Abstract
Renal medullary endothelin-B (ETB) receptors contribute to blood pressure regulation by facilitating salt excretion. Pre-menopausal females have relatively less hypertension than males; therefore, we examined whether there is a sex difference in the natriuretic response to renal medullary infusion of endothelin peptides in the rat. All experiments were conducted in anesthetized wild type (wt) or ETB deficient (sl/sl) rats. Infusion of ET-1 significantly increased sodium excretion (UNaV) in female, but not male, wt rats (ΔUNaV 0.41±0.07 vs -0.04±0.06 μmol/min, respectively). The ETB receptor agonist, sarafotoxin 6c, produced similar increases in UNaV in both male (Δ0.58±0.15 μmol/min) and female (Δ0.67±0.18 Δmol/min) wt rats. Surprisingly, ET-1 markedly increased UNaV in female (Δ0.70±0.11 μmol/min) but not male sl/sl rats (Δ0.00±0.05 μmol/min). ET-1 had no effect on medullary blood flow (MBF) in females although MBF was significantly reduced to a similar extent in males of both strains. These results suggest that the lack of a natriuretic response to ET-1 in male rats is due to reductions in MBF. Treatment with ABT-627, an ETA antagonist, or NG-propyl-L-arginine, a NOS1 inhibitor, prevented the increase in UNaV observed in female rats. Gonadectomy eliminated the sex difference in the UNaV and MBF response to ET-1. These findings demonstrate that 1) there is no sex difference in ETB-dependent natriuresis, and 2) the ETA receptor contributes to ET-1-dependent natriuresis in female rats, an effect that requires NOS1. These findings provide a possible mechanism for why pre-menopausal women are more resistant to salt-dependent hypertension.
Keywords: sex, endothelin-1, sodium excretion, ETA, ETB, medullary blood flow
Introduction
Both animal and human studies have shown that the pre-menopausal female is relatively protected against the development of hypertension.1, 2 Experiments in normal Sprague Dawley or spontaneously hypertensive rats have shown that the pressure-natriuresis relationship of females is shifted towards lower blood pressures for a given level of sodium intake compared to males, suggesting that females have greater defense system against salt retention.3-5 Kawanishi et al. recently reported that female rats were relatively resistant to hypertension produced by DOCA-salt treatment compared to male rats, and that the sex difference in DOCA-salt hypertension was abolished in the rats that do not have a functional renal endothelin (ET) B (ETB) receptor.6 These findings indicate that the ETB receptor contributes to the sex difference in hypertension produced by mineralocorticoid and salt treatment.
Studies over the past decade have established ET-1 and the ETB receptor within the renal collecting duct system and perhaps the thick ascending limb as an important pro-natriuretic mechanism that serves to maintain the pressure-natriuresis relationship.7-9 Selective deletion of ET-1 or ETB receptor expression in collecting duct cells induces salt retention and blood pressure elevation in mice, clearly demonstrating the importance of the ET-1/ETB system in the control of salt balance.10, 11 We have recently demonstrated that intrarenal medullary infusion of sarafotoxin 6c (S6c), a selective ETB agonist, resulted in diuretic and natriuretic responses that were inhibited by selective NOS1 inhibition in anesthetized male rats,12 indicating that ET-1/ETB receptor signaling contributes to a decrease in salt and water reabsorption through a NOS1-dependent mechanism in renal medulla.
The first aim of the current study was to test the hypothesis that female rats have facilitated ETB-dependent natriuretic activity to prevent salt retention compared to male rats. In contrast to the ETB receptor, the physiological significance of the renal medullary ETA receptor is poorly understood despite the fact that the ETA receptor is expressed in medullary structures such as vasa recta, interstitial cells and collecting duct cells.13 Therefore, we also sought to determine the influence of ETA receptors using both male and female ETB receptor deficient rats on the natriuretic responses to intramedullary infusion of ET-1.
Methods
Animals
12-17 week old wild type (wt) and homozygous (sl/sl) rats deficient of ETB receptors on a Wister-Kyoto genetic background were obtained from our local breeding colony. Both wt and sl/sl rats carry the transgene for dopamine-ß-hydroxylase that rescues the ETB deficient rats from a lethal phenotype by expressing a functional ETB receptor in adrenergic tissues.14, 15 Experimental protocols and animal care methods were approved by the Medical College of Georgia Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Surgical preparation
Rats were anesthetized with inactin (100 mg/kg ip) and placed on a servo-controlled heating table to maintain rectal temperature constant at 37°C. The trachea was cannulated (PE-205) to facilitate respiration. The right femoral artery was catheterized for monitoring blood pressure using a MacLab data acquisition system (AD Instruments, Milford, MA), and the right jugular vein was catheterized for infusion of phosphate buffered 0.9% NaCl containing 6.2% bovine serum albumin (BSA) at a rate of 1.8 ml/h to replace fluid loss. A midline incision was made and a stretched PE10 catheter was inserted into the left kidney to a depth of 5 mm for male and 4.5 mm for female rats. After inserting the catheter, saline was infused directly into the renal medulla (0.5 ml/h) for the duration of the experiment. Medullary blood flow (MBF) and cortical blood flow (CBF) were measured by single-fiber, laser Doppler flowmetry as previously described.12, 16 Urine was collected separately from the left and right kidneys via catheters placed in each ureter. Experiments were started after an 80 min equilibration period. At the end of each experiment, the kidneys were dissected to ensure the catheter was in the appropriate position within the medulla.
Protocol
After two 20-min control periods, saline or increasing dosages of ET-1 or S6c (0.15 and 0.45 μg/kg/h; American Peptide, Inc., Sunnyvale, CA) were infused into the renal medullary interstitium of sl/sl or wt rats for two 20-min experimental periods at each dose. A separate group of rats received the ETA selective antagonist, ABT-627 (10 mg/kg, i.v.; Abbott Laboratories, Abbott Park, IL) 10 min prior to control periods. In third groups of rats, an adjustable aortic clamp was placed around the aorta upstream of the renal arteries and tightened 10 min prior to the control period such that renal perfusion pressure was maintained at an equivalent level to that of the ABT-627-treated rats. In another group of rats, NG-propyl-L-arginine (NPA; Calbiochem, Inc., La Jolla, CA) was co-infused with ET-1 into the renal medulla. NPA was infused beginning 40 min prior to ET-1 infusion in female wt and sl/sl rats.
Gonadectomy
A separate group of rats underwent gonadectomy (ovariectomy for female rats and orchidectomy for male rats) at 6 weeks of age under anesthesia using an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg). 6-8 weeks later, rats were prepared for intramedullary infusion and blood flow measurements as described above.
Statistical analysis
All values were expressed as the mean ± S.E.M. For statistical analysis, we used one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison tests. Student's t-test was used to determine the effect of gonadectomy in separate groups of male or female rats. Differences were considered significant at p<0.05.
Results
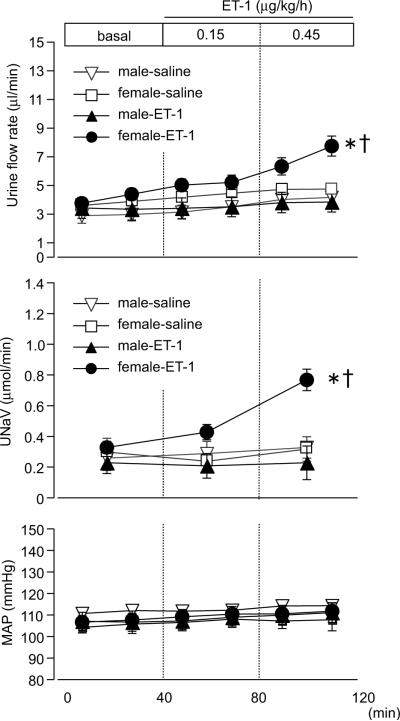
Intramedullary infusion of ET-1 (0.15 and 0.45 μg/kg/h) into the left kidney of female wt rats induced significant diuretic and natriuretic responses compared to saline infusion or compared with those in male rats (Fig 1). Infusion of ET-1 did not increase urine flow or sodium excretion in male rats compared to saline infusion. Urine flow and sodium excretion from the contralateral kidney were not significantly increased by ET-1 infusion compared to saline infusion (data not shown). Mean arterial pressure was not changed by infusion of ET-1, eliminating the possibility that increasing water and sodium excretion was a result of pressure-induced changes.
Figure 1.
Urine flow rate, sodium excretion (UNaV), and mean arterial pressure (MAP) during medullary interstitial infusion of ET-1 or saline (0.9% NaCl) in wild type rats. Data are expressed as the mean ± SE. Number of rats: male-saline, n=7; female-saline, n=5; male-ET-1, n=10; female-ET-1, n=8. *p<0.05 versus saline. †p<0.05 versus male rats.
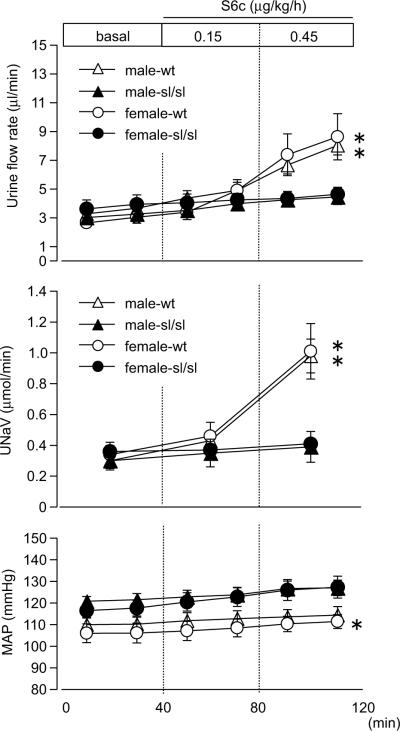
S6c, a selective ETB agonist, significantly increased urine flow rate and sodium excretion in wt rats compared to sl/sl (Fig 2). No difference was observed in the response to S6c between male and female rats, suggesting that the sex difference in the response to ET-1 is not dependent upon the ETB receptor. As previously reported,12, 14 mean arterial pressure of sl/sl rats were higher compared to wt rats. Intramedullary infusion of S6c had no effect on mean arterial pressure.
Figure 2.
Urine flow rate, sodium excretion (UNaV), and mean arterial pressure (MAP) during medullary interstitial infusion of the ETB receptor agonist, sarafotoxin 6c (S6c), in wild type or ETB-deficient rats. Data are expressed as the mean ± SE. Number of rats: male-wt, n=7; male-sl/sl, n=6; female-wt, n=8; female-sl/sl, n=6. *p<0.05 versus genotype.
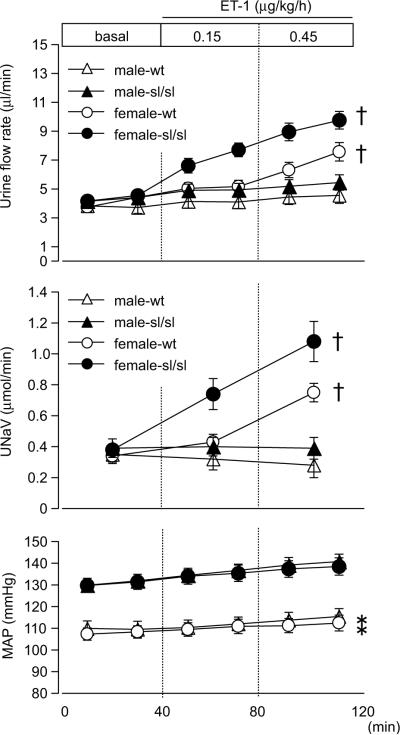
Infusion of ET-1 in female wt, and surprisingly, sl/sl rats markedly increased urine flow and sodium excretion (Fig 3) compared to baseline, yet no increase was observed in male rats, indicating the existence of ETB-independent effects. The diuretic and natriuretic responses in female sl/sl rats were greater than those in female wt rats. Again, infusion of ET-1 did not significantly change the mean arterial pressure.
Figure 3.
Urine flow rate, sodium excretion (UNaV), and mean arterial pressure (MAP) during medullary interstitial infusion of ET-1 in wild type or ETB-deficient rats. Data are expressed as the mean ± SE. Number of rats: male-wt, n=10; male-sl/sl, n=13; female-wt, n=8; female-sl/sl, n=13. *p<0.05 versus genotype. †p<0.05 versus male rats.
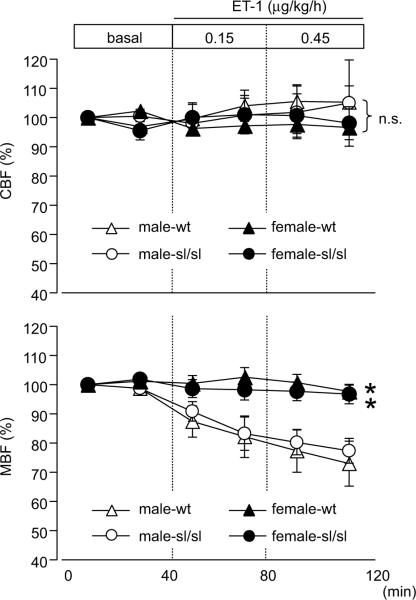
A long-standing theory suggests that changes in renal MBF affect the sodium reabsorption in the renal medulla.17 Since ET-1 is a potent vasoactive peptide, we evaluated the effect of ET-1 infusion on MBF. Intramedullary infusion of ET-1 significantly decreased the MBF in male rats but not in female rats (Fig 4). The presence of a functional ETB receptor did not affect these responses since identical changes were observed in wt and sl/sl rats. Medullary infusion of ET-1 did not induce significant effects on CBF.
Figure 4.
Cortical and medullary blood flow (CBF and MBF, respectively) during renal medullary infusion of ET-1. Flow is presented as a percent of the average flow for each animal during the initial 20 min baseline period. Data are expressed as the mean ± SE. Number of rats: male-wt, n=7; male-sl/sl, n=6; female-wt, n=6; female-sl/sl, n=8. *p<0.05 versus male rats. n.s.; not significant.
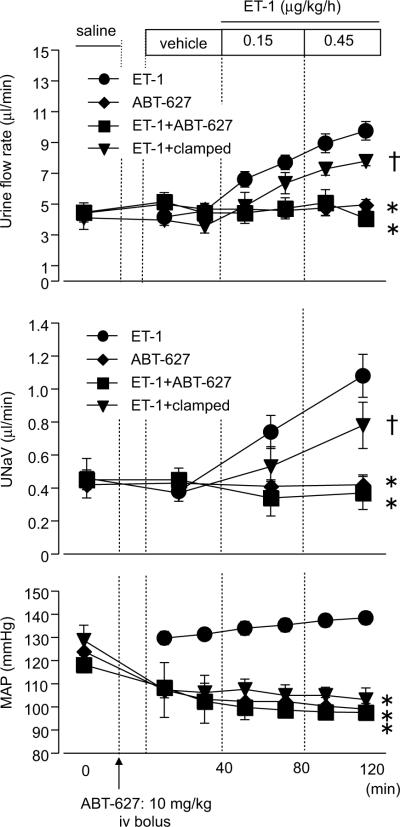
Because significant ET-1-dependent natriuretic responses were observed in female sl/sl rats, we assessed the contribution of ETA receptors by administration of a selective ETA antagonist, ABT-627 prior to ET-1 infusion. ABT-627 prevented the ET-1-induced increase in urine flow and sodium excretion observed of female sl/sl rats (Fig 5). Since injection of ABT-627 produced a slight, but significant decrease in mean arterial pressure in sl/sl rats, renal perfusion pressure was controlled by an aortic clamp to mimic the ABT-627 group in a separate group of rats. ET-1-induced responses were still observed in the clamped group, providing further support for the idea that ET-1-induced diuretic and natriuretic responses are, at least in part, due to an ETA-dependent pathway.
Figure 5.
Urine flow rate, sodium excretion (UNaV), and mean arterial pressure (MAP) during medullary interstitial infusion of ET-1 in female sl/sl rats following ETA receptor blockade (ABT-627). ABT-627 was injected intravenously 10 min prior to control period (10 mg/kg). In a separate group of rats, renal perfusion pressure was manually lowered (clamped) to mimic the decrease in pressure induced by ABT-627. Data are expressed as the mean ± SE. Number of rats: ET-1, n=13; ABT-627, n=3; ET-1+ABT-627, n=6; ET-1+clamped, n=5. *p<0.05 versus ET-1 group. †p<0.05 versus ET-1+ABT-627 group.
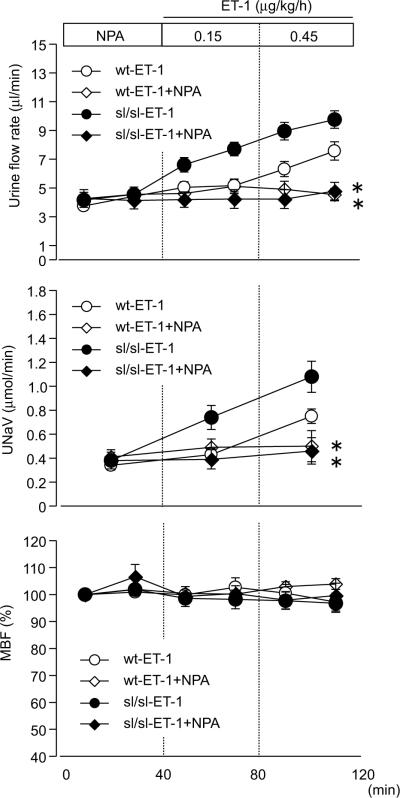
We also examined whether NOS1 mediates the ETA-dependent increase in sodium and water excretion, similar to ETB-dependent responses.12 The diuretic and natriuretic response to ET-1 in female rats was suppressed by co-infusion of NPA, a selective NOS1 inhibitor, indicating that ETA-dependent salt and water excretion is induced by a NOS1-dependent pathway (Fig 6).
Figure 6.
Urine flow rate, sodium excretion (UNaV), and changes in MBF during medullary interstitial infusion of ET-1 in female rats. A separate group was co-infused with the NOS1 selective inhibitor, NPA. NPA was infused into the renal medulla beginning 40 min prior to ET-1 infusion. Data are expressed as the mean ± SE. Number of rats: wt-ET-1, n=8; wt-ET-1+NPA, n=6; sl/sl-ET-1, n=13; sl/sl-ET-1+NPA, n=5. *p<0.05 versus ET-1 group.
A final series of experiments determined whether sex steroids affect the sex difference in renal medullary ET-1-dependent sodium excretion (Table 1). Orchidectomy (ORX) potentiated the urinary sodium excretion in response to ET-1 and suppressed the ET-1-induced decrease in MBF in wt, but not in sl/sl rats. Ovariectomy (OVX) suppressed the natriuretic response to ET-1 in both wt and sl/sl rats, and enhanced the ET-1-induced decreases in MBF only in sl/sl rats. Overall, gonadectomy diminished the sex difference in ET-1-dependent natriuresis and changes in MBF in each strain.
Table 1.
Effects of gonadectomy on the changes in natriuretic and medullary vasocontractile responses to ET-1 infusion (0.45 μg/kg/min). Data are expressed as the mean±SE. (n=6-13).
| male |
female |
|||
|---|---|---|---|---|
| genotype | intact | ORX | intact | OVX |
| ΔUNaV (μmol/min) | ||||
| wt | -0.04 ± 0.06 | 0.20 ± 0.10* | 0.41 ± 0.07† | 0.09 ± 0.04* |
| sl/sl | 0.00 ± 0.05 | -0.08 ± 0.03 | 0.70 ±0.11† | 0.04 ± 0.06* |
| ΔMBF (%) | ||||
| wt | -32.9 ± 5.9 | -0.6 ± 5.5* | -2.4 ± 2.2† | -1.6 ± 4.7 |
| sl/sl | -22.7 ± 4.3 | -31.8 ± 6.0 | -3.2 ± 3.3† | -30.0 ± 5.4* |
P < 0.05 compared with intact.
P < 0.05 compared with male.
ΔUNaV, changes in urinary sodium excretion from baseline value; ΔMBF, % changes in MBF from baseline value
Discussion
The physiological importance of ET-1 in the kidney has been the subject of investigation ever since its identification in 1988. Kitamura et al. first observed that the expression level of ET-1 in the renal medulla is markedly higher than that in the other organs.18 In addition, considerable in vitro and in vivo studies have shown that renal ET-1 facilitates urine production by inhibition of tubular reabsorption.8, 9, 19, 20 Chronic blockade of ETB receptors or genetic deletion of functional ETB receptors either generally or specifically in the renal collecting duct results in salt-dependent increases in arterial pressure.11, 14, 21 Our laboratory recently demonstrated that infusion of S6c into the renal medullary interstitium produces a significant diuresis and natriuresis in normal rats, indicating that activation of ETB receptor in the renal medulla facilitates the excretion of salt and water.12 However, the role of the renal medullary ETA receptor has been uncertain despite its known presence in the renal medulla.
Results from the current study demonstrated that intramedullary infusion of ET-1 produced greater diuretic and natriuretic responses in female rats compared to male rats, and that this sex difference is primarily due to an ETA receptor-dependent mechanism. To our knowledge, this study is the first evidence to show the involvement of renal medullary ETA receptors in the control of salt balance by ET-1. Considerable evidence indicates that females are more resistant in the development of hypertension compare to males,2, 5, 22, 23 and that reduced sodium retention in females is likely to account for this sex difference.24 The presence of an ETA receptor-mediated natriuretic pathway combined with ETB receptor dependent natriuretic pathway might explain why female rats are more resistant to salt-induced hypertension. We also provide evidence that the ETA-dependent diuresis and natriuresis in female rats is dependent upon NOS1, similar to previous reports of ETB-mediated actions in the renal medulla,12, 25 and appears to be maintained by ovarian hormones.
Infusion of ET-1 significantly decreased medullary blood flow in male rats regardless of the presence of ETB receptor, indicating the involvement of ETA-dependent vasoconstriction. It is well accepted that a decrease in MBF could facilitate sodium reabsorption in the renal medulla and suppress urinary sodium excretion.17, 26 Therefore, larger ET-1-induced vasoconstriction may provide an explanation for why ET-1 did not induce diuretic and natriuretic responses in male wt rats despite the presence of the ETB receptor. A contribution of hemodynamic changes in ET-1 dependent responses in females seem to be insignificant since we did not observe any changes in MBF in response to ET-1 in intact female rats. In addition, we recently observed that renal medullary infusion of the selective NOS1 inhibitor, NPA, did not change MBF, but infusion of the non-selective NOS inhibitor, L-NAME, decreases MBF,12 suggesting that basal MBF was regulated by NOS3 and/or NOS2, but not NOS1. Taken together with the current results, we speculate that ET-1/NOS1-dependent responses observed in the current study are primary mediated through changes in tubular reabsorption that do not involve changes in medullary hemodynamics. It is also possible that ET-1-dependent inhibition of tubular sodium reabsorption is similar between male and female rats, but that the sex difference in ET-1-dependent increases in sodium excretion may be due to the counteracting effects of vasoconstriction within the renal medulla in males. Future studies will have to determine whether there is renal tubular action of ETA receptors in males independent of hemodynamic changes. The distribution of ETA and ETB receptors within the renal medulla of male and female rats should also provide further insight into the receptor specific mechanisms.
The difference in the diuretic and natriuretic actions of ET-1 in male versus female rats could be partly due to sex differences in receptor expression level within renal tubular and vascular structures in the renal medulla. ETB receptors are expressed in tubular cells, endothelial cells and collecting duct cells, while ETA receptors are expressed in vasa recta, pericytes, interstitial cells and collecting duct cells.13 However, to our knowledge, there have been no investigations into whether a sex difference exists in the distribution or expression level of each receptor. Our group has previously shown that there is no sex difference in urinary ET-1 excretion, which is generally used as an indicator of renal ET-1 production, in the same strain of wt rats used in the current study.27 Thus it appears as though the sex difference in responsiveness to intramedullary ET-1 cannot be explained by endogenous peptide production. However, this may not be the best evidence for determining whether there is a sex difference in renal production of ET-1 since urinary ET-1 most likely includes production originating from both the renal cortex and medulla.
Another possible factor contributing to the sex difference in ETA-dependent diuretic and natriuretic responses is that the ETA receptor may regulate the balance of other hormonal factors that can influence the water and sodium reabsorption. It has been demonstrated that ET-1 antagonizes the urine concentrating effects of vasopressin (AVP), thereby facilitating water excretion.28, 29 However, a recent study demonstrated that deletion of ETA receptor in collecting duct decreases the sensitivity of water reabsorption in response to AVP,30 indicating that ETA signaling would interfere the ET-1-induced inhibition of AVP response. Moreover, men appear to have increased sensitivity to AVP-induced urine concentrating capability compared to women.3 Therefore, the greater AVP-induced anti-diuretic effects, which could be facilitated by collecting duct ETA receptor stimulation in males, may contribute the sex difference in ET-1- and ETA-dependent urine excretion.
The ET-1-dependent diuretic and natriuretic responses in wild type rats were less than those in sl/sl rats despite the presence of both ETA and ETB receptors. However, it is important to note that the ETB receptor also functions to clear ET-1 from the circulation,31 and so infusion of ET-1 in the renal medulla of wild type rats might be cleared by functional ETB receptors there, resulting in reduced ETA receptor stimulation in wt rats. Alternatively, ET-1 might be accumulated in the renal medulla because of no clearance receptor in sl/sl rats. This could account for the larger ET-1-induced natriuretic response in female sl/sl rats compared to female wild type rats, but this possibility has yet to be explored.
Sex hormones are known to account for a majority of functional differences between males and females in the cardiovascular and renal systems.5, 32 Results from gonadectomy experiments suggest that testicular hormones enhance ET-1-induced medullary vasoconstriction by suppressing the ETB receptor-dependent pathway, thereby inhibiting sodium excretion, and that ovarian hormones weaken ET-1-induced medullary vasoconstriction by suppressing the ETA receptor-dependent pathway, thereby facilitating sodium excretion. ETA-dependent vasoconstriction may be masked by ETB-dependent vasodilation in female wt rats. Most importantly, the sex difference in the ET-1-dependent natriuretic response in intact rats was diminished by gonadectomy in each strain, indicating the importance of sex steroids on the sex difference of ET-1-induced responses in the renal medulla.
NOS1 inhibition suppressed ETA-dependent diuresis and natriuresis in female rats. Both ETA receptors and NOS1 are expressed in the IMCD.13, 33 However, our results would appear to conflict with findings in cultured IMCD cells which showed that ET-1-dependent NO release was inhibited by ETB antagonist, but not by ETA antagonist.25 Aside from the obvious differences in expression that could exist in cultured cells versus intact kidneys, ETA-dependent NO production could occur within any number of cell types within the renal medulla such as thick ascending limb or interstitial cells. Another possibility is that cell-cell interaction with surrounding cells in the renal medulla may be essential to induce ETA-dependent NOS1 stimulation.
Hypertension produced by DOCA-salt treatment is blunted in female rats compared to male rats.6 This difference was abolished in the absence of ETB receptor. Therefore, we hypothesized that female might have greater ETB-dependent sodium excreting activity and that this sex difference might contribute to prevent salt retention and salt-induced blood pressure elevation. However, we could not observe the difference in the responses to S6c between male and female rats, indicating that there is no sex difference in ETB-dependent diuretic and natriuretic activities at least via receptors located in the renal medulla. Therefore, the ETB-dependent sex difference in DOCA-salt-induced hypertension appears to result from the differences in non-medullary tissues, such as brain, heart, vasculature and renal cortex.
Perspectives
In summary, female rats have greater renal medullary ET-1-dependent diuretic and natriuretic activity compared to male rats and involves both ETA and ETB receptor dependent activation of NOS1. The sum of tubular and hemodynamic mechanisms results in increased ET-1-dependent natriuresis in female compared to male. These sex differences in response to ET-1 are, at least partly, mediated by sex hormones. This increased ET-1 dependent natriuretic capacity in females may be an important mechanism for protection from salt-dependent hypertension in pre-menopausal women. Furthermore, to our knowledge, this study is the first to show the existence of ETA-dependent natriuresis. This novel role of ETA receptors in the renal medulla may provide an explanation for edema observed in patients given ETA/B or ETA-selective antagonists.
Acknowledgments
The authors wish to thank Dr. Erika Boesen for her valuable scientific advice.
Source(s) of Funding These studies have been supported by grants from the National Institutes of Health: HL64776, HL60653, HL69999, and HL74167. Dr. Pollock is an Established Investigator of the American Heart Association.
Footnotes
Conflict(s) of Interest/Disclosure (s)none
References
- 1.Jazbutyte V, Hu K, Kruchten P, Bey E, Maier SK, Fritzemeier KH, Prelle K, Hegele-Hartung C, Hartmann RW, Neyses L, Ertl G, Pelzer T. Aging reduces the efficacy of estrogen substitution to attenuate cardiac hypertrophy in female spontaneously hypertensive rats. Hypertension. 2006;48:579–586. doi: 10.1161/01.HYP.0000240053.48517.c7. [DOI] [PubMed] [Google Scholar]
- 2.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 3.Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol. 2001;91:1893–1901. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 4.Khraibi AA. Renal interstitial hydrostatic pressure and pressure natriuresis in pregnant rats. Am J Physiol Renal Physiol. 2000;279:F353–F357. doi: 10.1152/ajprenal.2000.279.2.F353. [DOI] [PubMed] [Google Scholar]
- 5.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 6.Kawanishi H, Hasegawa Y, Nakano D, Ohkita M, Takaoka M, Ohno Y, Matsumura Y. Involvement of the endothelin ETB receptor in gender differences in deoxycorticosterone acetate-salt-induced hypertension. Clin Exp Pharmacol Physiol. 2007;34:280–285. doi: 10.1111/j.1440-1681.2007.04580.x. [DOI] [PubMed] [Google Scholar]
- 7.Gallego MS, Ling BN. Regulation of amiloride-sensitive Na+ channels by endothelin-1 in distal nephron cells. Am J Physiol. 1996;271:F451–F460. doi: 10.1152/ajprenal.1996.271.2.F451. [DOI] [PubMed] [Google Scholar]
- 8.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol. 2000;279:F326–F333. doi: 10.1152/ajprenal.2000.279.2.F326. [DOI] [PubMed] [Google Scholar]
- 9.Sandgaard NC, Bie P. Natriuretic effect of non-pressor doses of endothelin-1 in conscious dogs. J Physiol. 1996;494:809–818. doi: 10.1113/jphysiol.1996.sp021534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–F1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 12.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol. 2008;294:F1205–F1211. doi: 10.1152/ajprenal.00578.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens. 2006;15:34–40. doi: 10.1097/01.mnh.0000186852.15889.1a. [DOI] [PubMed] [Google Scholar]
- 14.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–933. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung's disease. J Clin Invest. 1998;102:1092–1101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassileva I, Mountain C, Pollock DM. Functional role of ETB receptors in the renal medulla. Hypertension. 2003;41:1359–1363. doi: 10.1161/01.HYP.0000070958.39174.7E. [DOI] [PubMed] [Google Scholar]
- 17.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–R27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura K, Tanaka T, Kato J, Ogawa T, Eto T, Tanaka K. Immunoreactive endothelin in rat kidney inner medulla: marked decrease in spontaneously hypertensive rats. Biochem Biophys Res Commun. 1989;162:38–44. doi: 10.1016/0006-291x(89)91958-x. [DOI] [PubMed] [Google Scholar]
- 19.Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K+-ATPase in intact renal tubular epithelial cells. Am J Physiol. 1989;257:C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- 20.Schnermann J, Lorenz JN, Briggs JP, Keiser JA. Induction of water diuresis by endothelin in rats. Am J Physiol. 1992;263:F516–F526. doi: 10.1152/ajprenal.1992.263.3.F516. [DOI] [PubMed] [Google Scholar]
- 21.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high sal. Am J Physiol Renal Physiol. 2001;281:F144–F150. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R764–R768. doi: 10.1152/ajpregu.00322.2006. [DOI] [PubMed] [Google Scholar]
- 23.Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, Carvalho MH. Endothelin, sex and hypertension. Clin Sci (Lond) 2008;114:85–97. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- 24.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of Dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 25.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol. 2006;290:F1315–F1319. doi: 10.1152/ajprenal.00450.2005. [DOI] [PubMed] [Google Scholar]
- 26.Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol. 2005;32:400–409. doi: 10.1111/j.1440-1681.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan JC, Pollock JS, Pollock DM. Superoxide-dependent hypertension in male and female endothelin B receptor-deficient rats. Exp Biol Med (Maywood) 2006;231:818–823. [PubMed] [Google Scholar]
- 28.Takemoto F, Uchida S, Ogata E, Kurokawa K. Endothelin-1 and endothelin-3 binding to rat nephrons. Am J Physiol. 1993;264:F827–F832. doi: 10.1152/ajprenal.1993.264.5.F827. [DOI] [PubMed] [Google Scholar]
- 29.Kohan DE, Hughes AK. Autocrine role of endothelin in rat IMCD: inhibition of AVP-induced cAMP accumulation. Am J Physiol. 1993;265:F126–F129. doi: 10.1152/ajprenal.1993.265.1.F126. [DOI] [PubMed] [Google Scholar]
- 30.Ge Y, Stricklett PK, Hughes AK, Yanagisawa M, Kohan DE. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am J Physiol Renal Physiol. 2005;289:F692–F698. doi: 10.1152/ajprenal.00100.2005. [DOI] [PubMed] [Google Scholar]
- 31.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 32.Baylis C. Sexual dimorphism of the aging kidney: role of nitric oxide deficiency. Physiology (Bethesda) 2008;23:142–150. doi: 10.1152/physiol.00001.2008. [DOI] [PubMed] [Google Scholar]
- 33.Cowley AW, Jr., Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355–R1369. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]