Abstract
An essential function of the innate immune system is to directly trigger antimicrobial mechanisms to defend against invading pathogens. In humans, one such pathway involves activation by TLR2/1L leading to the vitamin D-dependent induction of antimicrobial peptides. In this study, we found that TLR2/1-induced IL-15 was required for induction of CYP27b1, the VDR and the downstream antimicrobial peptide cathelicidin. Although both IL-15 and IL-4 triggered macrophage differentiation, only IL-15 was sufficient by itself to induce CYP27b1 and subsequent bioconversion of 25-hydroxyvitamin D3 (25D3) into bioactive 1,25D3, leading to VDR activation and induction of cathelicidin. Finally, IL-15-differentiated macrophages could be triggered by 25D3 to induce an antimicrobial activity against intracellular Mycobacterium tuberculosis. Therefore, IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway.
The ability of the innate immune system to recognize microbial pathogens is mediated by highly conserved families of pattern recognition receptors that activate host defense pathways. One such family, the TLRs, expressed by a variety of cells of the innate immune system, with each family member germline encoded to recognize a distinct biochemical class of conserved microbial molecules. Activation of TLRs triggers the release of immunomodulatory cytokines, which contribute to inflammation and cell differentiation. In particular, the release of GM-CSF and induction of the GM-CSF receptor triggers differentiation of monocytes into immature dendritic cells (iDC),2 which instruct the adaptive T cell response (1, 2). TLR2/1-mediated induction of IL-15/IL-15Rα triggers differentiation of monocytes into macrophages (Mϕ) expressing CD209, a C-type lectin that mediates phagocytosis of bacteria, viruses, and fungi (1, 3–6).
A key function of TLRs in innate immunity is the activation of direct antimicrobial pathways (7), which in human monocytes and Mϕ is mediated by the vitamin D-dependent production of the antimicrobial peptide cathelicidin (8, 9). Activation of this antimicrobial pathway has been shown to require three events. First, TLR2/1 mediates induction of the 25D-1α-hydroxylase CYP27b1, which converts 25D to bioactive 1,25D, which triggers antimicrobial activity against Mycobacterium tuberculosis (10, 11). Second, TLR2/1 activation up-regulates expression of the vitamin D receptor (VDR), required for transcription of the cathelicidin gene, which contains cis-acting vitamin D response elements (8). Third, the induction of cathelicidin, an antimicrobial peptide with activity against intracellular M. tuberculosis, is required for the vitamin D-dependent antimicrobial activity (12).
The aforementioned studies identify two key pathways of the human innate immune system: the TLR2/1-induced differentiation of monocytes into distinct populations of iDC and Mϕ, and a TLR2/1-induced vitamin D-dependent antimicrobial activity. We postulated that these two pathways were not distinct, but unified by a common mediator. Therefore, the mechanism of TLR2/1-induced vitamin D-dependent antimicrobial activity was investigated in the context of TLR2/1-induced monocyte differentiation.
Materials and Methods
TLR2/1 ligand, Abs, and cytokines
The TLR2/1L, a triacylated lipopeptide of the M. tuberculosis 19 kDa Ag, was purchased (EMC Microcollections). Abs for cell surface molecules: CD14 (Zymed), CD1b (OKT6, Bcd3.1, ATCC), CD16, CD32, CD64, CD209 (DC-SIGN), CD206 (BD Pharmingen), TLR2 (gift from Genentech), and IgG controls (Sigma-Aldrich). Recombinant cytokines used: rIL-4 (PeproTech), rIL-15 (R&D Systems), and rGM-CSF (Immunex). IL-15 blocking Ab was obtained from R&D systems (clone 34559).
Mϕ and DC differentiation
We obtained whole blood from healthy donors (UCLA I.R.B. no. 92−10−591−31) with informed consent. Peripheral monocytes were isolated via plastic adherence for 2 h in 1% FCS, washed, and then cultured with either rIL-15 (IL15-Mϕ), rIL-4 (IL4-Mϕ), or rGM-CSF (iDC) for 48 h in RPMI 1640 and 10% FCS (Omega Scientific). IL15-Mϕ and IL4-Mϕ were CD209+ while iDC were CD1b+.
Cell surface labeling
Surface expression was determined using epitope-specific Abs. A PE-conjugated secondary Ab (Caltag) was used for CD1b. Cells were acquired and analyzed as described (1). For blocking, monocytes were stimulated with TLR ligands in the presence of media, a IL-15-specific Ab (R&D Systems) or an isotype control.
Measurement of 25D3 to 1,25D3 bioconversion by Mϕ and iDC
IL15-Mϕ, IL4-Mϕ, and iDC were differentiated as above. 1α-hydroxylase (CYP27b1) activity in these cells was then assessed by quantifying the conversion of radiolabeled 25D3 to 1,25D3 in serum-free cultures. For each assay 50 nM [3H]-25D (a 1 in 10 mixture of unlabelled 25OHD and [3H]-25D, (180 Ci/mmol, Amersham Biosciences) was added to 106 cells in 200 μl of serum-free medium and then incubated for 5 h at 37°C, with the reaction being terminated by freezing at −20°C. Protein from these samples was initially precipitated with added acetonitrile (1/1). Vitamin D metabolites were then extracted from the reaction mixtures by elution on C18-OH columns according to manufacturer's instructions (Diasorin). The resulting eluent was resuspended in 25 μl of elution solvent hexane:methanol:isopropanol (90:5:5), vortexed for 15 s, and individual metabolites separated by HPLC using a Beckman Gold system with an Agilent Technologies Zobax Sil normal phase column (Agilent Technologies) eluted at a rate of 1.5 ml/min for 20 min. Elution profiles for standard vitamin D metabolites (25D, 24,25D, 1,25D) were determined by UV absorbance at 264 nm. Elution of metabolites of [3H]-25D was assessed using a β-Ram Model 4 in-flow detector (IN/US) in conjunction with Ultima-Flo M scintillation fluid (PerkinElmer) at a 2:1 ratio with a 5 s dwell time to designate the increments for data collection. Lauralite 3 software (LabLogic) was used to quantitate peaks of radioactivity corresponding to 25D or 1,25D. Data were reported as mean fmoles metabolite synthesized/h/106 cells ± SD.
Real time qPCR
Monocytes were activated as above for 24 h. RNA was isolated using TRIzol (Invitrogen). cDNA was synthesized using the SuperScript Choice System Kit (Invitrogen). h36B4, CYP27b1, VDR, and cathelicidin primer sequences were used as described (8). Reactions use Sybr Green PCR Master Mix (Bio-Rad). The relative quantities of GM-CSF and IL-15 per sample were calculated as described (1). The data was normalized by fold change to media control. For cytokine induction of cathelicidin, values were log10 transformed before Student's t test.
Infection with M. tuberculosis and activation with vitamin D
IL15-Mϕ IL4-Mϕ and iDC were obtained as described above. On day 2, cells were washed and replated with 10% FCS (Omega Scientific). Cells were then infected with M. tuberculosis (H37Ra) at a multiplicity of infection of one for 16 h at 37°C. One set of cells was harvested for the CFU assay to obtain day 0 counts and the remaining sets of cells were cultured with 25D3 (10−7, 10−8; BIOMOL), 1,25D3 (10−8 M; BIOMOL), or left unstimulated. Infected cells were harvested three days after infection and lysed for the CFU assay (8).
Results
TLR2/1-mediated induction of CYP27b1, VDR, and cathelicidin is dependent on IL-15
We hypothesized that the TLR2/1-induced Mϕ differentiation (1) and antimicrobial pathways (8) intersect, and therefore investigated whether IL-15 was a common link. Human peripheral monocytes were stimulated with a TLR2/1L, the triacylated lipopeptide of the M. tuberculosis 19 kDa lipoprotein (7), which resulted in the cell surface expression of IL-15 (Fig. 1a). Addition of an IL-15-specific mAb significantly reduced the TLR2/1-mediated gene induction of CYP27b1, the VDR and cathelicidin (Fig. 1, b–d; p < 0.01, p < 0.05, p < 0.005, respectively), while the control Ab had no significant effect on gene induction. Although anti-IL-15 blocked the TLR2/1-mediated induction of CYP27b1 and the VDR by ∼60−70%, the inhibition of cathelicidin expression was approximately 80% (Fig. 1e). These data indicate that the TLR2/1-mediated induction of the vitamin D-dependent antimicrobial pathway genes requires IL-15 expression and activity.
FIGURE 1.
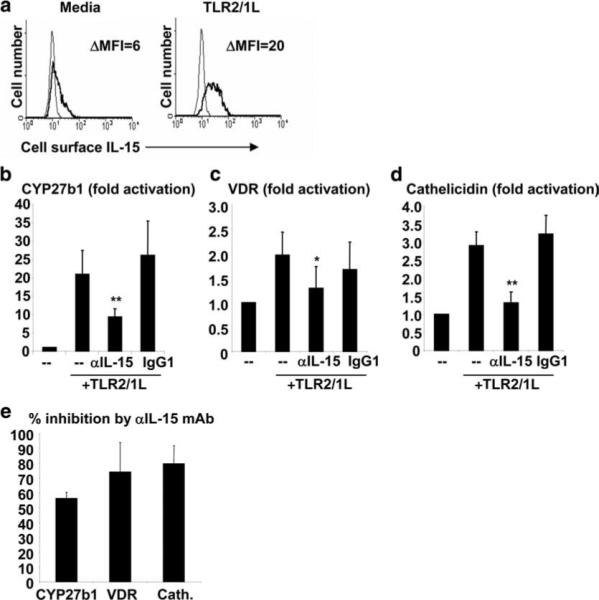
IL-15 mediates TLR2/1L-induced expression of CYP27b1, the VDR and cathelicidin. a, Cell surface expression of IL-15 following activation of monocytes with a TLR2/1L. b and c, Human peripheral monocytes were stimulated in 10% FCS with a TLR2/1L in the presence of medium, an IL-15 blocking Ab, or an isotype control Ab. Levels of CYP27b1 (n = 10) and VDR (n = 5) mRNA were measured after 24 h using qPCR. d, Monocytes were stimulated as above in vitamin D-sufficient serum (100 nM). Levels of cathelicidin (n = 6) mRNA were measured after 24 h using qPCR. Fold activation was calculated as ratio to media control cells (−). Data (b–d) are shown as the average ± SEM. e, Percent inhibition of TLR2/1-induced expression of CYP27b1, VDR, and cathelicidin by the IL-15 mAb. *, p < 0.05, **, p < 0.01.
IL-15 is sufficient to trigger the induction of CYP27b1, VDR, and cathelicidin
The TLR2/1-mediated induction of IL-15 has also been shown to drive the differentiation of monocytes into CD209+ Mϕ (1). Similarly, rIL-4 has been shown to induce CD209 expression on monocytes (13). To compare the effects of IL-15 and IL-4 on monocyte differentiation, we treated monocytes with rIL-15 and rIL-4 and assessed their cell surface phenotype. Both rIL-15 and rIL-4 induced differentiation of monocytes into CD209+ CD14+CD16+Mϕ (1), yet with distinct FcR expression patterns (Fig. 2). These Mϕ differentiation pathways are therefore distinct from each other, and from the TLR2/1-induced and/or GM-CSF-dependent monocyte differentiation into CD1b+ iDC (Fig. 2) (1, 2).
FIGURE 2.
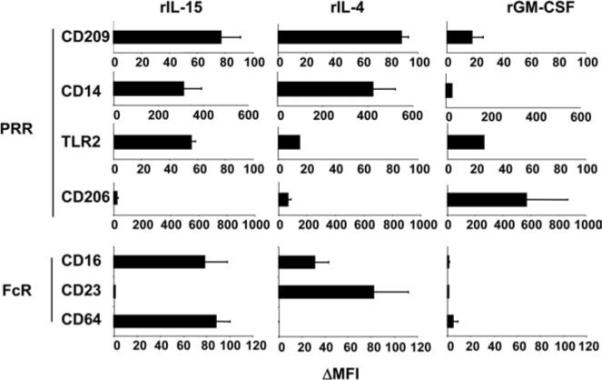
Phenotype of rIL-15, rIL-4, and GM-CSF differentiated cells. Peripheral monocytes were differentiated with either rIL-15, rIL-4, or rGM-CSF for 48 h. Cell surface markers were measured using marker-specific Abs and analyzed by flow cytometry. Data represent the change in mean fluorescence intensity (MFI) relative to the isotype control and are the average ± SEM of four experiments.
In this context, we asked whether the direct actions of these cytokines on monocyte differentiation were sufficient for the induction of key vitamin D-dependent antimicrobial pathway elements. Activation of monocytes with rIL-15 triggered gene induction of CYP27b1 and the VDR by 55- and 2.8-fold (p < 0.01, p < 0.05), respectively (Fig. 3, a and b). In contrast, rIL-4 and rGM-CSF were comparatively less effective at induction of CYP27b1 (1.9- and 3.4-fold; p > 0.05, p < 0.05) and VDR (1.6- and 1.1-fold; p > 0.05, p > 0.05). Importantly, activation of monocytes with rIL-15 in 25D3-sufficient serum triggered cathelicidin gene expression (11.2-fold; p < 0.05) while rIL-4 and rGM-CSF did not (p > 0.05) (Fig. 3c). These data indicate that activation of peripheral monocytes with rIL-15 is sufficient to trigger Mϕ differentiation and an antimicrobial gene program that includes the up-regulation of CYP27b1, VDR, and cathelicidin.
FIGURE 3.
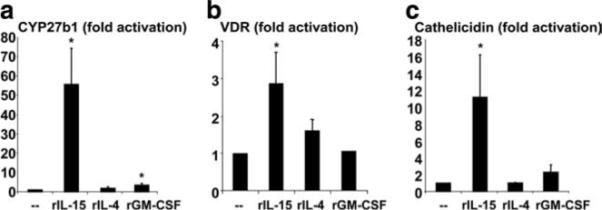
Induction of a vitamin D-dependent antimicrobial pathway by IL-15. Monocytes were activated with either rIL-15, rIL-4, rGM-CSF, or cultured with media alone for 24 h. RNA was isolated and levels of CYP27b1 (n = 10) (a), VDR (n = 10) (b) and cathelicidin (n = 4) (c) were measured by qPCR. Fold activation was calculated as ratio to media control cells (−). Data are shown as the average ± SEM. *, p < 0.05, **, p < 0.01.
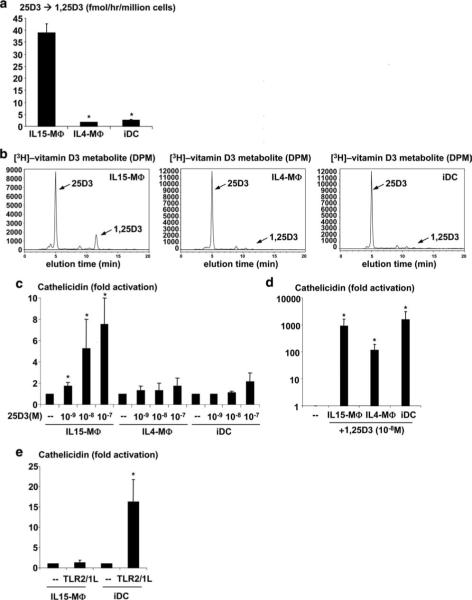
IL15-Mϕ convert 25D3 to 1,25D3 leading to cathelicidin gene expression
A key feature of the vitamin D-dependent antimicrobial pathway is that it requires the CYP27b1-dependent bioconversion of 25D3 to 1,25D3 as part of an intracrine loop permitting the extra-renal conversion and utilization of serum 25D3. To determine whether the IL-15-induced up-regulation of CYP27b1 gene expression leads to enhanced bioactivity, monocytes were first differentiated into Mϕ using rIL-15 (IL15-Mϕ) or rIL-4 (IL4-Mϕ) and into iDC using rGM-CSF (1, 2). Followed by the addition of radio-labeled 25D3, bioactive conversion of 25D3 to 1,25D3 was measured by high pressure liquid chromatography. IL15-Mϕ converted 25D3 to 1,25D3 at a significantly higher rate than IL4-Mϕ and iDC (35 fmol/min/million cells compared with 5.5 and 8.4 fmol/min/million cells, respectively, p < 0.05) (Fig. 4, a and b). Therefore, the ability of rIL-15 to induce CYP27b1 gene expression as part of the Mϕ gene program also results in bioconversion of 25D3 into bioactive 1,25D3.
FIGURE 4.
CYP27b1 bioactivity in Mϕ subsets and iDC. IL15-Mϕ, IL4-Mϕ, and iDC were obtained as described. Cells were then cultured with [3H]-25D3 and the amount of conversion to [3H]-1,25D3 was measured by HPLC. a, The rate of [3H]-25D3 to [3H]-1,25D3 conversion was calculated (n = 3). b, Representative HPLC plots demonstrating that only IL15-Mϕ convert 25D3 into 1,25D3. Arrow indicates the peak for 1,25D3. c and d, Mϕ and iDC were cultured with 25D3 (10−7 to 10−9 M) or 1,25D3 (10−8 M) and cathelicidin mRNA levels were determined by qPCR. Fold activation was calculated as ratio to media control cells (−). Data represent average ± SEM from between four and eight independent experiments. *, p < 0.05. e, IL15-Mϕ and iDC were activated with a TLR2/1L in the presence of vitamin D-sufficient serum and cathelicidin mRNA levels were determined by qPCR. Fold activation was calculated as above (n = 4). *, p < 0.05.
To determine whether the ability of IL15-Mϕ to bioconvert 25D3 to 1,25D3 leads to antimicrobial peptide gene expression, IL15-Mϕ, IL4-Mϕ, and iDC were cultured with 25D3 at a range of concentrations, and levels of cathelicidin mRNA were measured by qPCR. The addition of 25D3 (10−9 M to 10−7 M) was sufficient to trigger cathelicidin gene expression in IL15-Mϕ by 1.7-, 5.2-, and 7.5-fold (p < 0.05) compared with unstimulated controls, respectively (Fig. 4c). In contrast, in IL4-Mϕ and iDC, 25D3 did not significantly induce cathelicidin gene expression (Fig. 4c). However, the active 1,25D3 significantly induced cathelicidin gene expression in all three cell types (Fig. 4d; p < 0.05), indicating that they all have a functional VDR. Finally, activation of IL15-Mϕ with a TLR2/1 ligand in the presence of vitamin D sufficient serum did not induce cathelicidin gene expression compared with media control (1.2-fold, p = 0.67), whereas TLR2/1 activation of iDC induced cathelicidin by ∼16-fold (p < 0.05) (Fig. 4e). These data suggest that IL15-Mϕ, because of their unique capacity to bioconvert 25D3 to 1,25D3, utilize circulating 25D3 to trigger an antimicrobial gene program that includes the antimicrobial peptide cathelicidin.
IL15-Mϕ utilize 25D3 to trigger an antimicrobial activity
To determine the contribution of IL-15-induced Mϕ differentiation to the vitamin D-dependent antimicrobial pathway, the ability of IL15-Mϕ to utilize 25D3 to trigger antimicrobial activity was investigated. IL15-Mϕ were first infected with attenuated M. tuberculosis (H37Ra) in media containing FCS, which we previously reported was vitamin D insufficient, to minimize activation of the VDR. After 18 h, the cells were washed to remove extracellular mycobacteria. The infected cells were then cultured with media, media plus 25D3, or media plus 1,25D3 as a positive control, and the number of viable intracellular bacteria was measured after 3 days using the colony forming unit assay. The result of one representative experiment is shown in Fig. 5a. In IL15-Mϕ, cultured in media alone, the number of viable M. tuberculosis increased over the course of the 3-day incubation (Fig. 5a). We have reported similar growth of M. tuberculosis in primary monocytes and Mϕ cultured in media alone (8, 12, 32). These data suggest that infection of monocytes, Mϕ, or IL15-Mϕ with M. tuberculosis does not directly activate a mycobacterial growth inhibition pathway.
FIGURE 5.
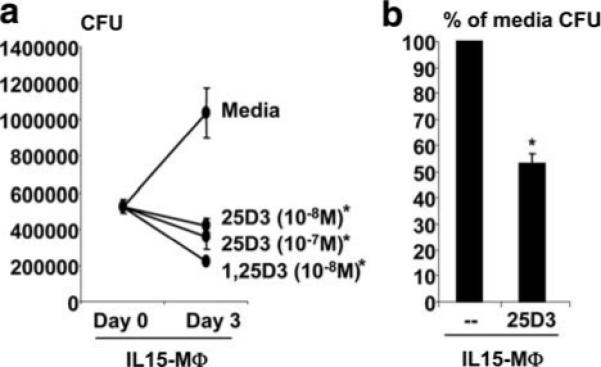
IL15-Mϕ have a vitamin D-inducible antimicrobial activity against M. tuberculosis. IL15-Mϕ were infected with M. tuberculosis H37Ra for 16 h and subsequently cultured with either medium, 25D3 (10−8 M), or 1,25D3 (10−8 M). After 3 days, cells were lysed and colonies of mycobacteria were enumerated using the CFU assay. Data are representative (a) and average (b) of three experiments ± SEM. *, p < 0.05.
The addition of 1,25D3 to M. tuberculosis infected IL15-Mϕ resulted in a 70% reduction in viable bacteria compared with cells cultured in media alone (p < 0.05) (Fig. 5a). The key finding was that the addition of 25D3 (10−7 M to 10−8 M) resulted in a 55−60% reduction in the number of viable bacteria in the IL15-Mϕ compared with cells cultured in media alone (p < 0.05) (Fig. 5a). In the three experiments performed, addition of 25D3 (10−8 M; 100 nM) to IL15-Mϕ triggered an ∼50% reduction (58−63%) in viable M. tuberculosis compared with treatment for 3 days with media alone (Fig. 5b). Together, the data suggest that IL15-Mϕ have the functional capacity to utilize 25D3 to trigger a direct antimicrobial activity against M. tuberculosis.
Discussion
As part of the innate immune response, cells of the monocyte/Mϕ lineage must recognize, then rapidly respond to and destroy, invading pathogens. A key antimicrobial pathway in human monocytes involves the TLR2/1-mediated activation of the vitamin D-dependent antimicrobial pathway. In this study, we demonstrate that IL-15, which is produced after TLR2/1 activation of human monocytes, up-regulates CYP27b1 and the VDR, allowing the bioconversion of 25D3 to 1,25D3, triggering expression of the antimicrobial peptide cathelicidin as well as antimicrobial activity against intracellular M. tuberculosis. At the same time, IL-15 induces differentiation of monocytes into CD209+ Mϕ with enhanced phagocytic activity for M. tuberculosis (1). Therefore, IL-15 amplifies the innate immune response by triggering a Mϕ differentiation program that includes the vitamin D-dependent antimicrobial pathway.
The classic function of IL-15 in host immune responses is defined by its ability to promote differentiation and activation of lymphocytes, including NK, B, and T cells, with regulation of CD8+ T cell homeostasis and the induction of T cell memory (14–20). IL-15 is less well known to have functional activity in cells of the myeloid lineage, reported to activate monocytes/Mϕ resulting in enhanced Ag presentation, cytokine/chemokine production and a superoxide-mediated anti-candida activity (21–23). Previously, we found that IL-15 differentiated human monocytes into Mϕ expressing the C-type lectin CD209, which mediates the phagocytosis of a diverse array of microbial pathogens expressing cell-surface carbohydrate-rich ligands including mycobacteria and HIV (3–6). In this study, we have linked the IL15-Mϕ differentiation program to the induction of the vitamin D-dependent antimicrobial pathway, by demonstrating that IL-15 induces CYP27b1 and VDR, bioconversion of 25D3 into 1,25D3, up-regulation of cathelicidin and antimicrobial activity against attenuated M. tuberculosis. Future studies will need to address the role of IL-15 in triggering the vitamin D-dependent antimicrobial activity against virulent M. tuberculosis. Nevertheless, the ability of IL-15 to trigger a Mϕ differentiation program linked to the phagocytosis and subsequent intracellular destruction of a microbial pathogen represents a powerful innate immune pathway. Therefore, IL-15 has a central functional role in host immunity, by programming both innate and adaptive cell differentiation programs.
The elements of the TLR2/1-induced vitamin D-dependent antimicrobial pathway define an intracrine loop, by which TLR2/1-regulated extra-renal utilization of 25D in Mϕ is required for antimicrobial activity (8). We found that IL-15 triggered induction of the 1α-hydroxylase CYP27b1, and demonstrated enhanced bioconversion of 25D3 into 1,25D3. This is a key step in the vitamin D antimicrobial pathway, given that specific pharmacologic inhibition of this p450 enzyme blocked TLR2/1-mediated induction of cathelicidin, which in turn is required for the antimicrobial response (8, 12). In a clinical correlation, serum from human donors with sufficient, but not insufficient levels of 25D3, was able to facilitate TLR2/1-induced cathelicidin expression. Similarly, we found that sufficient levels of 25D3 were required for induction of cathelicidin in IL15-Mϕ. Given that people of African and Asian descent, who are deficient in UV-dependent production of vitamin D, are known to have a high incidence of tuberculosis, the potential for therapeutic intervention by vitamin D supplementation to restore Mϕ function needs to be evaluated (8, 24–26). There are likely other pathways by which CYP27b1 can be induced; IFN-γ activation of human Mϕ also triggers conversion of 25D3 to 1,25D3 (27), although this may involve up-regulation of IL-15 and IL-15Rα (28, 29).
IL-15 is utilized by both human and murine Mϕ to trigger host defense against mycobacteria. In murine Mϕ, IL-15 activates iNOS/NO, a key mediator in this species for antimicrobial activity against M. tuberculosis (30–35). In murine models of mycobacterial infection, IL-15 also mediates the induction and maintenance of mycobacteria-specific T cell responses (33, 34). We demonstrate in this study that IL-15 was required in human monocytes for TLR2/1-mediated induction of cathelicidin gene expression, and sufficient by itself to trigger induction of cathelicidin gene expression and antimicrobial activity. Interestingly, the human but not mouse cathelicidin gene contains vitamin D response elements in its promoter region, suggesting an evolutionary divergence in host defense mechanisms. Importantly, both IL-15 and CD209+ Mϕ, identical in phenotype to IL15-Mϕ, were detected in lesions of the resistant tuberculoid form of leprosy as well as in lung biopsies of patients with tuberculosis (1, 36). Together these data suggest that the IL-15-triggered Mϕ differentiation program is relevant at the site of infection in mycobacterial diseases.
In this context, the identification of IL-15 as a mediator of the TLR2/1-induced vitamin D-dependent antimicrobial pathway provides a new paradigm for innate immune responses against intracellular pathogens. Infection of monocytes and macrophages with M. tuberculosis alone does not provide a sufficient endogenous signal to the innate immune response to generate an antimicrobial activity, but requires addition of exogenous TLR2/1L (8, 12, 32). These data suggest a model in which each infected cell requires activation by exogenous TLR2/1L to inhibit the growth of the intracellular mycobacteria, thereby requiring release of mycobacterial lipoproteins during the course of the infection. However, the present data demonstrate that IL-15 was required for TLR2/1L induction of cathelicidin and that IL-15 was sufficient to trigger activation of a vitamin D-dependent antimicrobial pathway. These data suggest an alternative model that does not require TLR activation of each infected cell, but instead TLR2/1L-induced IL-15 expression in a small number of cells could lead to activation of a vitamin D-dependent antimicrobial pathway in neighboring monocytes and Mϕs (17). The transpresentation of IL-15 provides a mechanism for amplification of the innate immune response, but does not preclude a direct role for TLR2/1 activation in infected cells.
Beyond the innate immune system, it is known that activated T cells, by their expression of CD40L, TRANCE, and IFN-γ trigger dendritic cells to release IL-15 and/or express IL-15 on the cell surface (37, 38). Therefore, it may be possible for the adaptive immune response to trigger Mϕ antimicrobial activity against intracellular pathogens in the absence of pattern recognition by the innate immune response. IL-15 then represents a possible common pathway, by which the innate and adaptive immune responses can directly trigger a Mϕ differentiation program linked to a vitamin D-dependent antimicrobial pathway, enhancing both phagocytosis and antimicrobial activity against invading pathogens. But in addition to its ability to trigger a Mϕ host defense mechanism, IL-15 is a lymphocyte differentiation factor, implicating IL-15 as potential therapeutic target for synergistically enhancing both innate and adaptive immune responses in humans.
Acknowledgments
We thank Dr. Genhong Cheng for scientific discussion and Kellie Corcoran for technical assistance.
Footnotes
Abbreviations used in this paper: iDC, immature dendritic cell; Mϕ, macrophage; VDR, vitamin D receptor.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DJ, Sieling PA, Ochoa MT, Krutzik SR, Guo B, Hernandez M, Rea TH, Cheng G, Colonna M, Modlin RL. LILRA2 activation inhibits dendritic cell differentiation and antigen presentation to T cells. J. Immunol. 2007;179:8128–8136. doi: 10.4049/jimmunol.179.12.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geijtenbeek TB, Kwon DS, Torensma R, Van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 4.Geijtenbeek TB, Torensma R, Van Vliet SJ, van Duijnhoven GC, Adema GJ, Van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 5.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 8.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 9.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ. IFN-γ- and TNF-independent vitamin D-inducible human suppression of Mycobacteria: the role of cathelicidin LL-37. J. Immunol. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 10.Rook GAW, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J. Vitamin D3, γ interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 11.Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect. Immun. 1987;55:2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 13.Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, Navarro J, Munoz-Fernandez MA, Sanchez-Mateos P, Corbi AL. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-β, and anti-inflammatory agents. J. Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 14.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, Caligiuri MA. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J. Clin. Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 17.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 18.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J. Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 19.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc. Natl. Acad. Sci. USA. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J. Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- 21.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J. Exp. Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saikh KU, Khan AS, Kissner T, Ulrich RG. IL-15-induced conversion of monocytes to mature dendritic cells. Clin. Exp. Immunol. 2001;126:447–455. doi: 10.1046/j.1365-2249.2001.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez N, Walsh TJ, Friedman D, Chanock SJ, Lyman CA. Interleukin-15 augments superoxide production and microbicidal activity of human monocytes against Candida albicans. Infect. Immun. 1998;66:145–150. doi: 10.1128/iai.66.1.145-150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, et al. A single dose of vitamin D enhances immunity to Mycobacteria. Am. J. Respir. Crit Care Med. 2007 doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 25.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J. Steroid Biochem. Mol. Biol. 2007;103:793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 27.Adams JS, Gacad MA. Characterization of 1 α-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J. Exp. Med. 1985;161:755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3–1α-hydroxylase in human monocytes. J. Bone Miner. Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 29.Yamaji K, Nabeshima S, Murata M, Chong Y, Furusyo N, Ikematsu H, Hayashi J. Interferon-α/β upregulate IL-15 expression in vitro and in vivo: analysis in human hepatocellular carcinoma cell lines and in chronic hepatitis C patients during interferon-α/β treatment. Cancer Immunol. Immunother. 2006;55:394–403. doi: 10.1007/s00262-005-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 31.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 32.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 33.Umemura M, Nishimura H, Hirose K, Matsuguchi T, Yoshikai Y. Overexpression of IL-15 in vivo enhances protection against Mycobacterium bovis bacillus Calmette-Guerin infection via augmentation of NK and T cytotoxic 1 responses. J. Immunol. 2001;167:946–956. doi: 10.4049/jimmunol.167.2.946. [DOI] [PubMed] [Google Scholar]
- 34.Lazarevic V, Yankura DJ, DiVito SJ, Flynn JL. Induction of Mycobacterium tuberculosis-specific primary and secondary T-cell responses in interleukin-15-deficient mice. Infect. Immun. 2005;73:2910–2922. doi: 10.1128/IAI.73.5.2910-2922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Zhai Q, Schaffner D, Bradburne C, Wu A, Hayford A, Popov S, Grene E, Bailey C, Alibek K. IL-15 induces IFN-β and iNOS gene expression, and antiviral activity of murine macrophage RAW 264.7 cells. Immunol. Lett. 2004;91:171–178. doi: 10.1016/j.imlet.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, Herrmann JL, Charles P, Schwartz O, Scheinmann P, Lagrange PH, de Blic J, Tazi A, Gicquel B, Neyrolles O. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005;2:e381. doi: 10.1371/journal.pmed.0020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josien R, Wong BR, Li HL, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J. Immunol. 1999;162:2562–2568. [PubMed] [Google Scholar]
- 38.Kuniyoshi JS, Kuniyoshi CJ, Lim AM, Wang FY, Bade ER, Lau R, Thomas EK, Weber JS. Dendritic cell secretion of IL-15 is induced by recombinant huCD40LT and augments the stimulation of antigen-specific cytolytic T cells. Cell. Immunol. 1999;193:48–58. doi: 10.1006/cimm.1999.1469. [DOI] [PubMed] [Google Scholar]