Abstract
Skeletal muscle protein synthesis rate decreases during contractions but the underlying regulatory mechanisms are poorly understood. It was hypothesized that there would be a coordinated regulation of eukaryotic elongation factor 2 (eEF2) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1) phosphorylation by signalling cascades downstream of rises in intracellular [Ca2+] and decreased energy charge via AMP-activated protein kinase (AMPK) in contracting skeletal muscle. When fast-twitch skeletal muscles were contracted ex vivo using different protocols, the suppression of protein synthesis correlated more closely with changes in eEF2 than 4EBP1 phosphorylation. Using a combination of Ca2+ release agents and ATPase inhibitors it was shown that the 60–70% suppression of fast-twitch skeletal muscle protein synthesis during contraction was equally distributed between Ca2+ and energy turnover-related mechanisms. Furthermore, eEF2 kinase (eEF2K) inhibition completely blunted increases in eEF2 phosphorylation and partially blunted (i.e. 30–40%) the suppression of protein synthesis during contractions. The 3- to 5-fold increase in skeletal muscle eEF2 phosphorylation during contractions in situ was rapid and sustained and restricted to working muscle. The increase in eEF2 phosphorylation and eEF2K activation were downstream of Ca2+–calmodulin (CaM) but not other putative activating factors such as a fall in intracellular pH or phosphorylation by protein kinases. Furthermore, blunted protein synthesis and 4EBP1 dephosphorylation were unrelated to AMPK activity during contractions, which was exemplified by normal blunting of protein synthesis during contractions in muscles overexpressing kinase-dead AMPK. In summary, in fast-twitch skeletal muscle, the inhibition of eEF2 activity by phosphorylation downstream of Ca2+–CaM–eEF2K signalling partially contributes to the suppression of protein synthesis during exercise/contractions.
It is recognized that the understanding of the mechanisms controlling skeletal muscle protein turnover may aid in the development of novel therapies to combat skeletal muscle diseases (Rennie, 2005). While the control of protein turnover in skeletal muscle during exercise is poorly understood (Rose & Richter, 2008), it is known that skeletal muscle protein synthesis is blunted during exercise (Pain & Manchester, 1970; Dohm et al. 1982; Dreyer et al. 2006). A recent study (Miranda et al. 2008) demonstrated that contractile activity was a potent stimulus to blunt net protein synthesis rate of rat skeletal muscle ex vivo and can even override the anabolic effect of insulin. Studies by Bylund-Fellenius et al. (1984) using perfused rat hindquarters showed that protein synthesis rates were lower in contracting compared with resting skeletal muscle, particularly in fast-twitch muscles. In that study (Bylund-Fellenius et al. 1984), it was shown that the magnitude of the blunting of protein synthesis was related to the magnitude of changes in high energy phosphagens indicating that the signalling downstream of metabolic stress is a likely mechanism behind the fall in protein synthesis in working muscle. Despite this, the signalling mechanisms responsible for the decrease in skeletal muscle protein synthesis during exercise are not well understood.
While the synthesis of individual proteins is likely to be under the control of specific gene transcription, global protein synthesis is determined by the rate of messenger RNA translation. Translation is conventionally divided into three phases: initiation, elongation and termination controlled by proteins called eukaryotic initiation, elongation and release factors, respectively (Proud, 2007). Recent studies (Rose et al. 2005,2008; Miranda et al. 2008) showed that the phosphorylation of eukaryotic elongation factor 2 at Thr56, which decreases its activity (Ryazanov et al. 1988; Ryazanov & Davydova, 1989; Carlberg et al. 1990; Redpath et al. 1993), increased rapidly in contracting skeletal muscle and it was hypothesized that it is a mechanism by which exercise may blunt skeletal muscle protein synthesis downstream of activated eEF2 kinase. Indeed, eEF2K can be activated by factors such as Ca2+, low pH, protein kinase A and AMP-activated protein kinase (AMPK) (Browne & Proud, 2002) which are all upregulated in skeletal muscle during exercise. On the other hand, Williamson et al. (2006b) demonstrated that there was polyribosome disaggregation in working murine skeletal muscle and provided evidence for a blunting of mRNA translation initiation. In particular, a dephosphorylation of eukaryotic initiation factor 4E(eIF4E)-binding protein 1 (4EBP1), which would bind eIF4E and inhibit initiation by preventing its association with eIF4G (Proud, 2007), was observed in skeletal muscle during exercise (Williamson et al. 2006b; Rose et al. 2008). In addition, cell culture studies consistently showed dephosphorylation of 4EBP1 by several different cellular stressors (Patel et al. 2002). The dephosphorylation of 4EBP1 during exercise was accompanied by changes in mammalian target of rapamycin complex 1 (mTORC1) complex formation and AMPK phosphorylation in skeletal muscle during exercise suggesting that the depression of initiation is mediated, at least in part, by an AMPK–mTORC1–4EBP1 signalling cascade (Williamson et al. 2006b; Rose et al. 2008).
The present series of studies sought to investigate the functional role and regulation of changes in eEF2 and 4EBP1 phosphorylation in contracting rodent skeletal muscle. It was hypothesized that there would be a coordinated regulation of these events by signalling cascades downstream of rises in intracellular [Ca2+] and decreased energy charge via AMPK.
Methods
Materials
All materials were from Sigma-Aldrich (USA) unless stated otherwise.
Animals
Male Sprague–Dawley rats and C57BL/6 mice were used for experimentation. C57BL/6 mice overexpressing a kinase-dead Lys45Arg mutant α2-AMPK protein (KD-AMPK), driven by the heart- and skeletal muscle-specific creatine kinase promoter, have been described previously (Mu et al. 2001) and founder mice were a kind gift from Morris J. Birnbaum (Pennsylvania School of Medicine). Hemizygous transgenic mice and wild-type mice used were littermates from intercross breeding of hemizygous transgenic mice and wild-type mice. The animals were maintained on a 10 h : 14 h light–dark cycle and received standard rodent diet (Altromin no. 1324; Chr. Pedersen, Ringsted, Denmark) and water ad libitum. All experiments were approved by the Danish Animal Experimental Inspectorate and complied with the ‘European Convention for the Protection of Vertebrate Animals Used for Experiments and Other Scientific Purposes’ (council of Europe no. 123, Strasbourg, France, 1985).
In situ experiments
Rats (190–230 g) were anaesthetized by intraperitoneal injection of sodium pentobarbital (5 mg (100 g body wt)–1). In brief, an in situ stimulation protocol was applied as a model of exercise, as described previously (Richter et al. 1987; Rose et al. 2007), to examine the time effect of contractions on signalling proteins. This protocol of muscle contraction was used rather than exercise as it results in recruitment of the entire fibre population of the stimulated muscles, the effect of local versus humoral factors can be accounted for by comparing the stimulated versus the resting contralateral hindlimb muscles, and it allows rapid collection of muscle tissue during the stimulation. Also, the gastrocnemius muscle was sampled as it contains mainly fast-twitch muscle fibres in which protein synthesis has been shown to be blunted by contractions (Bylund-Fellenius et al. 1984) and allows for rapid sampling due to its superficial anatomical location. The sciatic nerve of one hindlimb was electrically stimulated (pulse (0.1 ms) frequency: 100 Hz; 200 ms trains every 2 s) to elicit contractions while the contralateral hindlimb served as a resting control and muscles from different rats were freeze-clamped and resected at rest or at selected time points during stimulation (Rose et al. 2007). A similar procedure was carried out on wild-type and transgenic kinase-dead AMPK mice (n= 5 per group) with the stimulation protocol being 10 min with 200 ms trains every 5 s.
All muscle samples were placed in liquid nitrogen after resection and later stored at −80°C until required. After experimentation, the animals were killed by cervical dislocation while unconscious.
Ex vivo experiments
For these experiments Sprague–Dawley rats (50–65 g) were anaesthetized by intraperitoneal injection of sodium pentobarbital (5 mg (100 g body wt)–1). These small rats were used so that the muscles would be smaller to minimize the diffusion distance in vitro. Excised muscles were placed in incubation chambers (Multi Myograph System organ bath 700MO; Danish Myo Technology A/S, Århus, Denmark) and suspended from the tendons at resting tension. The extensor digitorum longus and epitrochlearis muscles were used for this work as they contain mainly fast-twitch muscle fibres (Nesher et al. 1980; Armstrong & Phelps, 1984) and it is in these types of muscles that protein synthesis has been shown to be blunted by contractions (Pain & Manchester, 1970; Bylund-Fellenius et al. 1984; Miranda et al. 2008). After muscle excision, the rodents were killed by cervical dislocation while unconscious and the muscles were incubated for 40–50 min in Krebs–Ringer–Henseleit buffer containing 5.5 mm glucose, 2 mm sodium pyruvate, 5 mm hydroxyethyl piperazine-ethanesulphonic acid (Hepes) pH 7.4 and 0.1% bovine serum albumin. All incubations were performed at 30°C and 95% O2–5% CO2 was gassed continuously through the incubation buffer. The muscles were then preincubated for 45 min in the buffer with 1 mm leucine.
An experiment was performed to examine the effect of net stimulation time on muscle protein synthesis rate and related signalling. To do this, epitrochlearis muscles were excised from rats and after the preincubation period were stimulated (100 Hz) to contract for 30 min at either 200 ms every 2 s (10% net stimulation time) or 200 ms every 10 s (2% net stimulation time) with the contralateral muscle from each animal serving as the non-stimulated resting control. Importantly, these protocols result in stark differences in metabolic rate as observed by much larger changes in glycogen breakdown, high energy phosphagens and glucose uptake rate (Aslesen et al. 2001). For some muscles (n= 10 per group), protein synthesis was measured over this 30 min incubation (see below) whereas other muscles (n= 5 per group) were rapidly frozen and stored for immunoblot analyses (see below).
Another experiment was conducted to examine the effect of eEF2 kinase inhibition on muscle signalling and protein synthesis rate during contractions. To do this, extensor digitorum longus (EDL) muscles were excised from rats and after the preincubation period were stimulated to contract for 30 min at 200 ms every 2 s with the contralateral muscle from each animal serving as the non-stimulated resting control. Muscles were either preincubated with 1-benzyl-3-cetyl-2-methylimidazolium iodide (NH125), or 2-((3,5-di-tert-butyl-4-hydroxyphenyl)- methylene)-4-cyclopentene-1,3-dione (TX-1123; Calbiochem, UK), or vehicle (0.1% DMSO) for 45 min and stimulated to contract or allowed to rest after a change of equivalent buffer solution. Both of these compounds are somewhat specific and potent inhibitors of eEF2 kinase (Hori et al. 2002; Arora et al. 2003). For the NH125 work, an initial experiment was performed to examine the dose–response of NH125 on muscle signalling (n= 5 per group) and then an additional experiment was performed to examine the effect of NH125 on muscle protein synthesis (n= 10 per group). For the TX-1123 work, both protein synthesis and signalling were measured from lysates prepared from muscle samples from the same experiment (n= 10 per group).
Another experiment was performed in order to assess the relative contribution of Ca2+ and energy turnover on skeletal muscle protein synthesis and signalling during contractions. The aim of this work was to specifically inhibit the major ATPases contributing to ATP turnover in skeletal muscle (Zhang et al. 2006; Barclay et al. 2007,2008) while raising intracellular Ca2+ concentrations from intracellular stores to levels that elicit contraction. To do this, extensor digitorum longus muscles were excised from rats and after the preincubation period were incubated with Krebs–Henseleit buffer containing 5 mm caffeine (a.k.a. 1,3,7-trimethylxanthine) and 100 μm cyclopiazonic acid (CPA) to raise intracellular Ca2+ concentrations (Duke & Steele, 1998; Terada et al. 2003) and elicit sustained contraction (Watt et al. 2003). Both compounds act on the sarcoplasmic reticulum (SR) with caffeine increasing the open probability of the Ca2+ release channel (Herrmann-Frank et al. 1999) and CPA specifically inhibiting the activity of the SR Ca2+-ATPase (SERCA; Seidler et al. 1989; Plenge-Tellechea et al. 1997). Muscles were either preincubated with 75 μmN-benzyl-p-toluene sulfonamide (BTS), a specific inhibitor of myosin-II (Cheung et al. 2002; Shaw et al. 2003; Young et al. 2003; Macdonald et al. 2005; Pinniger et al. 2005), or vehicle (0.2% DMSO) for 60 min prior to changing buffer with or without caffeine and CPA which also contained BTS where appropriate. This concentration of BTS was chosen as preliminary experiments demonstrated no substantial differences in the blunting of tetanic force development of EDL with 50 (93 ± 4%) or 100 (96 ± 5%) μm BTS (n= 3 data not shown). Tension development was recorded at specific times and muscles were carefully taken and rapidly frozen after 15 min and stored at −80°C until required. A separate study was also performed (n= 6 per group) to examine the effect of these protocols on EDL muscle protein synthesis during the period of addition of the Ca2+ release agents. As before, contralateral muscles served as controls but the treatment time was 25 min.
Another experiment was conducted to examine the potential role of AMPK as an upstream signalling intermediate involved in contraction-stimulated blunting of protein synthesis and related signalling. Soleus and EDL muscles from male wild-type (WT) and KD-AMPK mice (Mu et al. 2001; Jensen et al. 2007; n= 6–7 per group; C57BL/6) were excised and stimulated to contract for 25 min at 200 ms every 2 s (pulse frequency: 50 Hz) with the contralateral muscle from each animal serving as the non-stimulated resting control. Muscles were stimulated at 50 Hz, and not 100 Hz, as stimulating at 50 Hz has been shown to produce equal force outputs between WT and KD-AMPK muscles, whereas 100 Hz did not (Lefort et al. 2008). Afterwards muscles were carefully taken and frozen and processed for protein synthesis and immunoblot analysis.
Analytical techniques
The muscle samples were extracted according to Rose et al. (2007). Protein concentration of tissue extracts was determined in triplicate using the bicinchoninic acid (BCA) method using bovine serum albumin (BSA) standards (Pierce Biotech., USA) and BCA assay reagents (Pierce Biotech., USA). A maximal coefficient of variance of 5% was accepted between replicates.
To measure protein expression and phosphorylation, equal amounts of muscle lysate proteins were resolved by SDS-PAGE, transferred to PVDF membrane (Millipore, USA), after which membranes were incubated in a blocking buffer (2% skimmed milk or 3% BSA, pH 7.4) to reduce background signal. The membranes were then incubated with primary and secondary antibodies for optimized times and concentrations, and washed with Tris buffered (pH 7.4) saline containing 0.2% Tween-20 (TBST). Proteins were visualized by chemiluminescence (ECL plus; Amersham Biosciences, UK) and light detection under conditions of negligible ambient light (Kodak Image Station 2000MM, USA). The primary antibodies used were eEF2 (Santa Cruz Biotechnology Inc., USA; sc-13003), anti-phospho-Thr56-eEF2 (Cell Signaling Technology, Inc., USA), anti-eEF2 kinase (US Biological, USA), anti-phospho-Thr172-AMPKα (Cell Signaling Technology, Inc., USA), anti-phospho-Ser221-ACC-β (Cell Signalling Technology, Inc., USA), anti-phospho-Thr-x-Tyr-ERK1/2 (Cell Signaling Technology, Inc., USA) and anti-phospho-Thr-x-Tyr-p38 MAPK (Cell Signaling Technology, Inc., USA). Secondary antibodies were from DakoCytomation (Denmark). Band intensities were quantified by imaging software (Kodak 1D 3.5, USA). Preliminary experiments demonstrated that the amounts of protein loaded were within the dynamic range for the conditions used and the results obtained (data not shown). Preliminary experiments also showed that when tissue proteins were extracted in the absence of phosphatase inhibitors and lysates were incubated for 30 min at 30°C, the band intensities using the phosphospecific antibodies were negligible when compared with an equivalent sample prepared normally (data not shown). All antigens studied migrated at expected molecular weights.
eEF2 kinase activity was measured according to Rose et al. (2005). Briefly, eEF2 kinase was immunoprecipitated from 50 μg of lysate protein by incubating with 3 μg of eEF2 kinase antibodies (US Biological, USA) and the immune complex was assayed in a buffer containing 1 μg eEF2 (purified from HeLa cells; S. G. Finn, University of Dundee, UK) and 100 μm ATP (1.0 Ci mmol−1 5′[γ32P]ATP; Amersham Biosciences, UK), 12 μm calmodulin (Upstate Biotechnology, USA) with or without 1.2 mm CaCl2. After 10 min at 30°C the reactions were stopped and subjected to SDS-PAGE followed by gel drying and autoradiography (Molecular Dynamics, USA). Samples on each gel were adjusted to an aliquot of an internal control reaction (i.e. immunopurification from rat heart extract).
Muscle metabolite concentrations were measured fluorometrically using standard techniques (Lowry & Passonneu, 1972). Muscle pH (Sahlin et al. 1975; Dudley & Terjung, 1985) and free AMP concentrations (Lawson & Veech, 1979) were estimated based upon calculations previously validated.
Muscle protein synthesis rate was measured over a 30 min incubation period in buffer solution containing 1 mm leucine with added 0.5 μCi ml−1[U-14C]leucine (specific radioactivity 300 mCi mmol−1, NEN, USA) as described in detail previously (Miranda et al. 2008). In brief, after incubation muscles were washed free of contaminating buffer, frozen and stored. Muscles were then homogenized and homogenate proteins were precipitated, washed, resolubilized and then counted using liquid scintillation counting.
Calculations and statistics
Statistical testing was done with descriptive analyses (MS-Excel), t tests (MS-Excel) or one-way or two-way
ANOVA with repeated measures, and post hoc testing performed when differences were significant as appropriate (Sigma.Stat v.3.5 and SPSS v.16). Data are expressed as mean ±s.e.m. and differences were considered to be significant when P < 0.05.
Results
Blunting of muscle protein synthesis with contraction correlates with changes in eEF2 phosphorylation
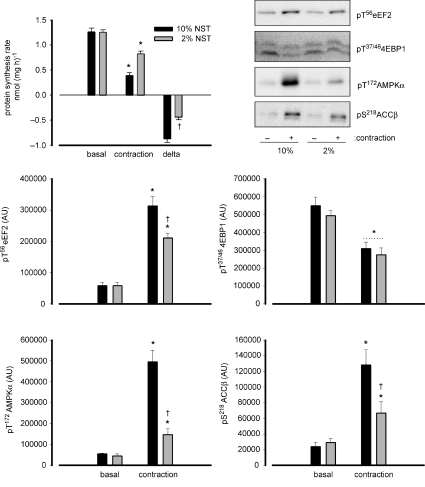
To examine the potential role of contraction intensity in the blunting of skeletal muscle protein synthesis with contractions, a study was performed to examine the effect of net stimulation time (NST) of contractions. As can be seen in Fig. 1, there was a 69 ± 3% reduction of muscle protein synthesis rate with contractions of 10% NST whereas there was an ∼50% lower reduction (i.e. 34 ± 3%) with 2% NST. Similar to the lower blunting of protein synthesis, there was a lower increase in eEF2 (∼25%), AMPK α-subunit (∼80%) and ACCβ phosphorylation (∼50%) with 2%versus 10% NST. In contrast, there was a similar magnitude of decrease in 4EBP1 phosphorylation (∼50%vs. basal) with 10 and 2% NST. Of note, there were no differences in eEF2 expression between groups (data not shown).
Figure 1. Effect of net stimulation time of contractile stimulation on the blunting of protein synthesis rate and associated signalling in rat skeletal muscle ex vivo.
Excised rat epitrochlearis muscles were incubated at rest (basal) or electrically stimulated to contract ex vivo (contraction) at 2% (200 ms trains every 10 s) or 10% (200 ms trains every 2 s) net stimulation time (NST). Protein synthesis rate was measured in muscles over a 30 min period of rest or stimulation. delta = difference between basal and contraction values. Data are mean ±s.e.m., n= 10; *P < 0.01 vs. basal. Samples from basal or contracted muscles were frozen rapidly after stimulation, processed and immunoblotted for phospho-Thr56 eukaryotic elongation factor 2 (eEF2), phospho-Thr37/46 eIF4 binding protein 1 (4EBP1), phospho-Thr172-AMP-activated protein kinase (AMPK) α-subunit and phospho-Ser218-acetyl-CoA-carboxylase-β (ACCβ). Data are mean ±s.e.m., n= 5; *P < 0.05 vs. basal; †P < 0.05 vs. 10%. Representative immunoblots are shown.
Changes in eEF2 phosphorylation and protein synthesis with contraction are blunted by eEF2 kinase inhibition
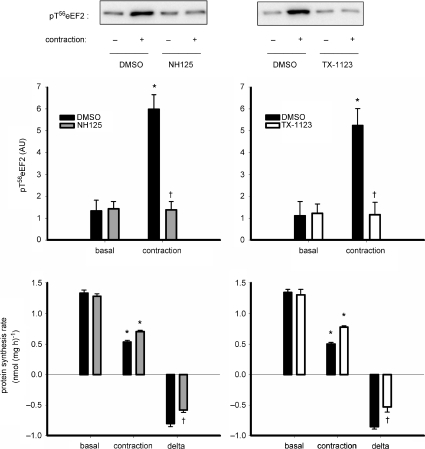
To examine the role and regulation of eEF2 phosphorylation with contractions experiments were performed to examine the effect of the eEF2 kinase inhibitors on skeletal muscle protein synthesis and eEF2 phosphorylation. As shown in Fig. 2 (upper panels), there was a complete blunting of the increase in eEF2 phosphorylation with contractions with 10 μm NH125 and 10 μm TX-1123. Of note, neither AMPK nor 4EBP1 phosphorylation was affected by addition of either inhibitor and there were no differences in eEF2 expression between groups (data not shown). Concerning skeletal muscle protein synthesis, as shown in Fig. 2 (lower panels), there was an ∼28% lower suppression of protein synthesis rate with 10 μm NH125 (−45 ± 2%) versus vehicle (−60 ± 2%). There was an ∼38% lower suppression with 10 μm TX-1123 (–39 ± 3%) versus vehicle (−63 ± 2%).
Figure 2. Inhibition of eEF2 kinase blunts contraction-stimulated changes in skeletal muscle eEF2 phosphorylation and protein synthesis.
Top panels: excised rat extensor digitorum longus muscles were preincubated ex vivo with and without (DMSO) of eEF2 kinase inhibitors 1-benzyl-3-cetyl-2-methylimidazolium iodide (NH125; left panel) or 2-((3,5-di-tert-butyl-4-hydroxyphenyl)-methylene)-4-cyclopentene-1,3-dione (TX-1123), and basal or contracted muscles were processed and immunoblotted for phospho-Thr56 eukaryotic elongation factor 2 (eEF2). Data are mean ±s.e.m., n= 6 (NH125), n= 10 (TX-1123); *P < 0.001 vs. basal; †P < 0.001 vs. NH125 or TX-1123. Representative immunoblots are shown. Bottom panels: protein synthesis rate was measured in muscles over a 30 min period of rest or stimulation with or without preincubation of 10 μm NH125 or 10 μm TX-1123. delta = difference between basal and contraction values. AU, arbitrary units. Data are mean ±s.e.m., n= 10; *P < 0.01 vs. basal; †P < 0.01 vs. DMSO.
Effects of in situ contractions on skeletal muscle phosphoproteins and metabolites
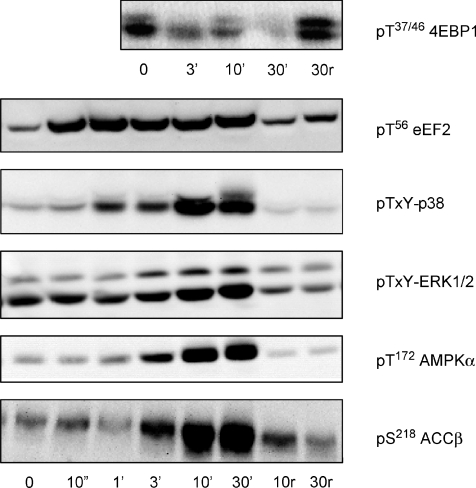
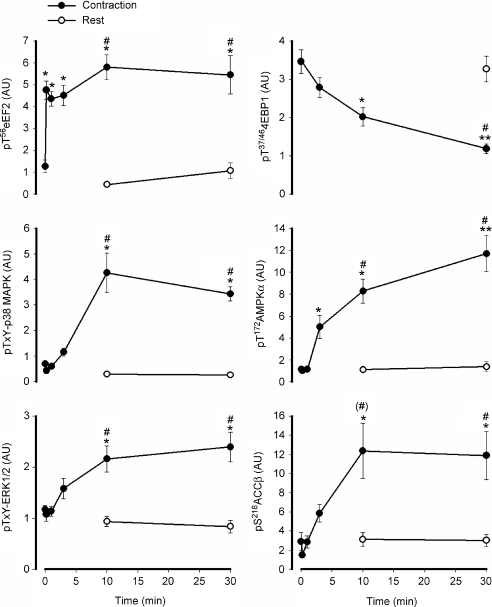
In response to in situ contractions (representative blots shown in Fig. 3), there was a rapid and sustained 4- to 5-fold increase in eEF2 phosphorylation, with this increase occurring after just 10 s (i.e. 5 trains) of stimulation (Fig. 4). In contrast, there was a relatively slow and progressive decrease in 4EBP1 phosphorylation with contractions, with no changes detected at 3 min, but significantly lower levels at 10 and 30 min of contractions (Fig. 4). The increase in AMPK α-subunit phosphorylation was relatively slow and progressive with a 4- to 8-fold increase in AMPK-α phosphorylation at 3 and 10 min and a further increase (i.e. ∼10-fold above basal) at 30 min. A similar response occurred with ACCβ phosphorylation, with an ∼6-fold increase at 10 and 30 min of contractions. The mitogen-activated protein kinases also showed a similar activation pattern, with ∼1- and 6-fold increases in ERK1/2 and p38 phosphorylation respectively, at both 10 and 30 min of contractions. Importantly, no changes were detected in any of the phosphoproteins in the resting contralateral muscle during stimulation (Fig. 4). There were no differences in eEF2 expression between groups (data not shown).
Figure 3. Time effect of in situ contractions on phosphoproteins of rat gastrocnemius skeletal muscle.
Rat gastrocnemius muscle samples before and during in situ contractions were processed and immunoblotted for phospho-Thr56 eukaryotic elongation factor 2 (eEF2), phospho-Thr37/46 eIF4-binding protein 1 (4EBP1), phospho-Thr-X-Tyr-p38 mitogen-activated protein kinase (p38-MAPK), phospho-Thr-X-Tyr-extracellular regulated protein kinase 1/2 (ERK1/2), phospho-Thr172-AMP-activated protein kinase (AMPK) α-subunit and phospho-Ser218-acetyl-CoA-carboxylase-β (ACCβ). r, samples of the resting muscle from the contralateral limb during stimulation. Representative immunoblots are shown.
Figure 4. Time effect of in situ contractions on phosphoproteins of rat gastrocnemius skeletal muscle.
Rat gastrocnemius muscle samples before and during in situ contractions were processed and immunoblotted for phospho-Thr56 eukaryotic elongation factor 2 (eEF2), phospho-Thr37/46 eIF4 binding protein 1 (4EBP1), phospho-Thr-X-Tyr-p38 mitogen-activated protein kinase (p38-MAPK), phospho-Thr-X-Tyr-extracellular regulated protein kinase 1/2 (ERK1/2), phospho-Thr172-AMP-activated protein kinase (AMPK) α-subunit and phospho-Ser218-acetyl-CoA-carboxylase-β (ACCβ). •, samples from stimulated muscles (contraction); ○, samples of the resting (Rest) muscle from the contralateral limb during stimulation. AU, arbitrary units. Data are mean ±s.e.m. from 8–11 samples. *Different from time 0, P < 0.05. **Different from all other times, P < 0.05. #Different from rest at corresponding time, P < 0.05. (#)Borderline different from rest at corresponding time, P= 0.06.
The effect of in situ stimulation on skeletal muscle metabolites is shown in Table 1. Of note, there was a progressive decline in glycogen concentration until 10 min after which there was no change. Also of note, there was a significant decline in pH after 1 min, but not 10 s, of contraction which was maintained throughout the remaining 29 min. Importantly, no changes were detected in any of the metabolites in the resting contralateral muscle during stimulation (Table 1).
Table 1.
Time effect of in situ contractions on metabolites of rat gastrocnemius skeletal muscle
| 0 | 10 s | 1 min | 3 min | 10 min | 30 min | 10 min r | 30 min r | |
|---|---|---|---|---|---|---|---|---|
| Glycogen (mmol (kg ww)−1) | 33.7 ± 1.6 | 33.9 ± 1.2 | 29.7 ± 1.9 | 18.5 ± 0.9* | 9.7 ± 0.7**† | 7.6 ± 0.3**† | 35.3 ± 2.2 | 38.2 ± 2.0 |
| Lactate (mmol (kg ww)−1) | 2.3 ± 0.3 | 3.3 ± 0.3 | 9.9 ± 1.0* | 15.5 ± 1.5** | 11.6 ± 0.8*† | 4.5 ± 0.7*† | 1.2 ± 0.2 | 1.0 ± 0.1 |
| PCr (mmol (kg ww)−1) | 14.5 ± 1.8 | 10.7 ± 0.5 | 5.2 ± 0.8* | 2.7 ± 0.5** | 2.9 ± 0.6**† | 4.1 ± 0.4**† | 17.0 ± 1.7 | 18.0 ± 1.7 |
| PCr:TCr (%) | 60 ± 10 | 37 ± 4* | 18 ± 8** | 11 ± 7** | 11 ± 7**† | 16 ± 5**† | 62 ± 7 | 59 ± 9 |
| ATP (mmol (kg ww)−1) | 3.97 ± 0.36 | 3.95 ± 0.11 | 4.56 ± 0.37 | 2.94 ± 0.40 | 1.89 ± 0.19*† | 2.44 ± 0.18*† | 3.94 ± 0.46 | 4.36 ± 0.25 |
| AMPf (nmol (kg ww)−1) | 0.07 ± 0.02 | 0.38 ± 0.08 | 2.11 ± 0.61* | 2.72 ± 1.21* | 3.71 ± 1.17*† | 2.72 ± 0.90*† | 0.06 ± 0.01 | 0.10 ± 0.02 |
| AMPf:ATP × 10−2 (%) | 0.2 ± 0.2 | 1.0 ± 0.4 | 4.8 ± 0.4* | 6.4 ± 0.4* | 17.1 ± 2.0*† | 8.2 ± 0.6*† | 0.2 ± 0.1 | 0.2 ± 0.1 |
| pH | 7.01 ± 0.01 | 6.99 ± 0.01 | 6.84 ± 0.02* | 6.71 ± 0.03* | 6.80 ± 0.02*† | 6.96 ± 0.02*† | 7.03 ± 0.00 | 7.04 ± 0.00 |
Rat gastrocnemius muscle samples before and during in situ contractions were processed and analysed for glycogen, lactate, phosphocreatine (PCr) and adenosine triphosphate (ATP). Total creatine (TCr), free adenosine monophosphate (AMPf) and pH were calculated (see Methods). r = samples of the resting muscle from the contralateral limb during stimulation. ww = wet weight. Data are mean ±s.e.m. from 8–11 samples.
Different from time 0, P < 0.05.
Different from all preceding times, P < 0.05.
Different from resting contralateral muscle (r) at corresponding time, P < 0.05.
In vitro eEF2 kinase activity
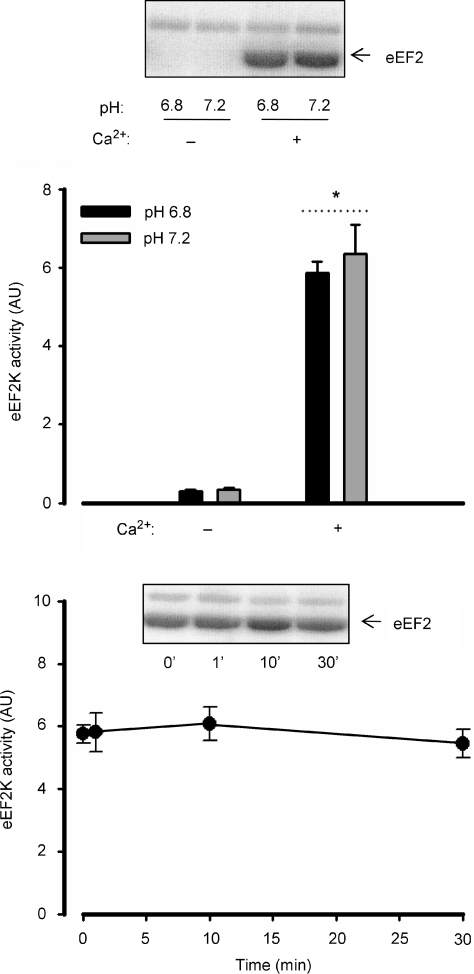
As shown in the upper panel of Fig. 5, there was a much higher skeletal muscle eEF2 kinase activity when Ca2+ was added to the assay medium when measured in vitro. However, there was no difference in skeletal muscle eEF2 kinase activity in vitro when assaying at pH 6.8 versus pH 7.2 (Fig. 5, upper panel). There was no difference in skeletal muscle eEF2 kinase activity between rested and contracted muscle at any time point measured (Fig. 5, lower panel).
Figure 5. Effects of Ca2+, pH and contraction on in vitro eEF2 kinase activity.
Top panel: eukaryotic elongation factor 2 (eEF2) kinase was immunoprecipitated from rat skeletal muscle samples and activity was measured in vitro in the absence and presence of Ca2+ at pH 7.2 or 6.8. Data are mean ±s.e.m. from 3 samples. *Main effect of Ca2+, P < 0.01. Bottom panel: rat gastrocnemius muscle samples before and during in situ contractions were processed and immunoprecipitated eEF2 kinase activity was measured in vitro. AU, arbitrary units. Data are mean ±s.e.m. from 5 samples.
Influence of increased intracellular Ca2+ and subsequent energy turnover on skeletal muscle protein synthesis and related signalling
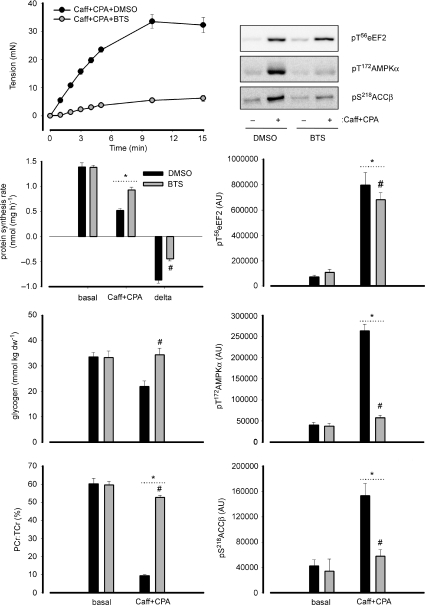
As shown in Fig. 6, upon the addition of caffeine and cyclopiazonic acid (CPA), there was a progressive increase in tension development until 5–10 min after which it levelled off at 30–35 mN. Incubation with BTS blunted this effect by ∼85%, with an increase of about 4–5 mN by 15 min. The contraction elicited by the addition of caffeine and CPA blunted protein synthesis in EDL muscles by 63 ± 2%, and this was 48% lower with the addition of BTS which resulted in a 33 ± 2% blunting compared with basal (Fig. 6).
Figure 6. Influence of increased intracellular Ca2+ and subsequent energy turnover on skeletal muscle protein synthesis and related signalling.
Isolated rat extensor digitorum longus muscles were incubated ex vivo with 5 mm caffeine (Caff) and 100 μm cyclopiazonic acid (CPA) to induce Ca2+ release from the sarcoplasmic reticulum. Some muscles were preincubated with N-benzyl-p-toluene sulfonamide (BTS) to inhibit myosin-II and subsequently prevent tension development and energy turnover by this process, or vehicle (DMSO). The resulting changes in tension development are shown (n= 5; top panel). Protein synthesis rate was measured in a subset of muscles under basal conditions as well as Caff + CPA treatment with or without BTS. delta = difference between basal and Caff + CPA values. Data are mean ±s.e.m., n= 5; *P < 0.05 vs. basal; #P < 0.05 vs. DMSO. In another subset, samples from basal or Caff + CPA muscles were frozen rapidly after treatment, processed and immunoblotted for phospho-Thr56 eukaryotic elongation factor 2 (eEF2), phospho-Thr37/46 eIF4-binding protein 1 (4EBP1), phospho-Thr172-AMP activated protein kinase (AMPK) α-subunit and phospho-Ser218-acetyl-CoA-carboxylase-β (ACCβ). In addition, glycogen and lactate concentrations were measured. AU, arbitrary units. Data are mean ±s.e.m., n= 5; *P < 0.05 vs. basal; #P < 0.05 vs. DMSO. Representative immunoblots are shown in the top, right panel.
As shown in Fig. 6 and Table 2, there was a marked increase in energy turnover as evident by differences in muscle glycogen and high energy phosphagen concentrations, which were largely blunted by the addition of BTS. In addition, the AMPK α-subunit and ACCβ phosphorylation were ∼10-fold and ∼4-fold, respectively, higher than basal with caffeine (Caff) + CPA, and these were blunted by ∼90% and ∼75%, respectively, with BTS. On the otherhand, there was an ∼10-fold higher eEF2 phosphorylation in muscles treated with Caff + CPA, which was only ∼10% lower with the addition of BTS.
Table 2.
Influence of increased intracellular Ca2+ and subsequent energy turnover on skeletal muscle metabolites
| Basal |
Caff + CPA |
|||
|---|---|---|---|---|
| DMSO | BTS | DMSO | BTS | |
| PCr (mmol (kg ww)−1) | 16.4 ± 1.0 | 15.2 ± 1.0 | 2.5 ± 0.2* | 13.6 ± 0.5*† |
| ATP (mmol (kg ww)−1) | 3.90 ± 0.3 | 4.03 ± 0.3 | 1.64 ± 0.4* | 3.96 ± 0.3† |
| AMPf (mmol (kg ww)−1) | 0.13 ± 0.4 | 0.16 ± 0.4 | 1.58 ± 1.4* | 0.32 ± 0.7*† |
| AMPf:ATP × 10−2 (%) | 0.3 ± 0.2 | 0.4 ± 0.2 | 7.1 ± 1.2* | 0.7 ± 0.2*† |
| pH | 7.05 ± 0.01 | 7.04 ± 0.01 | 6.83 ± 0.03* | 7.04 ± 0.01† |
Isolated rat extensor digitorum longus muscles were incubated ex vivo with 5 mm caffeine (Caff) and 100 μm cyclopiazonic acid (CPA) to induce Ca2+ release from the sarcoplasmic reticulum and elicit contraction. Some muscles were preincubated with 75 μmN-benzyl-p-toluene sulfonamide (BTS) to inhibit myosin-II and subsequently prevent tension development and energy turnover by this process, or vehicle (DMSO). Samples from basal or Caff + CPA muscles were frozen rapidly after treatment, processed and analysed for phosphocreatine (PCr) and adenosine triphosphate (ATP). Total creatine (TCr), free adenosine monophosphate (AMPf) and pH were calculated (see Methods). ww, wet weight. Data are mean ±s.e.m., n= 5; *
P < 0.05 vs. basal.
P< 0.05 vs. DMSO.
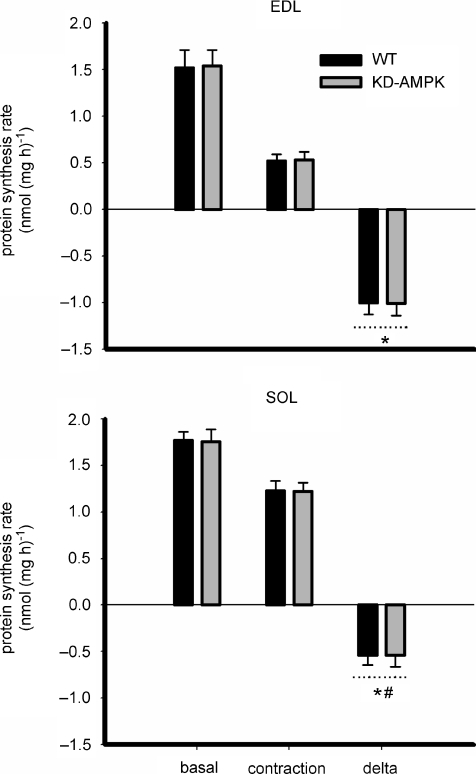
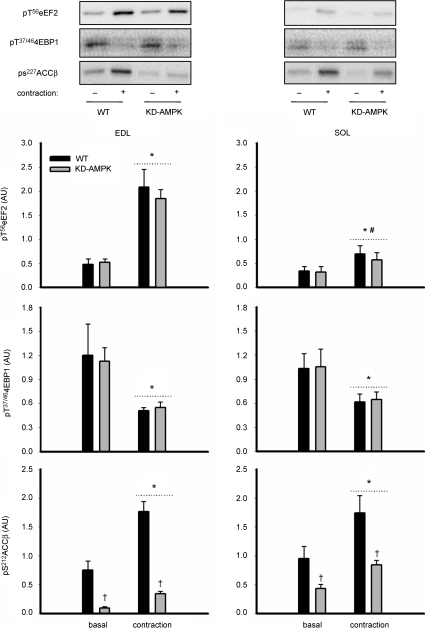
To examine the precise role of AMPK in the regulation of changes in protein synthesis and related signalling with contraction the muscle-specific KD-AMPK mice were used. As can be seen in Fig. 7, there were no differences in basal protein synthesis or ex vivo contraction-induced blunting of protein synthesis in EDL (WT: −65 ± 5%, KD-AMPK: −65 ± 3%; P < 0.1) or soleus (WT: −30 ± 6%, KD-AMPK: −29 ± 6%; P < 0.1) muscles. There were no differences in basal or contraction-induced changes in either eEF2 or 4EBP1 phosphorylation between muscles of WT and KD-AMPK mice, despite clear differences in ACCβ phosphorylation between genotypes (Fig. 8). However, there was an ∼15% higher basal and lower (∼50%) contraction-induced blunting of protein synthesis in soleus compared with EDL muscle irrespective of genotype. There was a lower basal (∼30%; P < 0.05) and contraction-induced increase (P < 0.01) in eEF2 phosphorylation in SOL (∼1-fold increase) versus EDL (∼3-fold increase) muscles. There were no differences in eEF2 expression between genotypes or muscle types (P= 0.3, data not shown), but there was an ∼3-fold higher (P < 0.01, n= 6–7) expression of eEF2 kinase in murine EDL (3.1 ± 0.4 arbitrary units (AU)) versus soleus (1.3 ± 0.2 AU), regardless of genotype. There was no difference in muscle eEF2K expression between genotypes (data not shown). Similar results for eEF2, 4EBP1 and ACCβ phosphorylation were found when gastrocnemius muscles from wild-type and transgenic animals were stimulated to contract in situ (data not shown).
Figure 7. Inhibition of AMPK does not influence contraction-stimulated changes in skeletal muscle protein synthesis.
Protein synthesis was measured in isolated mouse extensor digitorum longus (EDL) and soleus (SOL) muscles from wild-type (WT) and muscle-specific kinase-defective AMP-activated protein kinase overexpression (KD-AMPK) mice at rest (basal) or during contractions (contraction). delta = difference between basal and contraction values. Data are mean ±s.e.m., n= 6–7 (EDL) and n= 7 (SOL). *Main effect of treatment, P < 0.005 vs. basal. #Difference between muscle types, P < 0.05.
Figure 8. Inhibition of AMPK does not influence contraction-stimulated changes in skeletal muscle eEF2 and 4EBP1 phosphorylation.
Isolated mouse extensor digitorum longus (EDL) and soleus (SOL) muscles from wild-type (WT) and muscle-specific kinase-defective AMP-activated protein kinase overexpression (KD-AMPK) mice at rest (basal) or after contractions (contraction) were processed and immunoblotted for phospho-Thr56 eukaryotic elongation factor 2 (eEF2), phospho-Thr37/46 eIF4E-binding protein 1 (4EBP1) and phospho-Ser212-acetyl-CoA-carboxylase-β (ACCβ). AU, arbitrary units. Data are mean ±s.e.m., n= 6–7 (EDL) and n= 7 (SOL). *Main effect of treatment, P < 0.05 vs. basal. †Main effect of genotype, P < 0.05. #Difference between muscle types, P < 0.05. Representative immunoblots are shown.
Discussion
The results provide evidence that contraction-stimulated suppression of skeletal muscle protein synthesis rate is partially mediated by a Ca2+–calmodulin–eEF2K–eEF2 signalling cascade. Earlier work examining this showed that in the perfused rat hindlimb, protein synthesis rates were lower in contracting compared with resting skeletal muscles, particularly in fast-twitch muscles (Bylund-Fellenius et al. 1984). In that study (Bylund-Fellenius et al. 1984), it was shown that the magnitude of the blunting of protein synthesis was related to the magnitude of changes in high energy phosphagens indicating that signalling downstream of metabolic stress is a likely mechanism behind the fall in protein synthesis in working muscle. However, that study (Bylund-Fellenius et al. 1984) examined different skeletal muscles which varied in their ability to buffer changes in intracellular energy charge homeostasis to observe this effect. Here, using a combination of Ca2+ release agents and ATPase inhibitors, the 60–65% suppression of fast-twitch skeletal muscle protein synthesis during contraction is equally distributed between Ca2+ and energy turnover-related mechanisms (Fig. 6). In particular, protein synthesis was blunted by 30–35% during contractions, even when energy turnover was blocked (Fig. 6), indicating that Ca2+ signalling contributes substantially, which was not surprising since others have shown potent effects of Ca2+ on blunting cellular protein synthesis (Nairn et al. 2001). In addition, when rat epitrochlearis muscles were contracted ex vivo at 10%versus 2% net stimulation time (NST), which results in starkly different changes in metabolic rate (Aslesen et al. 2001), there was an ∼50% lower suppression of protein synthesis rate with 2%versus 10% NST (Fig. 1). Importantly, the ∼70% suppression of muscle protein synthesis with ex vivo contractions of 10% NST compared with basal is similar to what has been shown before (Miranda et al. 2008). Of the mRNA translational machinery, the changes in eEF2 but not 4EBP1 phosphorylation matched the changes in protein synthesis rate with these two situations, with an ∼33% lower increase in eEF2 phosphorylation with contractions of 2%versus 10% NST. Although correlative, this suggests that the increase in eEF2 phosphorylation may be responsible for the suppression of skeletal muscle protein synthesis during contractions given the well-described effect of phosphorylation of eEF2 depressing its activity mediating an inhibition of mRNA translation elongation (Ryazanov & Davydova, 1989; Carlberg et al. 1990; Redpath et al. 1993).
Next, the regulation and role of eEF2 phosphorylation was examined. Conceivably, the increase in skeletal muscle eEF2 phosphorylation with contractions could result from increased activity of the upstream eEF2 kinase or decreased activity of the upstream eEF2 phosphatase (i.e. PP2A), or a combination of both (Browne & Proud, 2002). Earlier work suggested that eEF2 kinase was the upstream effector of eEF2 phosphorylation in working skeletal muscle (Rose et al. 2005) and heart (Horman et al. 2003) but neither study could rule out the effect of upstream phosphatase activity. To examine this, isolated EDL muscles were contracted in the absence or presence of the recently described eEF2 kinase inhibitors NH125 (Arora et al. 2003) and TX-1123 (Hori et al. 2002). As shown in Fig. 2, treatment of muscles with 10 μm NH125 or TX-1123 completely blunted the contraction-induced increase in eEF2 phosphorylation, indicating that it is indeed activation of eEF2 kinase mediating this effect.
When isolated EDL muscles were contracted in the presence of 10 μm NH125 or TX-1123, the magnitude of suppression of protein synthesis was 30–40% less indicating that eEF2 phosphorylation by eEF2 kinase partially contributes to this process. The effect of the non-specific (Gschwendt et al. 1994; Davies et al. 2000) eEF2 kinase inhibitor rottlerin was also tested, but this resulted in a decrease in basal muscle protein synthesis (data not shown) and hence results from this work were non-conclusive. Nevertheless, these (Fig. 2) are the first data to describe a functional role of eEF2K–eEF2 signalling in skeletal muscle. Of note, the magnitude of the inhibition of the suppression of protein synthesis was incomplete with eEF2K blockade, indicating that there are other signalling events involved. Indeed, regulation of mRNA translation is a complex biochemical process involving many proteins (Proud, 2007) and other studies have shown that cellular stressors can affect protein synthesis independently of changes in eEF2 phosphorylation (Laitisus et al. 1998; Patel et al. 2002). In fairness it should be noted that increased skeletal eEF2 phosphorylation during contractile activity is not a universal observation (Rose & Richter, 2008), with other studies reporting no differences with resistance-type exercise despite a blunting of protein synthesis (Dreyer et al. 2006), highlighting that there are other mechanisms involved. Indeed, a study of running mice has shown that there is polyribosome disaggregation in working skeletal muscle (Williamson et al. 2006b) and there are several lines of evidence that contractions can blunt mRNA translation initiation enzymes via suppression of mTORC1 activity and downstream targets (present study; Atherton et al. 2005; Williamson et al. 2006b; Miranda et al. 2008; Rose et al. 2008). Clearly, further work is required to define the role of other mRNA translational mechanisms and associated signalling pathways involved in the suppression of skeletal muscle protein synthesis during exercise.
Given that there is a functional role for eEF2 kinase in working skeletal muscle the understanding of the regulation of this kinase is important. In particular, many factors which activate eEF2K such as a rise in intracellular Ca2+ (Nairn & Palfrey, 1987; Ryazanov 1987; Laitisus et al. 1998; Nairn et al. 2001) a fall in pH (Dorovkov et al. 2002) as well as AMPK (Horman et al. 2002,2003; Browne et al. 2004; Williamson et al. 2006a) and protein kinase A (Redpath & Proud, 1993; Diggle et al. 2001; McLeod et al. 2001) activity, are known to be upregulated in contracting skeletal muscle during exercise (Dudley & Terjung, 1985; Melzer et al. 1995; Winder & Hardie, 1996; Wojtaszewski et al. 2000; Rose et al. 2005; Williamson et al. 2006b). To examine this, an in situ nerve-induced contraction protocol was used in an attempt to resolve the likely factors contributing to eEF2K activation during exercise. As shown in Fig. 4, the increase in eEF2 phosphorylation was restricted to contracting muscle during stimulation and given that contractions ex vivo also result in increased skeletal muscle eEF2 phosphorylation (present study; Atherton et al. 2005; Miranda et al. 2008), eEF2K activation is mediated by local factors within contracting muscle and not by humoral factors. There are several lines of evidence that the activation mechanism is likely to be via Ca2+–calmodulin. Firstly, as shown previously (Rose et al. 2005; Miranda et al. 2008), the increase in eEF2 phosphorylation was rapid and sustained (Fig. 4) indicating that eEF2K activity was activated rapidly and this activation was continuous throughout the stimulation period. As a rise in intracellular Ca2+ is a pivotal event in excitation–contraction coupling (Melzer et al. 1995), Ca2+ signalling should be activated rapidly as is the case for eEF2 phosphorylation. Secondly, as shown previously for humans (Rose et al. 2005), in vitro activity of eEF2K immunopurified from rat skeletal muscle was only detectable when Ca2+ was present in the assay medium (Fig. 5). Thirdly, increases in intracellular Ca2+ that elicit contractions while not drastically disturbing other putative activating factors (i.e. pH and AMPK) results in near normal increases in eEF2 phosphorylation (Fig. 6). Altogether, these results show that eEF2K activation is largely dependent on a Ca2+-dependent mechanism which is not surprising since eEF2K is a Ca2+–calmodulin-activated kinase (Nairn & Palfrey, 1987; Ryazanov 1987).
A study has shown that a fall in intracellular pH below 7.0 may be an additive mechanism for Ca2+-induced activation of eEF2K (Dorovkov et al. 2002). However, while typical decreases (Dudley & Terjung, 1985) in intracellular pH were observed during contractions (Tables 1 and 2), the increases in eEF2 phosphorylation did not correlate with these changes (Figs 4 and 6). In particular, the increase in eEF2 phosphorylation occurred at 10 s of contractions (Fig. 4) a time at which no significant change in muscle pH was detected. Furthermore, there were no differences in skeletal muscle eEF2K activity when measured at pH 7.2 or 6.8 in vitro (Fig. 5). However, even though the fall in muscle pH may not affect eEF2K–eEF2 during contractions, it may still affect other steps of mRNA translation as discussed by Dorovkov et al. (2002).
Similar to other studies (Rose et al. 2005; Miranda et al. 2008), there was no effect of in situ contractions on skeletal muscle eEF2K activity when measured in vitro (Fig. 5). This demonstrates that there was no net change of eEF2K phosphorylation with contractions. Indeed, in contrast to the rapid and sustained eEF2 phosphorylation with contractions, there was a relatively slow and progressive increase in change in intracellular AMPK activity, as indicated by AMPK α-subunit and ACCβ phosphorylation (Fig. 4). Given that there are several lines of evidence indicating a role for AMPK in regulating protein translation inhibition (Fig. 1; Horman et al. 2002; Bolster et al. 2002; Browne et al. 2004; Williamson et al. 2006a; Deshmukh et al. 2008) this was investigated more carefully. In particular, increases in intracellular Ca2+ that elicit contractions while largely blocking the resulting ATP turnover substantially blunted the increase in AMPK activity without largely influencing the increase in eEF2 phosphorylation (Fig. 6).
There is a strong hypothesis that AMPK regulates the blunting of protein synthesis during contractions. Indeed, there are many lines of evidence indicating that energy charge can regulate cellular protein synthesis rate (Figs 1 and 6; Bylund-Fellenius et al. 1984; Laitisus et al. 1998; Horman et al. 2003) and that this could involve AMPK (Figs 1 and 6), perhaps by phosphorylating eEF2K (Horman et al. 2002; Browne et al. 2004; Williamson et al. 2006a) or decreasing mTORC1 activity (Bolster et al. 2002; Williamson et al. 2006b; Deshmukh et al. 2008). Thus, the role of AMPK on skeletal muscle protein synthesis and related signalling during contraction was also directly investigated. Contrary to the hypothesis, the suppression of protein synthesis in soleus and EDL muscles was not different when comparing muscles of mice overexpressing an inactive form of AMPKα2 with the corresponding wild-type mice. Furthermore, the increases in eEF2 phosphorylation were normal with in situ (data not shown) and ex vivo (Fig. 8) contractions in skeletal muscle of mice overexpressing a kinase-defective form of AMPKα2, which is in line with prior correlative evidence of a dissociation between eEF2 phosphorylation and AMPK activity during contractions (Figs 1, 4 and 6; Rose et al. 2005; Miranda et al. 2008; Rose et al. 2008). On the other hand, although the changes in muscle 4EBP1 and AMPK activity during exercise seem to correlate (Fig. 4; Williamson et al. 2006b; Rose et al. 2008) and other studies have suggested that the suppression of mTOR and subsequent 4EBP1 dephosphorylation is mediated by AMPK (Bolster et al. 2002; Williamson et al. 2006b; Deshmukh et al. 2008) during contractions, the present study shows no relationship between changes in AMPK and 4EBP1 phosphorylation with contractions (Figs 1 and 8). Importantly, a prior study (Lefort et al. 2008) has shown that unlike wild-type muscles, there was no activation of either α1 or α2 AMPK complexes in AMPK-KD muscles during stimulation. Taken together, these data suggest that AMPK does not regulate either eEF2 or 4EBP1 phosphorylation or the suppression of protein synthesis during contractions. However, given that there are many lines of evidence indicating that energy charge can regulate cellular protein synthesis rate (Figs 1 and 6; Bylund-Fellenius et al. 1984; Laitisus et al. 1998; Horman et al. 2003) it may be that a signalling mechanism downstream of altered energy charge could negatively regulate the mRNA translation machinery and further studies are warranted to investigate this.
Lastly, differences between muscles types were observed in that the magnitude of the suppression of protein synthesis was lower in slow-twitch soleus muscle compared with fast-twitch EDL muscle (Fig. 7). This is in agreement with a previous study which showed that the protein synthesis was selectively suppressed in fast-twitch but not slow-twitch skeletal muscles during contractions in situ (Bylund-Fellenius et al. 1984). This may be explained by differences in energy turnover-related signalling to translational control enzymes (see earlier discussion) as it is a well-known phenomenon that slow-twitch muscles are better at maintaining energy-charge homeostasis during contractions. On the other hand, it may also be explained by the evidence that there was a lower magnitude of increase in eEF2 phosphorylation in slow-twitch soleus versus fast-twitch EDL mouse muscles during contractions; given that eEF2 phosphorylation is partially involved in the suppression of protein synthesis with contractions (Fig. 2). The lower magnitude of increase in eEF2 phosphorylation in contracting soleus versus EDL is probably the result of a lower expression of eEF2 kinase in murine soleus versus EDL muscles (see Results).
In summary, in fast-twitch skeletal muscles, signalling downstream of Ca2+ and energy-turnover is involved in the suppression of protein synthesis during contractions. While AMPK signalling is not involved, the inhibition of eEF2 activity by phosphorylation downstream of Ca2+–CaM–eEF2K signalling partially contributes to the suppression of protein synthesis during exercise/contractile activity.
Acknowledgments
The authors acknowledge Betina Bolmgren and Ida Ingvaldsen for skilled technical assistance and Dr Peter Schjerling (Copenhagen, Denmark) for mouse genotyping. Gratitude is extended to Professor Christopher Proud and Stephen Finn (University of Dundee, UK) for the provision of purified eEF2 and to Morris Birnbaum (Pennsylvania School of Medicine, USA) for provision of the muscle-specific KD-AMPKα2 founder mice. Financial support was from the Copenhagen Muscle Research Centre, from the Danish Natural Science and Health Science Research Councils, the Lundbeck Foundation, The Novo-Nordisk Foundation and an Integrated Project (contract number LSHM-CT-2004-005272) from the European Union. A.J.R. was supported by a postdoctoral fellowship from the Carlsberg Foundation and from the European Union.
Glossary
Abbreviations
- AMPK
AMP-activated protein kinase
- AU
arbitrary units
- BTS
N-benzyl-p-toluene sulfonamide
- Caff
caffeine
- CaM
calmodulin
- CPA
cyclopiazonic acid
- DMSO
dimethyl sulfoxide
- 4EBP1
eukaryotic initiation factor 4E-binding protein 1
- EDL
extensor digitorum longus
- eEF2
eukaryotic elongation factor 2
- eEF2K
eukaryotic elongation factor 2 kinase
- mTORC1
mammalian target of rapamycin complex 1
- NH125
1-benzyl-3-cetyl-2-methylimidazolium iodide
- NST
net stimulation time
- SOL
soleus
References
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Arora S, Yang JM, Kinzy TG, Utsumi R, Okamoto T, Kitayama T, Ortiz PA, Hait WN. Identification and characterization of an inhibitor of eukaryotic elongation factor 2 kinase against human cancer cell lines. Cancer Res. 2003;63:6894–6899. [PubMed] [Google Scholar]
- Aslesen R, Engebretsen EM, Franch J, Jensen J. Glucose uptake and metabolic stress in rat muscles stimulated electrically with different protocols. J Appl Physiol. 2001;91:1237–1244. doi: 10.1152/jappl.2001.91.3.1237. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Barclay CJ, Lichtwark GA, Curtin NA. The energetic cost of activation in mouse fast-twitch muscle is the same whether measured using reduced filament overlap or N-benzyl-p-toluenesulphonamide. Acta Physiol (Oxf) 2008;193:381–391. doi: 10.1111/j.1748-1716.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- Barclay CJ, Woledge RC, Curtin NA. Energy turnover for Ca2+ cycling in skeletal muscle. J Muscle Res Cell Motil. 2007;28:259–274. doi: 10.1007/s10974-007-9116-7. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Bylund-Fellenius AC, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol Endocrinol Metab. 1984;246:E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Carlberg U, Nilsson A, Nygård O. Functional properties of phosphorylated elongation factor 2. Eur J Biochem. 1990;191:639–645. doi: 10.1111/j.1432-1033.1990.tb19169.x. [DOI] [PubMed] [Google Scholar]
- Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh AS, Treebak JT, Long YC, Viollet B, Wojtaszewski JF, Zierath JR. Role of adenosine 5′-monophosphate-activated protein kinase subunits in skeletal muscle mammalian target of rapamycin signaling. Mol Endocrinol. 2008;22:1105–1112. doi: 10.1210/me.2007-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle TA, Subkhankulova T, Lilley KS, Shikotra N, Willis AE, Redpath NT. Phosphorylation of elongation factor-2 kinase on serine 499 by cAMP-dependent protein kinase induces Ca2+/calmodulin-independent activity. Biochem J. 2001;353:621–626. doi: 10.1042/0264-6021:3530621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm GL, Tapscott EB, Barakat HA, Kasperek GJ. Measurement of in vivo protein synthesis in rats during an exercise bout. Biochem Med. 1982;27:367–373. doi: 10.1016/0006-2944(82)90042-4. [DOI] [PubMed] [Google Scholar]
- Dorovkov MV, Pavur KS, Petrov AN, Ryazanov AG. Regulation of elongation factor-2 kinase by pH. Biochemistry. 2002;41:13444–13450. doi: 10.1021/bi026494p. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J. Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Terjung RL. Influence of acidosis on AMP deaminase activity in contracting fast-twitch muscle. Am J Physiol Cell Physiol. 1985;248:C43–C50. doi: 10.1152/ajpcell.1985.248.1.C43. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of cyclopiazonic acid on Ca2+ regulation by the sarcoplasmic reticulum in saponin-permeabilized skeletal muscle fibres. Pflugers Arch. 1998;436:104–111. doi: 10.1007/s004240050610. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Kittstein W, Marks F. Elongation factor-2 kinase: effective inhibition by the novel protein kinase inhibitor rottlerin and relative insensitivity towards staurosporine. FEBS Lett. 1994;338:85–88. doi: 10.1016/0014-5793(94)80121-5. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A, Lüttgau HC, Stephenson DG. Caffeine and excitation-contraction coupling in skeletal muscle: a stimulating story. J Muscle Res Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- Hori H, Nagasawa H, Ishibashi M, Uto Y, Hirata A, Saijo K, Ohkura K, Kirk KL, Uehara Y. TX-1123: an antitumor 2-hydroxyarylidene-4-cyclopentene-1,3-dione as a protein tyrosine kinase inhibitor having low mitochondrial toxicity. Bioorg Med Chem. 2002;10:3257–3265. doi: 10.1016/s0968-0896(02)00160-8. [DOI] [PubMed] [Google Scholar]
- Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Jørgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Caffeine-induced Ca2+ release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab. 2007;292:E1308–E1317. [Google Scholar]
- Laitusis AL, Brostrom CO, Ryazanov AG, Brostrom MA. An examination of the role of increased cytosolic free Ca2+ concentrations in the inhibition of mRNA translation. Arch Biochem Biophys. 1998;354:270–280. doi: 10.1006/abbi.1998.0712. [DOI] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Lefort N, St-Amand E, Morasse S, Cote CH, Marette A. The α subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab. 2008;295:E1447–E1454. doi: 10.1152/ajpendo.90362.2008. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. London: Academic Press, Inc; 1972. [Google Scholar]
- Macdonald WA, Pedersen TH, Clausen T, Nielsen OB. N-Benzyl-p-toluene sulphonamide allows the recording of trains of intracellular action potentials from nerve-stimulated intact fast-twitch skeletal muscle of the rat. Exp Physiol. 2005;90:815–825. doi: 10.1113/expphysiol.2005.031435. [DOI] [PubMed] [Google Scholar]
- McLeod LE, Wang L, Proud CG. β-Adrenergic agonists increase phosphorylation of elongation factor 2 in cardiomyocytes without eliciting calcium-independent eEF2 kinase activity. FEBS Lett. 2001;489:225–228. doi: 10.1016/s0014-5793(01)02100-7. [DOI] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Miranda L, Horman S, De Potter I, Hue L, Jensen J, Rider MH. Effects of contraction and insulin on protein synthesis, AMP-activated protein kinase and phosphorylation state of translation factors in rat skeletal muscle. Pflugers Arch. 2008;455:1129–1140. doi: 10.1007/s00424-007-0368-2. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Matsushita M, Nastiuk K, Horiuchi A, Mitsui K, Shimizu Y, Palfrey HC. Elongation factor-2 phosphorylation and the regulation of protein synthesis by calcium. Prog Mol Subcell Biol. 2001;27:91–129. doi: 10.1007/978-3-662-09889-9_4. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Palfrey HC. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987;262:17299–17303. [PubMed] [Google Scholar]
- Nesher R, Karl IE, Kaiser KE, Kipnis DM. Epitrochlearis muscle. I. Mechanical performance, energetics, and fiber composition. Am J Physiol Endocrinol Metab. 1980;239:E454–E460. doi: 10.1152/ajpendo.1980.239.6.E454. [DOI] [PubMed] [Google Scholar]
- Pain VM, Manchester KL. The influence of electrical stimulation in vitro on protein synthesis and other metabolic parameters of rat extensor digitorum longus muscle. Biochem J. 1970;118:209–220. doi: 10.1042/bj1180209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem. 2002;269:3076–3085. doi: 10.1046/j.1432-1033.2002.02992.x. [DOI] [PubMed] [Google Scholar]
- Pinniger GJ, Bruton JD, Westerblad H, Ranatunga KW. Effects of a myosin-II inhibitor (N-benzyl-p-toluene sulphonamide, BTS) on contractile characteristics of intact fast-twitch mammalian muscle fibres. J Muscle Res Cell Motil. 2005;26:135–141. doi: 10.1007/s10974-005-2679-2. [DOI] [PubMed] [Google Scholar]
- Plenge-Tellechea F, Soler F, Fernandez-Belda F. On the inhibition mechanism of sarcoplasmic or endoplasmic reticulum Ca2+-ATPases by cyclopiazonic acid. J Biol Chem. 1997;272:2794–2800. doi: 10.1074/jbc.272.5.2794. [DOI] [PubMed] [Google Scholar]
- Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Redpath NT, Price NT, Severinov KV, Proud CG. Regulation of elongation factor-2 by multisite phosphorylation. Eur J Biochem. 1993;213:689–699. doi: 10.1111/j.1432-1033.1993.tb17809.x. [DOI] [PubMed] [Google Scholar]
- Redpath NT, Proud CG. Cyclic AMP-dependent protein kinase phosphorylates rabbit reticulocyte elongation factor-2 kinase and induces calcium-independent activity. Biochem J. 1993;293:31–34. doi: 10.1042/bj2930031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ. Why muscle stops building when it's working. J Physiol. 2005;569:3. doi: 10.1113/jphysiol.2005.099424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Cleland PJ, Rattigan S, Clark MG. FEBS Lett. 1987;217:232–236. doi: 10.1016/0014-5793(87)80669-5. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Alsted TJ, Kobberø JB, Richter EA. Regulation and function of Ca2+-calmodulin-dependent protein kinase II of fast-twitch rat skeletal muscle. J Physiol. 2007;580:993–1005. doi: 10.1113/jphysiol.2006.127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Bisiani B, Vistisen B, Kiens B, Richter EA. Skeletal muscle eEF2 and 4EBP1 phosphorylation during endurance exercise is dependent on intensity and muscle fiber type. Am J Physiol Reg Int Comp Physiol. 2008;296:R326–333. doi: 10.1152/ajpregu.90806.2008. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Killerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Richter EA. Regulatory mechanisms of skeletal muscle protein turnover during exercise. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.91375.2008. DOI 10.1152/japplphysiol.91375.2008. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG. Ca2+/calmodulin-dependent phosphorylation of elongation factor 2. FEBS Lett. 1987;214:331–334. doi: 10.1016/0014-5793(87)80081-9. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Davydova EK. Mechanism of elongation factor 2 (EF-2) inactivation upon phosphorylation. Phosphorylated EF-2 is unable to catalyze translocation. FEBS Lett. 1989;251:187–190. doi: 10.1016/0014-5793(89)81452-8. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Hultman E. Creatine kinase equilibrium and lactate content compared with muscle pH in tissue samples obtained after isometric exercise. Biochem J. 1975;152:173–180. doi: 10.1042/bj1520173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Shaw MA, Ostap EM, Goldman YE. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluenesulfonamide. Biochemistry. 2003;42:6128–6135. doi: 10.1021/bi026964f. [DOI] [PubMed] [Google Scholar]
- Terada S, Muraoka I, Tabata I. Changes in [Ca2+]i induced by several glucose transport-enhancing stimuli in rat epitrochlearis muscle. J Appl Physiol. 2003;94:1813–1820. doi: 10.1152/japplphysiol.00780.2002. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Heigenhauser GJ, Spriet LL, Dyck DJ. Hormone-sensitive lipase activity and triacylglycerol hydrolysis are decreased in rat soleus muscle by cyclopiazonic acid. Am J Physiol Endocrinol Metab. 2003;285:E412–E419. doi: 10.1152/ajpendo.00023.2003. [DOI] [PubMed] [Google Scholar]
- Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab. 2006a;291:E80–E89. doi: 10.1152/ajpendo.00566.2005. [DOI] [PubMed] [Google Scholar]
- Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mTOR) signalling to regulatory mechanisms of mRNA translation in mouse muscle. J Physiol. 2006b;573:497–510. doi: 10.1113/jphysiol.2005.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young IS, Harwood CL, Rome LC. Cross-bridge blocker BTS permits direct measurement of SR Ca2+ pump ATP utilization in toadfish swimbladder muscle fibers. Am J Physiol Cell Physiol. 2003;285:C781–C787. doi: 10.1152/ajpcell.00025.2003. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Andersson DC, Sandström ME, Westerblad H, Katz A. Cross bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol. 2006;291:C147–C154. doi: 10.1152/ajpcell.00578.2005. [DOI] [PubMed] [Google Scholar]