Abstract
Arsenic trioxide (ATO) has been recommended for the treatment of refractory cases of acute promyelocytic leukemia (APL). Recent studies in our laboratory indicated that oxidative stress plays a key role in ATO-induced cytotoxicity in human leukemia (HL-60) cells. In the present investigation, we performed the MTT assay and trypan blue exclusion test for cell viability. We also performed the thiobarbituric acid test to determine the levels of malondialdehyde (MDA) production in HL-60 cells co-exposed to either ascorbic acid (AA) and ATO or to n-acetyl-l-cysteine (NAC) and ATO. The results of MTT assay indicated that AA exposure potentiates the cytotoxicity of ATO in HL-60 cells, as evidenced by a gradual increase in MDA levels with increasing doses of AA. In contrary, the addition of NAC to ATO-treated HL-60 cells resulted in a dose dependent decrease of MDA production. From these results, we conclude that the addition of the ascorbic acid to ATO-treated HL-60 cells enhances the formation of reactive oxygen species (ROS) whereas the addition of NAC under the same experimental condition significantly (p<0.05) decreases the level of ROS formation. Based on these direct in vitro findings, our studies provide evidence that AA may extend the therapeutic spectrum of ATO. The co-administration of NAC with ATO shows a potential specificity for tumor cells, indicating it may not enhance the clinical outcome associated with ATO monotherapy in vivo.
Keywords: Arsenic trioxide, HL-60 cells, MDA, ascorbic acid, n-acetyl-l-cysteine
INTRODUCTION
Acute Promyelocytic Leukemia (APL) is a subtype of acute leukemia which can affect people of any age. It strikes about 1,500 patients in the United States each year. The standard treatment of this disease is chemotherapy and retinoic acid. Arsenic trioxide (ATO) is a new form of therapy that has recently been found to benefit APL patients. Both in vitro and in vivo studies have shown that ATO can induce a clinical remission in APL patients [1, 2]. It has been reported that APL patients that no longer respond to chemotherapy or retinoic acid, can achieve a complete remission with ATO with only few side effects [2].
Many antioxidants have been reported to enhance or inhibit ATO-mediated apoptosis in tumor cells [3]. Ascorbic acid (AA) is an anti-oxidant and free radical scavenger effective against peroxyl- and hydroxyl-radicals, superoxide, singlet oxygen and peroxynitrite. Many researchers believe that AA, known as vitamin C, prevents cancer by deactivating free radicals before they can damage DNA and initiate tumor growth [4]. Some scientists have claimed that AA can cure anything from the common cold to cancer by stimulating the immune system and protecting the body against free radicals [5]. However, other studies have reported that vitamin C may act as a pro-oxidant that helps the body's own free radical defense mechanism destroy tumors in their early stages [6, 7]. N-acetyl-L-cysteine (NAC) is an antioxidant/free radical scavenger or reducing agent that protects against cell death [8, 9]. The protective action of NAC, a thiol-containing compound that acts as a nucleophile, and a precursor of reduced glutathione, has been widely demonstrated [9].
Ascorbic acid and n-aceltyl-l-cysteine as well as other antioxidants have different reactivities for specific oxidative species, such as hydroxyl radicals, singlet oxygen, hydrogen peroxide, peroxyl radicals, or superoxide anion [10, 11]. Understanding how each of these two compounds reacts with ATO may provide new insights into their potential influence on the clinical outcome of APL patients receiving ATO chemotherapy. The specific aim of this research was to determine whether co-exposure to AA or NAC modulates oxidative stress associated with ATO toxicity in human leukemia (HL-60) cells.
MATERIALS AND METHODS
Chemicals and Test Media
Arsenic trioxide (As2O3), CASRN 1327-53-3, MW 197.84, with an active ingredient of 100% (w/v) arsenic in 10% nitric acid was purchased from Fisher Scientific in (Houston Texas). Growth medium RPMI 1640 containing 1 mmol/L L-glutamine was purchased from Gibco BRL products (Grand Island, NY). Ninety-six well plates were purchased from Costar (Cambridge, MA). Fetal bovine serum (FBS), ascorbic acid, n-aceltyl-l-cysteine, phosphate buffered saline (PBS), and MTT assay kit were obtained from Sigma Chemical Company (St. Louis, MO). Lipid peroxidation kit was purchased from Calbiochem-Novabiochem (San Diego, CA).
Tissue Culture
The HL-60 promyelocytic leukemia cell line was purchased from the American Type Culture Collection –ATCC (Manassas, VA). This cell line has been derived from peripheral blood cells of a 36-year old Caucasian female with acute promyelocytic leukemia (APL). The HL-60 cells grow as a suspension culture. The predominant cell population consists of neutrophilic promyelocytes [12, 13].
In the laboratory, cells were stored in the liquid nitrogen until use. They were next thawed by gentle agitation of their containers (vials) for 2 minutes in a water bath at 37°C. After thawing, the content of each vial of cell was transferred to a 25 cm2 tissue culture flask, diluted with up to 10 mL of RPMI 1640 containing 1 mmol/L L-glutamine (GIBCO/BRL, Gaithersburg, MD) and supplemented with 10% (v/v) fetal bovine serum (FBS), and 1% (w/v) penicillin/streptomycin. The 25 cm2 culture flasks, each containing 2 × 106 viable cells, were observed under the microscope, followed by incubation in a humidified 5 % CO2 incubator at 37° C. Three times a week, they were diluted under same conditions to maintain a density of 5 × 105/mL, and harvested in the exponential phase of growth.
Measurements of Cell Viability
The cell viability was assessed by both the trypan blue exclusion test (Life Technologies) using a hemocytometer to manually count the cells, and the ability of viable cells to reduce 3-[4, (5-dimethylthiasol-2-yl)-2, 4,-diphenyltetrazolium bromide] (MTT).
MTT Assay
To examine the effect of AA and NAC on the viability of HL-60 cells, 180 μL aliquots of cell suspension (5× 105/mL) were seeded to 96 well polystyrene tissue culture plates and treated with 20 μL of either AA or NAC solutions, respectively in separated culture plates to reach final doses of 0, 25, 50, and 100 μM AA or NAC. Cells were placed in the humidified 5% CO2 incubator at 37°C for 24 hr. Cells incubated in culture medium alone served as a control for cell viability (untreated wells). After incubation, 20 μL aliquots of MTT solution (5 mg/mL in PBS) were added to each well and re-incubated for 4 hr at 37° C, followed by low centrifugation at 800 rpm for 5 min. Then, the supernatants were carefully aspirated and 200 μL aliquots of dimethylsulfoxide (DMSO) were added to each well to dissolve the formazan crystals, followed by incubation for 10 min to dissolve air bubbles. The culture plates were placed on a Biotex Model micro-plate reader and the absorbance was measured at 550 nm. The amount of color produced is directly proportional to the number of viable cells. All assays were performed in six replicates for each AA, NAC, AA + ATO, or NAC + ATO concentration, and means ± SD values were calculated. Cell viability rate was calculated as the percentage of MTT absorption as follows: % survival = (mean experimental absorbance/mean control absorbance)×100.
From a recently published experiment, we reported that ATO is cytotoxic to HL-60 cells, showing a 24 hr LD50 of 6.4 ± 0.6 μg/mL [14]. Hence, to examine the effect of AA and NAC on ATO-induced cytotoxicity, cells exposed to 6 μg/mL ATO were co-exposed to 25, 50, or 100 μM of AA or NAC, incubated in humidified 5% CO2 incubator at 37°C for 24 hr, and tested for cell viability following the MTT assay protocol as described above.
Trypan Blue Exclusion Test
HL-60 cells were treated with AA, NAC, AA + ATO or NAC + ATO as described in the MTT assay. After incubation in a humidified 5% CO2 incubator at 37°C for 24 hr. Cells were washed twice with phosphate buffered saline (PBS), re-suspended in fresh RPMI 1640 medium and mixed. Briefly, ten μl of a 0.5% solution of the dye (trypan blue) was added to 100 μl of treated cells (1.0 × 105/ml). The suspension was then examined on a hemocytometer. Both viable and nonviable cells were counted. A minimum of 200 cells were counted for each data point in a total of eight microscopic fields.
Assay of Lipid Peroxidation
Aldehydes such as malondialdehydes (MDA) are formed during lipid peroxidation [15]. In this experiment, the concentration of MDA was measured using a lipid peroxidation assay kit (Calbiochem Novabiochem San Diego, CA). Briefly, 2 × 106 HL-60 cells/mL untreated as a control, or exposed to ATO, AA + ATO, or NAC + ATO, were incubated in a total volume of 10 ml growth medium for 24 hr. After the incubation period, cells were collected in 15 mL tubes, followed by low-speed centrifugation. The cell pellets were re-suspended in 0.5 ml of Tris-HCl, pH 7.4, and lysed using a sonicator (W-220; Ultrasonic, Farmingdale, NY) under the conditions of duty cycle-25% and output control-40% for 5 sec on ice. The protein concentration of the cell suspension was determined using a protein assay kit (BioRad, Hercules, California). A 200μl aliquot of the culture medium or 2 mg of cell lysate protein was assayed for MDA according to the lipid peroxidation assay kit protocol (Calbiochem-Novabiochem, San Diego, CA). The absorbance of the sample was read at 586 nm, and the concentration of MDA was determined from a standard curve.
Statistical Analysis
Data were presented as means ± SDs. Statistical analysis was done using one way analysis of variance (ANOVA Dunnett's test) for multiple samples and Student's t-test for comparing paired sample sets. P-values less than 0.05 were considered statistically significant. The percentages of cell viability and MDA levels were presented graphically in the form of histograms, using Microsoft Excel computer program.
RESULTS
Measurements of Cell Viability
Ascorbic acid enhances the cytotoxicity of arsenic trioxide in HL-60 cells
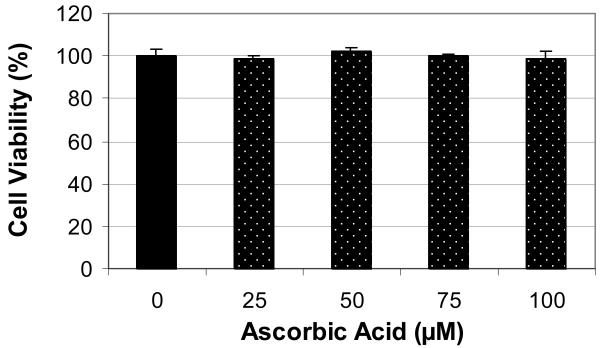
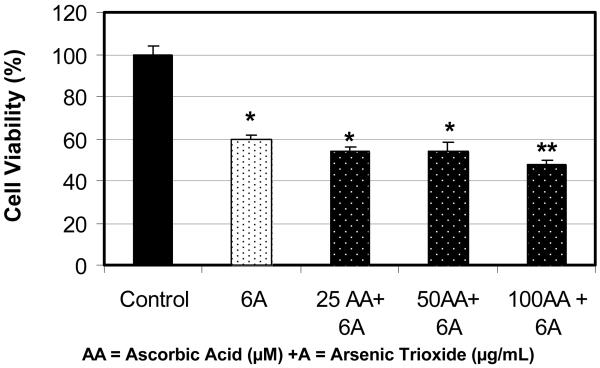
Figure 1 shows the effect of ascorbic acid (AA) on the viability of human leukemia (HL-60) cells using the MTT assay. As shown in this figure, physiological doses (10-100 μM) of AA have no effect on the viability of HL-60 cells. There were no significant (p>0.05) differences in cell viability between the control and AA-treated cells. Similar to this data, Zhang and his collaborators have tested the effect of vitamin C at different physiological concentrations ranging from 0 to 400 μM on the growth of gastric cancer cells and found that vitamin C, in this concentration range, has no effect on this cell line [16]. In contrary, at 24 hr of exposure to 6μg/mL dose of arsenic trioxide (ATO), the cell viability significantly decreased by 40% (Figure 2). Similarly, co-treatment of HL-60 cells with 6μg/mL ATO and AA at 25 and 50 μM concentrations did not significantly affects the viability of HL-60 cells compared to ATO alone. However, co-treatment of these cells using 100 μM AA with 6μg/mL ATO resulted in a higher level of cell death than did ATO alone. We found that the viability of HL-60 cells declined from 60% to 42% in cells treated with 100 μM AA and 6 μg/mL ATO compared with cells treated with ATO alone. Hence, co-exposure to AA (100 μM) and ATO resulted in higher level of cell death as compared to ATO alone. Similar results were also obtained using trypan blue exclusion test (data not shown).
Figure 1.
Effect of ascorbic acid (AA) to human leukemia (HL-60) cells. HL-60 cells were cultured with different doses of AA for 24 hr as indicated in the Materials and Methods. Cell viability was determined based on the MTT assay. Each point represents a mean value and standard deviation of 3 experiments with 6 replicates per dose.
Figure 2.
Potential effect of co-administration of ascorbic acid (AA) and arsenic trioxide (ATO) to human leukemia (HL-60) cells. HL-60 cells were cultured in the absence or presence of AA and ATO or in combination of AA and ATO for 24 hr as indicated in the Materials and Methods. Cell viability was determined based on the MTT assay. Each point represents a mean value and standard deviation of 3 experiments with 6 replicates per dose. *Significantly different from the control by ANOVA Dunnett's test; p < 0.05. **Significantly different from ATO alone by ANOVA Dunnett's test; p < 0.05.
N-acetyl-l-cysteine inhibits the cytotoxicity of arsenic trioxide in HL-60 cells
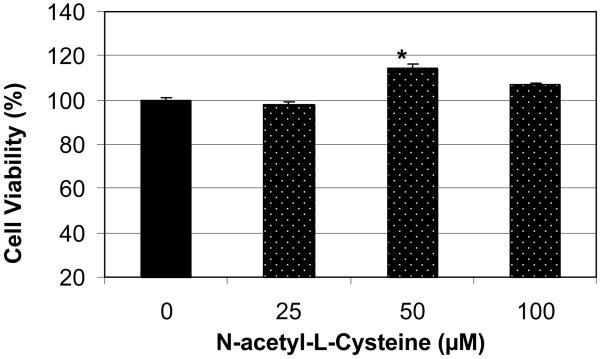
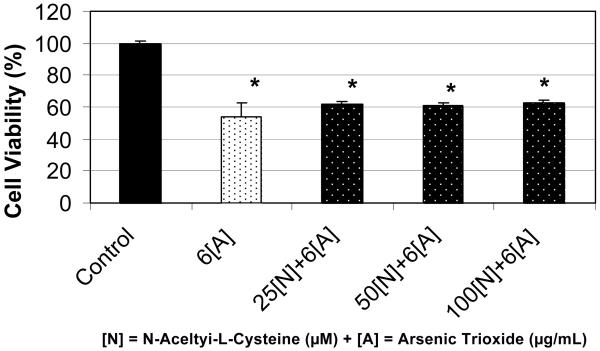
Using the MTT method, we found that treatment of HL-60 cells with n-acetyl-l-cysteine (NAC) at 25 μM did not affect cell viability compared to the control. However, the treatment of these cells with NAC at 50 and 100 μM demonstrated a marked increase in cell viability compared to the control, suggesting a stimulatory effect of this antioxidant especially at the 50 μM where there is a significant increase (p<0.05) in cell viability compared to the control (Figure 3). Although there was significant increase in cell viability at 50μM of NAC, the statistical analysis did not show a significant difference in cell proliferation at 100 μM of NAC. In contrary, the viability of HL-60 cells decreased by 46% when treated with 6μg/mL arsenic trioxide (ATO) compared to the control (Figure 4). Interestingly, co-treatment of these cells using 6μg/mL ATO with NAC at 25, 50, and 100 μM demonstrated a slight increase in cell viability compared to ATO treatment alone, indicating that NAC may afford protection against the toxicity associated with ATO exposure. For instance, in cells treated with 100 μM NAC and 6μg/mL ATO, the viability increased was 63% compared to 54% for cells treated with ATO alone. Similar results were also obtained using the trypan blue exclusion test (data not shown).
Figure 3.
Effect of n-acetyl-l-cysteine (NAC) on human leukemia (HL-60) cells. HL-60 cells were cultured with different doses of NAC for 24 hr as indicated in the Materials and Methods. Cell viability was determined based on the MTT assay. Each point represents a mean value and standard deviation of 3 experiments with 6 replicates per dose. *Significantly different from the control by ANOVA Dunnett's test; p < 0.05.
Figure 4.
Potential effect of co-administration of n-acetyl-l-cysteine (NAC) and arsenic trioxide (ATO) to human leukemia (HL-60) cells. HL-60 cells were cultured in the absence or presence of NAC and ATO or in combination of NAC and ATO for 24 hr as indicated in the Materials and Methods. Cell viability was determined based on the MTT assay. Each point represents a mean value and standard deviation of 3 experiments with 6 replicates per dose. *Significantly different from the control by ANOVA Dunnett's test; p < 0.05.
Lipid Peroxidation Assay
Ascorbic acid enhances MDA production in arsenic trioxide- treated HL-60 cells
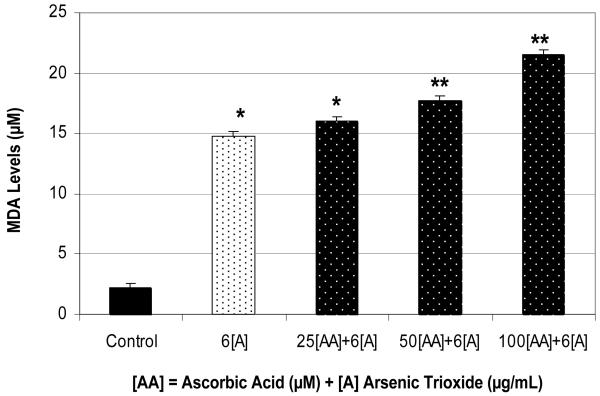
The treatment of HL-60 cells with arsenic trioxide (ATO) at 6 μg/mL significantly increases (p<0.05) MDA production compared to the control. Also, a concentration-dependent increase in MDA production in ATO-treated cells was associated with ascorbic acid (AA) exposure with the concentration range from 25-100 μM. A high level of MDA production was detected in HL-60 cells after 24 hr of ATO exposure compared to the control (Figure 5). Data presented in this figure also demonstrated that ascorbic acid co-treatment significantly potentiates the production of MDA, an indicator of lipid peroxidation. Taken together, co-administration of ascorbic acid and arsenic trioxide in culture cells resulted in a significant increase of lipid peroxidation (MDA) as result of oxidative stress, a biomarker of cellular injury. Finding from this experiment suggests that the pro-oxidant property of AA in vitro may increase ROS formation that potentiates the cytotoxicity of arsenic trioxide.
Figure 5.
Potentiation effect of AA on ATO-induced oxidative stress in HL-60 cells. Cells were incubated for 24 hr with 6 μg/mL ATO and various concentrations of AA (25, 50, and 100 μM). Malondialdehyde formation was determined as described in Materials and Methods. *Significantly different from the control by ANOVA Dunnett's test; p < 0.05. **Significantly different from ATO alone by ANOVA Dunnett's test; p < 0.05. Data are representative of 3 independent experiments.
N-acetyl-l-cysteine inhibits MDA production in arsenic trioxide treated HL-60 cells
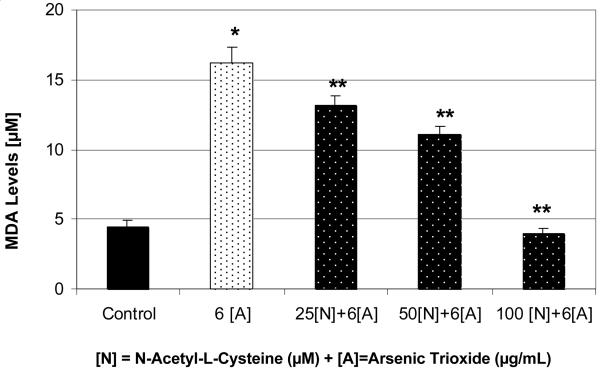
As in the case of ascorbic acid (AA), the treatment of HL-60 cells with 6 μg/mL of arsenic trioxide (ATO) resulted in a significant increase (p < 0.05) of MDA production compared to the control (Figure 6). Interestingly, co-treatment of these cells with n-acetyl-l-cysteine (NAC) resulted in a significant decrease (p<0.05) of MDA production compared to ATO alone (Figure 6). The MDA production level was greatly reduced when cells were treated with 100 μM NAC and 6μg/mL ATO compared to ATO alone. This experiment suggests that the protection of HL-60 cells from the toxicity of ATO by NAC is likely due to the inhibition of reactive oxygen species production and subsequent reduction of intracellular lipid peroxidation.
Figure 6.
Protective effect of NAC on ATO-induced oxidative stress in HL-60 cells. Cells were incubated for 24 hr with 6 μg/mL ATO and various concentrations of NAC (25, 50, and 100 μM). Malondialdehyde formation was determined as described in Materials and Methods. *Significantly different from the control by ANOVA Dunnett's test; p < 0.05. **Significantly different from ATO alone by ANOVA Dunnett's test; p < 0.05. Data are representative of 3 independent experiments.
DISCUSSION
Measurements of Cell Viability
Ascorbic acid enhances the cytotoxicity of arsenic trioxide in HL-60 cells
Arsenic trioxide (ATO) has previously been reported to be cytotoxic to various mammalian cancer cell lines [14, 17-20]. Data obtained from the present study indicate that the combination of ascorbic acid (AA) and ATO is highly cytotoxic to human promyelocytic leukemia (HL-60) cells. We found that AA enhances the cytotoxicity of ATO to HL-60 cells. AA treatment alone was not cytotoxic, suggesting that AA has the potential to be a safe and effective chemosensitizing agent in ATO-based chemotherapy. AA also known as vitamin C aids in the making of collagen, which is a big part of blood vessels, bones, joints, teeth, gums, and all connective tissues in the body [5]. Animal studies in rats using sodium arsenite indicated that vitamin C ameliorated arsenic-induced toxicity [21-22]. Recent publications have accumulated evidence showing that ascorbate enhanced the growth of leukemia colony forming cells derived from patients with acute myelocytic in 35% of the case only; 50% of cells were unresponsive; and the remaining 15% growth was inhibited [23].
Using the MTT and comet assays respectively, a recent report from our laboratory indicated that the pharmacology of ATO as an effective anti-cancer drug is associated with its cytotoxic and genotoxic effects in human leukemia cells [24]. These cytotoxic and genotoxic effects have been found to be mediated through oxidative stress [25]. We further demonstrated that the toxicity of ATO depends on the chemical dose, cell type, and exposure time [14, 25]. Current research has also reported many possible treatments for APL patients [26, 27]. However, the most prolific treatment remains ATO, or perhaps a combination of AA and ATO [17]. Interestingly, finding from our present studies suggest that the combination of these two compounds could be a more proficient treatment in killing cancer cells compared to ATO alone. Similar to our findings, previous studies have shown that AA potentiates ATO-mediated cytotoxicity in U266 cells [17, 28]. Others have demonstrated that AA enhances ATO-induced cytotoxicity in multiple myeloma cells [17].
N-acetyl-l-cysteine inhibits the cytotoxicity of arsenic trioxide in HL-60 cells
In the present study, we also investigated the protective effect of n-acetyl-l-cysteine (NAC) on arsenic trioxide-treated HL-60 cells. We found that cells co-exposed to both compounds and especially at 100 μM NAC resulted in a significant (p<0.05) increase in growth and proliferation compared to arsenic trioxide (ATO) alone. These findings showed a clear evidence that NAC acts as a potential chelator that attenuates ATO toxicity in HL-60 cells (Fig 5). This study is consistent with a recent in vitro finding from our laboratory indicating that NAC affords cellular protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells [29]. Further, previous studies have demonstrated that NAC has potential as an anti-tumorigenic agent with efficacy in preventing initial tumor take and metastasis [30, 31]. In vivo studies with animals and fetal cell cultures exposed to arsenic have shown that antioxidants such as NAC, vitamin E, and vitamin C, given in conjunction with a chelating agent (DMSA) are able to restore glutathione levels and reduce damage secondary to oxidative stress [32].
Lipid Peroxidation Assay
Ascorbic acid enhances MDA production in arsenic trioxide-treated HL-60 cells
The present study indicates that the treatment of HL-60 cells with arsenic trioxide (ATO) produces a significantly higher level of MDA. This significant increase in MDA formation was further exacerbated by the co-exposure to ascorbic acid (AA) within the concentration range of 25-100 uM. Because AA potentiated ATO-mediated cell death, it is possible that ATO treatment increased ROS production. Consistent with this finding, published reports indicate that arsenic induces the generation of reactive oxygen species (ROS) that contribute significantly to cell killing [23, 33, 34]. Recent studies reported that ascorbate-mediated killing in HL-60 cells depends on the levels of H2O2 produced by the reaction of AA within the cell culture medium, and direct addition of H2O2 to the cells reproduced these results [35]. Because ATO is the standard treatment of choice for refractory cases of acute promyelocytic leukemia (APL), these observations with HL-60 cells led us to postulate that the combination of AA and ATO might improve the clinical outcome in APL patients, based on its antiproliferative activity and induction of oxidative stress that may lead to apoptosis in tumor cells. A recent report demonstrated that ascorbic acid in the presence of transition metals stimulates the decomposition of products of lipid peroxidation to fatty acid metabolites such as HNE [36]. To our knowledge, the synergism between ascorbic acid and arsenic trioxide may, therefore, be responsible for enhancing ATO cytotoxicity. Although the mechanism by which AA enhances ATO-mediated cytotoxicity in HL-60 cells remains unknown, here we provide evidence that AA potentiates ATO-induced oxidative stress in human leukemia (HL-60) cells.
N-acetyl-l-cysteine inhibits MDA production in arsenic trioxide-treated HL-60 cells
We evaluated the induction of lipid peroxidation in arsenic trioxide-treated HL-60 cells in the absence or presence of n-acetyl-l-cysteine (NAC) by estimating the levels of malondialdehyde. We found that NAC attenuates the arsenic trioxide (ATO) effects on cytotoxicity and MDA formation; confirming that NAC exerts a protective effect against ATO- mediated oxidative stress. Similarly, other antioxidants such as vitamin E have been reported to inhibit arsenic cytotoxicity in vitro and in vivo [37], while other such as catalase have been implicated in the suppression on arsenic-induced apoptosis [38]. Our results suggest that NAC protects HL-60 cells from the cytotoxicity and oxidative stress induced by arsenic trioxide. We observed a maximum cell protection from oxidative stress and cell injury at 100 μM of NAC, indicating the inhibitory activity of NAC on arsenic trioxide toxicity in vitro. Findings from this study show that the combination of NAC and ATO may have potential specificity for tumor cells, indicating it may not enhance the clinical outcome associated with ATO monotherapy in vivo. Consistently, recent studies reported that antioxidant NAC co-treatment prevented arsenic trioxide-induced apoptosis in U937 cells and lowered the toxicity of certain chemotherapy agents [32, 39]
CONCLUSIONS
The mechanisms by which arsenic trioxide (ATO) induces cytotoxicity and oxidative stress in mammalian cell lines are not completely elucidated. However, several reports point to the potential role of oxidative stress in ATO mediated toxicity. In this research, we examine the role of oxidative stress and the modulatory effect of ascorbic acid (AA) and n-acetyl-l-cysteine (NAC) on ATO-mediated toxicity in human leukemia cells. Data generated from these studies showed that AA enhances ATO-induced MDA production in a dose-dependent manner. This antioxidant failed to prevent ATO cytotoxicity and acted instead as a pro-oxidant by increasing the levels of cytotoxicity and lipid peroxidation induced by ATO. These findings highlight the potential effect of AA in promoting the pharmacology of ATO, suggesting a possible future role of AA/ATO combination therapy in patients with acute promyelocytic leukemia (APL). Furthermore, the combination of ATO and ROS-producing agents may provide a new strategy to enhance therapeutic activity and overcome drug resistance. On the other hand, the antioxidant NAC attenuates the ATO effects on cytotoxicity and formation of MDA, confirming that oxidative stress is an important mechanism mediating ATO toxicity. Based on these studies, clinical trials are needed to test the use of AA to enhance ATO activity in the treatment of APL and other malignancies. In summary, results from this investigation demonstrate that incubation of HL-60 cells with AA and ATO is associated with an increase in lipid peroxidation and cell injury, and that antioxidant suppressors of lipid peroxidation and free radicals such as NAC protect against this form of cell injury.
ACKNOWLEDGEMENTS
This research was financially supported by a grant from the National Institutes of Health (Grant No. 1G12RR13459), through the RCMI-Center for Environmental Health at Jackson State University.
REFERENCES
- 1.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP. Complete Remission after Treatment of Acute Promyelocytic Leukemia with Arsenic Trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 2.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutré S, Dahlberg S, Ellison R, Warrell RP., Jr. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 3.Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.can-2820-2. [DOI] [PubMed] [Google Scholar]
- 4.Frei Balz. Reactive oxygen species and antioxidant vitamins: mechansims of action. American Journal of Medicine. 1994 September 26;97(Suppl 3A):5S–13S. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 5.Pauling L. Vitamin C and the Common Cold. Freeman; San Francisco, CA: 1970. [Google Scholar]
- 6.Uddin S, Ahmad S. Antioxidants protection against cancer and other human diseases. Comprehensive Therapy. 1995;21(1):41–45. [PubMed] [Google Scholar]
- 7.Schwartz JL. The dual roles of nutrients as antioxidants and prooxidants: Their effects on tumor cell growth. Journal of Nutrition April. 1996;126:1221–1227. doi: 10.1093/jn/126.suppl_4.1221S. [DOI] [PubMed] [Google Scholar]
- 8.Mayer M, Noble M. N-Acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sc USA. 1994;91:7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotgreave IA. N-acetylcysteine: Pharmacological considerations and experimental and clinical applications. Ad Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 10.Ramanathan K, Balakumar BS, Panneer-Selvam C. Effects of ascorbic acid and a-tocopherol on arsenic-induced oxidative stress. Hum Exp Toxicol. 2002;21:675–80. doi: 10.1191/0960327102ht307oa. [DOI] [PubMed] [Google Scholar]
- 11.Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 12.Paul J. Cell and Tissue Culture. Churchill Livingstone (CSL); 1975. [Google Scholar]
- 13.Freshney RI. A manual of basic techniques. Alan Liss Inc (University Library); 1983. Culture of animal cells. [Google Scholar]
- 14.Yedjou CG, Moore P, Tchounwou PB. Dose and time-dependent response of human acute promyelocytic leukemia (HL-60) cells to arsenic trioxide treatment. Int J Environ Res Public Health. 2006;3(2):136–140. doi: 10.3390/ijerph2006030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z-W, Abdullahi M, Farthing MJG. Effect of physiological concentrations of vitamin C on gastric cancer cells and Helicobacter pylori. Gut. 2002;50(2):165–169. doi: 10.1136/gut.50.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, Boise LH. Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells. Blood. 2001;98:805–813. doi: 10.1182/blood.v98.3.805. [DOI] [PubMed] [Google Scholar]
- 18.Bachleitner-Hofmann T, Gisslinger B, Grumbeck E, Gisslinger H. Arsenic trioxide and ascorbic acid: synergy with potential implications for the treatment of acute myeloid leukaemia? Br J Haematol. 2001;112:783–786. doi: 10.1046/j.1365-2141.2001.02608.x. [DOI] [PubMed] [Google Scholar]
- 19.Gao F, Yi J, Shi G, Li H, Shi X, Wang Z, Tang X. Ascorbic acid enhances the apoptosis of U937 cells induced by arsenic trioxide in combination with DMNQ and its mechanism. ChungHua Hsueh Yeh Hsueh Tsa Chih [Chinese Hematol] 2002;23:9–11. [PubMed] [Google Scholar]
- 20.Tchounwou PB, Yedjou CG, Dorsey WC. Arsenic trioxide induced transcriptional activation and expression of stress genes in human liver carcinoma cells (HepG2) Cellular and Molecular Biology™. 2003;49(7):1071–1079. [PubMed] [Google Scholar]
- 21.Ramanathan K, Balakumar BS, Panneerselvam C. Effects of ascorbic acid and alpha-tocopherol on arsenic-induced oxidative stress. Hum Exp Toxicol. 2002;21:675–680. doi: 10.1191/0960327102ht307oa. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan K, Shila S, Kumaran S, Panneerselvam C. Ascorbic acid and alpha-tocopherol as potent modulators on arsenic induced toxicity in mitochondria. J Nutr BioChem. 2003;14:416–420. doi: 10.1016/s0955-2863(03)00076-7. [DOI] [PubMed] [Google Scholar]
- 23.Park CH. The biological nature of the effect of ascorbic acid on the growth of human leukemic cells. Cancer Res. 1985;45:3969–73. [PubMed] [Google Scholar]
- 24.Yedjou CG, Tchounwou PB. In vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoreis (comet) assays. Mol Cell Biochem. 2007;301:123–130. doi: 10.1007/s11010-006-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yedjou C, Tchounwou P. Oxidative stress in human leukemia (HL-60), human liver carcinoma (HepG2), and human Jurkat-T cells exposed to arsenic trioxide. Metal Ions Biol Med. 2006;9:293–297. [PMC free article] [PubMed] [Google Scholar]
- 26.Berenson JR, Boccia R, Siegel D, Bozdech M, Bessudo A, Stadtmauer E, Pomeroy JT, Steis R, Flam M, Lutzky J, Jilani S, Volk J, Wong S-F, Moss R, Patel R, Ferretti D, Russell K, Louie R, Yeh HS, Swift RA. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: a prospective, multicentre, phase II, single-arm study. British Journal of Haematology. 2006;135:174–183. doi: 10.1111/j.1365-2141.2006.06280.x. [DOI] [PubMed] [Google Scholar]
- 27.Raffoux E, Rousselot P, Poupon J, Daniel M-T, Cassinat B, Delarue R, Taksin A-L, Rea D, Buzyn A, Tibi A, Lebbe G, Cimerman P, Chomienne C, Fermand J-P, de The H, Degos L, Hermine O, Dombret H. Combined Treatment With Arsenic Trioxide and All-Trans-Retinoic Acid in Patients With Relapsed Acute Promyelocytic Leukemia. JCO. 2003;21:2326–2334. doi: 10.1200/JCO.2003.01.149. [DOI] [PubMed] [Google Scholar]
- 28.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- 29.Yedjou CG, Tchounwou PB. N-acetyl-l-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int J Environ Res Public Health. 2007;4(2):1326–137. doi: 10.3390/ijerph2007040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai T, Fassina G, Morini M, Aluigi MG, Masiello L, Fontanini G, D'Agostini F, De Flora S, Noonan DM, Albini A. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab Invest. 1999;79:1151–1159. [PubMed] [Google Scholar]
- 31.Aluigi MG, De Flora S, D'Agostini F, Albini A, Fassina G. Antiapoptotic and antigenotoxic effects of N-acetylcysteine in human cells of endothelial origin. Anticancer Res. 2000;20:3183–3187. [PubMed] [Google Scholar]
- 32.De Flora S, D'Agostini F, Masiello L, Giunciuglio D, Albini A. Synergism between N-acetylcysteine and doxorubicin in the prevention of tumorigenicity and metastasis in murine models. Int J Cancer. 1996;67:842–848. doi: 10.1002/(SICI)1097-0215(19960917)67:6<842::AID-IJC14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Jing Y, Dai J, Charmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:102–2111. [PubMed] [Google Scholar]
- 35.Clement MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Redox Signal. 2001;3:157–163. doi: 10.1089/152308601750100687. [DOI] [PubMed] [Google Scholar]
- 36.Retsky K, Frei B. Vitamin C prevents metal ion-dependent initiation and propagation of lipid peroxidation in human low-density lipoprotein. Biochim Biophys Acta. 1995;1257:279–287. doi: 10.1016/0005-2760(95)00089-u. [DOI] [PubMed] [Google Scholar]
- 37.Chen A, Cao EH, Zhang TC, Qin JF. Arsenite-induced reactive oxygen species and the repression of alpha-tocopherol in the MGC-803 cells. Eur J Pharmacol. 2002;448:11–18. doi: 10.1016/s0014-2999(02)01901-5. [DOI] [PubMed] [Google Scholar]
- 38.Woo SH, Park IC, Park MJ, Lee HC, Lee SJ, Chun YJ, Lee SH, Hong SI, Rhee CH. Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in HeLa cells. Int J Oncol. 2002;21:57–63. [PubMed] [Google Scholar]
- 39.Choi YJ, Park JW, Suh SI, Mun KC, Bae JH, Song DK, Kim SP, Kwon TK. Arsenic trioxide-induced apoptosis in U937 cells involve generation of reactive oxygen species and inhibition of Akt. Int J Oncol. 2002;21:603–610. [PubMed] [Google Scholar]