SUMMARY
Knowledge about the ability of vitamin D to function outside its established role in skeletal homeostasis is not a new phenomenon. Nonclassical immunomodulatory and antiproliferative responses triggered by active 1,25-dihydroxyvitamin D were first reported more than a quarter of a century ago. It is only in recent years, however, that there has been a significant improvement in our understanding of how these nonclassical effects of vitamin D can influence the pathophysiology and possible prevention of human disease. Three particular strands of evidence have been prominent: firstly, population studies have revised our interpretation of normal vitamin D status in humans, suggesting, in turn, that vitamin D insufficiency is a clinical problem of global proportions; secondly, epidemiology has linked vitamin D status with disease susceptibility and/or mortality; and, thirdly, expression of the machinery required to synthesize 1,25-dihydroxyvitamin D in normal human tissue seems to be much more widespread than originally thought. Collectively, these observations suggest that nonclassical metabolism and response to vitamin D might have a significant role in human physiology beyond skeletal and calcium homeostasis. Specific examples of this will be detailed in the current Review, with particular emphasis on the immunomodulatory properties of vitamin D.
Keywords: adaptive immunity, autoimmune disease, infection, innate immunity, vitamin D
INTRODUCTION
Despite its long-standing association with rickets and osteoporosis, vitamin D has become increasing ly recognized as a pluripotent regulator of biological functions above and beyond its classical effects on bone and calcium homeostasis. Initial evidence for this arose from studies in the early 1980s demonstrating nonclassical effects of the active form of vitamin D—1,25(OH)2D (1,25-dihydroxyvitamin D). The active form is synthesized from the major circulating form of vitamin D—25OHD (25-hydroxyvitamin D). In 1981, it was shown that the elevated serum levels of 1,25(OH)2D that are frequently observed in patients with granuloma-forming diseases such as sarcoidosis were due to extrarenal expression of the enzyme that activates vitamin D—CP27B (25OHD-1α hydroxylase).1 In this case, synthesis of 1,25(OH)2D from 25OHD was due to expression of CP27B by disease-associated macrophages rather than the classical location of the hydroxylase in proximal tubule cells within the kidney.2 Consequently, subsequent studies have explored the potential immunomodulatory properties of 1,25(OH)2D.3
Since the 1980s, other groups have reported expression of the intracellular receptor for 1,25(OH)2D—the vitamin D receptor (VDR)—in a variety of cell types not directly involved in classical vitamin D endocrinology, leading in turn to the pivotal observation that 1,25(OH)2D was able to inhibit the proliferation of cells expressing the VDR (reviewed by Eelen et al.4 and Bouillon et al.5).
The antiproliferative properties of 1,25(OH)2D have been explored in considerable detail with respect to its potential use as therapy for various common cancers.6 The major limitation with this strategy stems from the potent hyper calcemic properties of 1,25(OH)2D in its classical endocrine setting. As a result, significant efforts have been made to generate synthetic analogs of 1,25(OH)2D that retain the parent compound's antiproliferative activity whilst minimizing calciotropic responses.4,5 Similar strategies have also predominated in the further exploration of immunomodulatory applications for vitamin D.7 Until recently, the non classical actions of vitamin D have, therefore, been character ized by two central concepts. The first concerns the pathophysiological impact of abnormal extrarenal synthesis of 1,25(OH)2D in diseases such as sarcoidosis. The second relates to the putative therapeutic application of 1,25(OH)2D analogs as anticancer agents or immunomodulatory agents.
In the last 2 years, this perception of non classical functions of vitamin D has changed signifi cantly, with emerging data demonstrating functional responses to the ‘inactive’ form of vitamin D—25OHD—that more closely reflect classical vitamin D physiology. This finding, coupled with increasing awareness of the global variability of vitamin D (i.e. 25OHD) status, has further emphasized the importance of vitamin D sufficiency as a key target in human health. The health implications for these new facets of vitamin D activity are diverse, and include a potential role in protection against common cancers and cardiovascular disease.8 In view of the extensive existing literature documenting nonclassical effects of vitamin D, the aim of this current Review will be to focus on recent studies that have described a previously unanticipated role for the localized metabolism of precursor 25OHD to active 1,25(OH)2D as a mechanism for potent coordination of innate and adaptive immune responses in humans.
VITAMIN D AND INNATE IMMUNITY
In 1986 Rook and colleagues described studies using cultured human macrophages in which they showed that 1,25(OH)2D inhibits the growth of Mycobacterium tuberculosis.9 Although this seminal report was widely cited, it is only in the last 2 years that more-comprehensive appraisals of the antimicrobial effects of vitamin D have been published. In the first of these recent studies, in silico screening of the human genome to identify potential 1,25(OH)2D target genes revealed the presence of a vitamin D response element in the promoter of the gene for cathelicidin, one of a class of antimicrobial peptides called defensins.10 Subsequent investigations confirmed the ability of 1,25(OH)2D to induce expression of cathelicidin in myeloid cell lines,11 bronchial epithelial cells12 and keratinocytes.13
Significantly, the report by Weber et al.13 also indicated that cathelicidin could be induced by 25OHD, highlighting the potential for autocrine induction of antimicrobial responses in cells that express the vitamin-D-activating enzyme, CP27B. Although detectable in many cell types, functionally significant expression and activity of CP27B seems to be dependent on cell-specific stimulation. This effect is perhaps best illustrated by studies that have characterized enhanced vitamin D metabolism, and enhanced sensitivity to 1,25(OH)2D, in cells exposed to pathogenic stimuli. Recognition of, and response to, pathogen-associated molecular patterns (PAMPs), which are structures on the pathogen that can trigger innate immune responses, are mediated via a broad spectrum of immune surveillance proteins such as the Toll-like receptors (TLRs)—an extended family of host noncatalytic transmembrane pathogen-recognition receptors (PRRs) that interact with specific PAMPs.14
To clarify innate immune responses to M. tuberculosis, Liu and colleagues used DNA array assays that characterized changes in gene expression following activation of macrophage TLR2 by one of the putative PRRs for M. tuberculosis.15 Human macrophages treated with mycobacterial 19 kDa lipoprotein, a TLR2-interacting PAMP, showed increased expression of both CP27B and VDR, and demonstrated autocrine induction of cathelicidin and bacterial killing in response to 25OHD.15
The importance of such a mechanism as part of the innate immune response to infection has been confirmed in experiments demonstrating 1,25(OH)2D-mediated modulation of cathelicidin expression in monocytes exposed to M. tuberculosis.16 Although this particular study showed that 1,25(OH)2D is also able to induce other factors associated with bacterial killing such as nitric oxide synthase, the authors concluded that activation of cathelicidin is the most likely pathway by which vitamin D interacts with the innate immune system. Further studies using short, interfering RNA to block 1,25(OH)2D-induced gene expression have endorsed this proposal, at least with respect to infection by M. tuberculosis.17
Despite a significant increase in our understanding of how vitamin D interacts with innate immunity in recent years, several key questions remain to be answered. The molecular mechanism that underpins TLR2-mediated induction of CP27B expression has yet to be fully elucidated. Similar induction of 1,25(OH)2D synthesis has been reported in macrophages treated with alternative PAMPs such as the TLR4 ligand, lipopolysaccharide,18 but the broader applicability of this pathway with respect to other TLR members is unclear.
Perhaps the most pertinent question to arise from these studies of innate immunity concerns the biological advantage of using a vitamin-D-mediated pathway to promote bacterial killing. One possible explanation is that 1,25(OH)2D synthesized locally by cells such as macrophages supports host immunity by enhancing expression of defensins such as cathelicidin that are routinely suppressed by specific pathogens. For example, Shigella infection of macrophages and epithelial cells inhibits expression of cathelicidin and human β-defensin 1 as part of an apparent mechanism for evading antibacterial innate immunity.19 Under these circumstances, enhanced localized synthesis of 1,25(OH)2D might act to ‘rescue’ cathelicidin expression and thereby maintain antibacterial surveillance.
Vitamin-D-mediated immunity also has the benefit of providing potential feedback control pathways that might act to limit antibacterial activity, thereby preventing potential inflammatory damage that arises from an over elaboration of immune responses. Recent studies have shown that 1,25(OH)2D can induce hypo responsiveness to PAMPs by downregulating expression of TLR2 and TLR4 on monocytes.20 In parallel with the induction of antimicrobial agents such as cathelicidin, the suppression of TLR expression in this fashion might act to limit inflamma tory T lymphocyte responses that would otherwise promote autoimmunity mediated by T-helper 1 (TH1) lymphocytes (see below).
The vitamin D pathway itself is also subject to feedback regulation as a consequence of 1,25(OH)2D-induced expression of the enzyme 1,25(OH)2D 24-hydroxylase (CP24A), which catalyzes synthesis of less active vitamin D metabolites.21
It is interesting to note, however, that CP24A activity is virtually undetectable in macrophages even after treatment with 1,25(OH)2D—the enzyme's principal stimulator. Instead, data from our group have shown that whilst 1,25(OH)2D readily stimulates expression of CP24A in macrophages, there is no concomitant increase in the activity of this enzyme. This seems to be due to the induction by 1,25(OH)2D of a splice variant form (CP24A-SV) that has a truncated amino-terminal protein sequence in which the mitochondrial targeting sequence is missing.22 Although CP24A-SV is readily able to bind 25OHD and/or 1,25(OH)2D, it is also confined to the cytoplasm in a meta bolically inactive state and would therefore seem to limit excessive vitamin D metabolism by functioning as a cytosolic ‘buffer’ for metabolites that would otherwise act as substrates for CP27B or CP24A.
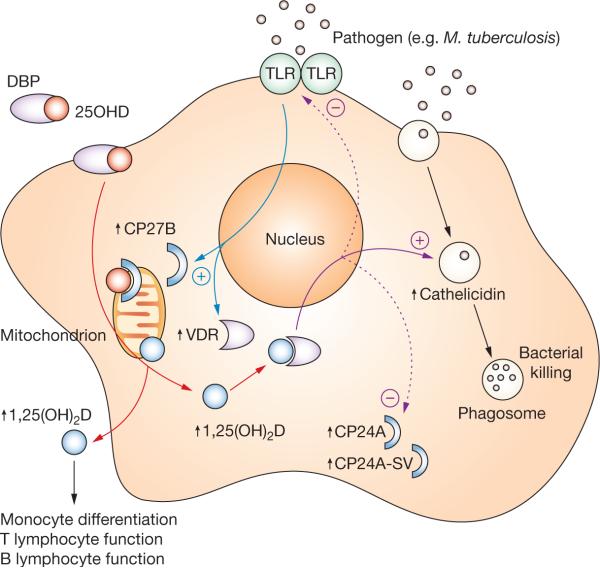
On the basis of the observations outlined above, it is possible to integrate metabolism of 25OHD with PRR responses to PAMPs to provide an autocrine mechanism for enhanced phagosomal bacterial killing by the host cell (Figure 1). Such a mechanism seems to be central to macrophage-mediated immune responses but is unlikely to be exclusive to these cells. Studies using keratinocytes have also demonstrated induction of cathelicidin in response to autocrine synthesis of 1,25(OH)2D.23
Figure 1.
Vitamin D and innate immunity. Activation of macrophage TLR (e.g. TLR2) signaling by pathogens such as as Mycobacterium tuberculosis results in the transcriptional induction of VDR and CP27B expression (blue arrows). Circulating 25OHD (red circles) bound to plasma DBP enters macrophages (red arrows) and is converted to 1,25(OH)2D (blue circles) by mitochondrial CP27B, and can bind to the VDR in the cell. Once bound to VDR, 1,25(OH)2D is able to act as a transcriptional factor leading to the induction of cathelicidin expression (solid purple arrow). Incorporation into phagosomes containing internalized pathogen enables cathelicidin to function as an antibacterial agent. As well as upregulating cathelicidin expression, macrophage synthesis of 1,25(OH)2D can also facilitate negative autoregulation (dashed purple arrows), firstly via increased expression of the feedback enzyme CP24A and its decoy CP24A-SV, and secondly via downregulation of TLR expression. In parallel with autocrine effects on innate antibacterial function, macrophage CP27B might also induce paracrine responses in monocytes, and T or B lymphocytes as a consequence of 1,25(OH)2D secretion. Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; CP24A, 1,25(OH)2D 24-hydroxylase; CP24A-SV, 1,25(OH)2D 24-hydroxylase splice variant; CP27B, 25OHD-1α hydroxylase; DBP, vitamin-D-binding protein; TLR, Toll-like receptor 2; VDR, vitamin D receptor.
As with macrophages, expression of CP27B by keratinocytes can be stimulated via TLR2-mediated recognition of PAMPs, although this mechanism requires initial induction of TLR2 expression (unlike keratinocytes, macrophages express TLR2 constitutively).23 Interestingly, it seems that 1,25(OH)2D itself can fulfill this function, in stark contrast to its suppression of TLR2 expression in macrophages.20 This finding then raises the question of how CP27B expression in keratinocytes is initiated in the absence of constitutive TLR2 signaling. The answer, it seems, is that production of 1,25(OH)2D by keratinocytes is also stimulated by transforming growth factor β1. Induction of CP27B via this alternative pathway appears to provide sufficient 1,25(OH)2D to upregulate expression of TLR2 by keratinocytes and this, in turn, amplifies cathelicidin production in a similar fashion to that described for macrophages.23
In human skin, expression of transforming growth factor β1 is closely associated with wound repair, and thus its interaction with vitamin-D-mediated cathelicidin production is postulated to be part of a mechanism linking wound repair with enhanced innate immune surveillance.23 The broader applicability of this mechanism for injury-induced enhancement of 1,25(OH)2D production with respect to barrier sites other than the skin remains unclear, and it is interesting to note that cells from the colon do not seem to induce cathelicidin in response to 1,25(OH)2D.24 The basis for regulation of vitamin-D-induced innate immunity in keratinocytes also requires clarification, particularly in view of the ‘feed-forward’ induction of TLR expression by 1,25(OH)2D. One possible explanation for this regulation in keratinocytes is that (unlike macrophages), they show suppression of CP27B activity and induction of CP24A activity in response to 1,25(OH)2D, and are thus capable of more-sensitive regulation of autocrine vitamin D responses.25
VITAMIN D, ANTIGEN PRESENTATION AND ADAPTIVE IMMUNITY
The presence of VDR in human lymphocytes was one of the first observations implicating vitamin D in noncalciotropic responses.26 The fact that expression of these receptors occurs in activated, but not resting, T lymphocytes27 and B lymphocytes28 provided evidence of a functional role for vitamin D as a modulator of the acquired (adaptive) arm of the immune system.
With respect to B lymphocytes, the ability of 1,25(OH)2D to suppress proliferation and immunoglobulin production was initially considered to be an indirect effect mediated via TH lymphocytes.29 A more recent report has, however, demonstrated that 1,25(OH)2D does indeed exert direct effects on B lymphocyte homeostasis.30 In addition to confirming direct VDR-mediated effects on B lymphocyte prolifera tion and immunoglobulin production, this study also highlighted the ability of 1,25(OH)2D to inhibit the differentiation of B lymphocytes to plasma cells and class-switched memory B cells (see Figure 2), suggesting a potential role for vitamin D in B-lymphocyte-related disorders such as systemic lupus erythematosus (SLE). Indeed the authors of this study showed that patients with SLE had significantly lower serum levels than controls of both 25OHD and 1,25(OH)2D.
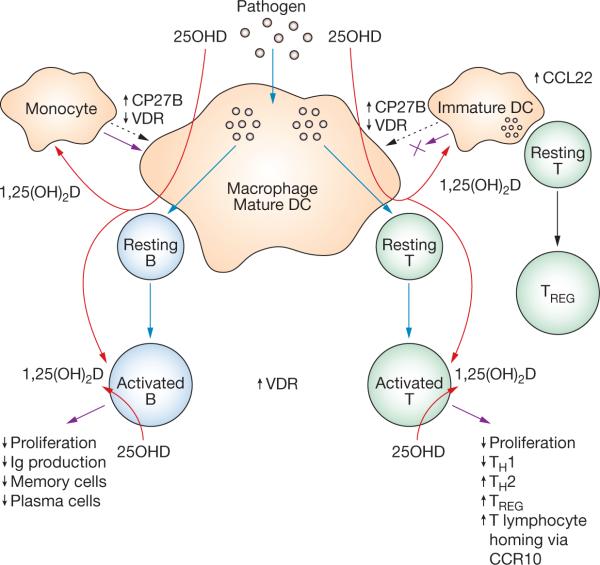
Figure 2.
Vitamin D, antigen presentation and adaptive immunity. Macrophages and mature DCs can induce both adaptive T-lymphocyte-mediated and B-lymphocyte-mediated immunity by internalizing and processing pathogens. Presentation of the resulting antigens to resting B lymphocytes or resting T lymphocytes leads to the activation of these cells and concomitant adaptive immune response (blue arrows). Macrophages and mature DCs also express the vitamin-D-activating enzyme CP27B and are thus able to synthesize 1,25(OH)2D from precursor 25OHD (red arrows). The 1,25(OH)2D synthesized in this way can act in a paracrine fashion on activated B lymphocytes and activated T lymphocytes, which express abundant VDR; local effects of 1,25(OH)2D are designated by purple arrows. The effects on B and T lymphocyte function are listed. Additional paracrine responses to 1,25(OH)2D also seem to be manifested via precursor monocytes and immature DCs, which express higher levels of VDR than their mature counterparts. In the case of monocytes, 1,25(OH)2D seems to stimulate further differentiation of macrophages (purple arrow at the upper left), possibly as an adjunct to the effects of vitamin D on innate immunity (see Figure 1). By contrast, 1,25(OH)2D suppresses DC maturation (blocked purple arrow at the upper right), providing a mechanism for attenuation of adaptive T lymphocyte responses coupled with enhancement of immunosuppression mediated by TREG. Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; CCL22, CC-chemokine ligand 22; CCR10, CC-chemokine receptor 10; CP27B, 25OHD-1α hydroxylase; DC, dendritic cell; Ig, immunoglobulin; TH1, type 1 T-helper lymphocyte; TH2, type 2 T-helper lymphocyte; TREG, T-regulatory lymphocyte; VDR, vitamin D receptor.
The most well-established function of vitamin D within the adaptive immune system concerns the ability of 1,25(OH)2D to modulate T lymphocyte proliferation and function. Following the detection of VDR in activated, proliferating T lymphocytes,27 several groups went on to demonstrate potent antiproliferative responses to 1,25(OH)2D in these cells.31 Amongst the various subgroups of T lymphocytes, the principal target for 1,25(OH)2D seems to be TH lymphocytes.
Initial studies showed that 1,25(OH)2D not only acts to suppress TH lymphocyte proliferation but also modulates their production of cytokines such as interleukin 2 (IL-2).32 Antigen-mediated activation of naive TH lymphocytes results in the generation of pluripotent TH0 lymphocytes that synthesize a broad spectrum of cytokines including IL-2, IL-4, IL-10 and interferon γ (IFN-γ).33 Proliferating TH0 lymphocytes are then able to differentiate into TH subgroups that exhibit a more distinct cytokine profile—TH1 (IL-2, IFN-γ, tumor necrosis factor) and TH2 (IL-3, IL-4, IL-5, IL-10), which respectively support cell-mediated and humoral immunity.33,34 A key immuno modulatory property of 1,25(OH)2D is its ability to inhibit expression of TH1 cytokines,35 whilst augmenting TH2 cytokines,36 with 1,25(OH)2D acting either directly via effects on T lymphocytes36 or indirectly via effects on antigen-presenting cells (APCs).37
The potent effects of 1,25(OH)2D in preferentially promoting cell-mediated TH1 immunity rather than antibody-mediated TH2 immunity have been proposed as one of the key mechanisms by which vitamin D can exert beneficial effects on autoimmune disease.38 Recent studies have, however, shown that the effects of 1,25(OH)2D on T lymphocytes are more complex and include the generation of IL-10-producing CD4+CD25+ T-regulatory lymphocytes (TREG). Previously known as suppressor T cells, TREG promote tolerance to self-antigens and are therefore a key consideration in combating autoimmune disease and host–graft rejection in transplantation.39 In addition to providing another avenue for vitamin D in the protection against autoimmune disease, the induction of TREG is therefore likely to be a key benefit of the proposed use of 1,25(OH)2D or its synthetic analogs to promote immune tolerance following organ transplantation.40
Although the ability of 1,25(OH)2D to support differentiation of TREG might involve direct effects on T lymphocytes, current data suggest that the most likely immune targets for adaptive immune responses to 1,25(OH)2D are dendritic cells (DCs). By suppressing maturation of DCs and enhancing their expression of specific cytokines such as IL-10, 1,25(OH)2D can enhance tolerogenesis through the suppression of TH1 lymphocyte development and the induction of TREG (see Figure 2).41 Similar DC responses might also be mediated through effects of 1,25(OH)2D on chemoattractant factors such as CC-chemokine ligand (CCL) 22, which is secreted by DCs and, like IL-10, supports the generation of TREG.42
Interestingly, these tolerogenic effects of 1,25(OH)2D on antigen presentation seem to be restricted to a specific class of DCs known as myeloid DCs.42 This class of DCs exhibits a repertoire of PRRs and cytokines that is distinct from the other class of DCs, known as plasmacytoid DCs, and the latter showed no apparent tolerogenic responses to 1,25(OH)2D. In myeloid DCs, but not plasmacytoid DCs, 1,25(OH)2D inhibited intracellular signaling of nuclear factor κB, suggesting that this pathway is crucial to vitamin D function in DCs.42
The role of vitamin D as a coordinator of other T lymphocyte phenotypes such as IL-17-secreting T lymphocytes (TH17 lymphocytes) has yet to been fully elucidated, but it is interesting to note that when nonobese diabetic (NOD) mice (a strain susceptible to auto immune disease) were treated with an analog of 1,25(OH)2D, they showed decreased expression of IL-17.43 In addition to proliferation and differentiation, a key component of T lymphocyte adaptive immunity is the manner in which lymphocytes ‘home’ to particular tissues and are retained or removed from these sites. Recent data have shown that 1,25(OH)2D can contribute to this process by stimulating T lymphocyte expression of CC-chemokine receptor (CCR) 10, which recognizes the chemokine CCL27 that is secreted by keratinocytes.44 In this way, 1,25(OH)2D might help to support translocation of T lymphocytes to the skin and/or the retention of these cells at this site.
IMMUNE RESPONSES INVOLVE LOCAL METABOLISM OF VITAMIN D
Although most of the data implicating vitamin D with innate and adaptive immunity have stemmed from studies in vitro using active 1,25(OH)2D, it is now apparent that in vivo the immune activity of vitamin D is unlikely to involve circulating levels of the active metabolite, in part because most immune responses to 1,25(OH)2D in vitro require concentrations of 1−100 nmol/l, despite circulating levels of the hormone being approximately 0.1 nmol/l. In addition, the ability of immune cells such as macrophages to synthesize 1,25(OH)2D from 25OHD was one of the first observations linking vitamin D with the immune system.2
With these two key factors in mind, the most probable mechanism by which vitamin D interacts with the immune system is via localized expression of CP27B, to support intracrine, autocrine or paracrine synthesis of 1,25(OH)2D. As has already been described, intracrine metabolism seems to be the prevalent mechanism by which 25OHD can influence innate immunity15 (see Figure 1). Similar activity might also, however, be important to adaptive immunity. Although macrophages can act as APCs, the process of T cell activation is primarily determined by ‘professional’ APCs such as DCs. It is therefore interesting to note that both macrophages and DCs show similar patterns of expression for CP27B and VDR as they differentiate.45,46
In the case of DCs, increased synthesis of 1,25(OH)2D and decreased expression of VDR in mature versus immature cells is consistent with a mechanism by which local metabolism of 25OHD can inhibit DC maturation.46 This process, in turn, will provide conditions that are conducive to the generation of TREG whilst suppressing possible TH1 activity. Analysis of the effects of vitamin D on T-cell homing via CCL27–CCR10 interaction has, moreover, shown that this activity also involves localized synthesis of 1,25(OH)2D.44 Intriguingly, although the authors of this study confirmed DCs as a source of CP27B activity, they showed that T cells can also express the enzyme. Similar observations have been reported for B cells,30 suggesting that at least some lymphocyte responses to vitamin D are mediated via autocrine or intracrine metabolism rather than paracrine metabolism of vitamin D (see Figure 2).
NONCLASSICAL VITAMIN D AND HUMAN DISEASE
The detection of VDR in tissues not immediately associated with calcium homeostasis and bone metabolism paved the way for the first re-evaluations of the physiological actions of 1,25(OH)2D.47,48 Here, investigations of the unexpected effects of vitamin D on human disease were very much focused on the potential clinical use of VDR agonists as therapy for common cancers, autoimmune disease or host–graft rejection. More recently, perception of the nonclassical effects of vitamin D has undergone a complete revision. In this case, recognition of the unexpected effects of vitamin D has stemmed from detection of the vitamin-D-activating enzyme CP27B in a much wider range of tissues than originally thought (reviewed by Townsend et al.49).
As a result, the new era of knowledge about nonclassical vitamin D function has had less to do with development of synthetic analogs of 1,25(OH)2D and more to do with revision of the parameters that define normal circulating levels of vitamin D, the potential clinical consequences of vitamin D insufficiency and the supplementation strategies that can be used to correct vitamin D status.8
Several recent studies have underlined this new approach to nonclassical vitamin D physiology. For example, CP27B expression and activity has been reported in normal breast,50 colon,51 pancreas52 and prostate53 tissue, as well as their respective tumors,50,51,54,55 providing a potential mechanism to underpin the higher mortality levels associated with these cancers in patients with low circulating levels of 25OHD.56-59 These observations are also consistent with data showing geographical variations in mortality from common cancers, which show a greater risk of death at higher latitudes where levels of sunlight-derived vitamin D in the body are diminished.60
Localized actions of nonclassical CP27B activity do not seem to be restricted to cancer prevention. Expression of CP27B and VDR has been reported in the human brain,61 and low maternal vitamin D status has been shown to affect neurogenesis in animals.62 The conclusion from these observations is that vitamin D exerts autocrine and paracrine effects on the brain that might possibly underpin the link between 25OHD status and psychological disorders such as schizophrenia and depression.63,64 Several other clinical conditions including hyper tension and cardiovascular disease have also been linked to low vitamin D status65,66 although, as yet, it is unclear whether these effects are due to autocrine rather than endocrine metabolism of 25OHD. Instead, the most powerful evidence that effects of altered vitamin D status are mediated via localized metabolism of 25OHD has arisen from studies of infection and autoimmune disease, and these are documented in greater detail in the following two sections.
VITAMIN D AND INFECTION
It is now clear that induction of extrarenal vitamin D metabolism is an integral component of normal human TLR-mediated immunity, and not simply a pathological phenomenon associated with inflammatory or granulomatous disease. The potent induction of antimicrobial cathelicidin and associated bacterial killing in response to 25OHD as well as 1,25(OH)2D is, furthermore, consistent with a cell-specific localized response that is distinct from the classical endocrine functions of vitamin D. The efficiency of this autocrine mechanism as a modulator of innate immunity will therefore be dependent on several factors, including the magnitude of gene induction in response to PAMP–PRR signaling. Regulation of both CP27B and VDR in response to a specific infection will, in turn, be influenced by inherited variations in either or both of these genes, and this is discussed in greater detail in a later section of this article.
Another significant variable within any intracrine, autocrine or indeed endocrine system is the availability of substrate for enzymic conversion to active product. In the case of vitamin D, 25OHD is not only the substrate for CP27B but is also the major circulating form of vitamin D. Serum levels of 25OHD are a direct reflection of the vitamin D status of any given individual and are an amalgam of the related metabolites 25OHD3 (the most abundant circulating form of vitamin D, derived predominantly from the action of sunlight on skin) and 25OHD2 (a form of vitamin D found in food).
With this is mind, perhaps the most striking of all the recent information linking vitamin D with innate immunity is the profound variation in cathelicidin expression observed when macrophages are cultured in serum from human populations with varying vitamin D status.15 In these studies, circulating levels of 25OHD in a white American population were shown to be three-times greater than levels of 25OHD in a group of African Americans. Significantly, macrophages cultured in sera from these two different populations showed TLR2-mediated induction of cathelicidin that was proportional to 25OHD levels, with white individuals exhibiting a threefold higher level of cathelicidin expression compared with their African American counterparts despite being subject to the same innate immune challenge. The singular importance of 25OHD as a determinant of this racial difference in antimicrobial responsiveness was underlined by ‘rescue’ experiments in which the addition in vitro of supplementary 25OHD to African American serum restored TLR-induced cathelicidin expression to levels similar to those observed with serum from white Americans.
As well as confirming the functional importance of localized 25OHD metabolism as a determinant of normal immunity, the studies of cathelicidin induction also provided the first clear example of how variations in vitamin D status have the potential to influence physiological responses. Unlike serum concentrations of 1,25(OH)2D, which are primarily defined by the endocrine regulators of renal CP27B activity, circulating levels of 25OHD are a direct reflection of vitamin D status, which for any given individual will depend on nutritional access to vitamin D either through exposure to sunlight or through dietary intake. The net effect of this differential access is that vitamin D status can vary significantly in populations as a consequence of geographical, social or economic factors. Until recently, vitamin-D-deficient rickets was considered to be the only significant clinical consequence of this variation.
Although absolute ranges have still yet to be agreed, there has been a broad acceptance that vitamin D deficiency can be defined by serum concentrations of 25OHD that are less than 50 nmol/l, with rachitic patients showing much lower levels than this.67 An entirely new perspective on what constitutes vitamin D deficiency has, however, arisen from the seminal observation that serum 25OHD levels correlate inversely with serum parathyroid hormone levels, but only below a threshold 25OHD concentration of approximately 80 nmol/l.68 This observation has led to a complete re-evaluation of the optimal circulating level of 25OHD required for normal bone homeostasis, so that serum concentrations of 25OHD up to 75 nmol/l are now considered to be inadequate and are more commonly referred to as vitamin D ‘insufficiency’ as opposed to ‘deficiency’.67 As a consequence, a recent consensus statement from the 13th Workshop on Vitamin D concluded that vitamin D insufficiency was a global epidemic.69
The potential implications of insufficiency for vitamin D physiology per se include proposed effects on skeletal homeostasis, muscle strength, cancer risk, autoimmune disease, cardiovascular disease and pregnancy outcome. Although these aspects are comprehensively detailed elsewhere,8 it is worth highlighting several specific recent studies that serve to illustrate best the developing clinical relevance of the immunomodulatory mechanisms outlined earlier in this article.
With respect to innate antibacterial effects of vitamin D, attention has focused primarily on potential protection against prevalent infectious diseases such as tuberculosis. Comprehensive clinical trials to assess the impact of 25OHD status and/or vitamin D therapy on tuberculosis have yet to be carried out. Initial studies to assess the effects of 25OHD status on macrophage function in healthy adults have, however, shown that supplementation with a single oral dose of 2.5 mg vitamin D, taken before macrophages are removed for testing, enhances the ability of the macrophages to combat infection with BCG (bacillus Calmette–Guérin) in vitro.70 The potential benefits of vitamin D supplementation as treatment for tuberculosis have been further endorsed by a study which showed that 100% of patients receiving supplementation with 0.25 mg vitamin D per day exhibited sputum conversion from acid-fast bacteria (AFB) positive to AFB-negative status, compared with a conversion rate of only 77% in non-supplemented patients.71
In other studies, a role for 25OHD in counteracting infection in the upper respiratory tract has been proposed.72,73 These observations were based in part on epidemiological data linking seasonal variations in vitamin D status with the seasonality of infections such as influenza,72 as well as randomized clinical studies demonstrating protective effects of vitamin D supplementation against colds and influenza.73 Unlike the model described for tuberculosis, a mechanism by which vitamin D can combat respiratory infection due to colds or influenza has yet to be proposed; however, in view of the fact that cathelicidin has been shown to exhibit antiviral properties,74 it is exciting to speculate that vitamin D therapy may provide a novel strategy for preventing these prevalent clinical problems.
VITAMIN D AND AUTOIMMUNE DISEASE
The proposed role of vitamin D as a regulator of adaptive immunity has been most closely studied in relation to autoimmune diseases. As with the common cancers, diseases such as multiple sclerosis, Crohn's disease and type 1 diabetes are all more prevalent at higher latitudes.75,76 Likewise, vitamin D supplementation has been shown to reduce the risk of multiple sclerosis,77 rheumatoid arthritis78 and type 1 diabetes.79,80 Protection against type 1 diabetes seems to be mediated via the potent effects of vitamin D on innate and adaptive immunity outlined earlier in this article. For example, chronic administration of a 1,25(OH)2D analog has been shown to inhibit the development of type 1 diabetes in NOD mice, and this seems to be associated, at least in part, with restoration of TREG function.81
In other studies using NOD mice, vitamin D deficiency and decreased serum levels of 25OHD during pregnancy and early neonatal life were shown to increase the incidence of diabetes, suggesting an additional role for vitamin D in utero.82 Effects of vitamin D on diabetes might not only be mediated via cells from the immune system. Both CP27B52 and VDR83 have been detected in pancreatic islets, providing a potential autocrine or paracrine system for vitamin D in this organ. The role of this system in protection against type 1 diabetes is unclear. Given the reported link between serum 25OHD status and glucose homeostasis,84 however, it is tempting to speculate that localized metabolism of vitamin D might also be involved in the progression of type 2 diabetes.
Another strand of evidence linking vitamin D with type 1 diabetes stems from the extensive genetic analyses that have explored the physiological impact of inherited variations in the genes for various components of the vitamin D metabolic and signaling system. Previous studies have indicated that some VDR gene haplotypes confer protection against diabetes85 and, more recently, evidence has been found that genetic variants of the CP27B gene also affect susceptibility to type 1 diabetes.86
Collectively these observations suggest that the impact of vitamin D on nonclassical aspects of human health is subject to the same genetic influences observed for classical vitamin D functions such as skeletal homeostasis. What is not clear is how variations in vitamin D status in the form of serum 25OHD concentrations will impact on classical versus nonclassical responses to vitamin D. Whilst the parameters for sufficiency or in sufficiency in vitamin D endocrinology have been partially addressed through studies of the impact of 25OHD status on parathyroid hormone and calcium homeostasis,8,67,68 the relevance of vitamin D insufficiency to nonclassical vitamin D function remains unclear. The elucidation of this relationship is unquestionably one of the key objectives for vitamin D research in coming years, and in addressing this issue it is possible that still further unexpected roles for vitamin D will be defined.
CONCLUSIONS
There is now growing acceptance that the so-called nonclassical effects of vitamin D are in fact a key component of its role in human physiology. In particular, studies over the last 2 years have provided compelling evidence that tissue-specific synthesis of active 1,25(OH)2D from precursor 25OHD is important to both the innate and adaptive immune systems. This evidence—coupled with population data documenting the prevalence of vitamin D insufficiency, and epidemiology detailing the associated immune-related diseases—has underlined the clinical significance of these previously unanticipated actions of vitamin D.
In a meta-analysis published in 2007, data were presented that showed that vitamin D supplementation was associated with decreased risk of mortality from any cause.87 The challenge now faced by vitamin D researchers and clinicians is to provide more-comprehensive mechanisms that will more clearly explain the underlying basis for these beneficial effects of vitamin D, and which will also better define the parameters for management of vitamin D status. The recent dissection of vitamin D effects on innate and adaptive immunity presented here represents the first major step in this direction.
KEY POINTS.
■ Nonclassical effects of vitamin D have been recognized for many years, but it is only recently that these have been accepted as a potentially significant component of vitamin D physiology
■ Immune cells such as macrophages contain all of the machinery required to synthesize and respond to active vitamin D, 1,25-dihydroxyvitamin D, and these functions are enhanced by challenge to the immune system
■ 1,25-Dihydroxyvitamin D stimulates innate (macrophage) immunity by enhancing bacterial killing but it also modulates adaptive (lymphocyte) immunity to minimize inflammation and autoimmune disease
■ Vitamin D insufficiency is now a global health issue—even in developed countries
■ Vitamin D insufficiency is associated with compromised immunity, leading to increased infectious diseases such as tuberculosis, and increased susceptibility to autoimmune diseases such as type 1 diabetes
Footnotes
REVIEW CRITERIA
PubMed was searched for papers published between January 1980 and October 2007 using the terms “vitamin D”, “innate immunity”, “autoimmune disease”, “lymphocyte”, “macrophage”, “dendritic cell” and “vitamin D deficiency”.
Competing interests
The authors declared no competing interests.
References
- 1.Barbour GL, et al. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981;305:440–443. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 2.Adams JS, et al. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 4.Eelen G, et al. Mechanism and potential of the growth-inhibitory actions of vitamin D and analogs. Curr Med Chem. 2007;14:1893–1910. doi: 10.2174/092986707781058823. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon R, et al. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CS, et al. The antitumor efficacy of calcitriol: preclinical studies. Anticancer Res. 2006;26:2543–2549. [PubMed] [Google Scholar]
- 7.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 9.Rook GA, et al. Vitamin D3, γ interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang TT, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 11.Gombart AF, et al. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 12.Yim S, et al. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber G, et al. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 15.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 16.Martineau AR, et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 17.Liu PT, et al. Cutting Edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 18.Reichel H, et al. 25-Hydroxyvitamin D3 metabolism by lipopolysaccharide-stimulated normal human macrophages. J Clin Endocrinol Metab. 1987;64:1–9. doi: 10.1210/jcem-64-1-1. [DOI] [PubMed] [Google Scholar]
- 19.Islam D, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghi K, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 21.Sakaki T, et al. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 22.Ren S, et al. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280:20604–20611. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 23.Schauber J, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schauber J, et al. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikle DD, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocr Rev. 1993;14:3–19. doi: 10.1210/edrv-14-1-3. [DOI] [PubMed] [Google Scholar]
- 26.Provvedini DM, et al. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 27.Bhalla AK, et al. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 28.Provvedini DM, et al. 1 α,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. J Immunol. 1986;136:2734–2740. [PubMed] [Google Scholar]
- 29.Lemire JM, et al. 1 α,25-Dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 31.Rigby WF, et al. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemire JM, et al. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–3035. [PubMed] [Google Scholar]
- 33.Abbas AK, et al. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 34.Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 35.Lemire JM, et al. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 36.Boonstra A, et al. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 37.Piemonti L, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 38.Overbergh L, et al. 1α,25-Dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524−543). Diabetes. 2000;49:1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 39.Barrat FJ, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregori S, et al. Regulatory T cells induced by 1 α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 41.Penna G, Adorini L. 1 α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 42.Penna G, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 43.Penna G, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504–8511. doi: 10.4049/jimmunol.177.12.8504. [DOI] [PubMed] [Google Scholar]
- 44.Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 45.Kreutz M, et al. 1,25-Dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–1307. [PubMed] [Google Scholar]
- 46.Hewison M, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 47.Jones G, et al. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 48.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 49.Townsend K, et al. Biological actions of extrarenal 25-hydroxyvitamin D-1α-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol. 2005;97:103–109. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Townsend K, et al. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res. 2005;11:3579–3586. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 51.Bises G, et al. 25-Hydroxyvitamin D3−1α-hydroxylase expression in normal and malignant human colon. J Histochem Cytochem. 2004;52:985–989. doi: 10.1369/jhc.4B6271.2004. [DOI] [PubMed] [Google Scholar]
- 52.Bland R, et al. Expression of 25-hydroxyvitamin D3−1α-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89−90:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz GG, et al. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7:391–395. [PubMed] [Google Scholar]
- 54.Schwartz GG, et al. Pancreatic cancer cells express 25-hydroxyvitamin D-1 α-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis. 2004;25:1015–1026. doi: 10.1093/carcin/bgh086. [DOI] [PubMed] [Google Scholar]
- 55.Whitlatch LW, et al. 25-Hydroxyvitamin D-1α-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transfer. J Steroid Biochem Mol Biol. 2002;81:135–140. doi: 10.1016/s0960-0760(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 56.Giovannucci E, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 57.Ahonen MH, et al. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000;11:847–852. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 58.Feskanich D, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 59.Garland CF, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 61.Eyles DW, et al. Distribution of the vitamin D receptor and 1 α-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 62.O'Loan J, et al. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology. 2007;32:227–234. doi: 10.1016/j.psyneuen.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 63.McGrath J, et al. Long-term trends in sunshine duration and its association with schizophrenia birth rates and age at first registration—data from Australia and the Netherlands. Schizophr Res. 2002;54:199–212. doi: 10.1016/s0920-9964(01)00259-6. [DOI] [PubMed] [Google Scholar]
- 64.Gloth FM, III, et al. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3:5–7. [PubMed] [Google Scholar]
- 65.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Zittermann A, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 67.Dawson-Hughes B, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 68.Chapuy MC, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 69.Norman AW, et al. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–205. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martineau AR, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 71.Nursyam EW, et al. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 72.Cannell JJ, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aloia JF, Li-Ng M. Correspondence. Epidemiol Infect. 2007;135:1095–1098. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergman P, et al. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 75.Ponsonby AL, et al. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267–1275. doi: 10.1562/2005-02-15-IR-441. [DOI] [PubMed] [Google Scholar]
- 76.Ponsonby AL, et al. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;181−182:71–78. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 77.Munger KL, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 78.Merlino LA, et al. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 79.Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr. 2005;135:323–325. doi: 10.1093/jn/135.2.323. [DOI] [PubMed] [Google Scholar]
- 80.Hypponen E, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 81.Gregori S, et al. A 1α,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 82.Giulietti A, et al. Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451–462. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 83.Pike JW, et al. Biochemical evidence for 1,25-dihydroxyvitamin D receptor macromolecules in parathyroid, pancreatic, pituitary, and placental tissues. Life Sci. 1980;26:407–414. doi: 10.1016/0024-3205(80)90158-7. [DOI] [PubMed] [Google Scholar]
- 84.Need AG, et al. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) 2005;62:738–741. doi: 10.1111/j.1365-2265.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- 85.Ramos-Lopez E, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann N Y Acad Sci. 2006;1079:327–334. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 86.Bailey R, et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007;56:2616–2621. doi: 10.2337/db07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]