.
Human leucocyte antigen (HLA) expression of primary villous trophoblast cells. Trophoblast cells were isolated from normal human pregnancies in the first trimester and analysed by triple-colour flow cytometry. (a) Villous trophoblast cells were identified using the following gates. Live cells selected by scatter (R1), placental macrophages labelled with monoclonal antibody (mAb) to ILT3 and excluded from subsequent analysis (R2), and villous trophoblast identified by staining for epidermal growth factor receptor (EGFR) (R3). (b) HLA-I (BBM.1, W6/32, MA2.1, Tu155) and HLA-II (L243) expression was investigated using the indicated mAb. Each histogram uses the gates R1 AND R3 AND NOT R2. Staining of the indicated mAb is shown as a filled trace with isotype controls as open plots. The results are representative of staining for at least three independent pregnancies for each mAb.
Villous mesenchymal cells
Besides Hofbauer cells that were gated out with anti-ILT3, the other major contaminating cells in preparations of first-trimester placentae are villous mesenchymal cells.3 These contaminating cells do express classical HLA-I molecules (Figs 2 and 3). For example, in Fig. 2(d–f), there is a population of HLA-G− cells that showed reactivity for MA2.1, Tu155 and 22E-1. These findings indicate that placental mesenchymal cells express HLA-A and HLA-B molecules similar to somatic cells.
HLA expression on choriocarcinoma cells
JEG-3 and JAR choriocarcinoma cell lines were generated from gestational choriocarcinomas46,47 and are routinely used as models for in vitro studies of trophoblast. Flow cytometry using our panel of HLA antibodies confirmed previous studies demonstrating that JAR cells do not express any HLA molecules and JEG-3 express HLA-G and HLA-C (Fig. 4). The JAR cells therefore resemble villous trophoblast and JEG-3 cells resemble EVT in terms of their HLA expression.
Figure 4.
Human leucocyte antigen (HLA) expression of choriocarcinoma cell lines. The cell lines JEG-3 and JAR were analysed by single-colour flow cytometry using mAb to classical HLA-I (W6/32, B1.23.2, 22E-1, MA2.1, Tu155), HLA-G (G233, MEM-G/9) and HLA-II (L243). Histograms use the single scatter gate, show binding of the indicated mAb (filled trace) and isotype control (open trace) and are representative of at least three independent experiments.
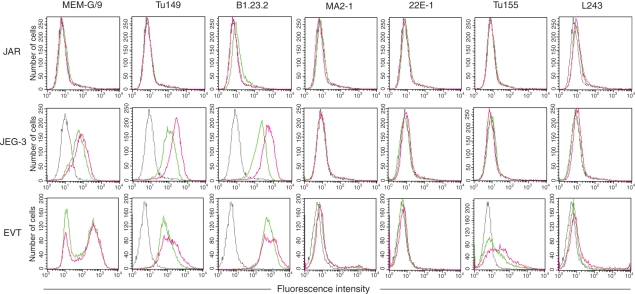
HLA expression by trophoblast after exposure to IFN-γ
HLA expression was next investigated after culture with IFN-γ (Fig. 5). On both JEG-3 and primary EVT cells HLA-C showed slight upregulation after exposure to IFN-γ, but there was no induction of HLA-A or HLA-B expression nor a significant effect on the level of HLA-G. In the sample of EVT shown in Fig. 5 the donor possessed an HLA-C allele weakly reactive with Tu155, HLA-Cw*1203, underlining the importance of combining genotyping with screening of mAbs to identify the HLA bound on primary cells. JAR cells remained negative for all HLA-I and HLA-II molecules. Transcription of the HLA class II genes in antigen-presenting cells, as well as fibroblasts, endothelial and epithelial cells exposed to IFN-γ, requires a transcription factor termed CIITA.7 We and others previously demonstrated that CIITA is not expressed in human or rodent trophoblast cells, even after exposure to IFN-γ.36,43,48 Importantly, no HLA-DR expression was seen on primary EVT or JEG-3 cells after IFN-γ treatment.
Figure 5.
Human leucocyte antigen (HLA) expression of primary trophoblast and choriocarcinoma cell lines after exposure to interferon-γ (IFN-γ). HLA expression of the choriocarcinoma cell lines JAR and JEG-3, and primary extravillous trophoblast was measured. Histograms show staining of the indicated monoclonal antibody (mAb) after culture with IFN-γ (red traces), culture without IFN-γ (green traces) and staining of isotype control mAb (broken trace) which was the same ±IFN-γ. Results are representative of at least three independent experiments with each mAb.
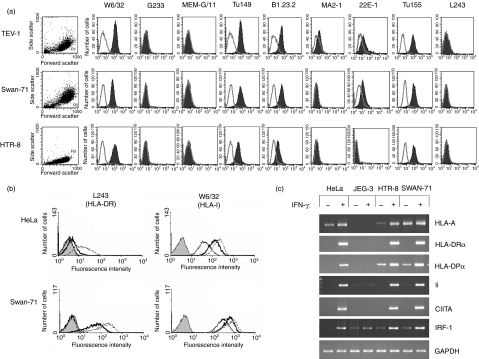
HLA surface expression on placental cell lines
We next examined cell surface expression of HLA-I and HLA-II molecules on three recently derived placental cell lines, HTR-8/SVneo, Swan-71 and TEV-1, using the same panel of antibodies. All three placental cell lines express HLA-I molecules as demonstrated by reactivity with W6/32 (Fig. 6a). Importantly, in contrast to normal trophoblast and the JEG-3 choriocarcinoma cell line, we were not able to detect HLA-G expression on any of these cell lines using two different mAbs, G233 and MEMG/11, even after culture on fibronectin or laminin, which upregulate the expression of HLA-G in primary trophoblast (data not shown).
Figure 6.
Human leucocyte antigen (HLA) expression of placental cell lines transformed in vitro. (a) Constitutive surface HLA expression of the placental cell lines TEV-1, Swan-71 and HTR-8 was analysed by single-colour flow cytometry. Classical HLA-I (W6/32, Tu149, B1.23.2, MA2.1, 22E-1, Tu155), HLA-G (G233, MEM-G/11) and HLA-DR (L243) expression was measured using the monoclonal antibody (mAb) shown. Histograms use the single scatter gate shown for each cell line and include staining of the indicated mAb (filled trace) and isotype control (open trace). (b) HLA surface protein expression after interferon-γ (IFN-γ) stimulation of Swan-71 and HeLa cells. Filled traces show binding of the isotype control. Staining of the indicated mAb is shown for unstimulated cells (single line), cells treated with IFN-γ for 24 hr (heavy trace) and 48 hr (dashed trace). (c) Messenger RNA (mRNA) expression of HLA and related genes after 24 hr IFN-γ stimulation of HeLa, JEG-3, HTR-8 and Swan-71 cells. Each of these results are representative of at least three replicate experiments and the identity of HLA-A mRNA was confirmed by sequencing of the polymerase chain reaction product.
As with normal EVT cells, binding of mAbs Tu149 and B1.23.2 was positive on all three lines, although we cannot determine which HLA-I allotypes are bound as these mAbs recognize several of the HLA-A, HLA-B and HLA-C allotypes expressed by the lines. Unlike normal trophoblast we detected consistent binding to the placental cell lines of the mAbs specific to more limited numbers of HLA-A and HLA-B allotypes. The mAb Tu155 bound to each cell line, mAb 22E-1 bound both TEV-1 and Swan-71 and the mAb MA2-1 bound TEV-1 cells (Fig. 6a). We have genotyped HTR-8/SVneo and TEV-1 and found that these mAbs recognize HLA-A and HLA-B molecules on these cell lines. For example HLA-B*5801 is the only one of the six classical HLA-I molecules encoded by TEV-1 which is recognized by the mAbs MA2.1 or 22E-1. We confirmed these findings by sequencing RT-PCR products to show the presence of HLA-A mRNA in HTR-8/SVneo and Swan-71 cells.
HLA-II expression of HTR-8/SVneo and Swan-71 cells was investigated after culture in the presence of IFN-γ. Although none of the cell lines expressed HLA-DR constitutively, IFN-γ induced HLA-DR mRNA and surface protein (Fig. 6). The RT-PCR showed that HLA-DRα, HLA-DPα, Ii and CIITA mRNA expression were co-ordinately upregulated by IFN-γ in both HTR-8/SVneo and Swan-71 cells, but not JEG-3 cells (Fig. 6c). Similar results were observed for the TEV-1, IST-1, HP-WT and HP-A1 placental cell lines (data not shown). Lastly, we previously demonstrated that IFN-γ-inducible IRF-1 gene expression is dampened in human trophoblast cells.29 However, HTR-8/SVneo and Swan-71 cells cultured in IFN-γ for 24 hr expressed high levels of IRF-1 mRNA (Fig. 6).
To summarize, our findings indicate that the placental cell lines show three major differences from normal trophoblast: they do not express HLA-G, they do express classical HLA-A and HLA-B molecules and they can be induced to express HLA-II molecules after exposure to IFN-γ (Table 2).
Table 2.
Summary of constitutive human leucocyte antigen (HLA) expression on normal primary placental cells, choriocarcinoma-derived cell lines and cell lines derived in vitro
| Monoclonal antibody | Primary extravillous trophoblast | Primary villous trophoblast | Primary mesenchyme | JEG-3 | JAR | TEV-1 | Swan-71 | HTR-8 |
|---|---|---|---|---|---|---|---|---|
| W6/32 | + | − | + | + | − | + | + | + |
| BBM.1 | + | − | + | + | − | + | + | + |
| G233 | + | − | − | + | − | − | − | − |
| MEM-G/9 | + | − | − | + | − | − | − | − |
| MEM-G/11 | + | − | − | + | − | − | − | − |
| Tu149 | + | − | + | + | − | + | + | + |
| B1.23.2 | + | − | + | + | − | + | + | + |
| Tu155 | − | − | + | − | − | + | + | + |
| 22E-1 | − | − | + | − | − | + | + | − |
| MA2.1 | − | − | + | − | − | − | + | − |
| L243 | − | − | − | − | − | − | − | − |
| Interpreted HLA expression | HLA-C, -E, -G | No HLA | HLA-A, -B, -C, -E | HLA-C, -E, -G | No HLA | HLA-A, -B, -C, -E | HLA-A, -B, -C, -E | HLA-A, -B, -C, -E |
Discussion
In this paper we have provided definitive evidence that normal EVT expresses HLA-C and HLA-G but not HLA-A or HLA-B molecules. It has been difficult to analyse specific HLA-I locus molecules because of the close similarity of the several thousand HLA-I allotypes resulting in cross-reactivity of many mAbs. For this reason we used beads coated with a range of HLA-I allotypes common in Caucasians to define the reactivity of a panel of mAbs. In conjunction with HLA-I genotyping, the specific HLA-I molecules bound in a given trophoblast sample could then be defined. In this way we have identified placentae whose HLA-A or HLA-B (but not HLA-C) molecules bind to some particular mAbs. Using the contaminating cells in the primary placental cell preparations as an internal positive control, we demonstrate that the HLA-G+ trophoblast cells do not express HLA-A or HLA-B molecules at the cell surface. In agreement with previous results we show expression of HLA-G and HLA-C molecules by EVT.8–13,41,44 Our results using normal trophoblast cells also confirm that EGFR+ villous trophoblast cells are HLA null. Of the cell lines derived from gestational choriocarcinomas, JEG-3 are identical to EVT whereas JAR resemble villous trophoblast in terms of HLA phenotype. These choriocarcinoma lines are therefore useful in studying some aspects of trophoblast immunobiology although the effects of transformation in vivo and their subsequent culture will inevitably result in significant differences from primary cells. JEG-3 was derived from the cerebral metastasis of a choriocarcinoma in 1959, and has subsequently been cultured for hundreds of passages in hamster cheek pouches and laboratory tissue culture.46,49
Our findings indicate that the recently-derived placental cell lines do not exhibit a normal trophoblast phenotype with regard to HLA expression. Using several mAbs to HLA-G, which we confirm do not react with classical HLA-I, we show that no HLA-G surface protein is expressed on any of these cell lines. HLA-G is the definitive EVT-specific HLA-I molecule that is not found at the cell surface of any other cell from normal individuals. Reports that it may be expressed in pathological situations such as tumours and allografts have used assays or mAbs for which cross-reaction with other molecules was likely and biochemical confirmation of the antigens detected was not performed.5 We have also used this extended mAb characterization in combination with HLA-I genotyping to show that there is expression of classical HLA-A and HLA-B allotypes on the placental lines.
We found no evidence for expression of the HLA-II molecule, HLA-DR, on villous trophoblast, EVT, JEG-3 or JAR cells. This is the case even after stimulation with IFN-γ. The placental cell lines are also constitutively negative for HLA-DR, but significantly we report induction of HLA-DR mRNA and protein in these cell lines following exposure to IFN-γ. This is another difference from normal trophoblast and the choriocarcinoma cell lines.
The disparity in HLA expression between primary trophoblast and these cell lines could potentially be explained by repressor molecules such as cytokines that are present in the uterine microenvironment, which function to suppress HLA expression in vivo. De-repression of HLA expression might thus occur during the immortalization process or during in vitro culture. However this seems unlikely, because if repression occurs in vivo, we would anticipate detecting classical HLA-A and HLA-B expression in the primary trophoblast cells cultured in vitro. Furthermore, these cell lines were immortalized using several different genes, including SV40 T antigen (HTR-8/SVneo), HPV E6 (TEV-1) and human telomerase (Swan-71), each of which functions to immortalize cells by slightly different mechanisms. We believe that it is unlikely that repression of the HLA genes was alleviated during the immortalization process. We are unaware of any precedence for alteration of HLA expression during culturing such that a non-classical HLA-I gene (HLA-G) is repressed and simultaneously, classical HLA-I and HLA-II genes are expressed de novo. In addition, in our previous studies we demonstrated that silencing of IFN-γ-inducible expression of CIITA and HLA class-II is conserved in mouse and rat trophoblast cell lines.28,43 Further work is obviously needed to understand how the unique trophoblast HLA repertoire is regulated during the in vivo development of trophectoderm, and subsequent differentiation of HLA-null villous and HLA-C+, HLA-G+ extravillous trophoblast.
An additional possibility explaining these results is that the cells from which the lines were derived were not in fact trophoblast. Our primary cell preparations include contaminating placental or maternal cells which do express a normal array of HLA-I molecules similar to the cell lines, and are negative for HLA-G. Additional antigens consistent with trophoblast have been demonstrated on HTR-8 cells,50–52 although these are not specific to trophoblast (e.g. cytokeratin-7) or easily compared with in vivo expression (e.g. integrins). Our results do highlight the importance of rigorous characterization of putative trophoblast lines. HLA molecules are particularly useful markers for defining the two main trophoblast lineages in humans because villous trophoblast completely lack HLA-I and HLA-II and EVT have a unique HLA-I profile. In future an HLA profile using the techniques we have described here might be included as an essential prerequisite of models to study trophoblast in vitro.
Acknowledgments
Our laboratory is funded by the Wellcome Trust, with additional support from the Cambridge Centre for Trophoblast Research. We would also like to thank all the donors and staff at Addenbrookes Hospital for primary tissue, as well as Drs P. LeBouteiller, B. Uchanska-Ziegler, N. Holmes, N. Lapaque and I. Smith for supplying antibodies and Drs C. Graham, G. Mor and H. Feng for providing cell lines.
Glossary
Abbreviations
- ATCC
American Type Culture Collection
- β2m
β2-microglobulin
- CIITA
class II transactivator
- EGFR
epidermal growth factor receptor
- EVT
extravillous trophoblast
- FITC
fluorescein isothiocyanate
- HLA
human leucocyte antigen
- IFN-γ
interferon-γ
- IgG2a
immunoglobulin G2a
- Ii
invariant chain
- KIR
killer immunoglobulin-like receptor
- LILR
leucocyte immunoglobulin-like receptor
- mAb
monoclonal antibody
- mRNA
messenger RNA
- PBMC
peripheral blood mononuclear cell
- PE
phycoerythrin
References
- 1.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–94. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 2.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–14. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 3.Loke YW, King A. Human Implantation: Cell Biology and Immunology. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- 4.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 5.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–21. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109:S21–33. doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 7.Harton JA, Ting JP. Class II transactivator: mastering the art of major histocompatibility complex expression. Mol Cell Biol. 2000;20:6185–94. doi: 10.1128/mcb.20.17.6185-6194.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–3. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 9.Loke YW, King A, Burrows T, et al. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens. 1997;50:135–46. doi: 10.1111/j.1399-0039.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 10.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–37. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King A, Boocock C, Sharkey AM, Gardner L, Beretta A, Siccardi AG, Loke YW. Evidence for the expression of HLA-C class I mRNA and protein by human first trimester trophoblast. J Immunol. 1996;156:2068–76. [PubMed] [Google Scholar]
- 12.King A, Burrows TD, Hiby SE, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–87. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- 13.Apps R, Gardner L, Hiby SE, Sharkey AM, Moffett A. Conformation of human leucocyte antigen-C molecules at the surface of human trophoblast cells. Immunology. 2008;124:322–8. doi: 10.1111/j.1365-2567.2007.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King A, Allan DS, Bowen M, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–31. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.King A, Thomas L, Bischof P. Cell culture models of trophoblast II: trophoblast cell lines; a workshop report. Placenta. 2000;21:S113–9. doi: 10.1053/plac.1999.0526. [DOI] [PubMed] [Google Scholar]
- 16.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens – new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky FM, Bodmer WF, Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979;9:536–45. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- 18.Rebai N, Malissen B. Structural and genetic analyses of HLA class I molecules using monoclonal xenoantibodies. Tissue Antigens. 1983;22:107–17. doi: 10.1111/j.1399-0039.1983.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 19.Uchanska-Ziegler B, Nossner E, Schenk A, Ziegler A, Schendel DJ. Soluble T cell receptor-like properties of an HLA-B35-specific monoclonal antibody (TU165) Eur J Immunol. 1993;23:734–8. doi: 10.1002/eji.1830230325. [DOI] [PubMed] [Google Scholar]
- 20.Hutter H, Hammer A, Blaschitz A, Hartmann M, Ebbesen P, Dohr G, Ziegler A, Uchanska-Ziegler B. Expression of HLA class I molecules in human first trimester and term placenta trophoblast. Cell Tissue Res. 1996;286:439–47. doi: 10.1007/s004410050713. [DOI] [PubMed] [Google Scholar]
- 21.Parham P, Bodmer WF. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978;276:397–9. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- 22.Ways JP, Parham P. The binding of monoclonal antibodies to cell-surface molecules. A quantitative analysis with immunoglobulin G against two alloantigenic determinants of the human transplantation antigen HLA-A2. Biochem J. 1983;216:423–32. doi: 10.1042/bj2160423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz CT, Smith KD, Greazel NS, Mace BE, Jensen DA, McCutcheon JA, Goeken NE. Bw4-reactive and Bw6-reactive antibodies recognize multiple distinct HLA structures that partially overlap in the alpha-1 helix. J Immunol. 1994;153:4099–110. [PubMed] [Google Scholar]
- 24.Tahara T, Yang SY, Khan R, Abish S, Hämmerling GJ, Hämmerling U. HLA antibody responses in HLA class I transgenic mice. Immunogenetics. 1990;32:351–60. doi: 10.1007/BF00211650. [DOI] [PubMed] [Google Scholar]
- 25.Menier C, Saez B, Horejsi V, et al. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: new tools to analyze the expression of nonclassical HLA class I molecules. Hum Immunol. 2003;64:315–26. doi: 10.1016/s0198-8859(02)00821-2. [DOI] [PubMed] [Google Scholar]
- 26.Lampson LA, Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980;125:293–9. [PubMed] [Google Scholar]
- 27.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43–9. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Holtz R, Choi JC, Petroff MG, Piskurich JF, Murphy SP. Class II transactivator (CIITA) promoter methylation does not correlate with silencing of CIITA transcription in trophoblasts. Biol Reprod. 2003;69:915–24. doi: 10.1095/biolreprod.103.017103. [DOI] [PubMed] [Google Scholar]
- 29.Choi JC, Holtz R, Petroff MG, Alfaidy N, Murphy SP. Dampening of IFN-gamma-inducible gene expression in human choriocarcinoma cells is due to phosphatase-mediated inhibition of the JAK/STAT-1 pathway. J Immunol. 2007;178:1598–607. doi: 10.4049/jimmunol.178.3.1598. [DOI] [PubMed] [Google Scholar]
- 30.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–11. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 31.Aplin JD, Straszewski-Chavez SL, Kalionis B, et al. Trophoblast differentiation: progenitor cells, fusion and migration – a workshop report. Placenta. 2006;27:S141–43. doi: 10.1016/j.placenta.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Feng HC, Choy MY, Deng W, Wong HL, Lau WM, Cheung AN, Ngan HY, Tsao SW. Establishment and characterization of a human first-trimester extravillous trophoblast cell line (TEV-1) J Soc Gynecol Investig. 2005;12:e21–32. doi: 10.1016/j.jsgi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Trundley A, Gardner L, Northfield J, Chang C, Moffett A. Methods for isolation of cells from the human fetal–maternal interface. Methods Mol Med. 2006;122:109–22. doi: 10.1385/1-59259-989-3:109. [DOI] [PubMed] [Google Scholar]
- 34.Jokhi PP, King A, Loke YW. Reciprocal expression of epidermal growth factor receptor (EGF-R) and c-erbB2 by non-invasive and invasive human trophoblast populations. Cytokine. 1994;6:433–42. doi: 10.1016/1043-4666(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 35.Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994;180:1367–74. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris AC, Riley JL, Fleming WH, Boss JM. MHC class II gene silencing in trophoblast cells is caused by inhibition of CIITA expression. Am J Reprod Immunol. 1998;40:385–94. doi: 10.1111/j.1600-0897.1998.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 37.Morris AC, Beresford GW, Mooney MR, Boss JM. Kinetics of a gamma interferon response: expression and assembly of CIITA promoter IV and inhibition by methylation. Mol Cell Biol. 2002;22:4781–91. doi: 10.1128/MCB.22.13.4781-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnett KL, Moses JH, Williams F, Marsh SG, Bodmer JG, Parham P, Middleton D. HLA-A *2607: sequence of a novel A*26 subtype predicted by DNA typing which shares the MA2.1 epitope with A*02, B*57 and B*58. Tissue Antigens. 1996;47:422–5. doi: 10.1111/j.1399-0039.1996.tb02578.x. [DOI] [PubMed] [Google Scholar]
- 39.El-Awar N, Lee J, Terasaki PI. HLA antibody identification with single antigen beads compared to conventional methods. Hum Immunol. 2005;66:989–97. doi: 10.1016/j.humimm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Heikkinen J, Möttönen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apps R, Gardner L, Traherne J, Male V, Moffett A. Natural-killer cell ligands at the maternal–fetal interface: UL-16 binding proteins, MHC class-I chain related molecules, HLA-F and CD48. Hum Reprod. 2008;23:2535–48. doi: 10.1093/humrep/den223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal–placental immune recognition. J Immunol. 2003;171:1376–84. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 43.Murphy SP, Tomasi TB. Absence of MHC class II antigen expression in trophoblast cells results from a lack of class II transactivator (CIITA) gene expression. Mol Reprod Dev. 1998;51:1–12. doi: 10.1002/(SICI)1098-2795(199809)51:1<1::AID-MRD1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 44.Blaschitz A, Hutter H, Dohr G. HLA class I protein expression in the human placenta. Early Pregnancy. 2001;5:67–9. [PubMed] [Google Scholar]
- 45.Blaschitz A, Juch H, Volz A, Hutter H, Daxboeck C, Desoye G, Dohr G. The soluble pool of HLA-G produced by human trophoblasts does not include detectable levels of the intron 4-containing HLA-G5 and HLA-G6 isoforms. Mol Hum Reprod. 2005;11:699–710. doi: 10.1093/molehr/gah185. [DOI] [PubMed] [Google Scholar]
- 46.Kohler PO, Bridsen WE. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol. 1971;32:683. doi: 10.1210/jcem-32-5-683. [DOI] [PubMed] [Google Scholar]
- 47.Pattillo RA, Ruckert A, Hussa R, Bernstein R, Delfs E. The Jar cell line – continuous human multihormone production and controls. In Vitro. 1971;6:398–399. [Google Scholar]
- 48.van den Elsen PJ, Gobin SJ, van der Stoep N, Datema G, Viëtor HE. Transcriptional control of MHC genes in fetal trophoblast cells. J Reprod Immunol. 2001;52:129–45. doi: 10.1016/s0165-0378(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 49.Hertz R. Choriocarcinoma of women maintained in serial passage in hamster and rat. Proc Soc Exp Biol Med. 1959;102:77. doi: 10.3181/00379727-102-25149. [DOI] [PubMed] [Google Scholar]
- 50.Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-beta at the human fetal–maternal interface: role in trophoblast growth and differentiation. Biol Reprod. 1992;46:561–72. doi: 10.1095/biolreprod46.4.561. [DOI] [PubMed] [Google Scholar]
- 51.Graham CH, Connelly I, MacDougall JR, Kerbel RS, Stetler-Stevenson WG, Lala PK. Resistance of malignant trophoblast cells to both the anti-proliferative and anti-invasive effects of transforming growth factor-beta. Exp Cell Res. 1994;214:93–9. doi: 10.1006/excr.1994.1237. [DOI] [PubMed] [Google Scholar]
- 52.Iacob D, Cai J, Tsonis M, Babwah A, Chakraborty C, Bhattacharjee RN, Lala PK. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors. Endocrinology. 2008;149:6187–97. doi: 10.1210/en.2008-0780. [DOI] [PubMed] [Google Scholar]