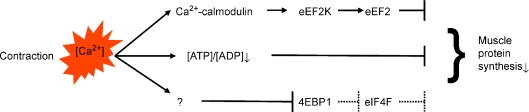
In skeletal muscles, both contraction and the synthesis of proteins consume energy: thus when muscles undergo contractile activity it makes sense, teleologically, for the fuelling of actomyosin by ATP to have a higher place in muscle's metabolic priorities than synthesizing new proteins; the net result is a blunting of muscle protein synthesis (MPS). Although this phenomenon and the above explanation were first identified 25 years ago by Christine Bylund-Fellenius and colleagues (Bylund-Fellenius et al. 1984) the mechanisms have remained poorly defined with the mechanistic links between Ca2+ activation of contraction, the inhibition of anabolic signalling pathways (and the consequent shutting off of MPS) inferred but not linked robustly (Rose et al. 2009b). It has long seemed likely that activation of AMP-dependent protein kinase (AMPK) by contraction-induced increases in [AMP] and/or reduced [glycogen] was involved through targeted deactivation of mammalian target of rapamycin complex 1 (mTORC1), resulting in a drop in the phosphorylation of binding proteins of eukaryotic initiation factor 4E (eIF4E-BP1s) necessary for initiation of MPS (Fig. 1).
Figure 1.
Mechanisms regulating inhibition of muscle protein synthesis during contraction
However, this would inevitably be a relatively sluggish mechanism. In contrast, rapid increases in the phosphorylation of the regulator of peptide elongation, eukaryotic elongation factor 2 (eEF2), which renders it less active, would arguably provide a more globally effective and a near instantaneous mechanism since it follows from increases in [Ca2+]i and resulting Ca2+–calmodulin-dependent activation of the kinase regulating eEF2 phosphorylation, eEF2K (Rose et al. 2005). In this issue of The Journal of Physiology Rose and colleagues (Rose et al. 2009a) now provide a convincing confirmation of the shut-down of MPS during contraction but also elegant explanations of how this happens, and the relative importance of the major mechanisms involving AMPK, 4E-BP1, Ca2+ and eEF2.
First, Rose and colleagues showed that the extent of inhibition of both MPS and anabolic signalling were dependent on the ‘duty cycle’ of contraction of a muscle stimulated in situ. With a high duty cycle (200 ms every 2 s) there was a ∼70% decrease in MPS and a marked increase in ‘inhibitory’ phosphorylations of eEF2, AMPK and its substrate acetyl-CoA carboxylase (ACCβ), which were all less inhibited at a less intense duty cycle (200 ms every 10 s). On the other hand, the inhibitory effect on eIF4E-BP1 was identical with both protocols, hinting at its minor importance for the suppression of MPS. Thus, the inferred ATP demand of contracting muscles seems to reflect the magnitude of inhibitory signalling and the extent of the consequent blunting in MPS. Indeed this notion is strengthened by further experiments showing that Ca2+-releasing agents, when used in combination with ATPase inhibitors, lessen the suppression of MPS seen with Ca2+-releasing agents alone.
In an attempt to uncover a role for the inhibition of peptide elongation by eEF2, the Scandinavian workers incubated contracting muscles in the presence of compounds selectively inhibiting the eEF2 Thr56 kinase and thereby completely prevented the previous ∼5-fold increase in eEF2 phosphorylation. Yet, despite this, the suppression of MPS was only attenuated by 30–40%, suggesting that blunting of MPS by contraction is not merely a consequence of deactivation in peptide elongation. Could then AMPK be influential in the negative regulation of initiation of protein synthesis? This long held belief was questioned in additional experiments showing that contraction of muscle from α2 kinase-dead transgenic mice resulted in no relief of the suppression of MPS compared to that in wild-type mice. Moreover, the profiles of inhibitory eIF4E-BP1 and eEF2 phosphorylations were unaffected. These data clearly demonstrate not only that AMPK activity is not necessary for the suppression of mTORC1 signalling or stimulatory eEF2K phosphorylation, but that other (as yet undetected) negative influences upon 4EBP1 are involved. Muscle acidity can increase substantially with contraction but it appears that use of exogenous substances to buffer muscle pH during resistance exercise produces no benefit in hypertrophy after training (Kendrick et al. 2008). Rose and colleagues produce results showing why this is may be so – the increases in eEF2 phosphorylation and eEF2 kinase activation are independent of pH. Where do we go from here? A slew of important questions arise. Is the suppression of protein synthesis global or targeted to cellular compartments or individual proteins, e.g. myofibrillar or mitochondrial proteins? How quick is the rebound of MPS, what does it depend upon and what are its mechanisms? It appears from our recent work (Kumar et al. 2009) that there is a latent period for myofibrillar protein synthesis in human muscle of about an hour, which suggests that long after the Ca2+-determined changes are reversed, other changes take longer to normalize – and indeed become supernormal. What is the effect of stretch? For every contraction of a muscle an antagonistic muscle is stretched, and certain activities cause muscles to simultaneously lengthen and contract (i.e. running). It would be odd if the stretch component had no effect on MPS in muscles involved in a cycle of locomotor contractions.
References
- Bylund-Fellenius AC, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Am J Physiol Endocrinol Metab. 1984;246:E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Kendrick IP, Harris RC, Kim HJ, Kim CK, Dang VH, Lam TQ, Bui TT, Smith M, Wise JA. Amino Acids. 2008;34:547–554. doi: 10.1007/s00726-007-0008-3. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Alsted TJ, Jensen TE, Kobberø JB, Maarbjerg SJ, Jensen J, Richter EA. J Physiol. 2009a;587:1547–1563. doi: 10.1113/jphysiol.2008.167528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Bisiani B, Vistisen B, Kiens B, Richter EA. Am J Physiol Regul Integr Comp Physiol. 2009b;296:R326–R333. doi: 10.1152/ajpregu.90806.2008. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]