Abstract
Nitric oxide (NO) is a potent molecule involved in the cytotoxic effects mediated by macrophages (MØ) against microorganisms. We previously reported that Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells generate a greater amount of NO than wild-type cells in response to interferon-γ (IFN-γ). We also reported that the Leishmania-induced MØ SHP-1 activity is needed for the survival of the parasite within phagocytes through the attenuation of NO-dependent and NO-independent mechanisms. In the present study, we investigated the role of SHP-1 in regulating key signalling molecules important in MØ NO generation. Janus tyrosine kinase 2 (JAK2), mitogen-activated extracellular signal-regulated protein kinase kinase (MEK), extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2) mitogen-activated protein kinases, p38 and stress-activated mitogen-activated protein kinases/c-Jun NH2-terminal kinase (SAPK/JNK) were examined in immortalized bone marrow-derived MØ (BMDM) from both SHP-1-deficient motheaten mice (me-3) and their respective littermates (LM-1). The results indicated that Erk1/Erk2 and SAPK/JNK are the main kinases regulated by SHP-1 because the absence of SHP-1 caused an increase in their phosphorylation. Moreover, only Apigenin, the specific inhibitor of Erk1/Erk2, was able to block IFN-γ-induced inducible nitric oxide synthase (iNOS) transcription and translation in me-3 cells. Transcription factor analyses revealed that in the absence of SHP-1, activator protein-1 (AP-1) was activated. The activation of AP-1, and not nuclear factor-κB (NF-κB) or signal transducer and activator of transcription-1α (STAT-1α), may explain the enhanced NO generation in SHP-1-deficent cells. These observations emphasize the involvement of the MAPKs Erk1/Erk2 and SAPK/JNK in NO generation via AP-1 activation. Collectively, our findings suggest that SHP-1 plays a pivotal role in the negative regulation of signalling events leading to iNOS expression and NO generation. Furthermore, our observations underline the importance of SHP-1-mediated negative regulation in maintaining NO homeostasis and thus preventing the abnormal generation of NO that can be detrimental to the host.
Keywords: activator protein-1 (AP-1), interferon-γ, mitogen-activated protein kinase, nitric oxide, Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)
Introduction
Nitric oxide (NO) is a gaseous free radical that mediates intercellular communication in most mammalian organs. It is also implicated in vascular homeostasis, neurotransmission and antimicrobial defense, including cytotoxic functions of rodent macrophages (MØ) against intracellular pathogens,1–3 viruses4,5 and tumours.6 This simple chemical mediator is produced by nitric oxide synthase (NOS), which converts l-arginine to l-citrulline and NO.7,8 Three isoforms of the enzyme have been cloned to date: two Ca2+-calmodulin-dependent isoforms known as neuronal NOS (nNOS)9 and endothelial NOS (eNOS),10 and a third Ca2+-independent–calmodulin-dependent isoform found in different cell types, such as macrophages,8,11–13 and known as inducible NOS (iNOS).
Nitric oxide plays an integral role in the control of Leishmania infections.14–17 To survive and propagate within its mammalian host, Leishmania needs to be effective at inhibiting NO production in MØ.18 Of interest is our observation that cells deficient for Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1) generate a greater amount of NO than wild-type cells at the basal level and in response to interferon-γ (IFN-γ).15 Experiments carried out using both the protein tyrosine phosphatase (PTP) inhibitor peroxovanadium (pV) and SHP-1-deficient motheaten mice showed that Leishmania infections cannot normally progress in vitro and in vivo in the absence of host PTP, particularly SHP-1.15,16,19 We and others demonstrated that Leishmania can selectively inactivate some members of the Janus tyrosine kinase (JAK) family,18,20 and that SHP-1 plays an important role in this selective inactivation20 and is therefore a key regulator of the JAK–signal transducer and activator of transcription (STAT) pathway. Furthermore, other PTPs [e.g. PTP1B and T-cell PTP (TCPTP)] have also been reported to bind to and negatively regulate several members of this pathway.21–23
Protein tyrosine phosphatases are key regulatory components in signal transduction pathways and have been identified in a wide variety of species.24,25 Some PTPs contain Src homology 2 (SH2) domains, which are specific amino acid sequences that mediate protein–protein interactions between signalling molecules. Two vertebrate SH2 domain-containing PTPs have been cloned and characterized: SHP-1 (a.k.a. PTP1C, HCP, SHPTP1 and SHP); and SHP-2.25 SHP-1 is an enzyme abundantly expressed in MØ and has been implicated in the negative regulation of many activation and growth-promoting haemopoietic signalling cascades.26–32
In the present study, we evaluated the role of the PTP SHP-1 in the modulation of signalling events leading to NO generation in IFN-γ-stimulated MØ using SHP-1-deficient cells (me-3) and their wild-type counterparts (LM-1). Our data revealed that the enhanced IFN-γ-induced NO generation in SHP-1-deficient cells (me-3) could be attributed to a selective activation of the extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2) and stress-activated mitogen-activated protein kinases/c-Jun NH2-terminal kinase (SAPK/JNK), leading to activator protein-1 (AP-1) activation. By contrast, JAK2, mitogen-activated or extracellular signal-regulated protein kinase kinase (MEK), p38 kinase, as well as the transcription factor signal transducer and activator of transcription (STAT-1α) were shown not to be involved in the enhanced NO generation. Finally, although nuclear factor-κB (NF-κB) was strongly detected at basal level and in response to IFN-γ in SHP-1-deficient cells, compared with wild-type LM-1 cells, the activation of this transcription factor was not associated with an increase in iNOS gene expression or the subsequent NO production in any of the cell lines used.
Experimental procedures
Reagents
Isotopes were obtained from ICN Pharmaceuticals Canada Ltd (Montreal, QC, Canada). Recombinant murine IFN-γ (2 × 105 U/ml) was purchased from Invitrogen (Invitrogen Canada Inc., Burlington, ON, Canada). The anti-iNOS Ig was purchased from Cedarlane Laboratories Limited (Hornby, ON, Canada). The Erk1/Erk2 mitogen-activated protein (MAP) kinase inhibitor (Apigenin), the MEK inhibitor (PD98059), the MAP kinase p38 inhibitor (SB203580) and the NF-κB inhibitor (BAY 11-7082) were purchased from Calbiochem (EMD Biosciences, San Diego, CA). The JAK2 inhibitor (AG490) was purchased from BioMol (BioMol International, Plymouth Meeting, PA) and the NF-κB inhibitor sodium salicylate (NaS) was purchased from Sigma-Aldrich (Oakville, Ontario, Canada). The oligonucleotides specific for AP-1 and NF-κB binding were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and the STAT-1α–iNOS specific gamma-activated sequence (GAS)-containing oligonucleotide (GAS/ISRE/iNOS) was synthesized in our laboratory.33
Cell culture
The immortalized me-3 and LM-1 BMDM used in this study were generated from motheaten mice (me; C3HeBFeJ me/me) and their respective wild-type littermates (LMme; C3HeBFeJ me/+), as previously described.15
NO production
Briefly, cells were seeded in 24-well dishes (5 × 105 cells/well) prior to stimulation with IFN-γ (100 U/ml, 24 hr). In some experiments, cells were treated with the various second-messenger inhibitors described above for 1 hr prior to stimulation with IFN-γ, and inhibitors were retained throughout the stimulation period. Nitric oxide generation was evaluated by measuring the accumulation of nitrite in the culture medium using the Griess reaction, as previously described.19
Western blot analysis
Cells (106–107) were collected and lysed in cold lysis buffer 20 mm Tris–HCl (pH 8·0), 0·14 m NaCl, 10% glycerol (v/v), 1% Nonidet P-40 (NP-40) (v/v), 10 μm NaF, 1 mm sodium ortho-vanadate and protease inhibitors: 100 μg/ml of phenylmethylsulfonyl fluoride (PMSF) and 25 μg/ml of aprotinin and leupeptin]. The lysates (30 μg per lane) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA), as previously described.19 Specific proteins and their state of phosphorylation were detected using antibodies directed against iNOS (Cedarlane), phosphotyrosine–JAK2, phosphotyrosine–Erk1/Erk2 (p42/p44), phophotyrosine–MEK (p43), phosphotyrosine–p38 (all BioSource International, Camarillo, CA), p-Y-STAT-1 and p-Ser727-STAT-1 (kindly provided by Dr David Frank, Harvard Medical School, Boston, MA). To monitor equal loading of proteins, membranes were stripped and reprobed with anti-JAK2 IgG (C-20 rabbit polyclonal IgG), anti-STAT-1 IgG (C-111 mouse monoclonal IgG) (both Santa Cruz Biotechnology), or antibodies to p42/p44, MEK (p43) or p38 (all Biosource International). Proteins were detected by probing with anti-mouse or anti-rabbit horseradish peroxidase (HRP) conjugates (Amersham, Montréal, Quebec, Canada) and visualized using enhanced chemoluminescence (ECL Western blotting detection system; Amersham).
Northern blot analysis
Expression of the iNOS gene in IFN-γ-stimulated me-3 and LM-1 cell lines (100 U/ml, 0–8 hr), either treated or not treated with specific inhibitors (1 hr prior to stimulation with IFN-γ and retained throughout the stimulation period) was evaluated by Northern blot of total mRNA, as previously described.19 Briefly, after stimulation, cells were washed twice with phosphate-buffered saline (PBS) and total RNA was extracted using Trizol (Invitrogen). RNA (10 μg) was then subjected to electrophoresis on a 1% agarose gel, transferred onto Hybond-N filter paper (Amersham) and hybridized with random primer-labeled cDNA probes. Loading of equal amounts of RNA was confirmed by hybridization with a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA probe. All washes were performed under stringent conditions, and transcripts were visualized by autoradiography.
Electrophoretic mobility shift assay (EMSA)
Cells grown to a density of 2 × 106 cells per flask were treated as indicated. Reactions were stopped by the addition of ice-cold PBS. In brief, sedimented cells were resuspended in 400 μl of cold buffer A [10 mm Hepes, pH 7·9, 1·5 mm MgCl2, 10 mm KCl, 0·5 mm dithiothreitol (DTT) and 0·2 mm PMSF]. After incubation for 15 min on ice, 25 μl of NP-40 (10%) was added to each sample. Samples were then vortexed for 10 seconds and centrifuged for 30 seconds at 12 000 g. The supernatants were discarded and the cell pellets were resuspended in 50 μl of cold buffer C (20 mm Hepes–KOH, pH 7·9, 25% glycerol, 420 mm NaCl, 1·5 mm MgCl2, 0·2 mm EDTA, 0·5 mm DTT and 0·2 mm PMSF) and incubated on ice for 15 min. Cell debris was removed by centrifugation at 12 000 g for 5 min at 4°, and the supernatants containing nuclear proteins were stored at −70° until further use. Oligonucleotide sequences specific in their binding to the transcription factors of interest were labeled with [γ-32P]ATP and incubated with the extracted nuclear proteins (6 μg), which were then subjected to electrophoresis on a 4% polyacrylamide gel. After migration, the gel was dried and exposed to Kodak BioMax MR film (Mandel Scientific Corporation, Guelph, Ontario, Canada). The oligonucleotide sequences used were as follows: NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′), AP-1 (5′-AGCTCGCGTGACTCAGCTG-3′) and STAT-1α-iNOS (GAS/ISRE/iNOS) (5′-CTTTTCCCCTAACAC-3′).33
Statistical analyses
Statistically significant differences were identified using the analysis of variance (anova) and the Fisher least significant difference test module of sas software (version 6.07; SAS Institute, Cary, NC). P-values of < 0·05 were deemed statistically significant. All data were presented as the mean ± standard error of the mean (SEM).
Results
Role of Erk1/Erk2 MAPKs and SAPK/JNK in the enhanced IFN-γ-induced NO generation in SHP-1-deficient cells
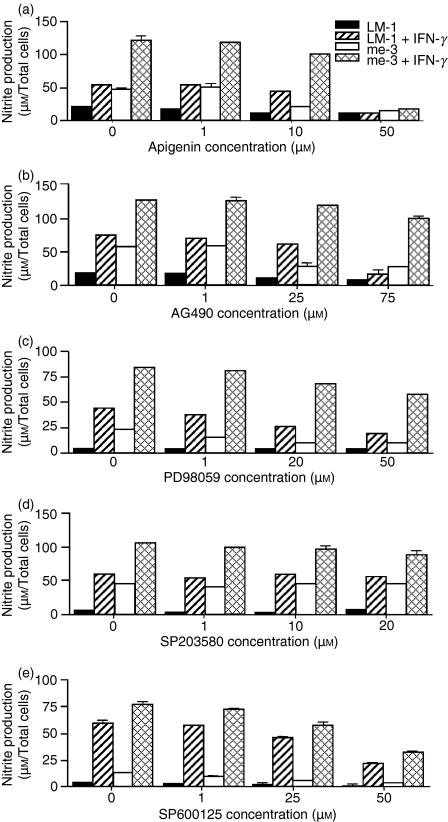
In the present study, we showed the capacity of SHP-1-deficient cells (me-3) to generate NO at a much higher level than their wild-type counterparts (LM-1) at the basal level and following IFN-γ stimulation (Fig. 1). This finding suggests that SHP-1 is a major negative regulator of signalling events that lead to NO generation. We and others have generated data suggesting an essential role for JAK2, Erk1/Erk2, SAPK/JNK and MEK in the signalling events leading to NO generation in response to IFN-γ.34 Based on those observations, we evaluated which of these kinases was responsible for the elevated NO production in SHP-1-deficient cells. Involvement of these second messengers in both cell lines was evaluated using increasing doses of selective inhibitors of JAK2 (AG490), Erk1/Erk2 (Apigenin), MEK (PD98059), p38 (SB203580), and SAPK/JNK (SP600125). As shown in Fig. 1(a,e), a significant inhibition of NO generation was observed in LM-1 and me-3 cells treated with the highest concentrations of the Erk1/Erk2 MAPK inhibitor (Apigenin) and the SAPK/JNK inhibitor (SP600125), both at the basal level and in response to IFN-γ. This observation suggests that the induced NO generation in SHP-1-deficient cells could be attributed to selective Erk1/Erk2 MAPK and SAPK/JNK activation in the absence of host SHP-1. Conversely, as shown in Fig. 1(b,c), JAK2 and MEK inhibitors were able to inhibit NO generation strongly, in a dose-dependent manner, in LM-1 cells with only a slight effect on me-3 cells. Finally, the p38 inhibitor (Fig. 1d) had no effect on NO generation in either cell line. Collectively, this suggests that it is the absence of SHP-1-negative regulation of Erk1/Erk2 kinases and SAPK/JNK that is responsible for the enhanced generation of NO in me-3 cells.
Figure 1.
Effect of the specific inhibitors of Janus tyrosine kinase 2 (JAK2), extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2), mitogen-activated extracellular signal-regulated protein kinase kinase (MEK), p38 and stress-activated mitogen-activated protein kinases/c-Jun NH2-terminal kinase (SAPK/JNK) on nitric oxide (NO) generation in Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells (me-3) and their wild-type counterparts (LM-1). Macrophages (MØ) were incubated with increasing doses of the Erk1/Erk2 inhibitor (Apigenin; 1–50 μm) (a), the JAK2 inhibitor (AG490; 1–75 μm) (b), the MEK inhibitor (PD98059, 1–40 μm) (c), the p38 inhibitor (SB203580; 1–20 μm) (d) and the SAPK/JNK inhibitor (SP600125), 1–50 μm) (e) for 1 hr prior to stimulation with interferon-γ (IFN-γ) (100 U/ml, 24 hr). Generation of NO was measured as described in the Experimental procedures. The results are representative of three independent experiments.
Selective Erk1/Erk2 MAPK-dependent regulation of iNOS expression in the absence of SHP-1
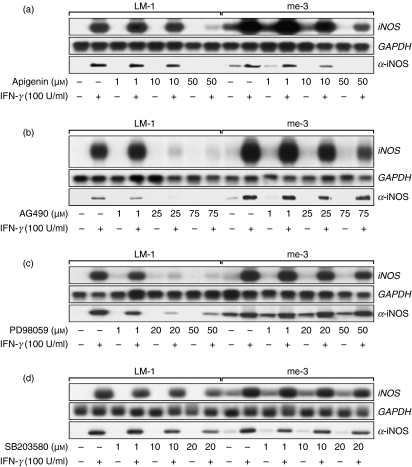
To understand in greater detail the signalling mechanism underlying the enhanced generation of NO by SHP-1-deficient cells, modulation of the expression of the iNOS gene and protein was monitored in the same experimental context described above. As depicted in Fig. 2, unlike LM-1 cells, me-3 cells were shown to express the iNOS gene and the iNOS protein15 at basal levels, in addition to producing significantly higher iNOS RNA levels in response to IFN-γ. This result suggests that the regulation of NO generation in SHP-1-deficient cells is achieved at the pretranscriptional level. Use of the JAK2, Erk1/Erk2, MEK and p38 inhibitors showed that the most significant blockage of iNOS expression in the absence of SHP-1 occurred using the Erk1/Erk2 inhibitor (Apigenin) (Fig. 2a). The other inhibitors were shown to inhibit, partially or totally, expression of the iNOS gene and protein in LM-1 MØ (Fig. 2a–c). Taken together, these results indicate that the enhanced NO generation by SHP-1-deficient MØ, whether at the basal level or in response to IFN-γ, was mediated by the MAP kinases Erk1/Erk2, resulting in enhanced iNOS expression (Fig. 2) and NO generation (Fig. 1).
Figure 2.
Effect of the kinase inhibitors on the expression of inducible nitric oxide synthase (iNOS) in Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells (me-3) in response to interferon-γ (IFN-γ). Cells were incubated with increasing doses of the extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2) inhibitor Apigenin (1–50 μm) (a), Janus tyrosine kinase 2 (JAK2) inhibitor AG490 (1–75 μm) (b), mitogen-activated extracellular signal-regulated protein kinase kinase (MEK) inhibitor PD98059 (1–40 μm) (c), or the p38 inhibitor SB203580 (1–20 μm) (d), for 1 hr prior to stimulation with IFN-γ. Expression of the iNOS gene (8 hr poststimulation) and iNOS protein (24 hr poststimulation) were monitored by Northern and western blot analyses, respectively, as described in the Experimental procedures. The results are representative of three independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Regulation of AP-1 and NF-κΒ nuclear translocation by SHP-1
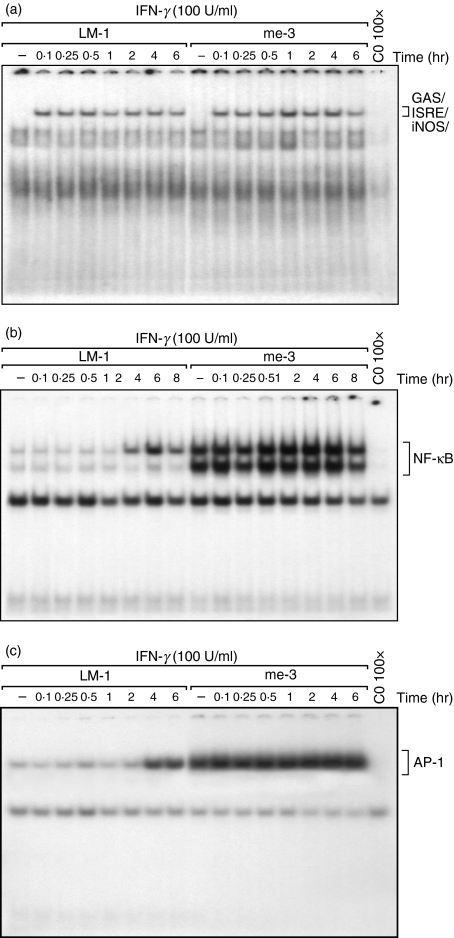
As described above, we observed that me-3 cells had an Erk1/Erk2-dependent effect on IFN-γ-induced iNOS expression and its consequent NO generation. Based on these observations, we were interested in investigating the role of SHP-1 in the regulation of various transcription factors (i.e. STAT-1α, NF-κB and AP-1), recognized for their involvement in iNOS expression in MØ subjected to IFN-γ stimulation alone or in combination with lipopolysaccharide (LPS).8,33,35,36 As shown in Fig. 3(a), STAT-1α was translocated to the nucleus to a similar extent in both wild-type and SHP-1-deficient cells upon stimulation with IFN-γ. By contrast, translocation of NF-κB and AP-1 to the nucleus, at the basal level and in response to IFN-γ, was considerably higher in me-3 cells than in their LM-1 counterparts (Fig. 3b,c, respectively). This raises the possibility that selective activation of AP-1 and/or NF-κB could be responsible for the enhanced iNOS expression observed in the SHP-1-deficient cells.
Figure 3.
Modulation of interferon-γ (IFN-γ)-mediated nuclear translocation of signal transducer and activator of transcription 1α (STAT-1α), nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) transcription factors in Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells (me-3) and their wild-type counterparts (LM-1). Cells were stimulated with IFN-γ for different periods of time (0–8 hr). Activation of STAT-1α was monitored using an oligonucleotide for the specific binding site on inducible nitric oxide synthase (iNOS) (GAS/ISRE/iNOS) (a). Nuclear translocation was also monitored using specific oligonucleotides for NF-κB (b) and AP-1 (c). The last lane in each panel represents nuclear extracts of me-3 cells stimulated with IFN-γ for 1 hr and subjected to competition with the respective cold oligonucleotide to confirm signal specificity. The results are representative of three independent experiments.
The increased iNOS expression and NO generation by SHP-1-deficient cells is NF-κB independent
To determine whether NF-κB was responsible for the increased iNOS gene and iNOS protein expression and NO generation in SHP-1-deficient cells, we used two different NF-κB inhibitors (i.e. NaS and Bay 11-7082). As depicted in Fig. 4, the different doses used of the NF-κB inhibitors, known to completely block NF-κB nuclear translocation (data not shown), did not significantly affect NO generation (Fig. 4a) and iNOS mRNA levels (Bay 11-7082, Fig. 4b; and NaS, Fig. 4c) in me-3 cells; however, a partial inhibition was observed in LM-1 cells. Although we observed a major increase in NF-κB nuclear translocation in SHP-1-deficient cells (Fig. 3b), the results of the experiments with the NF-κB inhibitors suggest that the increased translocation observed previously does not explain the increased iNOS expression and NO generation reported in Fig. 4.
Figure 4.
Role of nuclear factor-κB (NF-κB) in nitric oxide (NO) generation and inducible nitric oxide synthase (iNOS) expression in interferon-γ (IFN-γ)-stimulated Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells (me-3) and their wild-type counterparts (LM-1). (a) Both cell lines were treated with two different NF-κB inhibitors, namely sodium salicylate (NaS, 5 mm) and BAY 11-7082 (5 μm) for 1 hr prior to stimulation with IFN-γ (100 U/ml). After 24 hr of incubation in the presence of the inhibitor, supernatants were collected and the Greiss reaction was performed to evaluate nitrite generation. The results with both inhibitors represent three independent experiments (mean ± standard error of the mean, n = 3). (b) Expression of the iNOS gene was monitored in cells treated for 1 hr with increasing doses of both inhibitors (1–5 mm NaS and 1–5 μm BAY 11-7082) prior to stimulation with IFN-γ (100 U/ml, 8 hr). Inhibitors were retained throughout the IFN-γ stimulation period. The results are representative of three independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Second-messenger phosphorylation profiles in LM-1 and me-3 cells confirm the role of Erk1/Erk2 and introduce a role for SAPK/JNK in iNOS expression and NO generation
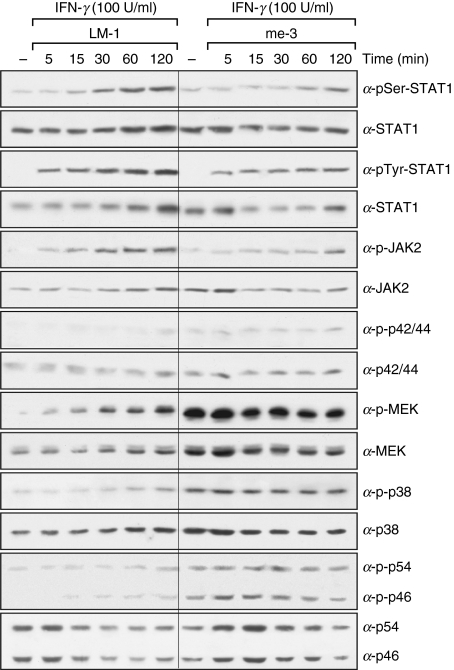
As suggested by the previous results, iNOS expression in SHP-1-deficient cells is Erk1/Erk2 dependent, and possibly AP-1 dependent. To characterize in greater detail the role of specific second messengers in the suggested AP-1 activation, the phosphorylation states of JAK2, STAT1α, Erk1/Erk2 (p44/p42), MEK, p38 and SAPK/JNK (p54/p46) were investigated. As shown in Fig. 5, the absence of SHP-1 in me-3 cells did not significantly alter the phosphorylation profiles of either JAK2 or STAT-1α on their critical tyrosyl or seryl residues in comparison to LM-1 cells. These results correlate with our previous observations showing that the JAK2 inhibitor was unable to block iNOS expression in me-3 cells (Fig. 2), and that the translocation of STAT-1α to the nucleus in me-3 cells was similar to that of LM-1 cells (Fig. 3). However, higher phosphorylation states of Erk1/Erk2, MEK and p38 were detected in me-3 cells compared with LM-1 MØ. Phospho-Erk band densitometry showed that in me-3 cells the p-Erk signal was 64 ± 13% higher at basal levels and 49 ± 10% higher in response to IFN-γ (2 hr) than in LM-1 cells. In addition, p-Erk was augmented in both cell lines by treatment with IFN-γ (2 hr) (51 ± 9% increase in LM-1 cells and 31 ± 11% increase in me-3 cells). These results support our previous finding that Erk1/Erk2 MAP kinases are key players in the elevated NO production in SHP-1-deficient cells and further confirm the role of IFN-γ in Erk activation. Although higher phosphorylation levels were detected for MEK and p38, our previous data, using the specific inhibitors of these signalling molecules, showed that the inhibition of these kinases did not affect the capacity of SHP-1-deficient cells to enhance iNOS expression and NO generation at basal levels or in response to IFN-γ (Fig. 2). Finally, SAPK/JNK (p46/p54), known to be involved in AP-1 activation,37 was shown to have increased phosphorylation in me-3 cells compared with LM-1 MØ (Fig. 5). Collectively, these results demonstrate that in the absence of SHP-1, Erk1/Erk2 and SAPK/JNK phosphorylation is significantly enhanced, and that the increased activity of these two kinases (known to be responsible for AP-1 activation)4,17 could lead to the increased iNOS expression and NO generation observed in SHP-1-deficient cells.
Figure 5.
Time-dependent activation of signal transducer and activator of transcription 1α (STAT-1α), Janus tyrosine kinase 2 (JAK2), extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2), mitogen-activated extracellular signal-regulated protein kinase kinase (MEK), p38 and stress-activated mitogen-activated protein kinases/c-Jun NH2-terminal kinase (SAPK/JNK) in Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells (me-3) and their wild-type counterparts (LM-1) stimulated with interferon-γ (IFN-γ). Cells were treated with IFN-γ (100 U/ml) for various periods of time (5–120 min). Cell lysates were subjected to western blot analyses using antibodies specific for the phosphorylated forms of STAT-1α seryl 727 and tyrosyl 701 residues (α-pSer-STAT1 and α-pTyr-STAT1, respectively), JAK2 (α-p-JAK2), Erk1/Erk2 MAPKs (α-p-p44 and α-p-p42), MEK (α-p-MEK), p38 (α-p-p38) and SAPK/JNK MAPK (α-p-p54 and α-p-p46). Loading of equal amounts of protein was monitored by stripping the membranes and reblotting with antibodies specific for the non-phosphorylated form of the various kinases analyzed. The results are representative of three independent experiments.
Erk1/Erk2 are involved in AP-1 nuclear translocation in SHP-1-deficient cells
To confirm that the Erk1/Erk2 activation observed in SHP-1-deficient cells is an important event leading to AP-1 nuclear translocation, we monitored the effect of the Erk1/Erk2 inhibitor Apigenin on AP-1 nuclear translocation in SHP-1-deficient cells, with or without IFN-γ stimulation. As depicted in Fig. 6, treatment of SHP-1-deficient MØ with Apigenin (50 μm) completely inhibited the nuclear translocation of AP-1, both at the basal level and in response to IFN-γ. No effect on AP-1 translocation was observed when the p38 inhibitor SB203580 was used as a control. This result suggests that the SHP-1-mediated negative regulation of Erk-/Erk2 MAPKs is an important mechanism whereby the regulation of iNOS expression and the consequent NO generation by AP-1 is selectively controlled.
Figure 6.
Effect of the extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2) and p38 inhibitors on activator protein-1 (AP-1) nuclear translocation in interferon-γ (IFN-γ)-stimulated Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells (me-3) and their wild-type counterparts (LM-1). The effect of the Erk1/Erk2 inhibitor Apigenin (50 μm) and p38 inhibitor SB203580 (20 μm) (both added for 1 hr prior to IFN-γ and retained throughout the IFN-γ stimulation period) on AP-1 nuclear translocation was evaluated in cells stimulated for 4 hr with IFN-γ or left unstimulated. AP-1 activation was monitored as described in the Experimental procedures. The results are representative of three independent experiments. CO, cold oligo.
Discussion
Nitric oxide plays an important microbicidal role in the host immune response (for a review, see reference).5 As with other potent effector molecules, the signalling pathways implied in NO generation, such as cytokine signalling pathways, have to be carefully regulated. Among these regulators, the SOCS and PIAS protein families38 as well the PTP SHP-1,15,19,20 PTP-1B21,22 and TCPTP23 phosphatases have been described. A role for the PTP-mediated negative regulation of iNOS expression has been reported in multiple cells, including rat hepatocytes,39 rat vascular smooth muscle tissue40 and MØ.15,16,41–43 Furthermore, the effect of PTPs on NO generation has been studied in our laboratory through the use of the PTP inhibitor pV compounds bpV(phen) and bpV(pic), which led to NO generation and protection against visceral and cutaneous leishmaniasis.16,19 The specific role of SHP-1 in the process of NO generation has been suggested to be of paramount importance because iNOS expression and NO production have been shown to be elevated in me-3 cells lacking SHP-1.15 In addition to SHP-1, other PTPs have been reported to play a role in the regulation of certain cytokine-signalling pathways.21–23 Results from different studies suggested a role for PTP-1B in the dephosphorylation of STAT5, TYK2 and JAK2.21,22 Neither of these studies, however, investigated the effect of the absence of PTP-1B on iNOS expression or NO generation. Simoncic et al.23 suggested a role for TCPTP in the negative regulation of JAK1 and JAK3. Although iNOS expression was shown to be upregulated upon IFN-γ stimulation in BMDM in the absence of TCPTP, NO generation was not evaluated and the overall in vivo effect of the absence of TCPTP on cytokine signalling remains to be elucidated.
Despite the established role of SHP-1 in the negative regulation of cytokine signaling,15,19,20 the molecular events regulated by SHP-1 leading to NO generation remain largely unknown. To understand these events in greater detail, we performed experiments using SHP-1-deficient cells (me-3) and their wild-type counterparts (LM-1). In agreement with our previous findings,15 we showed that SHP-1-deficient me-3 cells generate NO at a much higher level than LM-1 cells in response to IFN-γ (see Fig. 1) and that this enhanced NO generation involves a selective activation of Erk1/Erk2 and SAPK/JNK MAP kinases, leading to AP-1 activation.
Studies using various experimental systems have shown that SHP-1 is an important negative regulator of several kinases, such as the Erk1/Erk2 MAPKs,44–46 p38 MAPK47 and JNK,48 and that these kinases play a role in IFN-γ- and/or LPS-induced NO generation.49–53 In the present study, the use of specific inhibitors of JAK2 and members of the MAPK family revealed that Erk1/Erk2 MAPK and SAPK/JNK are the main players in the negative regulatory effects of SHP-1 on mechanisms responsible for IFN-γ-induced MØ NO generation. The inhibition of JAK2 and MEK, however, slightly affected NO production in me-3 cells, whereas LM-1 cells showed a partial or complete inhibition in response to IFN-γ. The fact that the JAK2 inhibitor only slightly affected NO production in me-3 cells suggests a minor role for the JAK/STAT pathway in NO generation in SHP-1-deficient cells (similar to that observed for NF-κB in this work). Furthermore, the minor effect observed with the MEK inhibitor in me-3 cells suggests that NO production in the absence of SHP-1 is mainly mediated by MEK-independent Erk activation. This activation could be a result of the fact that SHP-1 directly interacts with and regulates Erk and not MEK activity, or that Erk activation could be mediated by other kinases such as protein kinase C (PKC). The p38 inhibitor, on the other hand, did not affect NO generation in either cell line, suggesting that this second messenger is not regulated by SHP-1 and is not implicated in NO generation by MØ in response to IFN-γper se. The result of the effect of each inhibitor on iNOS expression supports our suggestion that Erk1/Erk2 and SAPK/JNK are the main kinases responsible for the capacity of SHP-1-deficient me-3 cells to generate high levels of NO in IFN-γ-stimulated or unstimulated cells. In the light of these findings, further experiments were performed to determine which transcription factors are involved in the negative regulation of NO generation by SHP-1. STAT-1α and NF-κB are recognized as the primary transcription factors involved in signalling events leading to iNOS expression and NO generation in MØ stimulated with IFN-γ alone or in combination with LPS.33,35,54 Of interest, our results indicated that SHP-1-deficient me-3 cells, whether stimulated or unstimulated with IFN-γ, showed a much higher nuclear translocation of the transcription factors NF-κB and AP-1 compared with wild-type counterparts. These results are consistent with previous studies where SHP-1 was implicated in the down-regulation of NF-κB and AP-1 nuclear translocation in astrocytes and vascular smooth-muscle cells, respectively.46,55 On the other hand, the translocation of STAT-1α was comparable in both cell lines, suggesting that this transcription factor is not implicated in the regulation of NO generation by SHP-1. Therefore, this set of data suggests that SHP-1 tightly regulates NF-κB and/or AP-1, and thus negatively regulates iNOS expression and NO generation in MØ. However, the use of NF-κB inhibitors did not significantly affect iNOS expression and NO generation in me-3 cells at doses known to block NF-κB translocation completely, suggesting that AP-1, and not NF-κB, is the main transcription factor implicated in iNOS expression and NO generation in SHP-1-deficient me-3 cells.
To reinforce these observations, the phosphorylation states of MEK, Erk1/Erk2, p38 and SAPK/JNK were monitored and it was found that they are markedly enhanced in the absence of host SHP-1. On the other hand, the phosphorylation states of JAK2 and STAT-1α were comparable in both cell lines at basal levels and following IFN-γ stimulation. Based on our results of the kinase inhibitor experiments in which we demonstrated that the inhibition of MEK and p38 kinases did not subsequently affect the capacity of SHP-1-deficient cells to enhance IFN-γ-mediated iNOS expression and the subsequent NO generation, we propose that the absence of MØ SHP-1 results in an activation process that although affecting the phosphorylation state of MEK and p38, bypasses them to signal selectively through Erk1/Erk2 and SAPK/JNK kinases, resulting in an AP-1-dependent NO generation in MØ lacking SHP-1. These findings are supported by previous studies demonstrating that the Erk1/Erk2 MAP kinases are involved in AP-1 activation in okadaic acid-treated cells,56 and that SAPK/JNK is pivotal in AP-1 nuclear translocation.37 Furthermore, we showed that the treatment of SHP-1-deficient cells with the Erk1/Erk2 inhibitor Apigenin, but not the p38 inhibitor SB203580, resulted in the inhibition of AP-1 nuclear translocation and the consequent generation of NO.
Collectively, the findings reported in the present study suggest that the host PTP SHP-1 negatively regulates IFN-γ-induced NO generation in MØ. These observations clearly demonstrate that in the absence of SHP-1, Erk1/Erk2 and SAPK/JNK kinases are selectively activated, which in turn is responsible for the enhanced AP-1 nuclear translocation observed in me-3 cells. This higher translocation helps to explain the increased expression of the iNOS gene and the subsequent generation of NO in SHP-1-deficient cells. Finally, a better understanding of the role played by the PTP SHP-1 in the negative regulation of MØ microbicidal functions, such as NO generation, would allow the development of selective molecules with immunomodulatory capacities that can help in the control of infectious pathogens.
Acknowledgments
This work was supported by grants from the Canadian Institute of Health Research (CIHR) to M. Olivier. M.O. is a member of the CIHR Group in Host Pathogen Interactions. J.B. is the recipient of a CIHR PhD studentship award, and I.A.D. is the recipient of an FRSQ PhD studentship award.
References
- 1.Green SJ, Nacy CA, Meltzer MS. Cytokine-induced synthesis of nitrogen-oxides in macrophages – a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Liew FY. The role of nitric-oxide in parasitic diseases. Ann Trop Med Parasitol. 1993;87:637–42. doi: 10.1080/00034983.1993.11812822. [DOI] [PubMed] [Google Scholar]
- 3.Lowenstein CJ, Dinerman JL, Snyder SH. Nitric-oxide – a physiological messenger. Ann Intern Med. 1994;120:227–37. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Croen KD. Evidence for an antiviral effect of nitric-oxide – inhibition of Herpes-Simplex Virus type-1 replication. J Clin Invest. 1993;91:2446–52. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karupiah G, Xie QW, Buller RML, Nathan C, Duarte C, Macmicking JD. Inhibition of viral replication by interferon-gamma-induced nitric-oxide synthase. Science. 1993;261:1445–8. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 6.Hibbs JB, Taintor RR, Vavrin Z, Rachlin EM. Nitric-oxide – a cyto-toxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 7.Lowenstein CJ, Snyder SH. Nitric-oxide, a novel biologic messenger. Cell. 1992;70:705–7. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- 8.Nathan C. Nitric-oxide as a secretory product of mammalian-cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 9.Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric-oxide synthase structurally resembles cytochrome-P-450 reductase. Nature. 1991;351:714–8. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 10.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric-oxide synthase - molecular-cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA. 1992;89:6348–52. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric-oxide synthase from macrophages. J Exp Med. 1992;176:599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowles RG, Moncada S. Nitric-oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric-oxide synthase from mouse macrophages. Science. 1992;256:225–8. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 14.Bogdan CT, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 15.Forget G, Siminovitch KA, Brochu S, Rivest S, Radzioch D, Olivier M. Role of host phosphotyrosine phosphatase SHP-1 in the development of murine leishmaniasis. Eur J Immunol. 2001;31:3185–96. doi: 10.1002/1521-4141(200111)31:11<3185::aid-immu3185>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Matte C, Marquis JF, Blanchette J, Gros P, Faure R, Posner BI, Olivier M. Peroxovanadium-mediated protection against murine leishmaniasis: role of the modulation of nitric oxide. Eur J Immunol. 2000;30:2555–64. doi: 10.1002/1521-4141(200009)30:9<2555::AID-IMMU2555>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.Proudfoot L, Nikolaev AV, Feng GJ, Wei XQ, Ferguson MAJ, Brimacombe JS, Liew FY. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc Natl Acad Sci USA. 1996;93:10984–9. doi: 10.1073/pnas.93.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandan D, Reiner NE. Attenuation of gamma-interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania-donovani – selective-inhibition of signaling through Janus kinases and STAT1. Infect Immun. 1995;63:4495–500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivier M, Romero-Gallo BJ, Matte C, Blanchette J, Posner BI, Tremblay MJ, Faure R. Modulation of interferon-gamma-induced macrophage activation by phosphotyrosine phosphatases inhibition – effect on murine Leishmaniasis progression. J Biol Chem. 1998;273:13944–9. doi: 10.1074/jbc.273.22.13944. [DOI] [PubMed] [Google Scholar]
- 20.Blanchette J, Racette N, Faure R, Siminovitch KA, Olivier M. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-gamma-triggered JAK2 activation. Eur J Immunol. 1999;29:3737–44. doi: 10.1002/(SICI)1521-4141(199911)29:11<3737::AID-IMMU3737>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Aoki N, Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J Biol Chem. 2000;275:39718–26. doi: 10.1074/jbc.M005615200. [DOI] [PubMed] [Google Scholar]
- 22.Myers MP, Andersen JN, Cheng A, et al. TYK2 and JAR2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–4. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 23.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12:446–53. doi: 10.1016/s0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 24.Feng GS, Pawson T. Phosphotyrosine phosphatases with SH2 domains – regulators of signal-transduction. Trends Genet. 1994;10:54–8. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 25.Kharitonenkov A, Chen ZJ, Sures I, Wang HY, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–6. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 26.Chen HE, Chang S, Trub T, Neel BG. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1996;16:3685–97. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of Sh-PTP1 to the erythropoietin receptor causes inactivation of Jak2 and termination of proliferative signals. Cell. 1995;80:729–38. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 28.Kozlowski M, Mlinaricrascan I, Feng GS, Shen R, Pawson T, Siminovitch KA. Expression and catalytic activity of the tyrosine phosphatase Ptp1c is severely impaired in moth-eaten and viable moth-eaten mice. J Exp Med. 1993;178:2157–63. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pani G, Fischer KD, MlinaricRascan I, Siminovitch KA. Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J Exp Med. 1996;184:839–52. doi: 10.1084/jem.184.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA. Identification of the tyrosine phosphatase PTP1c as a B-cell antigen receptor-associated protein involved in the regulation of B-cell signaling. J Exp Med. 1995;181:2077–84. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi TL, Mui ALF, Krystal G, Ihle JN. Hematopoietic-cell phosphatase associates with the interleukin-3 (IL-3) receptor-beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol Cell Biol. 1993;13:7577–86. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi TL, Zhang JL, Miura O, Ihle JN. Hematopoietic-cell phosphatase associates with erythropoietin (Epo) receptor after Epo-induced receptor tyrosine phosphorylation – identification of potential binding-sites. Blood. 1995;85:87–95. [PubMed] [Google Scholar]
- 33.Gao JJ, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272:1226–30. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 34.Blanchette J, Jaramillo M, Olivier M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology. 2003;108:513–22. doi: 10.1046/j.1365-2567.2003.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YM, Lee BS, Yi KY, Paik SG. Upstream NF-kappa B site is required for the maximal expression of mouse inducible nitric oxide synthase gene in interferon-gamma plus lipopolysaccharide-induced RAW 264·7 macrophages. Biochem Biophys Res Commun. 1997;236:655–60. doi: 10.1006/bbrc.1997.7031. [DOI] [PubMed] [Google Scholar]
- 36.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-Kappa-B/Rel in induction of nitric-oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 37.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 38.Greenhalgh CJ, Hilton DJ. Negative regulation of cytokine signaling. J Leukoc Biol. 2001;70:348–56. [PubMed] [Google Scholar]
- 39.Taylor BS, Liu SB, Villavicencio RT, Ganster RW, Geller DA. The role of protein phosphatases in the expression of inducible nitric oxide synthase in the rat hepatocyte. Hepatology. 1999;29:1199–207. doi: 10.1002/hep.510290419. [DOI] [PubMed] [Google Scholar]
- 40.Zheng XL, Gui Y, Sharkey KA, Hollenberg MD. Differential induction of nitric oxide synthase in rat gastric and vascular smooth muscle tissue: Distinct tissue distribution and distinctive signaling pathways. J Pharmacol Exp Ther. 1999;289:632–40. [PubMed] [Google Scholar]
- 41.Diaz-Guerra MJM, Castrillo A, Martin-Sanz P, Bosca L. Negative regulation by protein tyrosine phosphatase of IFN-gamma-dependent expression of inducible nitric oxide synthase. J Immunol. 1999;162:6776–83. [PubMed] [Google Scholar]
- 42.Pan JM, Burgher KL, Szczepanik AM, Ringheim GE. Tyrosine phosphorylation of inducible nitric oxide synthase: Implications for potential post-translational regulation. Biochem J. 1996;314:889–94. doi: 10.1042/bj3140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nandan D, Lo R, Reiner NE. Activation of phosphotyrosine phosphatase activity attenuates mitogen-activated protein kinase signaling and inhibits c-FOS and nitric oxide synthase expression in macrophages infected with Leishmania donovani. Infect Immun. 1999;67:4055–63. doi: 10.1128/iai.67.8.4055-4063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hennige AM, Lammers R, Hoppner W, et al. Inhibition of Ret oncogene activity by the protein tyrosine phosphatase SHP1. Endocrinology. 2001;142:4441–7. doi: 10.1210/endo.142.10.8453. [DOI] [PubMed] [Google Scholar]
- 45.Lehtonen JYA, Daviet L, Nahmias C, Horiuchi M, Dzau VJ. Analysis of functional domains of angiotensin II type 2 receptor involved in apoptosis. Mol Endocrinol. 1999;13:1051–60. doi: 10.1210/mend.13.7.0303. [DOI] [PubMed] [Google Scholar]
- 46.Shibasaki Y, Matsubara H, Nozawa Y, et al. Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase. Hypertension. 2001;38:367–72. doi: 10.1161/01.hyp.38.3.367. [DOI] [PubMed] [Google Scholar]
- 47.Hsu HC, Shultz LD, Su X, Shi J, Yang PA, Relyea MJ, Zhang HG, Mountz JD. Mutation of the hematopoietic cell phosphatase (Hcph) gene is associated with resistance to gamma-irradiation-induced apoptosis in Src homology protein tyrosine phosphatase (SHP)-1-deficient “motheaten” mutant mice. J Immunol. 2001;166:772–80. doi: 10.4049/jimmunol.166.2.772. [DOI] [PubMed] [Google Scholar]
- 48.Matsubara H, Shibasaki Y, Okigaki M, et al. Effect of angiotensin II type 2 receptor on tyrosine kinase Pyk2 and c-Jun NH2-terminal kinase via SHP-1 tyrosine phosphatase activity: evidence from vascular-targeted transgenic mice of AT2 receptor. Biochem Biophys Res Commun. 2001;282:1085–91. doi: 10.1006/bbrc.2001.4695. [DOI] [PubMed] [Google Scholar]
- 49.Bhat NR, Zhang PS, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caivano M. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett. 1998;429:249–53. doi: 10.1016/s0014-5793(98)00578-x. [DOI] [PubMed] [Google Scholar]
- 51.Chan ED, Riches DWH. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(MAPK) in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001;280:C441–50. doi: 10.1152/ajpcell.2001.280.3.C441. [DOI] [PubMed] [Google Scholar]
- 52.Chan ED, Winston BW, Uh ST, Wynes MW, Rose DM, Riches DWH. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-gamma and TNF-alpha in mouse macrophages. J Immunol. 1999;162:415–22. [PubMed] [Google Scholar]
- 53.Faure V, Hecquet C, Courtois Y, Goureau O. Role of interferon regulatory factor-1 and mitogen-activated protein kinase pathways in the induction of nitric oxide synthase-2 in retinal pigmented epithelial cells. J Biol Chem. 1999;274:4794–800. doi: 10.1074/jbc.274.8.4794. [DOI] [PubMed] [Google Scholar]
- 54.Nathan C, Xie QW. Regulation of biosynthesis of nitric-oxide. J Biol Chem. 1994;269:13725–8. [PubMed] [Google Scholar]
- 55.Massa PT, Wu C. Increased inducible activation of NF-kappa B and responsive genes in astrocytes deficient in the protein tyrosine phosphatase SHP-1. J Interferon Cytokine Res. 1998;18:499–507. doi: 10.1089/jir.1998.18.499. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberger SF, Finch JS, Gupta A, Bowden GT. Extracellular signal-regulated kinase 1/2-mediated phosphorylation of JunD and FosB is required for okadaic acid-induced activator protein 1 activation. J Biol Chem. 1999;274:1124–30. doi: 10.1074/jbc.274.2.1124. [DOI] [PubMed] [Google Scholar]