Abstract
The induction of persistent protective levels of pathogen-specific antibody is an important goal of immunization against childhood infections. However, antibody persistence is poor after immunization in infancy versus later in life. Serogroup C meningococci (MenC) are an important cause of bacteraemia and meningitis in children. The use of protein–polysaccharide conjugate vaccines against MenC has been associated with a significant decline in the incidence of invasive disease. However, vaccine effectiveness is negligible by more than 1 year after a three-dose priming series in infancy and corresponds to a rapid decline in antibody following an initial immune response. The cellular mechanisms underlying the generation of persistent antibody in this age group are unclear. An essential prelude to larger studies of peripheral blood B cells is an understanding of B-cell kinetics following immunization. We measured MenC- and diphtheria-specific plasma and memory B-cell kinetics in infants receiving a CRM197 (cross-reactive material; mutant diphtheria toxoid)-conjugated MenC vaccine at 2, 3 and 4 months of age. Plasma cell responses were more delayed after the first dose when compared with the rapid appearance of plasma cells after the third dose. Memory B cells were detectable at all time-points following the third dose as opposed to the low frequency seen following a first dose. This study provides data on B-cell kinetics following a primary schedule of immunization in young infants upon which to base further studies of the underlying cellular mechanism of humoral immunity.
Keywords: antibody, B cell, infant, memory, meningococcal, vaccine
Introduction
Two-thirds of deaths in children under 5 years of age can be attributed to infectious diseases, many of which can be prevented by routine infant immunization. For a vaccine to provide protection throughout childhood, the induction and persistence of pathogen-specific antibody is essential. Although antibody may persist for many years following immunization of adults and older children, the level wanes more rapidly after infant immunization. The mechanisms behind the persistence of antibody are not well understood.1–3
Neisseria meningitidis is an important cause of bacterial meningitis and septicaemia in children. Serogroups A, B, C, W135 and Y cause most disease.4 Infant immunization with serogroup C meningococcal polysaccharide–protein conjugate (MenCC) vaccines induces protective antibody against this organism.5–7 However, serogroup C meningococcal polysaccharide (MenCPS)-specific antibody concentrations decline in the months following a three-dose primary series of infant immunization despite evidence of immunological memory.5,6,8 This fall in antibody corresponds to a loss of vaccine effectiveness more than 1 year after immunization.9Neisseria meningitidis has the potential, in some cases, to cause invasive disease shortly after its acquisition in the nasopahrynx.10 In such cases pre-existing serum antibody may be important for protection against an invading organism prior to the mounting of secondary antibody responses. Antibody persistence is thought to be determined by long-lived plasma and memory B cells.11–13 Following immunization with a T-dependent antigen, naïve B cells enter the germinal centre reaction in secondary lymphoid tissues. During this reaction, B cells undergo several rounds of proliferation together with somatic hypermutation of the immunoglobulin genes followed by selection of B-cell clones of increasing avidity.14 The germinal centre is able to produce both plasma and memory B cells. The plasma cells then migrate to the bone marrow where they continue to secrete antibody. Studies in infant and adult rats given tetanus toxoid (TT) vaccine indicate that the poorer persistence of TT antibody in infants is attributable to a reduced capacity to retain long-lived plasma cells in bone marrow compared with adults.15,16 In mice the transfer of plasma cells in the absence of memory B cells results in a gradual decline in antibody levels in subsequent weeks, indicating a role for the memory B cells in renewing the plasma cell population in the long term.13 A similar dependence of antibody on memory B cells can be seen in humans treated with an anti-CD20 monoclonal antibody (rituximab) for a variety of inflammatory conditions.17,18 The anti-CD20 monoclonal antibody causes apoptosis of CD20-positive B cells, resulting in a depletion of memory B cells but the survival of plasma cells, which are CD20 negative. A decline in antibody levels is seen in the weeks following rituximab treatment as plasma cells die and cannot be replenished because of the absence of a memory B-cell population.
In humans it is difficult to access plasma and memory B cells in the lymphoid tissue and bone marrow. Most studies are undertaken on peripheral blood and used to infer what is occurring in these other tissues. Following immunization there are rapid changes in the frequency of plasma and memory B-cell populations in peripheral blood as these cells move from the tissue where they are produced to other sites in the body. The transit of these cell populations through peripheral blood following immunization may allow a more accurate estimation of the frequency of such cells in other tissues. However, the design and interpretation of such studies require an understanding of the kinetics of B-cell populations in peripheral blood. No such data are currently available for infants at the age at which the primary immunization schedule is given.
We have determined the kinetics of plasma and memory B cells following CRM197 (cross-reactive material; mutant diphtheria toxoid)-conjugated MenC polysaccharide vaccine given to infants as a primary course of immunization. MenC and CRM197 plasma and memory B-cell responses were measured following the first and third doses of the MenCC vaccine.
Materials and methods
Study participants
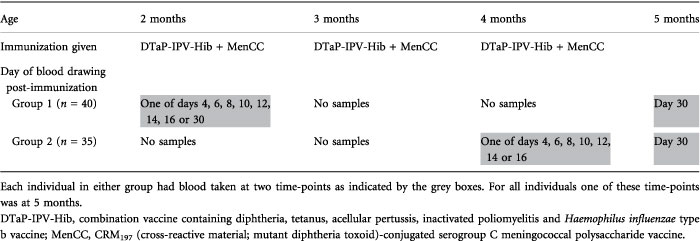
Two-month-old healthy infants were assigned to one of 15 groups to allow blood sampling on various days following administration of the first and third doses of routine immunizations (including MenCC) at 2 and 4 months of age (Table 1). Blood samples were also obtained from all children at day 30 after the third immunization. Ethical approval was obtained from the Oxfordshire Research Ethics Committee (OxREC number 04.OxA.030) and informed parental consent obtained. The trial is registered with clinicaltrials.gov (identifier NCT00262015). In order to measure antibody persistence, a further blood sample was obtained at 1 year of age as part of a follow-up study of the response to a booster dose of MenCC (OxREC number 06/Q1604/41).19
Table 1.
The schedule of immunization in relation to the blood sampling time-points
 |
Vaccines and blood sampling
All infants received routine vaccines in accordance with the UK schedule of immunization at the time of the study (Table 1). This consisted of diptheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliomyelitis as a combination vaccine (Pediacel®; Sanofi Pasteur MSD Ltd., Maidenhead, UK) and a CRM197 (mutant diphtheria toxoid)-conjugated MenC vaccine (Menjugate®; Novartis Vaccines, Sienna, Italy) each given at 2, 3 and 4 months of age. All vaccines were administered using a 23-gauge, 25-mm needle. At each time-point a maximum of 5–6 ml of blood was taken by venepuncture into heparinized tubes.
Serogroup C meningococcal polysaccharide enzyme-linked immunosorbent assay (ELISA)
Serum was separated from a maximum of 1 ml of blood and frozen at −80° prior to testing for MenC polysaccharide-specific immunoglobulin G (IgG) antibody concentration using standard ELISA methods described previously.20
Preparation of peripheral blood mononuclear cells (PBMCs)
A maximum volume of 4–5 ml of heparinized blood was available for the separation of PBMCs. The blood was diluted 1 : 2 with RPMI-1640 medium (Sigma-Aldrich, Poole, UK) to which penicillin–streptomycin solution (Sigma-Aldrich) and l-glutamine 200 mm (Sigma-Aldrich) had been added at a dilution of 1 : 100 (‘complete medium’). The PBMCs were then separated by density gradient centrifugation over Lymphoprep (Axis-Shield; PoC As, Oslo, Norway). PBMCs were washed once in complete medium prior to further preparation for enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) or cell culture.
Preparation of ELISPOT plates
MultiScreenTM-IP 96-well filter plates (Millipore, Bedford, MA) were coated with either 5 μg/ml MenC polysaccharide [National Institute for Biological Standards and Controls (NIBSC), Hertfordshire, UK] conjugated to methylated human albumin (NIBSC) or 10 μg/ml diphtheria toxoid (Statens Serum Institut, Copenhagen, Denmark) in sterile phosphate-buffered saline (PBS). PBS alone was added to the antigen blank wells. The ELISPOT plates were stored at 4° until use.
Detection of plasma cells
PBMCs prepared from peripheral blood were washed three times in complete medium with 10% fetal calf serum (Sigma-Aldrich) and re-suspended to a final concentration of 2 × 106 PBMCs/ml. Then 100 μl/well of the suspension was added to ELISPOT plates, coated with antigen as described above, and incubated overnight at 37° in 5% CO2. Antibody-secreting cells (ASCs) were detected with a 1 : 5000 dilution of goat anti-human IgG γ-chain-specific alkaline phosphatase conjugate (Calbiochem, San Diego, CA) in complete medium with 10% fetal calf serum, developed using 5-bromo-4-chloro-3-indolyl phosphate in nitroblue tetrazolium dissolved in aqueous dimethylformamide (Bio-Rad Laboratories, Hemel Hempstead, UK).
Detection of memory B cells
PBMCs prepared from peripheral blood were re-suspended in complete medium with 10% fetal calf serum (Sigma-Aldrich) at a final concentration of 2 × 106 PBMCs/ml. Two hundred microlitres was added to 96-well round-bottomed culture plates (Costar® 3799; Corning Life Sciences, Schiphol-Rijk, the Netherlands) with 1/10 000 Staphylococcus aureus Cowan (Calbiochem), 83 ng/ml poke weed mitogen (Sigma-Aldrich) and 2·5 μg/ml CpG (phosphorothioated TCG-TCG-TTT-TGT-CGT-TTT-GTC-GTT; Autogen-Bioclear UK Ltd, Calne, UK). The cells were incubated at 37° in 5% CO2 for 5·5–6·5 days before being re-suspended and washed four times in complete medium with 10% fetal calf serum. The cultured cells were plated onto pre-coated ELISPOT plates at 2 × 105 cells/well and then incubated and developed as described for plasma cells. For each individual assay at least five wells were used for MenC and at least two wells for other antigens.
ELISPOT counting
Spots were counted using an AID ELISpot reader ELR02 and software version 3.2.3 (Cadama Medical Ltd, Stourbridge, UK). Identical settings were used for all plates and antigens, and the operator was blinded to the sample being counted.
Sample size calculation
The sample size for the study was calculated on the assumption that B cells would be detectable between 4 and 16 days post-vaccination and would be present for a minimum of 48 hr in any individual. We assumed a priori an equal probability of B cells being detectable on any given day and calculated that recruiting five individuals for sampling at each of seven time-points from day 14 to 16 would give a greater than 96% chance of detecting at least two positive individuals for the time-point at which the cells appeared in the peripheral circulation.
Statistical analysis
For the purposes of analysis, ELISPOT assays in which less than four spots were detected were treated as having zero ASCs detected. Hence for MenCPS-specific B-cell assays in which at least five wells of 2 × 105 lymphocytes from culture were put on the detection plate, the minimum sensitivity of the assay was 0·8 ASCs per 2 × 105 lymphocytes from culture. For diphtheria-specific B cells where at least two wells of 2 × 105 lymphocytes were assayed the minimum sensitivity of the assay was 2 ASCs per 2 × 105 lymphocytes from culture. Any assay in which the antigen blank wells contained ≥ 2 ASCs per 2 × 105 lymphocytes was rejected. All data were entered into EXCEL (Microsoft, Redmond, WA). Statistical analysis was undertaken using stata (version 9.1; StataCorp, College Station, TX). For B-cell numbers at each time-point medians were calculated as for most time-points there were less than five data points available for analysis.
Results
Enrolment and sample availability
Seventy-five infants (34 female) were enrolled at 2 months of age and received a total of three doses of MenCC vaccine given at 2, 3 and 4 months of age. Because of the limitations in blood sample volume, plasma cell ELISPOTs were performed using cells from 33 infants 4–30 days after the first immunization and 29 infants 4–16 days after the third immunization. Memory B-cell ELISPOTs were performed on 31 infants 4–30 days after the first immunization and 17 infants 4–16 days after the third immunization. At the final time-point, 30 days after the third dose of vaccine, plasma cells were assayed from 19 infants and memory cells from 47 infants. The median age of infants at the time of first immunization was 8 completed weeks (range 7–10 weeks) and at the time of the third immunization the median age was 18 completed weeks (range 15–22 weeks).
Plasma cells
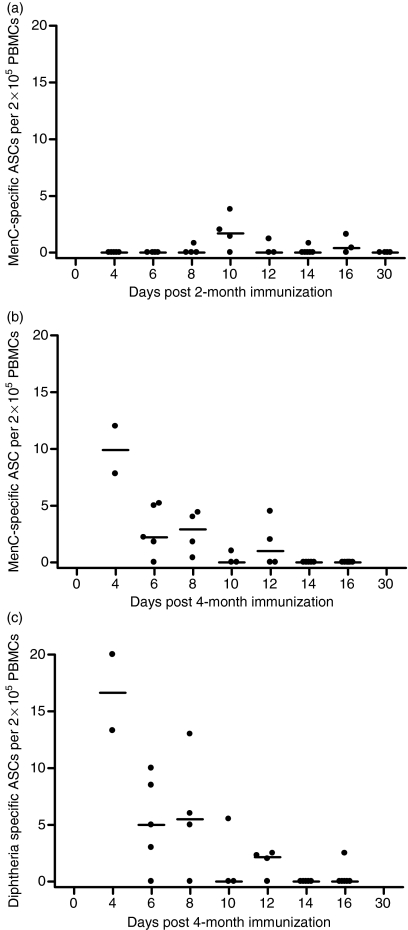
After the 2-month immunization the cell frequencies were close to the limit of detection of the assay. Between days 8 and 16 after the 2-month immunization eight of 20 individuals had detectable MenCPS specific ASCs (Fig. 1). Both the greatest proportion of responders and the highest magnitude of an individual response occurred on day 10 (three of four individuals and 3·8 MenCPS specific ASCs per 2 × 105 PBMCs, respectively). There were no individuals with MenCPS specific ASCs by day 30. No individuals had a diphtheria-specific plasma cell response at any of the time-points studied. The MenCPS specific plasma cell assay was of greater sensitivity than the diphtheria assay (mean sensitivity 0·7 versus 2·0 ASCs per 2 × 105 PBMCs, respectively) as a result of the greater number of PBMCs prioritized for use in the MenC assay.
Figure 1.
Serogroup C meningococcal (MenC) polysaccharide-specific plasma cell frequencies after the first dose (2 months of age) and MenC polysaccharide- and diphtheria-specific plasma cell frequencies after the third dose (4 months of age) of MenC polysaccharide/CRM197 (cross-reactive material; mutant diphtheria toxoid)-conjugated vaccine given as part of a 2-, 3- and 4-month schedule of infant immunization. MenC polysaccharide-specific plasma cell frequencies are shown in (a) for the 2-month dose and (b) for the 4-month dose. Diphtheria-specific plasma cell frequencies are shown in (c) for the 4-month dose. Each point represents one individual and lymphocytes from different individuals were used for each time-point. The bars indicate median values. ASCs, antibody-secreting cells; PBMCs, peripheral blood mononuclear cells.
The MenCPS specific plasma cell responses after the third immunization at 4 months of age were markedly greater and earlier than those following the first immunization at 2 months of age. The greatest magnitude of any individual response was seen for both MenC (10 ASCs per 2 × 105 cultured lymphocytes) and diphtheria (20 ASCs per 2 × 105 cultured lymphocytes) on day 4 after immunization. This was the first day after immunization that blood was taken. After day 4 the ASC frequency and the proportion of children with any detectable ASCs declined such that after day 12 only one individual had any antigen-specific plasma cells detected. The MenCPS- and diphtheria-specific responses appeared to be very similar in both timing and magnitude.
Memory B cells
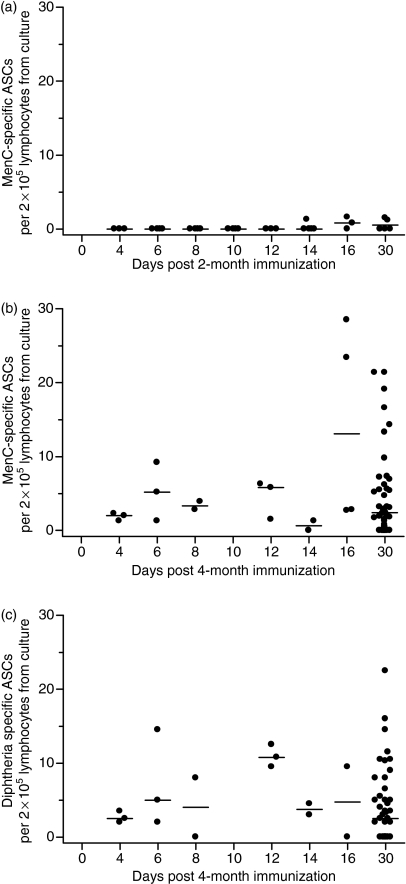
After the first immunization at 2 months of age, the cell frequencies were generally low in relation to the sensitivity of the assay. Memory B-cell responses were not detected until day 14 (Fig. 2). For MenCPS-specific memory B cells, three of eight individuals had detectable cells on days 14 and 16 and a further two of five individuals by day 30. Both the highest proportion of responders and the highest magnitude of individual response occurred on the same day, day 16 (two of three individuals and 1·6 MenCPS specific ASCs per 2 × 105 cultured lymphocytes, respectively). For diphtheria no individuals had detectable memory cells at any time-point after the first immunization at 2 months of age. The MenCPS specific memory B-cell assay was more sensitive than that for diphtheria (mean of 0·5 versus 2·0 ASCs per 2 × 105 cultured lymphocytes, respectively) because of the greater number of cultured lymphocytes prioritized for use in the MenC assay.
Figure 2.
Serogroup C meningococcal (MenC) polysaccharide-specific memory B-cell responses after the first dose (2 months of age) and MenC polysaccharide- and diphtheria-specific memory B-cell frequencies after the third dose (4 months of age) of MenC polysaccharide/CRM197 (cross-reactive material; mutant diphtheria toxoid)-conjugated vaccine given as part of a 2-, 3- and 4-month schedule of infant immunization. MenC polysaccharide-specific memory B-cell responses are shown in (a) for the 2-month dose and (b) for the 4-month dose. Diphtheria-specfic plasma cell frequencies are shown in (c) for the 4-month dose. Each point represents one individual and lymphocytes from different individuals were used for each time-point. The bars indicate median values. ASCs, antibody-secreting cells; PBMCs, peripheral blood mononuclear cells.
Following the third immunization at 4 months of age, memory B-cell responses for both MenCPS and diphtheria were detectable at all time-points tested up to and including day 30 (Fig. 2). The study was powered to detect the presence or absence of a B-cell response and not to detect differences in magnitude between responses on any two pairs of days. Hence, as memory B-cell responses were present at all time-points, it was not possible to demonstrate any changes in memory B-cell frequencies after the third vaccine dose. At day 30 the median MenCPS specific memory B-cell response was 2·5 ASCs per 2 × 105 cultured lymphocytes and that for diphtheria was 2·8 ASCs per 2 × 105 cultured lymphocytes.
MenC polysaccharide-specific antibody responses
The MenCPS specific geometric mean antibody concentration at 5 months of age (1 month after the third dose of vaccine) was 18·7 μg/ml [95% confidence interval (CI) 15·9–21·9 μg/ml; n = 59].
Correlation of MenCPS-specific memory B cells with MenCPS antibody responses
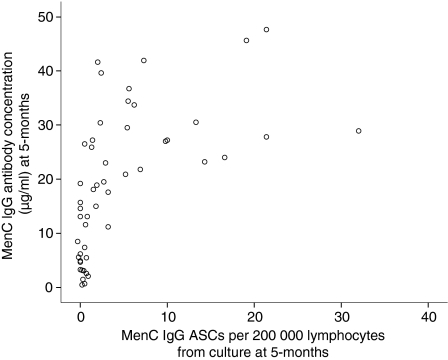
There was a significant positive correlation between the MenCPS specific memory B-cell response at 5 months (1 month after the third immunization) and MenCPS specific antibody concentration at 5 months (Spearman's rho = 0·58, P < 0·01) (Fig. 3).
Figure 3.
Scatterplot of the frequency of serogroup C meningococcal (MenC) polysaccharide-specific antibody-secreting cells (ASCs) per 2 × 105 lymphocytes from culture at 5 months of age against MenCPS specific antibody concentration (μg/ml) at 5 months of age (Spearman's rho = 0·58, P < 0·01). IgG, immunoglobulin G.
Discussion
This study provides the first description of the kinetics of plasma and memory B-cell responses in infants at the time of primary immunization during the first year of life to a polysaccharide antigen conjugated to a carrier protein.
Plasma cell kinetics
Ex vivo B-cell ELISPOTs, such as those used in the current study, detect cells with the phenotype of plasma cells.21 In studies of serum antibody responses in humans following primary immunization, antibody is first detected during the second week post-immunization with IgM being detected prior to IgG.22–24 Extrapolating from these studies, it is unlikely that plasma cells would be detected in peripheral blood much prior to these time-points. In murine models of primary immunization with T-dependent antigens, the plasma cells migrate from the site of production in lymphoid tissue to bone marrow through peripheral blood during the second week after immunization.25–28 Responses are more rapid for secondary responses and, following booster immunization of human adults, antigen-specific plasma cell are consistently detected in peripheral blood from day 5 to 8 after immunization but rapidly decline thereafter.21,29–36 In infants receiving a booster dose of MenCC vaccine at 1 year of age, plasma cells are detected from day 4 onwards at least until day 9.19
In the infants in the current study, MenCPS-specific plasma cells were detected during the second week after immunization from day 8 until day 16. The responses are consistent with murine data for a primary response and support the idea that the primary response occurs later and over a relatively prolonged period compared with the secondary response. Following a third dose of MenCC vaccine given at 4 months of age, plasma cells were detected from day 4 onwards as opposed to day 8 following a first dose. This pattern of kinetics with an earlier appearance of plasma cells is similar to that seen in primed infants receiving a booster dose of MenCC at 1 year of age.19 The kinetics of diphtheria-specific plasma cells followed a similar time–course. Although CRM197 was the carrier protein of the vaccine this was not available as a coating antigen for this study. Other work has shown that diphtheria-specific plasma cells are increased to 1·5–3 times the frequency of CRM197-specific plasma cells in response to a CRM197-conjugated MenC vaccine (G. Blanchard-Rohner, personal communication). Assuming that such kinetics are characteristic of a secondary response in infants, then the data suggest that memory B cells are available to participate in a secondary response from at least 8 weeks after primary immunization of infants. Adoptive transfer experiments in mice37,38 have demonstrated that memory B cells can participate in secondary responses much earlier than this and it would be interesting in further studies to investigate the B-cell kinetics around the second dose at 3 months of age (1 month after the first dose of vaccine).
Memory B-cell kinetics
The use of polyclonal B-cell activators in culture stimulates the CD27+ B-cell memory population to proliferate and secrete antibody, thereby allowing their detection by ELISPOT methods.39 Other investigators have shown that, in cell culture, plasma cells do not survive for more than a few days without appropriate supporting stromal cells (e.g. fibroblasts).40 Thus in this study B-cell culture with 5-day polyclonal stimulation followed by B-cell IgG ELISPOT is likely to be specific for the detection of CD27+ memory B cells alone.
A small number of human studies have investigated the kinetics of memory B cells in a primary response. In antigen-naïve adults receiving a priming dose of smallpox vaccine, IgG memory B cells were detected at 1 month post-immunization.2 A study of previously unvaccinated adults given tetanus vaccine demonstrated IgG memory B cells appearing in peripheral blood after day 7 and before day 28.34 Murine models of a primary response demonstrate that memory B cells are generated and disseminated during the second week of a primary immune response to a variety of T-dependent antigens. In these models memory B cells persist in the circulation after the initial period of the primary follicular response is over and B cells with a germinal centre phenotype can still be detected at low frequency after several weeks in the site of the initial germinal centre reaction.37 During a secondary response, IgG memory B cells appear in the circulation more rapidly, and in adolescents receiving a booster dose of MenCC vaccine memory B-cells were detected by day 6.41 For a tetanus booster in adults an increase in memory B cells was not seen by day 4 but by day 12 there was a significant rise.42 Whether these memory B cells are newly formed memory B cells or extant memory B cells that have been mobilized by the process of booster immunization is unknown.
In the primary response, following the first dose of vaccine given to infants in the current study, IgG memory B cells were first detected at day 14. The first appearance of memory B cells in peripheral blood is later than in the above described animal models of a primary response, but as the responses were low frequency and close to the limit of detection of the assay it is possible that earlier responses were not detected. Consistent with this possibility, the less sensitive diphtheria assay detected no memory cells in the primary response. Importantly, the MenC responses in the current study were clearly delayed in comparison to human secondary memory B-cell responses,41 consistent with the slower germinal centre formation of a primary response.43 After the third dose of vaccine the antigen-specific memory B cells were readily detectable at all time-points from day 4 to 30. The current study was not powered to compare the differences in magnitude between any 2 days for which cells were detected. To more clearly define the memory B-cell response characteristic of a secondary response would require a larger number of individuals.
Memory B-cell frequency and immunogenicity
There was a significant correlation between MenCPS-specific memory B cells and antibody at 5 months, 1 month after the third dose of vaccine (Fig. 3). As memory B cells are unlikely to be contributing significantly to serum antibody at this time-point post-immunization, the correlation may instead be related to a common origin of the class-switched IgG antibody and memory B cells in the germinal centre. After primary immunization in young infants any MenCPS-specific memory B cells can only have been generated in the recently active germinal centres and the germinal centres will also have been the site of plasma cell class-switching, to allow subsequent IgG production.44 Whilst individuals with a high frequency of memory B cells (≥ 5 MenCPS specific IgG antibody-secreting cells per 2 × 105 cultured lymphocytes) have higher levels of MenCPS specific antibody, there is a more variable relationship for those with lower memory B-cell frequencies. There are individuals with significant amounts of MenCPS specific antibody but with a low frequency of memory B cells. It may be that this is an issue of the assay operating at the lower limit of sensitivity where it is not possible to discriminate clearly between individuals with differing but low frequencies of memory B cells. However, it is also possible that this is a reflection of the fact that there are other factors that regulate these two aspects of the immune response independently of each other.45
Antibody persistence
Whilst antibody persistence ultimately depends on antibody production by plasma cells, there is uncertainty about the nature and origin of these plasma cells. In particular it is unclear to what extent long-lived plasma cells generated by the initial response are important versus their ongoing replenishment by plasma cells derived from memory B cells. In addition, the mechanisms by which memory B cells might be triggered to differentiate into plasma cells to maintain persistent levels of antibody are a matter of debate.11,46 Following MenCC vaccine the ability to generate persistent antibody is age dependent.8 Several studies have confirmed that, although infants initially develop significant MenCPS specific antibody levels, these wane rapidly by 1 year of age.5,6,47 In contrast, studies of adolescents indicate that from 1 to 3 years after immunization with MenCC there are higher levels of persistent antibody.48,49 The cellular basis for the lack of antibody persistence in infants as opposed to older children is unclear. Estimates of the half-life of long-lived plasma cells from animal and human studies are between 40 and 100 days and suggest a limit on the degree to which such cells can contribute to antibody persistence in the absence of continued replenishment from memory B cells.12,50,51 This view is supported by murine models which have shown a slow decline in antibody with the removal of antigen-specific memory B cells.13 Consistent with this are observations of individuals following treatment with an anti-CD20 monoclonal antibody (which removes memmory B cells but should leave plasma cells intact).17,18 Although a recent study in mice emphasized antibody and plasma cell persistence in the absence of antigen-specific memory B cells, the study did not investigate beyond 16 weeks from memory B-cell depletion.52
For the infants in the current study the only comparative data on memory B-cell frequency in other age groups come from studies of an identical MenCC vaccine given as a booster dose (rather than a primary course of immunization) to toddlers or adolescents.19,41 In the toddlers, a follow-up study of the infants reported in this study, there was no difference in circulating MenCPS-specific B-cell memory between that at 5 months of age (1 month after primary immunization) and that at 13 months of age (1 month after boosting).19 The degree of long-term antibody persistence following the booster dose in this situation is unknown. We have also previously reported MenCPS specific plasma and memory B-cell frequencies in a group of adolescents who had been boosted with an identical MenCC vaccine to that used in the current study.41 There is a significant difference between the median responses in infants in the current study 1 month after the third dose of vaccine (at 5 months of age) and adolescents 1 month post-immunization (2·5 versus 8·8 MenC ASCs per 2 × 105 cultured lymphocytes, respectively; P = 0·001). Antibody persistence was greater in the adolescents 1 year after immunization than in the infants 7 months after immunization.19,53 However, the memory B-cell assay used in the adolescent study was not identical to that used in the current study.
The hypothesis that there might be a relative lack of memory B-cell production in infants would not be in conflict with the observation that all infants given MenCC appear to have immunological memory. Whilst the degree of antibody persistence may be dependent on the total memory B-cell pool, the secondary antibody response may require only a proportion of the total memory B cells. There are some animal data to support this hypothesis. Using a murine model with defective production of memory B cells, it has been demonstrated that it is possible to impair long-term antibody production by reduced numbers of memory B cells whilst maintaining a normal secondary antibody response.54 In addition, studies of secondary responses in a different murine model have demonstrated that the memory response can be generated from a small proportion of the available memory B-cell pool.55 In order to determine the contribution of memory B cells to antibody persistence in infants, studies are needed to directly compare memory B-cell frequencies following primary immunization at varying ages with subsequent antibody persistence.
Conclusion
This study has demonstrated the kinetics of the responses of plasma and memory B cells to the first and third doses of a CRM197-conjugated MenCC vaccine given to infants according to a 2-, 3- and 4-month schedule. The B-cell responses to the MenCPS component of the vaccine are of relevance to other T-dependent antigens as the process of conjugating the polysaccharide to a carrier protein results in a T-dependent antigen. This is the first report of the B-cell kinetics of infants in their first year of life in response to any vaccine.
The information from this study will be important in allowing the design of further studies in this age group to elucidate the key features of vaccines that give rise to persistent antibody. The use of optimized infant immunization schedules or adjuvants specifically designed to increase memory B-cell production may improve antibody persistence, and in so doing provide enhanced and sustained protection from childhood infections.
Acknowledgments
The authors express their gratitude to the children and families who participated in the study described in this article and to the research team at the Oxford Vaccine Group. The study was funded by Novartis Vaccines and the authors acknowledge the role of Astrid Borkowski of Novartis Vaccines in this process.
Author contributions
DFK designed the study, undertook laboratory work, analysed the data and wrote the manuscript. MDS participated in study design and undertook planning and supervision of the clinical aspects of the study (including ethics submission, study protocol drafting, recruitment and sample collection). In addition, MDS reviewed drafts of the manuscript. KPP undertook laboratory work and reviewed drafts of the manuscript. SL undertook laboratory work and reviewed drafts of the manuscript. EAC advised on laboratory assays, undertook laboratory work and reviewed drafts of the manuscript. GB undertook laboratory work, participated in study design and reviewed drafts of the manuscript. MJ recruited children to the study, administered immunizations, undertook blood collection and reviewed drafts of the manuscript. LMY performed the statistical analysis and the sample size calculation. AJP supervised all aspects of the study, participated in the original design and took overall clinical responsibility for the study, in addition to reviewing drafts of the manuscript.
Conflict of interest
DFK, KPP, SL, EAC, GB, MJ and LY have no conflicting financial interests to declare. AJP is a Jenner Institute Investigator. AJP has conducted clinical trials on behalf of Oxford University, sponsored by the following vaccine manufacturers: Wyeth Vaccines, GlaxoSmithline Vaccines, Sanofi Pasteur, Sanofi Pasteur MSD and Novartis Vaccines. He has received assistance from vaccine manufacturers to attend scientific meetings. Industry-sourced honoraria for consultancy, lecturing or writing are paid directly to an independent charity or an educational/administrative fund held by the Department of Paediatrics, University of Oxford. MDS has received assistance to attend scientific meetings from Novartis Vaccines.
References
- 1.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 2.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 3.Kelly DF, Pollard AJ, Moxon ER. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. Jama. 2005;294:3019–23. doi: 10.1001/jama.294.23.3019. [DOI] [PubMed] [Google Scholar]
- 4.Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(12 Suppl):S274–9. [PubMed] [Google Scholar]
- 5.MacLennan JM, Shackley F, Heath PT, et al. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. Jama. 2000;283:2795–801. doi: 10.1001/jama.283.21.2795. [DOI] [PubMed] [Google Scholar]
- 6.Richmond P, Borrow R, Miller E, et al. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis. 1999;179:1569–72. doi: 10.1086/314753. [DOI] [PubMed] [Google Scholar]
- 7.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 8.Snape MD, Pollard AJ. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect Dis. 2005;5:21–30. doi: 10.1016/S1473-3099(04)01251-4. [DOI] [PubMed] [Google Scholar]
- 9.Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, Mallard RH, Kaczmarski EB. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–96. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 10.Edwards EA, Devine LF, Sengbusch GH, Ward HW. Immunological investigations of meningococcal disease. Scand J Infect Dis. 1977;9:105–10. doi: 10.3109/inf.1977.9.issue-2.09. III. Brevity of group C acquisition prior to disease occurrence. [DOI] [PubMed] [Google Scholar]
- 11.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Slifka MK, Ahmed R. Long-term antibody production is sustained by antibody-secreting cells in the bone marrow following acute viral infection. Ann N Y Acad Sci. 1996;797:166–76. doi: 10.1111/j.1749-6632.1996.tb52958.x. [DOI] [PubMed] [Google Scholar]
- 13.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–72. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 14.McHeyzer-Williams LJ, McHeyzer-Williams M. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 15.Pihlgren M, Schallert N, Tougne C, et al. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur J Immunol. 2001;31:939–46. doi: 10.1002/1521-4141(200103)31:3<939::aid-immu939>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Pihlgren M, Friedli M, Tougne C, Rochat AF, Lambert PH, Siegrist CA. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J Immunol. 2006;176:165–72. doi: 10.4049/jimmunol.176.1.165. [DOI] [PubMed] [Google Scholar]
- 17.Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146–54. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 18.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 19.Rohner GB, Snape MD, Kelly DF, et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol. 2008;180:2165–73. doi: 10.4049/jimmunol.180.4.2165. [DOI] [PubMed] [Google Scholar]
- 20.Gheesling LL, Carlone GM, Pais LB, et al. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J Clin Microbiol. 1994;32:1475–82. doi: 10.1128/jcm.32.6.1475-1482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 22.Brinkman DM, Jol-van der Zijde CM, ten Dam MM, Vossen JM, Osterhaus AD, Kroon FP, van Tol MJ. Vaccination with rabies to study the humoral and cellular immune response to a T-cell dependent neoantigen in man. J Clin Immunol. 2003;23:528–38. doi: 10.1023/b:joci.0000010429.36461.6b. [DOI] [PubMed] [Google Scholar]
- 23.Curtis JE, Hersh EM, Harris JE, McBride C, Freireich EJ. The human primary immune response to keyhole limpet haemocyanin: interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin Exp Immunol. 1970;6:473–91. [PMC free article] [PubMed] [Google Scholar]
- 24.Weits J, de Gast GC, The TH, Esselink MT, Deelder AM, Petrovic M, Mandema E. Class-specific antibody titres (ELISA) against the primary immunogen Helix pomatia haemocyanin (HPH) in man. Clin Exp Immunol. 1978;32:443–50. [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–8. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. J Exp Med. 1998;187:885–95. doi: 10.1084/jem.187.6.885. V. Affinity maturation develops in two stages of clonal selection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–7. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 28.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69:1895–902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiele CJ, Stevens RH. Expression of surface membrane IgG on pokeweed mitogen-reactive anti-tetanus toxoid antibody-producing cells. J Clin Immunol. 1981;1:174–80. doi: 10.1007/BF00922760. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Garcia I, Ocana E, Jimenez-Gomez G, Campos-Caro A, Brieva JA. Immunization-induced perturbation of human blood plasma cell pool: progressive maturation, IL-6 responsiveness, and high PRDI-BF1/BLIMP1 expression are critical distinctions between antigen-specific and nonspecific plasma cells. J Immunol. 2006;176:4042–50. doi: 10.4049/jimmunol.176.7.4042. [DOI] [PubMed] [Google Scholar]
- 31.Kehrl JH, Fauci AS. Activation of human B lymphocytes after immunization with pneumococcal polysaccharides. J Clin Invest. 1983;71:1032–40. doi: 10.1172/JCI110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens RH, Macy E, Morrow C, Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979;122:2498–504. [PubMed] [Google Scholar]
- 33.Geha RS. Dynamics of human circulating antigen reactive cells following secondary immunization with tetanus toxoid. Clin Immunol Immunopathol. 1981;19:196–205. doi: 10.1016/0090-1229(81)90063-5. [DOI] [PubMed] [Google Scholar]
- 34.Kodo H, Gale RP, Saxon A. Antibody synthesis by bone marrow cells in vitro following primary and booster tetanus toxoid immunization in humans. J Clin Invest. 1984;73:1377–84. doi: 10.1172/JCI111341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina F, Segundo C, Campos-Caro A, Gonzalez-Garcia I, Brieva JA. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 2002;99:2154–61. doi: 10.1182/blood.v99.6.2154. [DOI] [PubMed] [Google Scholar]
- 36.Odendahl M, Mei H, Hoyer BF, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–21. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 37.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201:545–54. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baine Y, Thorbecke GJ. Induction and persistence of local B cell memory in mice. J Immunol. 1982;128:639–43. [PubMed] [Google Scholar]
- 39.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–21. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 41.Kelly DF, Snape MD, Cutterbuck EA, et al. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood. 2006;108:2642–7. doi: 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 42.Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20:498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 43.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 44.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 45.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159:2652–7. [PubMed] [Google Scholar]
- 46.Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(Suppl 2):S35–7. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 47.Borrow R, Goldblatt D, Finn A, et al. Immunogenicity of, and immunologic memory to, a reduced primary schedule of meningococcal C-tetanus toxoid conjugate vaccine in infants in the United kingdom. Infect Immun. 2003;71:5549–55. doi: 10.1128/IAI.71.10.5549-5555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snape M, Kelly DF, Diggle L, Lewis S, Banner C, Schulze V, Borkowski X, Pollard AJ. A Sustained Increase in Meningococcal Serogroup C Specific IgG Following Glycoconjugate or Plain Polysaccharide Meningococcal Vaccines in Adolescents Previously Immunised With Serogroup C Meningococcal Glycoconjugate Vaccine. Carins, Australia: International Pathogenic Neisseria Conference; 2006. [Google Scholar]
- 49.Vu DM, Welsch JA, Zuno-Mitchell P, Dela Cruz JV, Granoff DM. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J Infect Dis. 2006;193:821–8. doi: 10.1086/500512. [DOI] [PubMed] [Google Scholar]
- 50.Lanzavecchia A, Bernasconi N, Traggiai E, Ruprecht CR, Corti D, Sallusto F. Understanding and making use of human memory B cells. Immunol Rev. 2006;211:303–9. doi: 10.1111/j.0105-2896.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–4. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 52.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci USA. 2008;105:4802–7. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snape MD, Kelly DF, Salt P, et al. Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin Infect Dis. 2006;43:1387–94. doi: 10.1086/508776. [DOI] [PubMed] [Google Scholar]
- 54.Ridderstad A, Nossal GJ, Tarlinton DM. The xid mutation diminishes memory B cell generation but does not affect somatic hypermutation and selection. J Immunol. 1996;157:3357–65. [PubMed] [Google Scholar]
- 55.Vora KA, Tumas-Brundage K, Manser T. Contrasting the in situ behavior of a memory B cell clone during primary and secondary immune responses. J Immunol. 1999;163:4315–27. [PubMed] [Google Scholar]