Abstract
The novel hypothalamic peptides avian gonadotropin inhibitory hormone (GnIH) and its mammalian analogue RFRP-3, are emerging as key negative regulators of reproductive functions across species. GnIH/RFRP-3 reduces gonadotropin release and may play an inhibitory role in ovulation and seasonal reproduction, actions opposite to that of the puberty-promoting kisspeptins. GnIH directly inhibits gonadotropin release from the anterior pituitary in birds. GnIH/RFRP-3-immunoreactive fibres also abut the preoptic-septal gonadotropin-releasing hormone (GnRH) neurons, suggesting an additional site of action that has not been studied at the cellular level. Using anatomical labelling and electrophysiological recordings in septal brain slices from GnRH-GFP, vGluT2-GFP and GAD67-GFP mice, we report inhibitory actions of GnIH/RFRP-3 on kisspeptin-activated vGluT2 (vesicular glutamate transporter 2)-GnRH neurons as well as on kisspeptin-insensitive GnRH neurons, but not on cholinergic or GABAergic neurons (n= 531). GnIH and RFRP-3 produced a strikingly similar non-desensitizing hyperpolarization following brief 15 s applications (GnIH: 9.3 ± 1.9 mV; RFRP-3: 9.0 ± 0.9 mV) with IC50 values of 34 and 37 nm, respectively. The inhibitory effect was mediated via a direct postsynaptic Ba2+-sensitive K+ current mechanism and could prevent or interrupt kisspeptin-induced activation of vGluT2-GnRH neurons. GnIH-immunoreactive fibres were in apparent contact with vGluT2-GFP neurons. Thus, GnIH/RFRP-3 could reduce GnRH and glutamate release in target brain regions and in the median eminence via a direct inhibition of vGluT2-GnRH neurons. This in turn could suppress gonadotropin release, influence reproductive development and alter sex behaviour.
Recent breakthroughs in neuroendocrinology have identified two novel hypothalamic RFamide peptides, kisspeptin (de Roux et al. 2003; Seminara et al. 2003) and gonadotropin-inhibitory hormone (GnIH; Tsutsui et al. 2000; Kriegsfeld et al. 2006), as important regulators of the reproductive axis. Initially discovered in the avian brain, GnIH was named after its unique property of directly inhibiting gonadotropin release from the anterior pituitary (Tsutsui et al. 2000; Ubuka et al. 2006). The gene sequence encoding avian GnIH is missing in the homologous human gene. However, mammalian RFRP-3 is structurally and functionally similar to avian GnIH; both peptides inhibit gonadotropin secretion (Tsutsui et al. 2000; Kriegsfeld et al. 2006) and suppress sex behaviour (Bentley et al. 2006; Johnson et al. 2007). RFRP-3 neurons are concentrated in the dorsomedial nucleus of the hypothalamus and express sex steroid receptors (Kriegsfeld, 2006). They are also under circadian control and show decreased immediate early gene expression at the time of the luteinizing hormone (LH) surge, suggesting a role in ovulation (Gibson et al. 2008).
Interest in GnIH/RFRP-3 has been heightened by the speculation that it may also act as a brake for the reproductive effects of kisspeptins and thus may be critical for precise regulation of the reproductive axis (Kriegsfeld, 2006). Inactivating mutations of the kisspeptin receptor, GPR54, prevent the onset of puberty and lead to hypogonadotropic hypogonadism and infertility both in humans and in mice (de Roux et al. 2003; Seminara et al. 2003; Funes et al. 2003). At the cellular level kisspeptin promotes gonadotropin-releasing hormone (GnRH) release via a direct and sustained activation of GnRH neurons (Han et al. 2005; Zhang et al. 2008; Pielecka-Fortuna et al. 2008; Liu et al. 2008; Dumalska et al. 2008). Within the medial septum/diagonal band of Broca (MSDB), kisspeptin activates GnRH neurons that co-localize vGluT2 (vGluT2-GnRH neurons) (Dumalska et al. 2008). Whether GnIH/RFRP-3 acts exclusively at the level of the pituitary or also has a direct action on the GnRH neurons remains to be determined. Mammalian RFRP-3-immunoreactive fibres and terminals are concentrated in the MSDB, preoptic area and anterior hypothalamus and make direct contact with >40% of GnRH neurons.
The goal of the present study was to examine the electrophysiological effects of GnIH/RFRP-3 on kisspeptin-activated vGluT2-GnRH neurons and on kisspeptin-insensitive MSDB vGluT2, GnRH, cholinergic and GABAergic neurons (Dumalska et al. 2008). We demonstrate that GnIH and RFRP-3 selectively inhibit kisspeptin-sensitive vGluT2-GnRH and kisspeptin-insensitive GnRH neurons, but have no effect on kisspeptin-insensitive vGluT2, cholinergic or GABAergic neurons. Additionally, we show that GnIH/RFRP-3 can prevent and/or interrupt the excitatory effects of kisspeptin directly at the level of the septal vGluT2-GnRH neuron.
Methods
GnRH-GFP, vGluT2-GFP and GAD67-GFP mice
Electrophysiological studies were conducted in brain slices prepared from transgenic mice that expressed green fluorescent protein (GFP) in GnRH, vGluT2 or GABAergic neurons. GABAergic neurons were recorded from a well established line of glutamic acid decarboxylase 67-GFP (GAD67-GFP) (kindly provided by Drs K. Obata and Y. Yanagawa; Tamamaki et al. 2003; Acuna-Goycolea et al. 2005). GnRH and vGluT2 neurons were recorded from well-established lines of GnRH-GFP (Spergel et al. 1999; Suter et al. 2000) or vGluT2-GFP (Huang et al. 2006; Fu & van den Pol, 2008; Dumalska et al. 2008) mice. MSDB cholinergic neurons were identified in mouse brain slices in the living state using a well-established fluorescent marker that is conjugated to an antibody against the NGF receptor (Cy3-labelled anti-NGFr). Cy3-labelled anti-NGFr (3–5 μl; 0.4 mg ml−1) was stereotaxically injected bilaterally into the lateral ventricle of anaesthetized mice with a Hamilton syringe (22 gauge needle) at a rate of 0.5 μl min−1. Two to five days later, slices were prepared from injected mice for electrophysiological recordings. A total of 110 mice was used and all experiments were carried out according to guidelines laid down by Yale University's animal welfare committee.
Slice preparation and electrophysiological recordings
Two- to four-week-old pre-pubertal male and female mice were anaesthetized with chloral hydrate (400 mg kg−1i.p.) and killed by decapitation. The ACSF (pH 7.35–7.38), equilibrated with 95%O2–5% CO2, contained (in mm): NaCl, 128; KCl, 3; NaH2PO4, 1.25; d-glucose, 10; NaHCO3, 26; CaCl2, 2; and MgSO4, 2. Following decapitation, brains were removed, placed in a Petri-dish containing ACSF and trimmed to yield a small block containing the MSDB. Coronal slices of thickness 300 μm were obtained with a Vibratome 1500 (Vibratome Co., St Louis, MO, USA) and transferred to a Plexiglas recording chamber (1.5 ml volume) on the fixed stage of an Olympus BX50WI microscope for visualized whole-cell recording. The chamber was continuously perfused with normal ACSF at a rate of 2–3 ml min−1 and its temperature maintained at 33 ± 0.5°C. One to two hours later the slice was used for recording.
Whole-cell current- and voltage-clamp recordings were performed using previously described methods (Wu et al. 2004). The low resistance (2.5–3.5 MΩ) patch pipettes were filled with a solution containing (in mm): potassium gluconate, 125; Hepes, 10; BAPTA-K4, 5; CaCl2, 2.38; Mg-ATP, 4; sodium phosphocreatine, 10 and Na2-GTP, 0.3 (pH – 7.32–7.35). Data were acquired using an Axoclamp-2B and pCLAMP 9 (Axon Instruments, Union City, CA, USA). No correction was made for the calculated liquid junction potential of ∼11 mV for our internal solution.
Immunocytochemistry
vGluT2-GFP mice were given an overdose of anaesthetic and perfused transcardially with saline followed by 4% paraformaldehyde (n= 5). Coronal sections of 30 μm thickness were incubated overnight in a rabbit anti-GnIH antiserum at 1 : 3000 (generously provided by Dr G. Bentley). The antiserum is selective for GnIH and absorption of the primary antiserum with the GnIH peptide blocks immunostaining (Kriegsfeld, 2006). Sections were then incubated in goat anti-rabbit secondary antiserum conjugated to Texas red, rhodamine, or Alexa 594. Sections were examined in an Olympus IX70 fluorescence microscope, and photomicrographs taken with a Spot camera (Diagnostic Imaging). Contrast and brightness were corrected in Adobe Photoshop; any correction was applied to the entire micrograph.
Drugs and drug application
GnIH (Sigma), kisspeptin (KiSS-1, metastin 45–54 amide, kisspeptin-10; Sigma), RFRP-3 (Phoenix Pharmaceuticals) and (S)-3,5 Dihydroxyphenylglycine (DHPG) (Tocris Bioscience, Ellisville, MO, USA) were diluted in ACSF from previously prepared stock solutions that were stored at −20°C. Agonists were applied using a Y-tube (Wu et al. 2003). TTX (Alomone Laboratories, Jerusalem, Israel) and BaCl2 were bath-applied.
Results
GnIH and RFRP-3 inhibit both kisspeptin-sensitive vGluT2-GnRH and kisspeptin-insensitive GnRH neurons
We tested the effect of GnIH and RFRP-3 on kisspeptin-activated vGluT2 and GnRH neurons as well as on kisspeptin-insensitive vGluT2, GnRH, cholinergic and GABAergic septal neurons using visualized whole-cell patch clamp recordings. In a recent study we specifically demonstrated that kisspeptin-activated MSDB neurons co-localize vGluT2 and GnRH mRNA and can therefore be visualized in brain slices prepared from either vGluT2-GFP or GnRH-GFP mice. It is estimated that 84–99% of GnRH neurons co-localize vGluT2-ir (Hrabovszky et al. 2004; Dumalska et al. 2008). However, only 25% of vGluT2-GFP neurons in the MSDB co-localize GnRH. In vGluT2-GFP mice, putative GnRH co-expressing neurons can be distinguished by their morphology (Sim et al. 2001; Dumalska et al. 2008). Given the importance of glutamate in reproduction (Kawakami et al. 1998), we examined the effects of GnIH and RFRP-3 on both vGluT2-GFP and GnRH-GFP neurons.
As detailed below, GnIH and RFRP-3 inhibited kisspeptin-insensitive GnRH neurons as well as kisspeptin-activated vGluT2-GnRH neurons, but had no effect on the kisspeptin-insensitive cholinergic (n= 17), vGluT2 (n= 22) or GABAergic neurons (n= 11) (not shown).
GnIH inhibited 50% of kisspeptin-insensitive GnRH-GFP neurons producing a mean hyperpolarization of 5.2 ± 0.8 mV (n= 22; Fig. 1A and C). Forty-five per cent of the recorded neurons fired spontaneously; the remaining had a mean resting membrane potential of −63.8 ± 1.3 mV. As shown in Fig. 1A, kisspeptin-insensitive GnRH-GFP neurons are strongly activated by the Group I metabotropic receptor agonist DHPG (Dumalska et al. 2008).
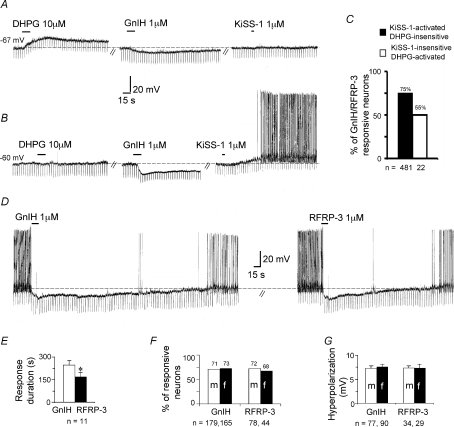
Figure 1. GnIH and RFRP-3 inhibit MSDB KiSS-1-activated and KiSS-1 insensitive pre-pubertal GnRH-GFP neurons.
A, current-clamp recording from a GnRH-GFP neuron shows that a DHPG-activated but KiSS-1-insensitive GnRH-GFP neuron responds to a 15 s application of 1 μm GnIH with a 7 mV hyperpolarization. B, a DHPG-insensitive neuron that also responds to GnIH with a hyperpolarization (15 mV) that lasted 3.5 min. Following washout the same neuron responded to a 5 s application of 1 μm KiSS-1 with a response that lasted for >10 min (washouts not shown). C, bar chart summarizing the percentage of KiSS-1-sensitive and −insensitive neurons that responded to GnIH/RFRP-3. D, equimolar concentrations of GnIH and RFRP-3 produced comparable peak hyperpolarizations of 17 and 19 mV, respectively. The GnIH inhibition returned close to baseline in 6.5 min and the RFRP-3 inhibition lasted only 4 min. These experiments were performed in pre-pubescent male and female mice. E–G, bar charts show that a similar percentage of neurons responded to GnIH and RFRP-3 in the two sexes with comparable hyperpolarizations, but GnIH produced a significantly longer response. Dashed line indicates base line and the number in mV denotes resting membrane potential in quiescent neurons. Downward vertical deflections are in response to −0.01 nA current pulses delivered every 4 s to monitor the input resistance of the recorded neuron.
To study the effect of GnIH/RFRP-3 on kisspeptin-activated vGluT2-GnRH neurons, recordings were performed in brain slices prepared from vGluT2-GFP or GnRH-GFP mice. Kisspeptin-activated vGluT2-GnRH neurons can be readily identified by their lack of response to DHPG (Dumalska et al. 2008). A total of 481 DHPG-insensitive vGluT2-GnRH neurons were tested in brain slices prepared from pre-pubertal male or female vGluT2-GFP (n= 386) or GnRH-GFP mice (n= 95). GnIH and RFRP-3 inhibited kisspeptin-activated vGluT2-GnRH neurons (Fig. 1B and C). At the end of the experiment, kisspeptin was applied to 116 vGluT2-GFP and 42 GnRH-GFP neurons; all 158 DHPG-insensitive neurons responded to kisspeptin with an excitation. Seventy-five per cent of the recorded neurons fired spontaneously; the remaining had a mean resting membrane potential of −62.4 ± 0.7 mV. In slices prepared from vGluT2-GFP mice, GnIH was applied to 336 neurons; 72% responded with an inhibition. A similar proportion of neurons were inhibited by RFRP-3 (70%; n= 95). Similarly, in slices prepared from GnRH-GFP mice, 68% of DHPG-insensitive neurons were inhibited by GnIH (51/75) and 67% by RFRP-3 (32/48). These experiments confirmed that similar to kisspeptin, GnIH/RFRP-3 inhibit both DHPG-insensitive vGluT2-GFP and GnRH-GFP neurons. Due to the similarity of responses in the vGluT2-GFP and GnRH-GFP mice, for further analysis on DHPG-insensitive neurons, data from the two transgenic mice were pooled.
The response to RFRP-3 and GnIH was comparable in amplitude when applied to the same neuron for 15 s at a 1 μm concentration (GnIH: 7.6 ± 1.1 mV; RFRP-3: 7.8 ± 1.3 mV; n= 11). However, the GnIH hyperpolarization was significantly longer in duration (GnIH: 247 ± 28 s; RFRP-3: 170 ± 27 s; n= 11, Student's paired t-test P= 0.03; Fig. 1D and E). The percentage of kisspeptin-activated vGluT2-GnRH neurons that responded to GnIH or RFRP-3 was statistically similar in the two sexes, ranging from 68 to 73% (Fig. 1F). The amplitude of the GnIH and RFRP-3 inhibition was also similar (GnIH – male: 7.3 ± 0.46 mV, n= 77; female: 7.6 ± 0.46 mV, n= 90; RFRP-3 – male: 7.3 ± 0.5 mV, n= 34; female: 7.3 ± 0.8 mV, n= 29; Student's unpaired t-test; Fig. 1G).
The inhibitory effect of GnIH and RFRP-3 is reproducible, concentration dependent and mediated via a direct postsynaptic mechanism
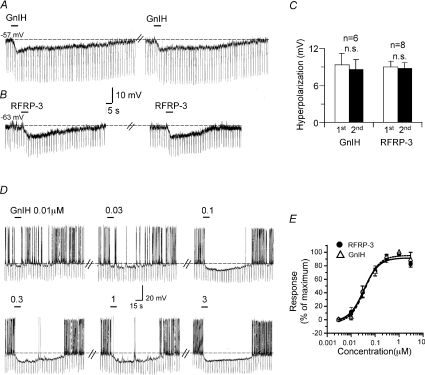
The GnIH/RFRP-3 inhibition showed little desensitization to a second application of agonist administered 5–15 min after the first (first GnIH application: 9.3 ± 1.9 mV, second GnIH application: 8.7 ± 1.6 mV, n= 6; first RFRP-3 application: 9.0 ± 0.9 mV, second RFRP-3 application: 8.9 ± 0.8 mV; n= 8; Student's paired t-test: not significant; Fig. 2A–C). This lack of desensitization is in striking contrast to the reported actions of kisspeptin that are more prolonged in duration, but show substantial desensitization. We also compared the amplitude of the GnIH/RFRP-3 inhibition in response to a 15 s vs. 60 s application of agonist in the same cell. The peak hyperpolarization was statistically similar under the two experimental conditions (GnIH/RFRP-3 15 s: 8 ± 1.6 mV; 60 s: 7 ± 1.5 mV; n= 5), suggesting equilibrium conditions even with a 15 s application. As such we applied the agonists for 15 s in the concentration–response studies.
Figure 2. GnIH and RFRP-3 produce a reproducible and concentration-dependent inhibition in vGluT2-GnRH neurons.
A, two consecutive applications of 1 μm GnIH at an interval of 8 min produced comparable hyperpolarizations of 8 mV. B, two consecutive applications of RFRP-3 at an interval of 6 min also produced comparable hyperpolarizations. C, bar charts summarizing the data for two consecutive 15 s applications of GnIH and RFRP-3 applied at intervals of 5–15 min. Note the reproducibility of the inhibitory effects and a lack of desensitization. D, the concentration dependence of the GnIH response. A maximal hyperpolarization of 18 mV was obtained with 1 and 3 μm GnIH. E, concentration–response curve summarizing GnIH data from 4 cells and RFRP-3 data from 6 cells. Both peptides inhibited with similar IC50 values of 37 and 34 nm and a near-maximal effect was obtained with 1 μm peptide concentration.
The GnIH/RFRP-3-induced hyperpolarization was concentration dependent with IC50 values of 37 nm (n= 4) and 34 nm (n= 6), respectively, as determined by testing the response to seven concentrations of agonist (0.003 to 10 μm). With both peptides near-maximal effects were obtained with 1 μm concentration (Fig. 2D and E). Thus, both GnIH and RFRP-3 were very similar in their effects on the vGluT2-GnRH neurons. As such data obtained with the two peptides were combined for the remaining studies.
The GnIH/RFRP-3 hyperpolarization persisted in ‘zero’ Ca2+/high Mg2+ ACSF (control: 8.9 ± 1.3 mV; ‘zero’ Ca2+/high Mg2+: 9.4 ± 1.4 mV; n= 12) and in TTX (control: 10.5 ± 1.0 mV; TTX: 11.5 ± 1.5 mV; n= 4) suggesting involvement of a direct postsynaptic mechanism (Fig. 3A, B and D). To prevent the confounding effects of small changes in membrane resistance, the conductance change associated with the GnIH/RFRP-3 inhibition was measured under voltage-clamp conditions. At a holding potential of −60 mV, GnIH/RFRP-3 produced an 11.5 ± 1.2 pA outward current that was associated with a significant increase in membrane conductance (control: 1.2 ± 0.2 nS; GnIH/RFRP-3: 1.5 ± 0.2 nS; P < 0.001; n= 13), suggesting a net opening of channels.
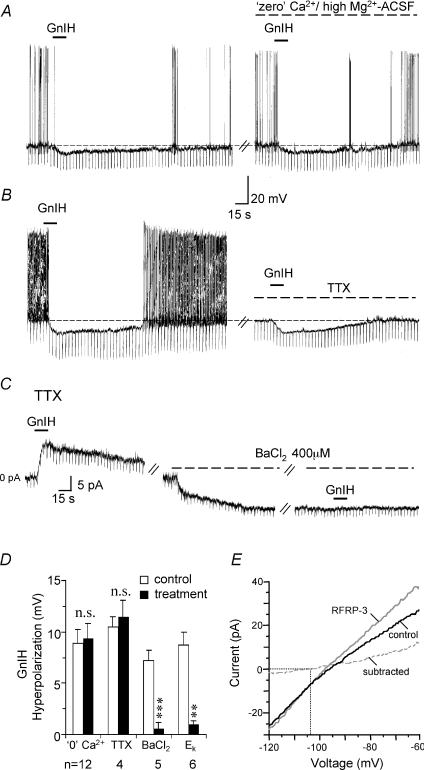
Figure 3. GnIH inhibits vGluT2-GnRH neurons via a direct postsynaptic potassium channel mechanism.
A and B, current-clamp recordings show that the hyperpolarizing response to GnIH (1 μm, 15 s) persists in ‘zero’ Ca2+/high Mg2+ ACSF and in TTX, suggesting involvement of a direct postsynaptic mechanism. In TTX (without the confounding effects of cell firing), the GnIH inhibition was associated with a decrease in input resistance. C, voltage-clamp recording (holding potential: −60 mV) done in the presence of TTX showing that GnIH produced a 11 pA outward current with an increase in membrane conductance. Bath-applied Ba2+ produced a 9 pA inward current and blocked GnIH inhibition. D, bar chart summarizing the statistically insignificant difference in the magnitude of GnIH hyperpolarization in control versus ‘zero’ Ca2+/high Mg2+ and TTX conditions. However, the GnIH response was significantly reduced in the presence of BaCl2. GnIH also failed to produce a significant inhibition when the cells were held near the calculated Ek of −101 mV as opposed to their resting membrane conditions (−60 ± 0.7 mV). E, I–V curves performed using slow steady-state ramps on a voltage-clamped GnRH-GFP neuron in the absence and presence of RFRP-3. Subtracted current is also shown. The RFRP-3 current reversed at −103 mV, close to the calculated EK of −101 mV.
The GnIH/RFRP-3 inhibition was significantly reduced in the presence of BaCl2, a blocker of K+ channels both in current-clamp (control: 7.2 ± 1.0 mV; Ba2+: 0.6 ± 0.6 mV; n= 5; Student's paired t-test; P < 0.001; Fig. 3D) and in voltage-clamp recordings (control: 10.5 ± 1.0 pA; Ba2+: 1 ± 1 pA; n= 4; Student's paired t-test; P < 0.01; Fig. 3C) done in the absence (n= 5) or presence of TTX (n= 4). Ba2+ alone produced a 7.2 ± 2 mV depolarization in current-clamp recordings (n= 5) and a 12 ± 4.5 pA inward current in voltage-clamp recordings (n= 4).
Consistent with the involvement of a K+ current, GnIH/RFRP-3 produced only a 1.3 ± 0.4 mV hyperpolarization near the calculated Ek of −101 mV, as compared to the 8.7 ± 1.3 mV hyperpolarization obtained under the control membrane potential of −60 ± 0.7 mV (Fig. 3D). Under voltage-clamp conditions using slow steady-state ramps (−60 mV to −120 mV), a mean reversal potential of −101 ± 1.2 mV was obtained in the six cells tested (Fig. 3E). Thus, GnIH/RFRP-3 inhibition of vGluT2-GnRH neurons involves opening of Ba2+-sensitive K+ channels.
GnIH/RFRP-3 opposes kisspeptin activation of vGluT2-GnRH neurons
Interactions between GnIH/RFRP-3 and kisspeptin (KiSS-1) were studied using two different protocols and agonists concentrations that were only a few-fold higher than their IC50 and EC50 values of 34 nm and 10 nm, respectively. Under control conditions KiSS-1 activation lasts an average of 16 ± 1.5 min (Dumalska et al. 2008). In eight cells in which a prior application of 1 μm GnIH produced a 10.6 ± 1.2 mV hyperpolarization, 100 nm KiSS-1 was applied for 5 s and GnIH re-applied for 15–30 s. As shown in Fig. 4Ba and b GnIH interrupted KiSS-1 activation in these neurons for an average of 135 ± 33 s (n= 8). Additionally, due to the non-desensitizing nature of the GnIH response, repeated applications of GnIH continued to reverse kisspeptin-activation (Fig. 4Bc).
Figure 4. GnIH opposes KiSS-1-induced activation of vGluT2-GnRH neurons.
A, chart record showing that KiSS-1 (100 nm, 5 s) induced a prolonged excitation that lasted >17 min. Ba, a KiSS-1 response (100 nm, 5 s) that was interrupted by GnIH (100 nm, 15 s) for 4 min 12 s. b, a second cell that gradually depolarized and started firing in response to KiSS-1 only to be interrupted by a subsequent GnIH application. c, a KiSS-1-activated neuron that was repeatedly inhibited by GnIH. Ca, a cell that responded to GnIH (1 μm, 60 s) with a 10 mV hyperpolarization; a subsequent cocktail of GnIH and KiSS-1 produced little activation, even though the neuron was exposed to 20 s of KiSS-1, 4 times longer than the 5 s applications above. b, in contrast, in a second cell that responded to GnIH with only a small decrease in firing rate, a subsequent application of KiSS-1 and GnIH (15 s) resulted in activation.
In a second set of experiments 1 μm GnIH was applied for 1 min, following which a cocktail of GnIH and KiSS-1 was given. In 6/8 cells tested, KiSS-1 failed to produce an excitatory response (Fig. 4Ca); in these six neurons the magnitude of the GnIH hyperpolarization ranged from 6 to 10 mV (mean hyperpolarization: 7.3 ± 0.84 mV). In two neurons, in which KiSS-1 excitation persisted even when co-applied with GnIH, the magnitude of the GnIH hyperpolarization was smaller (2 and 5 mV) (Fig. 4Cb). Thus, depending on its magnitude, GnIH can produce a weak to a complete block of KiSS-1 activation.
GnIH immunoreactive fibres are present in the vicinity of septal vGluT2-GFP neurons
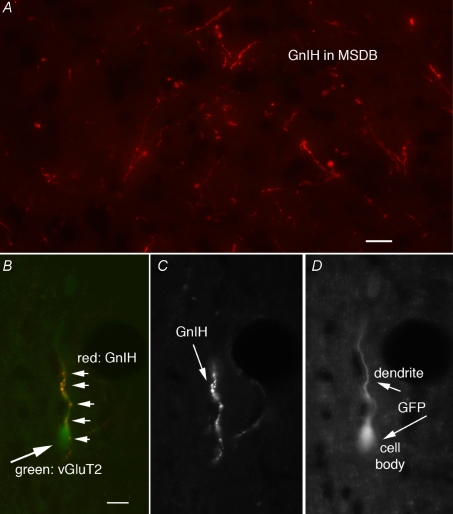
Since, GnIH/RFRP-3 inhibited kisspeptin-activated vGluT2-GnRH neurons, we used the vGluT2-GFP mouse (n= 5) to specifically determine the anatomical relationship between GnIH-immunoreactive fibres and vGluT2-GFP neurons. Parallel to previously published reports that focused on GnRH-GFP neurons (Kriegsfeld, 2006), GnIH immunoreactive cell bodies were found in the dorsomedial hypothalamus and GnIH-immunoreactive axons were found in abundance within the MSDB (Fig. 5A). More importantly, GnIH fibres were in close apparent apposition with a subpopulation of vGluT2-GFP neurons that had the general shape of GnRH neurons (Fig. 5B–D). Sections in which the primary antiserum was omitted showed no immunostaining, and sections incubated in a different rabbit antisera against hypocretin stained different cells and axons in the hypothalamus (van den Pol et al. 1998).
Figure 5. GnIH-immunoreactive fibres contact MSDB vGluT2-GFP neurons.
A, GnIH-ir fibres within the MSDB of a vGluT2-GFP mouse. B, GnIH-ir fibres with multiple boutons (small arrows) in close proximity to the dendrite of a vGluT2-GFP neuron (big arrow). Scale bar, 15 μm. C, GnIH-ir fibres alone. D, the vGluT2-GFP cell.
Discussion
In this study we report the electrophysiological effects of the novel avian peptide GnIH and its mammalian analogue, RFRP-3, on CNS neurons for the first time. We report that GnIH/RFRP-3, the first hypothalamic peptides to directly inhibit gonadotropin release from the anterior pituitary, also inhibit the GnRH neuron itself. The GnIH/RFRP-3 inhibition is reversible, reproducible, concentration dependent and non-desensitizing, and occurs in vGluT2-GnRH neurons that are activated by the puberty-promoting peptide, kisspeptin, as well as in GnRH neurons that are kisspeptin insensitive. GnIH is also able to prevent or interrupt kisspeptin-induced activation of GnRH neurons.
The actions of GnIH/RFRP-3 were highly selective for GnRH and vGluT2-GnRH neurons and showed no detectable effect on cholinergic or GABAergic neurons. Among the GnRH neurons, GnIH/RFRP-3 inhibited ∼50% of kisspeptin-insensitive GnRH neurons and ∼70% of kisspeptin-sensitive vGluT2-GnRH neurons. The response of GnRH neurons to a brief 15 s application of GnIH and RFRP-3 was strikingly similar in magnitude, albeit significantly longer in duration, following exposure to GnIH. Both peptides inhibited a similar proportion of neurons in male and female pre-pubertal mice and the IC50 values of GnIH and RFRP-3 were also comparable, 34 and 37 nm, respectively. The inhibitory action of GnIH/RFRP-3 persisted under conditions of synaptic blockade, was associated with an increase in membrane conductance, was blocked by BaCl2 and reversed near Ek, suggesting a mechanism based on potassium current.
The high density of GnIH immunoreactive fibres in the mouse MSDB underscores the physiological significance of the observed effects of GnIH/RFRP-3. In the hamster, GnIH fibres are also in the close vicinity of GnRH neurons (Kriegsfeld, 2006). A recent study in the rat has demonstrated the existence of RFRP-1/3 immunoreactive fibres in various brain regions including the septal-preoptic area, but interestingly, not in the media eminence. In the same study, systemically administered RFRP-3 failed to alter basal or GnRH-evoked plasma LH levels, suggesting that unlike in the birds, RFRP-3 may not act as a hypophysiotropic neuroendocrine hormone in the rat (Rizwan et al. 2009). These observations further strengthen the significance of our findings on the direct actions of GnIH/RFRP-3 in mouse GnRH neurons.
In the present study, using the GnIH antibody (Kriegsfeld et al. 2006) in the vGluT2-GFP mice we demonstrate GnIH fibres in close apposition with vGluT2-GFP neurons. This is consistent with the observed inhibitory effects of GnIH/RFRP-3 on vGluT2-GnRH neurons. Functionally the presence of vGluT2 confers a glutamatergic phenotype on neurons; GnIH/RFRP-3 inhibition of kisspeptin-activated vGluT2-GnRH neurons would thus reduce both glutamate and GnRH release into the pituitary portal blood system of the median eminence; in the median eminence, glutamate enhances GnRH release (Kawakami et al. 1998). Through these dual modes of action, GnIH/RFRP-3 could eventually suppress basal and kisspeptin-evoked gonadotropin release in the rodent brain. In addition to the above, GnIH/RFRP-3, through its actions on GnRH neurons, could participate in odour and pheromone processing, sexual behaviour and appetite and defensive behaviour (Boehm et al. 2005; Yoon et al. 2005). In fact, in rats GnIH/RFRP-3 stimulate food intake, suggesting that GnIH/RFRP-3 could also favour feeding and simultaneously reduce reproductive physiology and behaviour during negative metabolic states (Johnson et al. 2007).
Similar to GnIH, kisspeptin fibres are also found in proximity to GnRH neurons, thus providing an anatomical locus for physiological interaction between kisspeptin and GnIH/RFRP-3 that may be critical for precise regulation of the reproductive axis (Kriegsfeld et al. 2006). In the present study, by virtue of its opposing and non-desensitizing inhibitory action, GnIH was able to prevent and/or temporarily but repeatedly interrupt kisspeptin-induced activation of vGluT2-GnRH neurons. This interaction between kisspeptin and GnIH at the level of the GnRH neuron is particularly significant as kisspeptin may have little or no direct effect on pituitary gonadotropes (Shahab et al. 2005; Mason et al. 2007). Also, while both peptides and their receptors are localized in male and female gonads, their functional role at the gonadal level remains unknown (Greives et al. 2008).
In conclusion, this study demonstrates that GnRH neurons are direct targets of the inhibitory effects of GnIH/RFRP-3. Additionally, the opposing effects of GnIH/RFRP-3 on kisspeptin-induced activation of vGluT2-GnRH neurons may be important for the speculated interactions between these novel peptides in the control of puberty, sexual behaviour, ovulatory cyclicity and possibly metabolism.
Acknowledgments
This work was supported by NIH grants MH61465 to Meenakshi Alreja and NS41454 and NS48476 and the State of Connecticut, Department of Mental Health and Addiction Services. We thank Dr Allan Herbison for helpful suggestions and Y. Yang and V. Rogulin for technical help.
Note added in Proof
Using cell-attached electrophysiological recordings, Ducret et al. (Endocrinology, published online Jan 8, 2009) have recently reported that RFRP-3 inhibits 41% of GnRH-GFP neurons.
References
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, Van Den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–555. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Dumalska I, Wu M, Morozova E, Liu R, Van Den Pol A, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28:8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, Van Den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives TJ, Kriegsfeld LJ, Bentley GE, Tsutsui K, Demas GE. Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction? Proc Biol Sci. 2008;275:1943–1951. doi: 10.1098/rspb.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Turi GF, Kalló I, Liposits Z. Expression of vesicular glutamate transporter-2 in gonadotropin-releasing hormone neurons of the adult male rat. Endocrinology. 2004;145:4018–4021. doi: 10.1210/en.2004-0589. [DOI] [PubMed] [Google Scholar]
- Huang H, Ghosh P, Van Den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami S, Ichikawa M, Murahashi K, Hirunagi K, Tsukamura H, Maeda K. Excitatory amino acids act on the median eminence nerve terminals to induce gonadotropin-releasing hormone release in female rats. Gen Comp Endocrinol. 1998;112:372–382. doi: 10.1006/gcen.1998.7140. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Horm Behav. 2006;50:655–666. doi: 10.1016/j.yhbeh.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone (GnRH) neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFRP-1/3, the mammalian GnIH orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009 doi: 10.1210/en.2008-1287. in press. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Herbison AE. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci. 2001;21:1067–1075. doi: 10.1523/JNEUROSCI.21-03-01067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- Van Den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci. 2003;18:1155–1168. doi: 10.1046/j.1460-9568.2003.02847.x. [DOI] [PubMed] [Google Scholar]
- Wu M, Zaborszky L, Hajszan T, Van Den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]