Abstract
A gene encoding the nucleoprotein (N) of rabies virus was inserted into the genome of the baculovirus Autographa californica nuclear polyhedrosis virus. Recombinant gene expression was controlled by the strong polyhedrin gene promoter. Insect cells (Spodoptera frugiperda) infected by a baculovirus recombinant containing the rabies virus N gene produced abundant amounts of a novel 55-kilodalton protein of a size comparable to that of the rabies virus N protein, as demonstrated by polyacrylamide gel electrophoresis. This new gene product possessed the antigenic and immunogenic properties of native viral N protein, as shown by the ability of the new protein to react in immunoprecipitation and immunofluorescence assays with antirabies antibodies, to serve as a substitute for infectious rabies virus in adsorbing suspensions for diagnostic tests, and to induce high-titered antiserum. The baculovirus expression system provides a safe, convenient, and inexpensive source of rabies virus N protein for the production of both antiserum and adsorbing suspensions for use in rabies diagnoses.
Full text
PDF
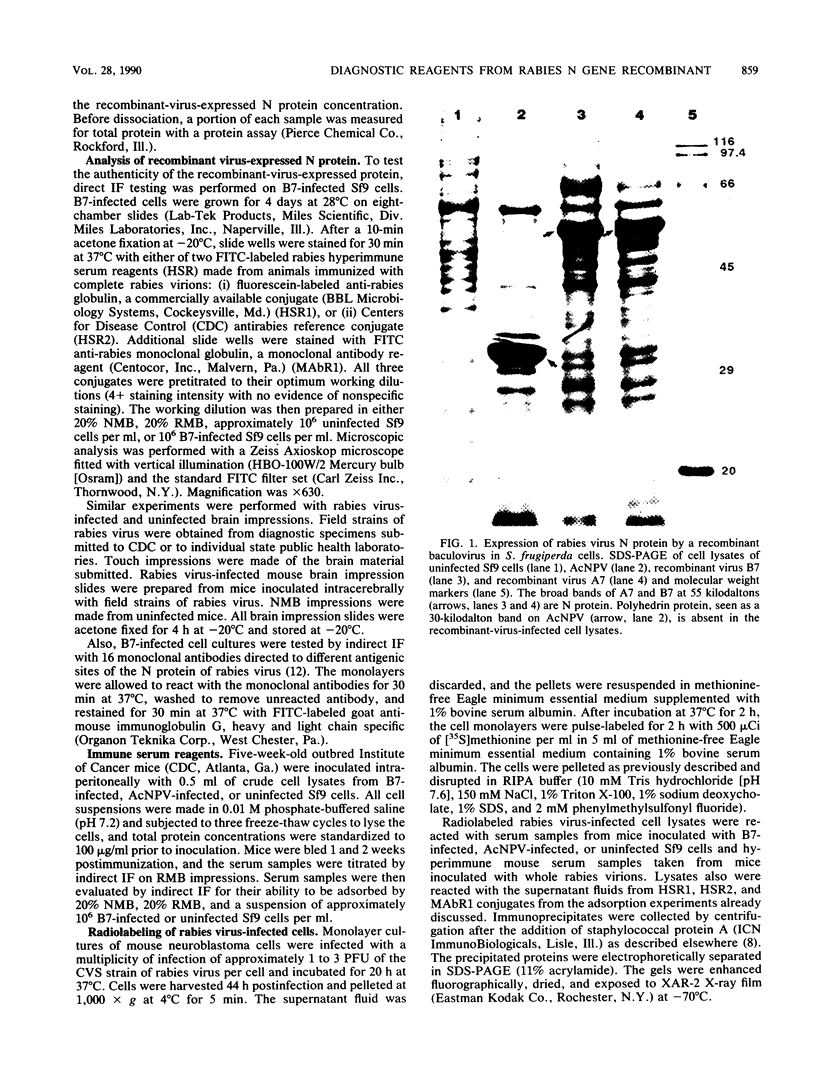
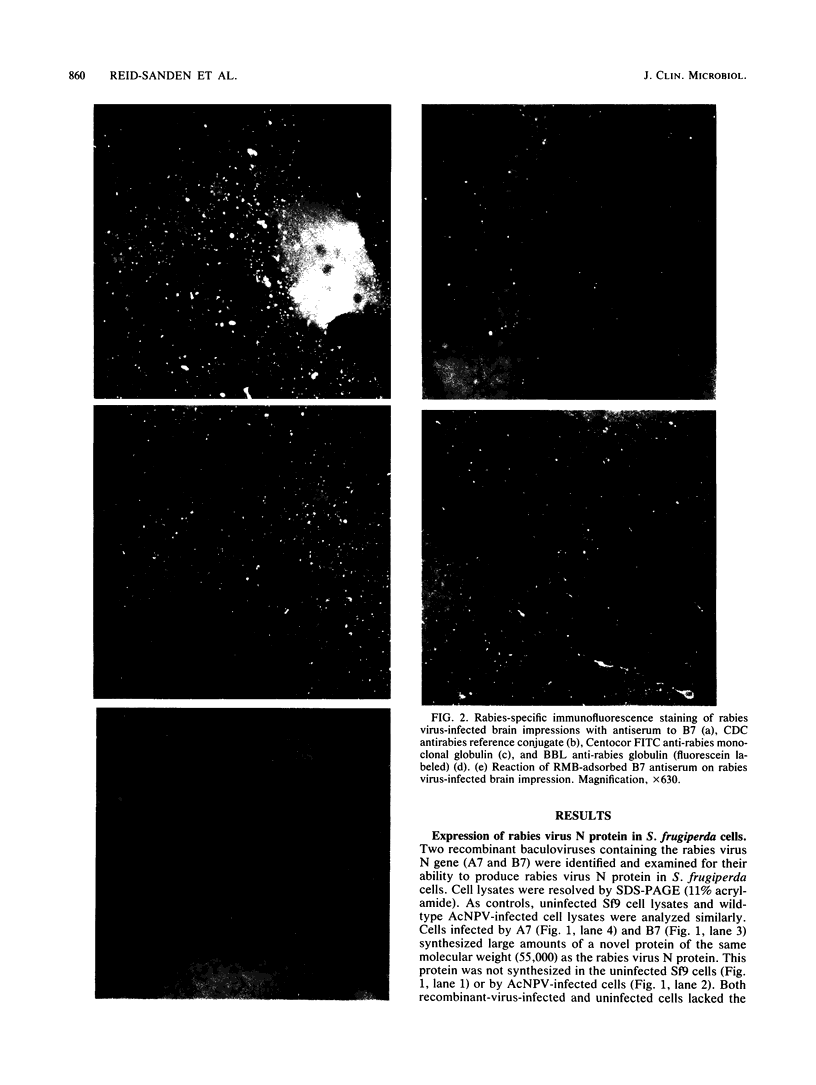


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durham T. M., Smith J. S., Reid F. L., Hale-Smith C. T., Fears M. B. Stability of immunofluorescence reactions produced by polyclonal and monoclonal antibody conjugates for rabies virus. J Clin Microbiol. 1986 Aug;24(2):301–303. doi: 10.1128/jcm.24.2.301-303.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein D. B., Belotto A. J., Pacer R. E., Smith J. S., Winkler W. G., Jenkins S. R., Porter K. M. Rabies in rodents and lagomorphs in the United States, 1971-1984: increased cases in the woodchuck (Marmota monax) in mid-Atlantic states. J Wildl Dis. 1986 Apr;22(2):151–155. doi: 10.7589/0090-3558-22.2.151. [DOI] [PubMed] [Google Scholar]
- Inumaru S., Ghiasi H., Roy P. Expression of bluetongue virus group-specific antigen VP3 in insect cells by a baculovirus vector: its use for the detection of bluetongue virus antibodies. J Gen Virol. 1987 Jun;68(Pt 6):1627–1635. doi: 10.1099/0022-1317-68-6-1627. [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Giam C. Z., Nerenberg M., Khoury G. Abundant synthesis of functional human T-cell leukemia virus type I p40x protein in eucaryotic cells by using a baculovirus expression vector. J Virol. 1987 Mar;61(3):708–713. doi: 10.1128/jvi.61.3.708-713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Bishop D. H. Expression of the S-coded genes of lymphocytic choriomeningitis arenavirus using a baculovirus vector. J Gen Virol. 1986 Aug;67(Pt 8):1515–1529. doi: 10.1099/0022-1317-67-8-1515. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Matsumoto S. The nature of the Negri body. J Cell Biol. 1965 Dec;27(3):677–682. doi: 10.1083/jcb.27.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L. G., Dietzschold B., Dierks R. E., Matthaeus W., Enzmann P. J., Strohmaier K. Rabies group-specific ribonucleoprotein antigen and a test system for grouping and typing of rhabdoviruses. J Virol. 1973 May;11(5):748–755. doi: 10.1128/jvi.11.5.748-755.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Reid-Sanden F. L., Roumillat L. F., Trimarchi C., Clark K., Baer G. M., Winkler W. G. Demonstration of antigenic variation among rabies virus isolates by using monoclonal antibodies to nucleocapsid proteins. J Clin Microbiol. 1986 Oct;24(4):573–580. doi: 10.1128/jcm.24.4.573-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]








