Abstract
Object
In this investigation the authors attempted to predict change in function following selective dorsal rhizotomy (SDR) and intensive physical therapy in patients with spastic diplegic cerebral palsy (CP) based on multidomain preintervention measures.
Methods
Data pertaining to 22 children with CP were collected before the SDR and again 20 months afterward. Although equations for predicting change in gait speed and function (such as the Gross Motor Function Measure) were derived, the 95% confidence interval (CI) widths were too broad to make accurate predictions that were clinically useful outside the study group.
Conclusions
Future work should be focused on developing additional measures such as lower-extremity motor control and balance in an attempt to reduce the CIs to more clinically relevant values.
Keywords: cerebral palsy, gait spasticity, strength, rhizotomy, predictive measure, pediatric neurosurgery
Selective dorsal rhizotomy is a surgical procedure used to treat patients with CP that involves partial sensory deafferentation at the L1–S2 levels.22 The effectiveness of the surgery has been reported in randomized19,28,30 and nonrandomized8 clinical studies. Despite the evidence of its effectiveness, it is important to note that variability exists within the patient groups and not all patients exhibit the same degree of improvement from the SDR. For example, although in one patient in our investigation the GMFM score improved from 75% preoperatively to 86% at 20 months postoperatively (an 11-point improvement), the GMFM score in another patient changed from 73% preoperatively to 72% at 20 months postoperatively (a one-point decrease).8 Relative to gait speed, one patient’s speed improved from 61 cm/second preoperatively to 121 cm/second at 20 months postoperatively (a 60-cm/second improvement), whereas another patient’s gait speed changed from 109 cm/second preoperatively to 102 cm/second at 20 months postoperatively (a 7-cm/second decrease).
Being able to predict preoperatively which patients will have the greatest improvements in GMFM, gait speed, or some other outcome measure would be extremely valuable for them and their parents when considering the SDR. Previously, authors of two investigations have attempted to predict postoperative outcomes based on preoperative data.2,16 Chicoine and colleagues2 attempted to predict walking ability after SDR using a series of preoperative variables (subjective gait score, dorsiflexion ability, and diagnosis). The results of their multivariate regression analysis indicated that gait score and diagnosis (spastic diplegia or quadriplegia) had significant predictive capability, explaining 78% of the variance in predicting the postoperative gait score. Despite the large amount of explained variance in their patients, the investigators were hesitant to suggest that their results could be used clinically and stated that additional work was necessary. In addition, they did not report 95% prediction CI widths from the regression analysis for their group or for patients not part of their cohort. They also did not report the pre- to postoperative change in gait score. Such a score may be extremely valuable in determining whether the surgery should be performed because it would give an indication of the expected improvement following the surgery.
Kim and colleagues16 recently attempted to predict poor surgical outcome using standard preoperative data. The data included CP type (spastic diplegia or quadriplegia), history of premature birth, previous orthopedic surgery, seizure history, dystonia, posturing, lumbar lordosis, truncal hypotonia, ambulatory function, GMFCS, and presence of intellectual and speech delay. It was concluded that the preoperative diagnosis of CP type was the strongest predictor of outcome (either acceptable or poor) after SDR, with a diagnosis of spastic diplegia related to better outcomes. However, this conclusion was based on a small number of poor outcomes (11 of the 174 patients in the study). From a clinical perspective, this small number of poor outcomes was an excellent result, but from a statistical perspective, it made the authors’ predictions more difficult. They presented a percentage expectation of achieving a poor outcome based on 95% CIs from their regression analysis. No CIs were presented for prediction outside their patient group.
The authors of the two previous publications have followed two different paths in their SDR prediction attempts. Chicoine et al.2 attempted to predict a subjective assessment of walking ability by using diagnosis and a series of subjective measurements. Kim et al.16 attempted to predict poor or acceptable outcomes using diagnosis and a series of standard clinical evaluation measures. Another prediction path would be to use multidimensional objective measures of impairment and function1 and only recruit patients with spastic diplegia to predict change in gait speed and function following SDR. It is possible that the objective data and the more homogeneous group might produce better predictor models than those previously reported. The purpose of this exploratory investigation was to use objective preintervention impairment and functional measures to predict change in gait speed and function following the SDR and intensive physical therapy. Our general hypothesis was that preintervention objective measures of spasticity, strength, function, and gait would permit a clinically relevant prediction of change in gait and function following the SDR.
Clinical Material and Methods
Patient Population
The methods of this investigation have been described elsewhere and will be briefly summarized here.8 Patients were recruited through the SDR clinic at St. Louis Children’s Hospital, Washington University, in St. Louis. Potential participants were identified, and parents were informed about the research project. The neurosurgeon screened the patient on the basis of the inclusion and exclusion criteria and determined whether the patient was an acceptable candidate for SDR. If the parents chose to have their child undergo the procedure, then the child was enrolled in the investigation.
Inclusion criteria for participants included the following: spastic diplegia CP; GMFCS Levels I to III; ability to walk (with or without orthoses including walkers, crutches, and canes); minimum cognitive skills for active participation; no surgical intervention within the preceding year; lower-extremity hypertonicity measured according to the modified Ashworth scale; ankle clonus; exaggerated deep tendon reflex in the legs; present Babinski sign; and abnormal postures while sitting, standing, and walking. Patients had to be able to perform six to eight repetitions of barefoot walking for approximately 8 m independently or using a walker, crutches, or canes. They were not permitted in the study until 6 months after any casting procedures or injections of botulinum toxin type A had been performed. We established a minimum participant age of 4 years to permit cooperation with the collection of gait, spasticity, and strength data.
Specifically excluded from the investigation were children with motor deficits that resulted from neurological injury or illness that began after the 1st month of life and children with malformations of the central nervous system. Other exclusion criteria included moderate to severe dystonia, athetosis, ataxia, and the presence of severe cognitive delay. Children were excluded if their parents reported that their children were unable to follow simple commands and understand concepts such as, “push as hard as you can” and “relax your muscles.”
Thirty-three patients (mean age 9.0 ± 5.3 years [SD]) were enrolled and underwent SDR. Thirty-one completed participation in the study (mean age 8.8 ± 4.8 years [SD]) by being tested preoperatively and 2 years postoperatively. Two patients were lost to attrition, one due to lack of cooperation during testing and one because travel was too difficult to continue participation. From this cohort of participants, complete data sets for all variables were obtained in 22, and these patients were used in the analysis (Table 1). Incomplete data sets were generally the result of the lack of time for data collection during the preoperative or postoperative visits. For example, a patient could have been late to the laboratory for testing, but could not be late to his or her next appointment. All study participants older than 17 years of age and/or patients’ parents signed an informed consent approved by the Washington University Human Studies Committee.
TABLE 1.
Demographic data, GMFCS level, and gait status in 22 patients undergoing SDR
| Variable | No. of Patients* |
|---|---|
| no. of patients | 22 |
| age (yrs) | 8.5 ± 3.9 |
| sex | |
| M | 10 |
| F | 12 |
| weight (kg) | 27.5 ± 15.7 |
| GMFCS level | |
| I | 11 |
| II | 5 |
| III | 6 |
| gait status | |
| independent walking | 19 |
| assisted walking | 3 |
Unless otherwise stated. Mean values are reported as the means ± SDs.
Surgical Procedure
Our surgical techniques for SDR have been previously described.22 A single-level laminectomy was performed at the L-1 vertebra, and ultrasonography was used to determine the location of the conus medullaris in relation to the laminectomy site. The L-1 nerve roots were identified at the foraminal exit, and the dorsal root was separated from the ventral root. Next, individual dorsal roots were identified at the level of the cauda equina. Each root was then subdivided into four to seven smaller rootlets, and these rootlets were individually suspended over rhizotomy probes. Electrical stimulation was used to grade a reflex response from six lower-extremity muscles. Rootlets were then cut according to the response. This procedure was repeated for the remaining L2–S2 dorsal roots, and the entire procedure was repeated on the contralateral side. Approximately 60 to 65% of the rootlets tested were cut, and one rootlet was left at each level regardless of the results of electrical stimulation. All patients received 1 week of postoperative inpatient physical therapy. Patients were discharged home on postoperative Day 5. After discharge from the hospital, the participants underwent physical therapy in their hometowns four times per week for 8 months. Subsequently, treatments were reduced to three times per week for an additional 12 to 16 months.
Symptom Tests
Spasticity, characterized as a velocity-dependent resistance to passive stretch,3,15,18 was measured using a KinCom isokinetic dynamometer (Isokinetic International) for the ankle plantar flexors, knee flexors, and hip adductors.5,6,9–12 For simplicity, only the test for the ankle plantar flexors will be described.9,11 Each patient sat on the KinCom dynamometer and had the ankle joint axis aligned with the center of the KinCom lever arm. The patient was instructed to remain as relaxed as possible as the joint was passively rotated by the dynamometer from a fully plantar-flexed to a fully dorsiflexed position, thereby stretching the ankle plantar flexors. Tests were conducted at speeds of 10, 30, 60, 90, and 120°/second bilaterally. Areas within the torque angle curves were calculated for each speed and joint, yielding work values. For each joint, linear regression was then used to determine the line of best fit for the five work values as a function of speed. The slope of the linear regression line was considered to measure the magnitude of the spasticity.
Strength tests were designed to measure the maximum active resultant torque-generating capacity that the patient could produce for the ankle plantar flexors and dorsiflexors, knee flexors and extensors, and hip adductors and abductors.5,7,9–12 Only the ankle tests will be described.9,11 Each patient actively moved the ankle at 10°/second from end-range ankle dorsiflexion to end-range plantar flexion, and vice versa, to obtain maximum concentric contractions of the ankle plantar flexors and dorsiflexors, respectively. The maximum torque values for both dorsiflexion and plantar flexion were recorded. The trapezoid rule was used to determine work values. The work was defined as the area bounded by the curve, the zero torque line, and the beginning and ending ROM for each dorsi- and plantar flexion torque-angle curve. All values were normalized by dividing by the patient’s mass to permit interpatient comparison.5–7, 9–12,17
Motor Function
The GMFM was recorded in all patients for each visit. The GMFM is a standard criterion-referenced test designed to assess change in gross motor function in children with CP.26 Recently, the GMFM-66 was developed using Rasch analysis to improve the sensitivity and interpretability of the test.25 The GMFM-66 produces a mean score or ability estimate (GMAE).
For the gait analysis, three 2.5-cm-diameter spherical reflective surface markers were placed on the trunk, thighs, legs, and feet of each participant.8 The patient then walked barefoot at a self-selected pace along a 9-m walkway, and video data were collected (six-camera HiRes system, Motion Analysis Corp.) during the middle 2 m. Temporal gait variables including speed, stride length, and cadence were determined. The kinematic data were tracked and uploaded into KinTrak software (Motion Analysis Corp.) for further processing. Nine variables were calculated: 1) ankle dorsiflexion at initial contact; 2) ankle dorsi- and plantar flexion ROM; 3) knee flexion at initial contact; 4) knee flexion/extension ROM; 5) hip flexion/extension ROM; 6) pelvic tilt ROM; 7) pelvic rotation ROM; 8) trunk rotation ROM; and 9) external foot progression angle at initial contact.
Statistical Analysis
Gait speed and function (GMAE) were chosen as primary outcome (predicted) variables given that keeping up with peers and gross motor abilities are very important to individuals with CP. The process for predicting gait speed and function (GMAE) consisted of two steps. The first step was to reduce the number of predictor variables. Although it has been suggested that six to 10 patients are needed for each predictor variable,20 the decision was made to use a greater number because the investigation was considered to be more exploratory than definitive. Thus, the previously mentioned potential predictor variables were reduced to nine (Table 2). The reduction was accomplished by the following: 1) considering the clinical importance of the variables; 2) eliminating variable duplication; and 3) averaging similar ankle, knee, and hip joint variables. For the strength variables, the work variables were eliminated and the maximum values were retained. For the kinematic variables, only one variable was permitted at each joint. The GMFM variable was retained and the GMAE was eliminated as a predictor. Next, ankle, knee, and hip joint spasticity values were averaged. Similarly, strength and joint ROM were averaged to produce a single variable of strength. We also believed that confusion would arise if too many factors were used to predict a single variable. Thus, it was decided that no more than four of the nine potential predictor variables would be used in the individual prediction of a specific predicted variable (GMAE change).
TABLE 2.
Means and SDs for the changes in gait speed, GMAE changes, variables in 22 patients, and preintervention predictor variables*
| Variable | Value |
|---|---|
| predicted | |
| gait speed change (cm/sec) | 20.7 ± 20.3 |
| GMAE change | 5.5 ± 5.7 |
| predictor | |
| LESpast0 | 0.0137 ± 0.0106 |
| LEStren0 (Nm/kg) | 0.56 ± 0.23 |
| Speed0 (cm/sec) | 81.0 ± 24.2 |
| Cad0 (steps/min) | 127.1 ± 27.2 |
| StrLen0 (cm) | 76.3 ± 19.3 |
| FTRotIC0 (°) | −3.8 ± 9.0 |
| LEGtROM0 (°) | 34.6 ± 7.5 |
| PlRotROM0 (°) | 19.4 ± 5.3 |
| GMFM0 (%) | 88.4 ± 10.1 |
Cad0 = preintervention gait cadence; FTRotIC0 = preintervention foot rotation at initial contact during gait; GMFM0 = preintervention GMFM percentage; LEGtROM0 = averaged preintervention ankle dorsi-/plantar flexion, knee flexion/extension, and hip abduction/adduction ROM during gait; LESpast0 = averaged preintervention ankle plantar flexor, knee flexor, and hip abductor spasticity; LEStren0 = averaged preintervention maximum ankle plantar flexor and dorsiflexor, knee flexor and extensor, and hip abduction and adduction strength; PlRotROM0 = preintervention pelvic rotation ROM during gait; Speed0 = preintervention gait speed; StrLen0 = preintervention gait stride length.
For the second step, stepwise multiple linear regression analysis was used to predict change in gait speed and function (GMAE) using preoperative impairment and functional data. In the stepwise regression, the threshold for using a variable for prediction was set at a probability value less than 0.10, and the threshold for excluding a variable for prediction was a probability value greater than 0.15. The focus was on four descriptors: the correlation (r), the explained variance (r2), and two types of 95% CI widths. Correlation and explained variance are typical descriptors indicating the strength of the relationship among the variables for the current data set. An r value of 0.90 to 1.00 was considered very high, 0.70 to 0.89 was high, 0.50 to 0.69 was moderate, 0.26 to 0.49 was fair (mild), and 0.00 to 0.25 indicated little to no relationship.4 A probability value less than 0.05 was considered significant.
The 95% CIs describe the potential variability of the predicted value calculated from the model.20 Larger CIs indicate greater prediction variability and less confidence in the accuracy of the prediction. The first 95% CI was derived from the data obtained in patients in the present investigation (that is, the within-set interval). In other words, the magnitude of this 95% CI quantified how well the model could predict a value for the patients who were already in the group used to develop the prediction. This method seems to be the one used in the previous investigations to predict SDR outcome.2,16 It should be noted that the 95% CIs and the prediction model derived from this first analysis are not intended for application in patients who were not part of the original group (that is, new patients considering undergoing SDR). Thus, the second 95% CI width (novel prediction interval) was developed. It can be used for hypothetically predicting change in function for a new patient outside the original study group and before undergoing SDR.20 In other words, the value for this 95% CI width quantified how well the model could predict a value for a patient who was not in the group that was used to develop the prediction. This second CI is larger than the within-set interval but would mimic that which could eventually be done clinically. Data obtained in a candidate for the SDR would be used as input for this second regression model to predict change in gait speed and GMAE following the SDR. The 95% CI width would describe the variability that could be expected from the prediction.
Results
There was a moderate significant correlation (r = 0.52, p = 0.013) between the change in gait speed and the predictor variables for the SDR group (Table 3). However, the explained variance for predicting change in gait speed, while being significant, only accounted for 27% of the variability. The total amount of the variance was accounted for by the preoperative stride length variable (StrLen0), indicating that the greater the preoperative stride length, the greater the change in postoperative gait speed.
TABLE 3.
Predictor variables and their contribution in predicting the gait speed and GMAE changes*
| Variance Contribution |
||
|---|---|---|
| Predictor Variable | Gait Speed Change | GMAE Change |
| LESpast0 | –– | –– |
| LEStren0 | –– | –– |
| Speed0 | –– | –– |
| Cad0 | –– | –– |
| StrLen0 | 0.27 | 0.25 |
| FTRotIC0 | –– | –– |
| LEGtROM0 (°) | –– | 0.22 |
| PlRotROM0 | –– | –– |
| GMFM0 | –– | 0.12 |
| predicted variable | –– | –– |
| correlation (r) | 0.52 | 0.77 |
| explained variance (r2) | 0.27 | 0.59 |
| p value | 0.013 | <0.001 |
–– = not applicable.
The strength of the relationship between GMAE change and the predictor variables for patients who underwent SDR was high (r = 0.77, p < 0.001). The explained variance for predicting the GMAE was 59% (Table 3). Three variables explained this variance with preoperative stride length (StrLen0, 25%), preoperative lower-extremity gait ROM (LEGtROM0, 22%) accounting for the greater part of the variance (47%), and the preoperative GMFM percentage (GMFM0) accounting for the remaining 12%. These were all positive correlations indicating that the greater the pre-operative stride length, lower-extremity gait ROM, and GMFM, the greater the improvement following SDR.
The regression analysis permitted the derivation of equations that could predict the change in gait speed and GMAE for the SDR cohort before intervention (Equations 1 and 2). For example, if a potential patient for SDR had a preintervention stride length (StrLen0) of 76.3 cm (the mean in Table 2), then Equation 1 could be used to predict a 20.4-cm/second increase in gait speed at 2 years postoperatively. The reported value presented in Table 2 is 20.7 cm/second (differences are due to rounding error). Similar calculations can also be made using Equation 2 in which the appropriate predictor values for predicting change in GMAE are used.
| (1) |
| (2) |
Ninety-five percent CIs can be used to understand the accuracy or variability of the predictions made using Equations 1 and 2. For a patient participating in the present investigation, the 95% CI for predicting gait speed change (r2 = 0.27) (that is, within-group prediction) was 10.9 cm/second (Fig. 1). Thus, if the participant in the aforementioned example was part of the investigation, the change in gait speed as a result of the SDR would be expected to be 20.7 ± 10.9 cm/second––somewhere between a 9.8- and 31.6-cm/second change. For the outside or novel prediction (that is, a patient not in the present study group) the 95% CI for predicting gait speed change for a patient undergoing an SDR was 38.6 cm/second. Thus, if the patient in the previous example was not part of the investigation, the change in gait speed as a result of the SDR would be expected to be 20.7 ± 38.6 cm/second––somewhere between a −17.9- to 59.3-cm/second change. These group prediction values far exceed our hypothesis values of ± 7 cm/second.
Fig. 1.
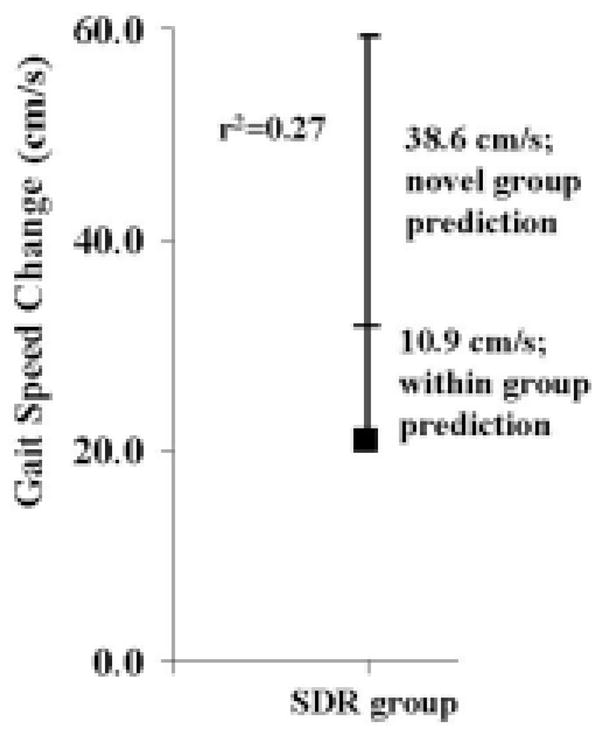
Graph showing the 95% CI widths from the regression analysis and explained variances for within- and outside (novel)–group predictions for change in gait speed. s = second.
The 95% CI for predicting GMAE change in a patient in the SDR group (r2 = 0.59) was 2.9 (Fig. 2). For the outside or novel prediction, the 95% CI for predicting GMAE change for a patient undergoing an SDR was 7.0. Although the within-group 95% CI was within our hypothesized value of ± 3 points, the outside-group prediction value far exceeds our hypothesis value.
Fig. 2.
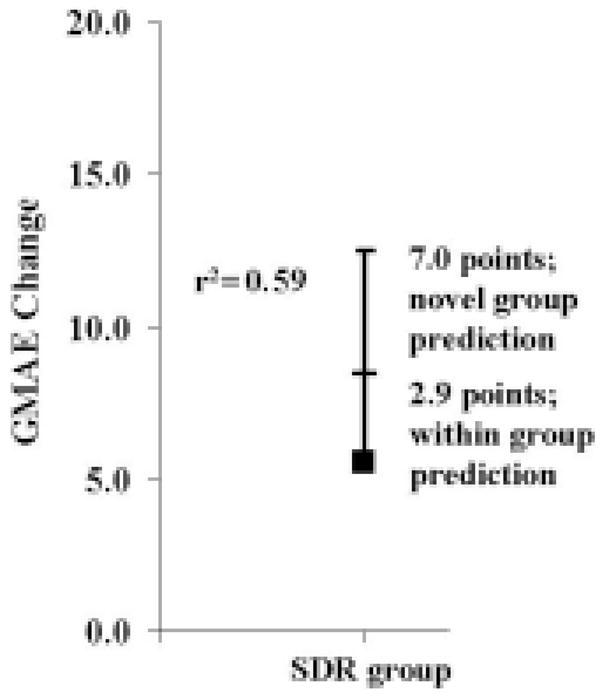
Graph showing the 95% CI widths from the regression analysis and explained variances for within- and outside (novel)–group predictions for change in GMAE.
Discussion
The purpose of this investigation was to use objective preoperative impairment and functional measures to predict change in gait speed and function (GMAE) following the SDR and intensive physical therapy. A key limitation associated with this investigation is the small sample size from which the predictions were made. However, the current study was considered more exploratory than definitive, taking a different path from that of previous work by using objective data instead of clinical and subjective data, and also only including patients with spastic diplegia. A second limitation is the potential use of the aforementioned prediction equations. Two items are noteworthy. First, it should be noted that although Equation 1 only has one predictor variable (StrLen0), Equation 2 has three predictor variables. Attempting to predict GMAE change without a complete set of predictor variables (that is, StrLen0, LEGtROM0, and GMFM0) is not valid because the regression coefficients change according to the number of predictor variables. Furthermore, the 95% CI width derived from a single variable for this prediction would also be much larger. The second noteworthy item relates to the clinical use of Equations 1 and 2. Their use is not recommended due to the large out-side-group 95% CIs. Such large CIs make the equations not very accurate in the clinical setting.
In the present investigation we have built on the previous two studies by taking a different approach to the prediction process. Our strategy was to use objective impairment and functional level data as predictors and objective data (gait speed and GMAE) to predict change (that is, improvement). Our results did not explain as much variance as those in the investigation by Chicoine et al.2 as we were able to explain 27% of the variance for change in gait speed and 59% of the variance for change in GMAE (Table 3). The lower explained variance could be due to a number of factors such as smaller patient numbers, including for study only those with spastic diplegia, predicting change instead of postoperative values, and lack of adequate predictor variables. The most interesting of these factors is the predictor variables. Originally, we speculated that spasticity and strength would be the key predictor variables, and we were quite surprised when they were not part of the final model (Table 3 and Equations 1 and 2). It is not so surprising that stride length would be a key predictor for gait speed change, but it was not expected to be a predictor for GMAE change. Certainly a gait kinematic variable (LEGtROM0) was not expected to be a key predictor for GMAE change. Authors of future work should consider other predictor variables such as lower-extremity motor control13,21,23 and balance14,24,27,29 measures.
Conclusions
In the present investigation we built on previous work by actually developing equations that could be used to predict gait speed and GMAE change and by reporting within-group and outside-group 95% CIs. The authors of the two previous investigations did not include these steps in their analysis. Relative to the equations, it is possible to see how simple the predictions could be made for use in the clinical setting. This is especially true for gait speed because it is relatively easy to determine that quantity. For predicting GMAE change, it would be necessary to conduct a gait analysis to calculate the LEGtROM0 variable. The complexity of this calculation depends on the resources of each individual center. Relative to the 95% CIs, Kim and coworkers16 reported an expectation of a poor outcome based on 95% CIs for their patient cohort. They did not make such a report for a participant outside their patient group. In the present study, we reported the actual expected variability of the predicted variable change based on 95% CIs for patients within our group and potential patients not part of the group (Figs. 1 and 2). It is clear from the figures that outside-group predictions are so large that they do not produce clinically relevant results.
Acknowledgments
Support was received from the National Institute of Neurological Disorders and Stroke (NINDS) at the National Institutes of Health (Grant No. R01 NS35830).
Abbreviations in this paper
- CI
confidence interval
- CP
cerebral palsy
- GMAE
gross motor ability estimate
- GMFCS
Gross Motor Function Classification System
- GMFM
Gross Motor Function Measure
- ROM
range of motion
- SD
standard deviation
- SDR
selective dorsal rhizotomy
References
- 1.Anonymous . Research Plan for the National Center for Medical Rehabilitation Research. Bethesda, MD: U.S. Department of Health and Human Services; 1993. [Google Scholar]
- 2.Chicoine MR, Park TS, Vogler GP, Kaufman BA. Predictors of ability to walk after selective dorsal rhizotomy in children with cerebral palsy. Neurosurgery. 1996;38:711–714. [PubMed] [Google Scholar]
- 3.Dimitrijevic MR. Spasticity. In: Swash M, Kennare C, editors. Scientific Basis of Clinical Neurology. Edinburgh: Churchill Livingstone; 1985. pp. 108–115. [Google Scholar]
- 4.Domholdt E. Statistical analysis of relationships: the basics correlation linear regression. In: Domholdt E, editor. Rehabilitation Research: Principles and Applications. Philadelphia: WB Saunders; 2004. p. 258. [Google Scholar]
- 5.Engsberg JR, Olree KS, Ross SA, Park TS. Maximum effort resultant knee joint torques in children with cerebral palsy. J Appl Biomech. 1998;14:52–61. [Google Scholar]
- 6.Engsberg JR, Olree KS, Ross SA, Park TS. Quantitative clinical measure of spasticity in children with cerebral palsy. Arch Phys Med Rehabil. 1996;77:594–599. doi: 10.1016/s0003-9993(96)90301-9. [DOI] [PubMed] [Google Scholar]
- 7.Engsberg JR, Olree KS, Ross SA, Park TS. Spasticity and strength changes as a function of selective dorsal rhizotomy. J Neurosurg. 1998;88:1020–1026. doi: 10.3171/jns.1998.88.6.1020. [DOI] [PubMed] [Google Scholar]
- 8.Engsberg JR, Ross SA, Collins DR, Park TS. Effect of selective dorsal rhizotomy in the treatment of children with cerebral palsy. J Neurosurg. 2006;105:8–15. doi: 10.3171/ped.2006.105.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engsberg JR, Ross SA, Olree KS, Park TS. Ankle spasticity and strength in children with spastic diplegic cerebral palsy. Dev Med Child Neurol. 2000;42:42–47. doi: 10.1017/s0012162200000086. [DOI] [PubMed] [Google Scholar]
- 10.Engsberg JR, Ross SA, Olree KS, Park TS. Hip spasticity and strength in children with spastic diplegia cerebral palsy. J Appl Biomech. 2000;16:221–233. doi: 10.1017/s0012162200000086. [DOI] [PubMed] [Google Scholar]
- 11.Engsberg JR, Ross SA, Park TS. Changes in ankle spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. J Neurosurg. 1999;91:727–732. doi: 10.3171/jns.1999.91.5.0727. [DOI] [PubMed] [Google Scholar]
- 12.Engsberg JR, Ross SA, Wagner JM, Park TS. Changes in hip spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. Dev Med Child Neurol. 2002;44:220–226. doi: 10.1017/s0012162201001980. [DOI] [PubMed] [Google Scholar]
- 13.Engsberg JR, Wagner JM, Reitenbach AK, Hollander KW. A measure of motor control at the knee in cerebral palsy. J Appl Biomech. 2001;17:334–342. [Google Scholar]
- 14.Ferdjallah M, Harris GF, Smith P, Wertsch JJ. Analysis of postural control synergies during quiet standing in healthy children and children with cerebral palsy. Clin Biomech (Bristol, Avon) 2002;17:203–210. doi: 10.1016/s0268-0033(01)00121-8. [DOI] [PubMed] [Google Scholar]
- 15.Jones EW, Mulley GP. The measurement of spasiticity. In: Rose FC, editor. Advances in Stroke Therapy. New York: Raven Press; 1982. pp. 187–195. [Google Scholar]
- 16.Kim HS, Steinbok P, Wickenheiser D. Predictors of poor outcome after selective dorsal rhizotomy in treatment of spastic cerebral palsy. Childs Nerv Syst. 2006;22:60–66. doi: 10.1007/s00381-005-1160-2. [DOI] [PubMed] [Google Scholar]
- 17.Kramer JF, MacPhail HE. Relationships among measures of walking efficiency, gross motor ability and isokinetic strength in adolescents with cerebral palsy. Pediatr Phys Ther. 1994;6:3–8. [Google Scholar]
- 18.Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: Disordered Motor Control. Chicago: Year Book; 1980. pp. 485–494. [Google Scholar]
- 19.McLaughlin J, Bjornson K, Temkin N, Steinbok P, Wright V, Reiner A, et al. Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol. 2002;44:17–25. doi: 10.1017/s0012162201001608. [DOI] [PubMed] [Google Scholar]
- 20.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. 4. St. Louis: McGraw-Hill; 1996. [Google Scholar]
- 21.Olree KS, Engsberg JR, Ross SA, Park TS. Changes in synergistic movement patterns after selective dorsal rhizotomy. Dev Med Child Neurol. 2000;42:297–303. doi: 10.1017/s0012162200000530. [DOI] [PubMed] [Google Scholar]
- 22.Park TS, Johnston JM. Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Technical note. Neurosurg Focus. 2006;21(2):E7. [PubMed] [Google Scholar]
- 23.Rose J, Martin JG, Torburn L, Rinsky LA, Gamble JG. Electromyographic differentiation of diplegic cerebral palsy from idiopathic toe walking: involuntary coactivation of the quadriceps and gastrocnemius. J Pediatr Orthop. 1999;19:677–682. [PubMed] [Google Scholar]
- 24.Rose J, Wolff DR, Jones VK, Bloch DA, Oehlert JW, Gamble JG. Postural balance in children with cerebral palsy. Dev Med Child Neurol. 2002;44:58–63. doi: 10.1017/s0012162201001669. [DOI] [PubMed] [Google Scholar]
- 25.Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther. 2000;80:873–885. [PubMed] [Google Scholar]
- 26.Russell DJ, Rosenbaum PL, Gowland C, Hardy C, Lane S, Plews M, et al. Manual for the Gross Motor Function Measure. 2. Hamilton, Ontario: University Press; 1993. [Google Scholar]
- 27.Shumway-Cook A, Hutchinson S, Kartin D, Price R, Woollacott M. Effect of balance training on recovery of stability in children with cerebral palsy. Dev Med Child Neurol. 2003;45:591–602. doi: 10.1017/s0012162203001099. [DOI] [PubMed] [Google Scholar]
- 28.Steinbok P, Reiner AM, Beauchamp R, Armstrong RW, Cochrane DD, Kestle J. A randomized clinical trial to compare selective posterior rhizotomy plus physiotherapy with physiotherapy alone in children with spastic diplegic cerebral palsy. Dev Med Child Neurol. 1997;39:178–184. doi: 10.1111/j.1469-8749.1997.tb07407.x. [DOI] [PubMed] [Google Scholar]
- 29.Woollacott MH, Burtner P. Neural and musculoskeletal contributions to the development of stance balance control in typical children and in children with cerebral palsy. Acta Paediatr Suppl. 1996;416:58–62. doi: 10.1111/j.1651-2227.1996.tb14279.x. [DOI] [PubMed] [Google Scholar]
- 30.Wright FV, Sheil EM, Drake JM, Wedge JH, Naumann S. Evaluation of selective dorsal rhizotomy for the reduction of spasticity in cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 1998;40:239–247. doi: 10.1111/j.1469-8749.1998.tb15456.x. [DOI] [PubMed] [Google Scholar]


