Abstract
The purpose of the present investigation was to explore the effects of exercise and adrenaline on the mRNA expression of PGC-1α, a master regulator of mitochondrial biogenesis, in rat abdominal adipose tissue. We hypothesized that (1) exercise training would increase PGC-1α mRNA expression in association with increases in mitochondrial marker enzymes, (2) adrenaline would increase PGC-1α mRNA expression and (3) the effect of exercise on PGC-1α mRNA expression in white adipose tissue would be attenuated by a β-blocker. Two hours of daily swim training for 4 weeks led to increases in mitochondrial marker proteins and PGC-1α mRNA expression in epididymal and retroperitoneal fat depots. Additionally, a single 2 h bout of exercise led to increases in PGC-1α mRNA expression immediately following exercise cessation. Adrenaline treatment of adipose tissue organ cultures led to dose-dependent increases in PGC-1α mRNA expression. A supra-physiological concentration of adrenaline increased PGC-1α mRNA expression in epididymal but not retroperitoneal adipose tissue. β-Blockade attenuated the effects of an acute bout of exercise on PGC-1α mRNA expression in epididymal but not retroperitoneal fat pads. In summary, this is the first investigation to demonstrate that exercise training, an acute bout of exercise and adrenaline all increase PGC-1α mRNA expression in rat white adipose tissue. Furthermore it would appear that increases in circulating catecholamine levels may be one potential mechanism mediating exercise induced increases in PGC-1α mRNA expression in rat abdominal adipose tissue.
In recent years a growing number of studies have focused on the regulation of adipose tissue mitochondrial biogenesis, in large part due to the purported role of adipose tissue mitochondria in the regulation of whole body fuel metabolism (Wilson-Fritch et al. 2004; Choo et al. 2006; Koh et al. 2007). For instance in rodent models of insulin resistance and type 2 diabetes, adipose tissue mitochondrial content is reduced (Wilson-Fritch et al. 2004; Choo et al. 2006; Valerio et al. 2006). Interestingly, peroxisome proliferator-activated receptor (PPAR)-γ agonists (e.g. thiazolidinediones) (Wilson-Fritch et al. 2004; Choo et al. 2006; Rong et al. 2007) and ciliary neurotrophic factor (CNTF) (Crowe et al. 2008) have been shown to induce mitochondrial biogenesis in adipose tissue from insulin resistant animals. These changes are associated with increases in the mRNA expression of PPARγ co-activator 1α (PGC-1α) (Wilson-Fritch et al. 2004; Crowe et al. 2008) and the related co-activator PGC-1β (Rong et al. 2007). PGC-1α and -β are master regulators of mitochondrial biogenesis that co-activate and induce the expression of transcription factors such as nuclear respiratory factors 1 and 2 and mitochondrial transcription factor A (Tfam), molecules involved in the coordinated regulation of nuclear and mitochondrial encoded genes, respectively (Scarpulla, 2008). When over-expressed in white adipocytes, PGC-1α leads to increases in the expression of mitochondrial respiratory chain proteins and enzymes involved in fatty acid oxidation (Tiraby et al. 2003) resulting in a phenotype similar to that of brown adipose tissue. Of interest from a clinical perspective, work from Smith's laboratory has shown a correlation between adipose tissue PGC-1α mRNA expression and whole body insulin sensitivity (Hammarstedt et al. 2003). Collectively these findings highlight the importance of PGC-1α in white adipose tissue.
Given the increasing interest surrounding adipose tissue mitochondrial biogenesis it is surprising that only one isolated report has examined the effects of exercise on the regulation of this process in adipose tissue (Stallknecht et al. 1991). Stallknecht and colleagues (1991) found that 6 h of daily swimming for 12 weeks led to increases in cytochrome c oxidase and malate dehydrogenase activities in rat epididymal adipose tissue. Given the close association between increases in PGC-1α mRNA expression and the induction of mitochondrial biogenesis it seems likely that exercise training could increase the mRNA expression of this key transcriptional co-activator in adipose tissue. While PGC-1α and -β regulate similar genes (Scarpulla, 2008), it would appear that they respond differently to external stimuli. For instance, in skeletal muscle, PGC-1β expression is not increased by exercise. It is yet to be determined if exercise induced increases in mitochondrial enzymes are paralleled by similar changes in PGC-1β mRNA expression in white adipose tissue.
In contrast to skeletal muscle (Winder et al. 2006; Wright, 2007) little is known regarding the specific mechanisms which may trigger exercise induced increases in PGC-1α mRNA expression and mitochondrial biogenesis in white adipose tissue. Given the recent findings that β-adrenergic agonists can increase PGC-1α mRNA expression in hepatocytes (Ding et al. 2006) and brown fat pre-adipocytes (Puigserver et al. 1998) it seems likely that increases in circulating catecholamine levels, as seen during exercise, could initiate exercise induced increases in adipose tissue PGC-1α mRNA expression. Within this context the purpose of the present investigation was to explore the regulation of adipose tissue PGC-1α mRNA expression by exercise. We hypothesized that both exercise training and an acute bout of exercise would lead to increases in PGC-1α mRNA expression. We further surmised that adrenaline, much like exercise, would lead to increases in PGC-1α mRNA expression. Lastly, we postulated that the acute effects of exercise on PGC-1α mRNA expression would be attenuated in the presence of β-blockade. To achieve these objectives we studied PGC-1α mRNA expression and markers of mitochondrial biogenesis in rat abdominal adipose tissue depots.
Methods
Materials
Reagents, molecular weight marker, and nitrocellulose membranaes for SDS-PAGE were purchased from Bio-Rad (Mississauga, Ontario). ECL Plus was a product of Amersham Pharmacia Biotech (Arlington Heights, IL,USA). Antibodies against COXIV, and CORE I were purchased from Molecular Probes (Eugene, OR, USA). An antibody against β-actin was a product of Sigma (St Louis, MO, USA). Horseradish peroxidase-conjugated donkey anti-rabbit and goat anti-mouse IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). SuperScript II Reverse Transcriptase, oligo(dT) and dNTP were purchased from Invitrogen (Carlsbad, CA, USA). Citrate Synthase activity kits were obtained from Sigma. All other chemicals were purchased from Sigma.
Treatment of rats
All protocols followed Canadian Council on Animal Care (CCAC) guidelines and were approved by the Animal Use and Welfare Committee at the University of Alberta. Male Wistar rats (Charles River, Wilmington, MA, USA) weighing ∼200 g were housed two per cage, with a 12 h–12 h light–dark cycle, and were provided with water and standard rat chow ad libitum. The 12 h light cycle was from 06.00 h to 18.00 h and all experimental protocols were performed between 07.00 h and 10.00 h. As we hypothesized that adrenaline would increase PGC-1α expression and since adrenaline levels would increase following an overnight fast, all animals were studied in the fed condition. After 1 week acclimatization, rats were randomly divided into two groups. Half of the animals were subjected to exercise training, starting at 15 min per day of swimming for 2 days and then 2 h per day, 7 days per week for 4 weeks. As described previously by Stallknecht et al. (1991), the remaining rats swam for 2 min per day and served as a sham control. Approximately twenty hours following the last bout of exercise, rats were anaesthesized with sodium pentabaritol (5 mg (100 g body weight)−1). Epididymal and retroperitoneal adipose tissue was dissected free of the testes and kidneys, respectively, immediately weighed and then clamp frozen in tongs cooled to the temperature of liquid nitrogen and stored at −80°C until further analysis.
Adipose tissue organ culture
Adipose tissue organ culture (ATOC) is a well characterized technique that has been used to determine changes in adipose tissue metabolism and gene expression (Fried et al. 1993; Trujillo et al. 2006; Lee et al. 2007). The major strength of this method is the maintenance of gene expression over prolonged periods (Fried & Moustaid-Moussa, 2001). Epididymal and retroperitoneal fat pads were removed from male Wistar rats (∼200 g), weighed, and immediately placed in 50 ml conical tubes containing sterile PBS with 1% antibiotic/antimycotic. Under sterile conditions, 500 mg of tissue was placed into culture dishes containing 15 ml of M199 supplemented with 1% antibiotic/antimycotic, 50 μU insulin and 2.5 nm dexamethasone added to the media. The tissue was then minced into ∼5–10 mg pieces and kept in an incubator at 37°C to equilibrate for 24 h. Approximately 3–4 g of adipose tissue was collected from each rat, which provided adequate adipose tissue to use a 500 mg sample from each individual rat in all treatment groups. For the dose–response experiment, adrenaline (1, 5, 10 or 50 μm) or vehicle (sterile dH2O) was added to the medium and the plates were returned to the incubator for 6 h. After 6 h, the culture medium containing the adipose tissue minces was poured into ice-cold phosphate-buffered saline (PBS) and then cell strainers were used to collect the adipose tissue minces from the media/PBS. The adipose tissue minces were then snap frozen in liquid nitrogen and stored at −80°C until further analysis. For the time course study, adipose tissue was allowed to equilibrate for 24 h and then 1 μm of adrenaline was added to the medium and plates were returned to the incubator for 2, 4, 6 or 12 h. In an additional experiment the effects of a 2 h treatment with 100 nm adrenaline was examined. After each time point, the adipose tissue minces were collected as described above and stored at −80°C until further analysis.
Acute exercise and β-blockade
Rats were acclimated to swim exercise as described above. Seventy and 10 min prior to the start of exercise, rats were injected (i.p.) with a weight adjusted bolus of propranolol hydrochloride (0.2 mg (100 g body weight)−1) or an equivalent volume of sterile saline. This protocol has previously been used to inhibit β-adrenergic signalling in rats during swim exercise (Nolte et al. 1994). Immediately following 2 h of swimming, rats were anaesthetized and adipose tissue harvested. There were six rats in the control group and nine rats each in the swim and swim + propranolol groups.
Western blotting
Clamp-frozen epididymal and retroperitoneal fat was homogenized in a 2 : 1 volume-to-weight ratio of ice cold cell lysis buffer supplemented with Protease Inhibitor Cocktail and phenylmethylsulfonyl fluoride using a motor driven glass on glass mortar and pestle. Homogenized samples were sonicated for 5 s and centrifuged for 15 min at 2500 g at 4°C. The protein concentration of the supernatant was determined using the BCA method (Smith et al. 1985). The CV for this assay is <5% in our laboratory. The protein content of CORE 1 and COXIV were determined by Western blot analysis as described previously (Wright et al. 2007; Sutherland et al. 2008). Briefly, equal amounts of protein were separated on either 10% (CORE 1) or 15% (COXIV) gels that were prepared in lab. Proteins were wet transferred to nitrocellulose membranes for 90 min at 200 mA per tank. Membranes were blocked in Tris buffered saline–0.01% tween (TBST) supplemented with 5% non-fat dry milk at room temperature for 1 h with gentle agitation. Membranes were incubated in TBST–5% non-fat dry milk supplemented with appropriate primary antibodies overnight at 4°C with gentle agitation. The following morning blots were briefly washed in TBST and then incubated in TBST–1% non-fat dry milk supplemented with HRP conjugated goat anti-mouse secondary antibody for 1 h at room temperature. Bands were visualized using ECL plus and captured using a Typhoon Imaging system (General Electric, Piscataway, NJ, USA). Imagequant software was used to quantify relative band intensities (General Electric). To control for equal loading and transfer of proteins, β-actin was used as an internal control. In preliminary experiments, we found that 4 weeks of swimming had no effect on the protein content of β-actin in epididymal and retroperitoneal adipose tissue (3.22 ± 0.09 control, 3.27 ± 0.12 trained in epididymal adipose tissue and 4.72 ± 0.33 control, 4.77 ± 0.39 trained in retroperitoneal adipose tissue, arbitrary densitometric units, n = 5–6).
Citrate synthase activity
Frozen adipose tissue samples were homogenized and protein extracted as described above, in the description of Western blotting. Citrate synthase activity was determined by measuring the formation of 5-thio-2-nitrobenzoic acid spectrophotometrically (412 nm) in a microplate reader (Molecular Devices, Sunnyvale, CA, USA) as described in the manufacturer's instructions.
Real time RT-PCR
RNA was isolated from epididymal and retroperitoneal adipose tissue and adipose tissue minces using an RNeasy lipid kit (Qiagen, Mississauga, ON, Canada) according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized from 1 μg of RNA using SuperScript II Reverse Transcriptase, oligo(dT) and dNTP. Real time PCR was performed using a 7900 HT fast real-time PCR system (Applied Biosystems, Streetsville, ON, Canada). Taqman gene expression assays (Applied Biosytems) were used to determine the mRNA expression of β-actin and Tfam. Primers and probes for PGC-1α and PGC-1β were designed using Primers Express 3.0 software (Applied Biosystems, Streetsville, ON, Canada) (Table 1). Samples were run in duplicate on a 96 well plate. Results were normalized to the mRNA expression of β-actin as we have found in preliminary experiments that this gene did not change with any of our experimental manipulations. Relative differences in gene expression between groups were determined using the 2−ΔΔCT method (Livak & Schmittgen, 2001). Standard curve assays were performed for β-actin and PGC-1α, PGC-1β, and Tfam. The amplification efficiencies of the gene of interest and β-actin were equivalent as determined using the equation 10(−1/slope)− 1. Similarly, when plotting log cDNA dilution versus ΔCT (ΔCT, CTgene-of-interest−CTβ-actin) the slope of this relationship was <0.1, indicating that the genes of interest were amplified with equal efficiency.
Table 1.
Sequences for the primers and probes used for real-time PCR procedures
| Gene of Interest | Forward | Reverse | Probe |
|---|---|---|---|
| PGC-1α | 5′-GTGCAGCCAAGACTCTGTATGG-3′ | 5′-GTCCAGGTCATTCACATCAAGTTC-3′ | 5′-AGTGACATAGAGTGTGCTGCC-3′ |
| PGC-1β | 5′-CCGATCCCGGCAAACC-3′ | 5′-CAGAAGTTCCCTTAGGATGGAGAA-3′ | 5′-CCAAAGCCTTCTGGACTG-3′ |
Statistical analysis
Data are presented as means ±s.e.m. Comparisons between the sham control and trained groups were made using Student's unpaired t test. Comparisons between the vehicle and treated groups during ATOC experiments were made using a one-way ANOVA followed by a post hoc comparison using Fisher's LSD test. Similarly, differences between control, swim and swim plus propranolol groups were made using a one-way ANOVA and Fisher's LSD test. Statistical significance was set at P < 0.05.
Results
Effect of exercise on body weight, fat pad mass and food intake
Despite a slight increase in food consumption, body weight gain in the swim trained group was significantly less than in the control group (Table 2). Fat pads from the swim trained rats weighed less than those in the control group.
Table 2.
The effect of exercise training on body weight, epididymal and retroperitoneal fat pad weight, and food intake in male Wistar rats
| Sedentary | Trained | |
|---|---|---|
| Initial body weight (g) | 248.5 ± 2.2 | 251.6 ± 2.7 |
| Final body weight (g) | 410.9 ± 4.8 | 389.4 ± 14.0 |
| Weight gain (g) | 162.4 ± 3.8 | 137.8 ± 13.8* |
| Epididymal fat pad (g) | 4.4 ± 0.2 | 3.4 ± 0.3* |
| Retroperitoneal fat pad (g) | 4.3 ± 0.4 | 2.0 ± 0.3* |
| Food intake (g (100 g body weight)−1) | 5.9 ± 0.2 | 6.4 ± 0.3 |
Data are presented as means ±s.e.m. for 8 per group.
P < 0.05 compared to sedentary values.
Exercise-induced increases in mitochondrial protein content and enzyme activity
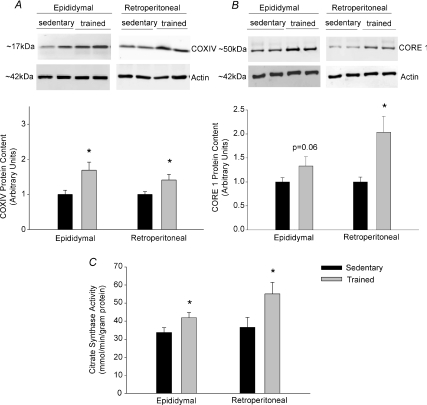
Four weeks of swim training led to increases in the protein content of COXIV and CORE 1, proteins of complex IV and complex III of the respiratory chain, respectively. Additionally citrate synthase activity was increased in epididymal and retroperitoneal adipose tissue from trained rats (Fig. 1). The measurement of these enzymes has previously been used as markers of mitochondrial content in skeletal muscle and adipose tissue (Garcia-Roves et al. 2006; Rong et al. 2007; Sutherland et al. 2008).
Figure 1. The effects of exercise training on COXIV protein content (A), CORE1 protein content (B) and citrate synthase activity (C) in rat epididymal and retroperitoneal adipose tissue.
Data are presented as means +s.e.m. for 10–14 samples per group. Representative western blots for CORE1, COXIV and β-actin are shown above the quantified data and results were normalized to actin protein content; *P < 0.05.
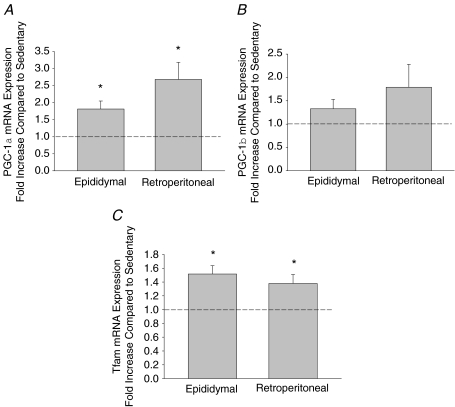
Exercise training increases the mRNA expression of PGC-1α and Tfam
PGC-1α and Tfam mRNA expression were increased in epididymal and retroperitoneal adipose tissue following training (Fig. 2). On the other hand, the mRNA expression of PGC-1β was not increased in either fat pad following swim training.
Figure 2. The effects of exercise training on the mRNA expression of PGC-1α (A), PGC-1β (B) and Tfam mRNA expression (C) in epididymal and retroperitoneal adipose tissue.
Data are presented as means +s.e.m. for 10–14 samples per group, normalized to actin mRNA expression, and expressed as fold differences compared to sedentary controls; *P < 0.05.
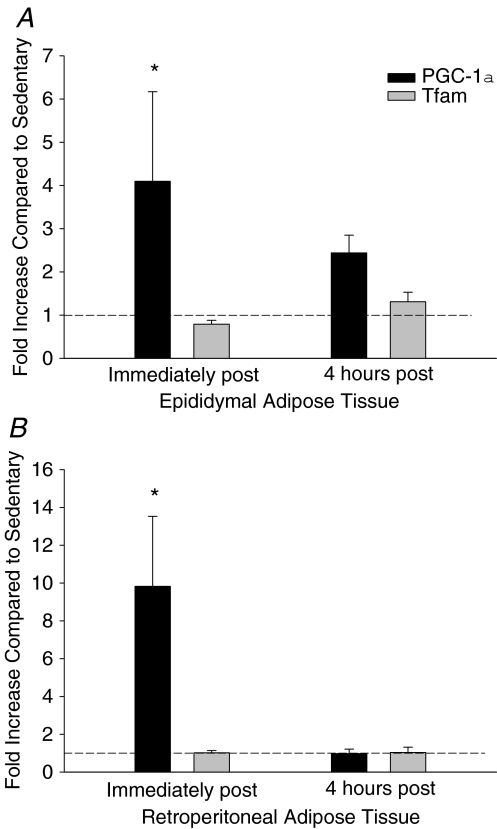
Acute exercise increases the mRNA expression of PGC-1α
Immediately following an acute, 2 h bout of exercise, PGC-1α mRNA expression was increased in both fat pads. Four hours following exercise cessation, PGC-1α mRNA expression was not different from control values (Fig. 3). Tfam mRNA expression was not significantly increased in either fat pad immediately, or 4 h following exercise cessation.
Figure 3. The time course of exercise induced increases in PGC-1α and Tfam mRNA expression in epididymal (A) and retroperitoneal (B) adipose tissue.
Data are presented as means +s.e.m. for 5–6 samples per group, normalized to actin mRNA expression, and expressed as fold differences compared to sedentary controls; *P < 0.05.
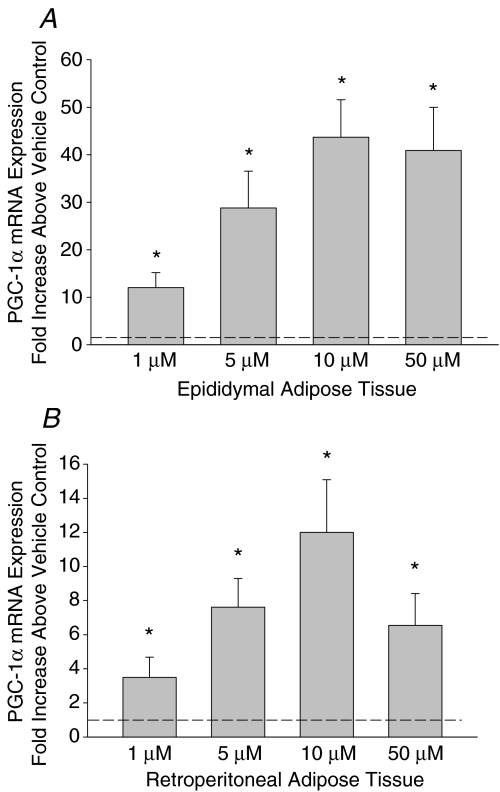
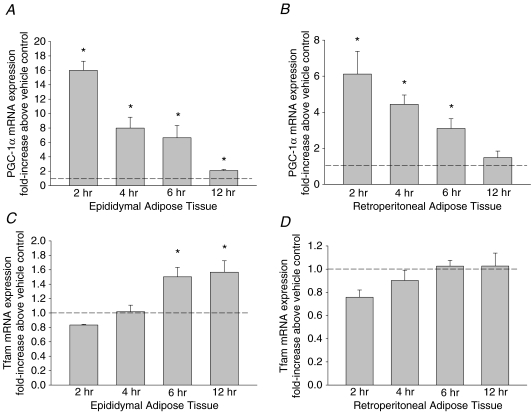
Adrenaline causes a dose and time dependent increase in PGC-1α mRNA expression
To observe the initial, direct effects of adrenaline on the induction of PGC-1α mRNA expression independent of systemic changes in other metabolites and hormones, we utilized adipose tissue organ culture. Adipose tissue minces were cultured and exposed to various concentrations of adrenaline over a number of time points. The initial adrenaline concentrations that we utilized were based on previous findings in hepatocytes (Ding et al. 2006). Treatment with pharmacological (1–50 μm) doses of adrenaline for 6 h resulted in a dose-dependent increase in PGC-1α mRNA expression in cultured epididymal and retroperitoneal adipose tissue (Fig. 4). Although 10 μm adrenaline resulted in the highest induction of PGC-1α mRNA expression, we used a 1 μm dose of adrenaline for the time course experiments to avoid any potential issues with toxicity at the high concentrations we were using. The highest measured increase in PGC-1α mRNA expression was observed following a 2 h exposure to 1 μm adrenaline with progressive decreases thereafter (Fig. 5). The increases in PGC-1α mRNA expression preceded the adrenaline mediated rise in Tfam mRNA expression in epididymal adipose tissue. Treatment of adipose tissue cultures for 2 h with 100 nm adrenaline, a concentration more representative of the levels of circulating catecholamines during exercise (adrenaline levels during swimming in rats are ∼15 nm; Higashida et al. 2008), led to significant increases in PGC-1α mRNA expression in epididymal adipose tissue (2.3 ± 0.6, P = 0.02), but not in retroperitoneal adipose tissue (1.4 ± 0.1, P > 0.05).
Figure 4. The dose–response relationship of adrenaline on PGC-1α mRNA expression in epididymal (A) and retroperitoneal (B) adipose tissue organ cultures.
Data are presented as means +s.e.m. for 4–6 samples per group, normalized to actin mRNA expression, and expressed as fold differences compared to vehicle treated controls; *P < 0.05.
Figure 5. The time course of adrenaline (1 μm) induced increases in PGC-1α and Tfam mRNA expression in rat epididymal (A and C) and retroperitoneal (B and D) organ cultures.
Data are presented as means +s.e.m. for 4–6 samples per group, normalized to actin mRNA expression, and expressed as fold differences compared to vehicle treated controls; *P < 0.05.
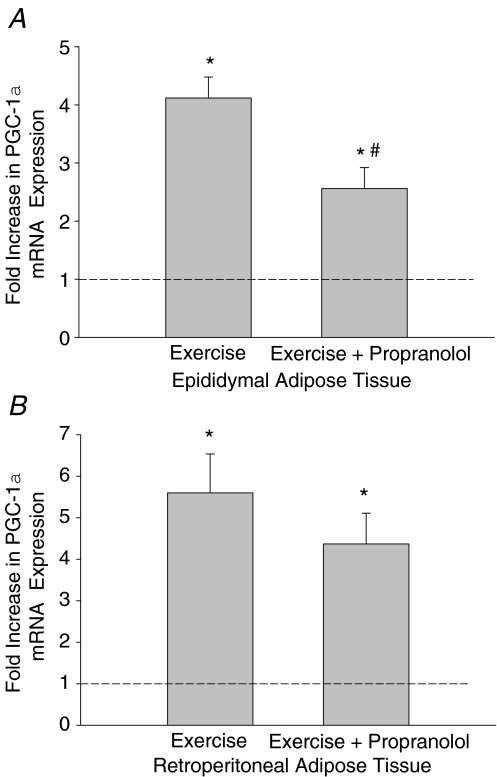
β-Blockade attenuates exercise induced increases in adipose tissue PGC-1α mRNA expression
Rats were treated with the β-blocker propranolol (200 μg (100 g body weight)−1) 70 and 10 min prior to 2 h of swim exercise. In preliminary experiments we found that this dosing protocol almost completely blocked the in vivo effects of adrenaline (20 μg (100 g body weight)−1) on PGC-1α mRNA expression in adipose tissue (adrenaline 3.69 ± 1.26-fold increase above control, adrenaline + propranolol 1.44 ± 0.36-fold increase above control). This adrenaline treatment results in increases in adrenaline (∼190 nm) (Fell et al. 1981) to levels much higher than seen during exercise (∼15 nm) (Higashida et al. 2008). The same propranolol treatment has previously been used in rat swim models and does not affect the ability of rats to complete the swim exercise (Nolte et al. 1994). As seen in Fig. 6, propranolol attenuated the exercise induced rise in PGC-1α mRNA expression in epididymal but not retroperitoneal adipose tissue.
Figure 6. The effects of propranolol on the exercise induced increases in PGC-1α mRNA expression in rat epididymal (A) and retroperitoneal (B) adipose tissue.
Data are presented as means +s.e.m. for 6–9 samples per group, normalized to actin mRNA expression, and are expressed as fold differences compared to non-exercised rats (*P < 0.05) or between the exercised groups with saline or propranolol treatment (#P < 0.05).
Discussion
Adipose tissue mitochondria are increasingly being recognized as key players in the regulation of whole body metabolism. Surprisingly, and in sharp contrast to skeletal muscle, few studies have explored the effects of exercise on mitochondrial biogenesis in adipose tissue. Consistent with one previous report (Stallknecht et al. 1991), we found that 2 h of daily swim exercise for 28 consecutive days led to increases in markers of adipose tissue mitochondrial biogenesis, such as CORE1 and COXIV protein content and citrate synthase activity.
Mitochondrial biogenesis is a complex process involving the coordinated regulation of both nuclear and mitochondrial encoded genes. A central cog in this process would appear to be PGC-1α. The over-expression of PGC-1α in skeletal muscle (Lin et al. 2002) or white adipocytes (Tiraby et al. 2003) induces mitochondrial biogenesis, whereas the deletion of this gene leads to reductions in mitochondria (Leone et al. 2005). Exercise has been shown to have both an acute (Baar et al. 2002; Miura et al. 2007; Mathai et al. 2008) and a chronic training related effect (Goto et al. 2000) on PGC-1α mRNA expression in skeletal muscle. Similar to these aforementioned findings we made the novel observation that 4 weeks of daily swim training led to increases in the mRNA expression of PGC-1α, in white adipose tissue. On the other hand training did not significantly increase the expression of PGC-1β in either fat depot. These findings are consistent with a previous report which demonstrated that cold exposure, fasting and exercise increased PGC-1α but not PGC-1β mRNA levels in brown adipose tissue, liver and skeletal muscle, respectively (Meirhaeghe et al. 2003). PGC-1α mRNA expression in white adipose tissue was increased immediately after exercise and returned to control levels 4 h following exercise cessation. Since tissue was harvested well after this time point, our results suggest that the long-term increases in PGC-1α mRNA expression were the result of a training effect and not related to the residual effects of the last bout of exercise. The rise in PGC-1α was associated with increases in the mRNA expression of Tfam, a transcription factor involved in the regulation of mitochondrial encoded genes whose expression is controlled, at least in part, by PGC-1α (Wu et al. 1999; Gleyzer et al. 2005). While our findings are consistent with the notion that PGC-1α could be involved in mediating exercise induced mitochondrial biogenesis in adipose tissue, future studies are needed to determine if the changes in PGC-1α mRNA expression are paralleled by increases in the protein content of this transcriptional co-activator.
Biochemical changes within the contracting muscle itself, such as perturbations in high energy phosphates (Bergeron et al. 2001; Baar et al. 2002; Zong et al. 2002), and increases in cytosolic calcium concentration (Ojuka et al. 2002, 2003), are believed to initiate, to a large extent, exercise induced mitochondrial biogenesis in skeletal muscle. On the other hand, the triggering mechanisms mediating this process in adipose tissue have not been established. Hormonal factors such as adrenaline are intimately involved in the acute regulation of adipose tissue metabolism during exercise (McMurray & Hackney, 2005). Moreover, micromolar concentrations of adrenaline have been shown to induce PGC-1α mRNA expression in hepatocytes (Ding et al. 2006). Given these findings it seems likely that adrenaline could induce PGC-1α mRNA expression in white adipose tissue and perhaps serve as an extracellular signal in the exercise mediated induction of PGC-1α.
As an initial approach to test this hypothesis we used adipose tissue organ culture and determined the dose–response relationship and time course of adrenaline induced increases in PGC-1α mRNA expression. Across a range of concentrations, we found that adrenaline markedly increased PGC-1α mRNA expression and that these changes preceded increases in Tfam mRNA expression. Interestingly, epididymal adipose tissue appeared much more responsive to the effects of pharmacological doses of adrenaline as witnessed by larger increases in PGC-1α mRNA expression in epididymal adipose tissue and the absence of adrenaline induced increases in Tfam mRNA expression in retroperitoneal adipose tissue. Consistent with these findings, PGC-1α mRNA expression in retroperitoneal adipose tissue organ cultures did not significantly increase when treated with supra-physiological (100 nm) concentrations of adrenaline. Our findings in epididymal adipose tissue are in line with recent results from Miura and colleagues (2007) who reported that β-adrenergic stimulation leads to increases in PGC-1α mRNA expression in murine skeletal muscle.
Having shown that both exercise and adrenaline treatment induce PGC-1α mRNA expression in white adipose tissue, we wanted to gain insight into a potential role of adrenaline in mediating the acute effects of exercise on the induction of PGC-1α mRNA expression in white adipose tissue. Rats were treated with the non-specific β-blocker propranolol prior to, and tissue harvested immediately following, 2 h of swimming. In epididymal adipose tissue, β-blockade led to a ∼40% reduction in the exercise induced increase in PGC-1α mRNA expression. It should be noted that the same propranolol treatment blocked the adrenaline induced rise in PGC-1α mRNA expression suggesting that this treatment was sufficient to block the effects of adrenaline on adipose tissue gene expression. The partial attenuation of the exercise induced increase in PGC-1α mRNA expression in combination with the results of our adrenaline experiments is consistent with the hypothesis that elevations in catecholamines may mediate a portion of the exercise induced increase in PGC-1α mRNA expression in epididymal adipose tissue.
In contrast to epididymal adipose tissue, β-blockade did not significantly attenuate the exercise induced increase in PGC-1α gene expression in the retroperitoneal fat pad. Given the greater effect of adrenaline on the induction of PGC-1α expression in epididymal compared to retroperitoneal adipose tissue, these results are not entirely surprising. Taken in combination with previous results showing enhanced lipolysis in epididymal versus retroperitoneal adipocytes (Tavernier et al. 1995), our findings would suggest the existence of depot specific differences in responsiveness to β-adrenergic stimulation. While the mechanisms underlying these apparent depot specific differences are not clear, our results suggest that multiple extracellular signals are likely to be involved in the exercise induced up-regulation of PGC-1α expression in white adipose tissue. For example exercise has been shown to increase circulating levels of thyroid hormone (Wirth et al. 1981; Limanova et al. 1983; Fortunato et al. 2008), a hormone that has been shown, at least in skeletal muscle, to increase PGC-1α protein content (Branvold et al. 2008). Along a similar line the expression and secretion of interleukin 6 (IL-6) from skeletal muscle has been shown to increase dramatically during exercise (Pedersen & Febbraio, 2008). Interestingly, IL-6 has been shown to activate 5′AMP activated protein kinase (Kelly et al. 2004), a reputed mediator of PGC-1α mRNA expression (Jager et al. 2007). The potential roles of IL-6 and/or thyroid hormone in the regulation of PGC-1α in white adipose tissue need to be explored in further detail.
The purpose of the present study was to examine the effects of exercise and adrenaline on PGC-1α mRNA expression in white adipose tissue. Although swimming may not be representative of all exercise models given the relatively large increases in catecholamines (Higashida et al. 2008), we have made the novel observations that both acute swim exercise and long term training lead to increases in PGC-1α mRNA expression in white adipose tissue. Interestingly, it does not appear that β-adrenergic agonists are the sole regulators of exercise induced increases in PGC-1α mRNA expression. A further elucidation of the specific extracellular signals which regulate exercise induced increases in PGC-1α mRNA expression is an area ripe for investigation and will lend much insight into the regulation of adipose tissue gene expression by exercise.
Acknowledgments
D.C.W. is a CIHR New Investigator, CDA Scholar and Alberta Heritage Foundation for Medical Research Scholar. L.S. was supported by an Alberta Heritage Foundation for Medical Research Studentship and a Natural Sciences and Engineering Research Council of Canada Graduate Student Scholarship. M.B. and S.B. were supported by NSERC summer studentships. This research was supported by an Operating Grant to D.C.W. from the Canadian Institutes of Health Research and a pilot project grant from the Alberta Diabetes Institute.
References
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Branvold DJ, Allred DR, Beckstead DJ, Kim HJ, Fillmore N, Condon BM, Brown JD, Sudweeks SN, Thomson DM, Winder WW. Thyroid hormone effects on LKB1, MO25, phospho-AMPK, phospho-CREB, and PGC-1α in rat muscle. J Appl Physiol. 2008;105:1218–1227. doi: 10.1152/japplphysiol.00997.2007. [DOI] [PubMed] [Google Scholar]
- Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- Crowe S, Turpin SM, Ke F, Kemp BE, Watt MJ. Metabolic remodeling in adipocytes promotes ciliary neurotrophic factor-mediated fat loss in obesity. Endocrinology. 2008;149:2546–2556. doi: 10.1210/en.2007-1447. [DOI] [PubMed] [Google Scholar]
- Ding X, Lichti K, Kim I, Gonzalez FJ, Staudinger JL. Regulation of constitutive androstane receptor and its target genes by fasting, cAMP, hepatocyte nuclear factor α, and the coactivator peroxisome proliferator-activated receptor γ coactivator-1α. J Biol Chem. 2006;281:26540–26551. doi: 10.1074/jbc.M600931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell RD, Terblanche SE, Winder WW, Holloszy JO. Adaptive responses of rats to prolonged treatment with epinephrine. Am J Physiol Cell Physiol. 1981;241:C55–C58. doi: 10.1152/ajpcell.1981.241.1.C55. [DOI] [PubMed] [Google Scholar]
- Fortunato RS, Ignacio DL, Padron AS, Pecanha R, Marassi MP, Rosenthal D, Werneck-de-Castro JP, Carvalho DP. The effect of acute exercise session on thyroid hormone economy in rats. J Endocrinol. 2008;198:347–353. doi: 10.1677/JOE-08-0174. [DOI] [PubMed] [Google Scholar]
- Fried SK, Moustaid-Moussa N. Culture of adipose tissue and isolated adipocytes. Methods Mol Biol. 2001;155:197–212. doi: 10.1385/1-59259-231-7:197. [DOI] [PubMed] [Google Scholar]
- Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest. 1993;92:2191–2198. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Roves PM, Huss J, Holloszy JO. Role of calcineurin in exercise-induced mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1172–E1179. doi: 10.1152/ajpendo.00633.2005. [DOI] [PubMed] [Google Scholar]
- Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun. 2000;274:350–354. doi: 10.1006/bbrc.2000.3134. [DOI] [PubMed] [Google Scholar]
- Hammarstedt A, Jansson PA, Wesslau C, Yang X, Smith U. Reduced expression of PGC-1 and insulin-signaling molecules in adipose tissue is associated with insulin resistance. Biochem Biophys Res Commun. 2003;301:578–582. doi: 10.1016/s0006-291x(03)00014-7. [DOI] [PubMed] [Google Scholar]
- Higashida K, Higuchi M, Terada S. Potential role of lipin-1 in exercise-induced mitochondrial biogenesis. Biochem Biophys Res Commun. 2008;374:587–591. doi: 10.1016/j.bbrc.2008.07.079. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, Youn JH, Lee KU. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56:2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Wang Y, Ricci MR, Sullivan S, Russell CD, Fried SK. Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am J Physiol Endocrinol Metab. 2007;292:E858–E864. doi: 10.1152/ajpendo.00439.2006. [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanova Z, Sonka J, Kratochvil O, Sonka K, Kanka J, Sprynarova S. Effects of exercise on serum cortisol and thyroid hormones. Exp Clin Endocrinol. 1983;81:308–314. doi: 10.1055/s-0029-1210241. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mathai AS, Bonen A, Benton CR, Robinson DL, Graham TE. Rapid exercise-induced changes in PGC-1α mRNA and protein in human skeletal muscle. J Appl Physiol. 2008;105:1098–1105. doi: 10.1152/japplphysiol.00847.2007. [DOI] [PubMed] [Google Scholar]
- McMurray RG, Hackney AC. Interactions of metabolic hormones, adipose tissue and exercise. Sports Med. 2005;35:393–412. doi: 10.2165/00007256-200535050-00003. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Crowley V, Lenaghan C, Lelliott C, Green K, Stewart A, Hart K, Schinner S, Sethi JK, Yeo G, Brand MD, Cortright RN, O’Rahilly S, Montague C, Vidal-Puig AJ. Characterization of the human, mouse and rat PGC1β (peroxisome-proliferator-activated receptor-γ co-activator 1β) gene in vitro and in vivo. Biochem J. 2003;373:155–165. doi: 10.1042/BJ20030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1alpha (PGC-1α) mRNA in response to exercise is mediated by β-adrenergic receptor activation. Endocrinology. 2007;148:3441–3448. doi: 10.1210/en.2006-1646. [DOI] [PubMed] [Google Scholar]
- Nolte LA, Gulve EA, Holloszy JO. Epinephrine-induced in vivo muscle glycogen depletion enhances insulin sensitivity of glucose transport. J Appl Physiol. 1994;76:2054–2058. doi: 10.1152/jappl.1994.76.5.2054. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J. 2003;17:675–681. doi: 10.1096/fj.02-0951com. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Han DH, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am J Physiol Endocrinol Metab. 2002;283:E1040–E1045. doi: 10.1152/ajpendo.00242.2002. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Vinten J, Ploug T, Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am J Physiol Endocrinol Metab. 1991;261:E410–E414. doi: 10.1152/ajpendo.1991.261.3.E410. [DOI] [PubMed] [Google Scholar]
- Sutherland LN, Capozzi LC, Turchinsky NJ, Bell RC, Wright DC. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab. 2008;295:E1076–E1083. doi: 10.1152/ajpendo.90408.2008. [DOI] [PubMed] [Google Scholar]
- Tavernier G, Galitzky J, Valet P, Remaury A, Bouloumie A, Lafontan M, Langin D. Molecular mechanisms underlying regional variations of catecholamine-induced lipolysis in rat adipocytes. Am J Physiol Endocrinol Metab. 1995;268:E1135–E1142. doi: 10.1152/ajpendo.1995.268.6.E1135. [DOI] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem. 2003;278:33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Lee MJ, Sullivan S, Feng J, Schneider SH, Greenberg AS, Fried SK. Tumor necrosis factor α and glucocorticoid synergistically increase leptin production in human adipose tissue: role for p38 mitogen-activated protein kinase. J Clin Endocrinol Metab. 2006;91:1484–1490. doi: 10.1210/jc.2005-1901. [DOI] [PubMed] [Google Scholar]
- Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116:2791–2798. doi: 10.1172/JCI28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Taylor EB, Thomson DM. Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc. 2006;38:1945–1949. doi: 10.1249/01.mss.0000233798.62153.50. [DOI] [PubMed] [Google Scholar]
- Wirth A, Holm G, Lindstedt G, Lundberg PA, Bjorntorp P. Thyroid hormones and lipolysis in physically trained rats. Metabolism. 1981;30:237–241. doi: 10.1016/0026-0495(81)90147-5. [DOI] [PubMed] [Google Scholar]
- Wright DC. Mechanisms of calcium-induced mitochondrial biogenesis and GLUT4 synthesis. Appl Physiol Nutr Metab. 2007;32:840–845. doi: 10.1139/H07-062. [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]