Abstract
Summary: Spinal cord injury (SCI) results in loss of nervous tissue and consequently loss of motor and sensory function. There is no treatment available that restores the injury-induced loss of function to a degree that an independent life can be guaranteed. Transplantation of stem cells or progenitors may support spinal cord repair. Stem cells are characterized by self-renewal and their ability to become any cell in an organism. Promising results have been obtained in experimental models of SCI. Stem cells can be directed to differentiate into neurons or glia in vitro, which can be used for replacement of neural cells lost after SCI. Neuroprotective and axon regeneration-promoting effects have also been credited to transplanted stem cells. There are still issues related to stem cell transplantation that need to be resolved, including ethical concerns. This paper reviews the current status of stem cell application for spinal cord repair.
Keywords: Spinal cord injuries, Stem cells, Totipotency, Pluripotency, Cell transplantation, Neuroprotection, Axon regeneration, Gene therapy, Ethics
INTRODUCTION
Stem cells proliferate, migrate, and differentiate to form organisms during embryogenesis. During adulthood, stem cells are present within tissues/organs including the central nervous system (1–5), where they may differentiate into neurons (6). Since the identification and characterization of stem cells, a great deal of interest has been given to their potential for treatment of spinal cord injury (SCI), traumatic brain injury, and degenerative brain diseases (7–12). Considering their characteristic abilities to self-renew and differentiate into any cell type in the body, the therapeutic promise of stem cells is justified. Before effective therapies can be developed, several issues need to be addressed and resolved. These issues range from increasing our basic knowledge about the stem cell's biology to prevailing over moral concerns fueled by religious and/or political ideas.
STEM CELL DEFINITIONS
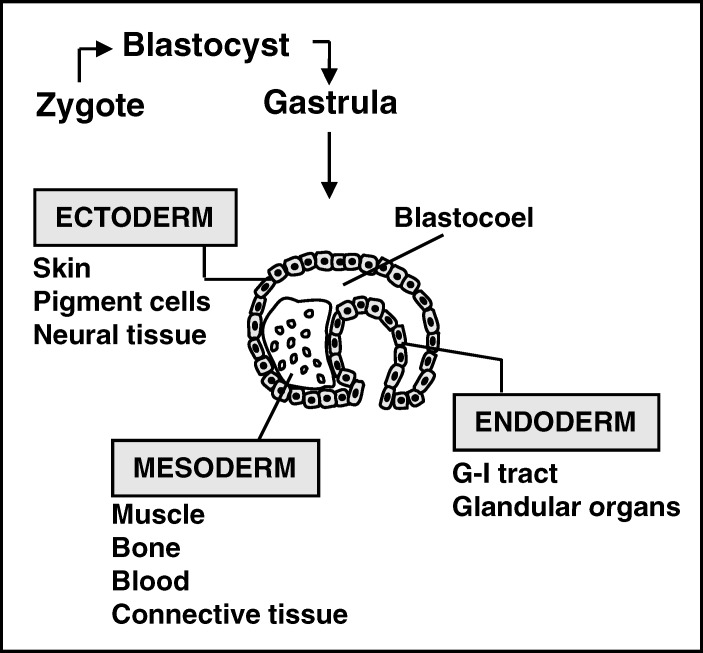
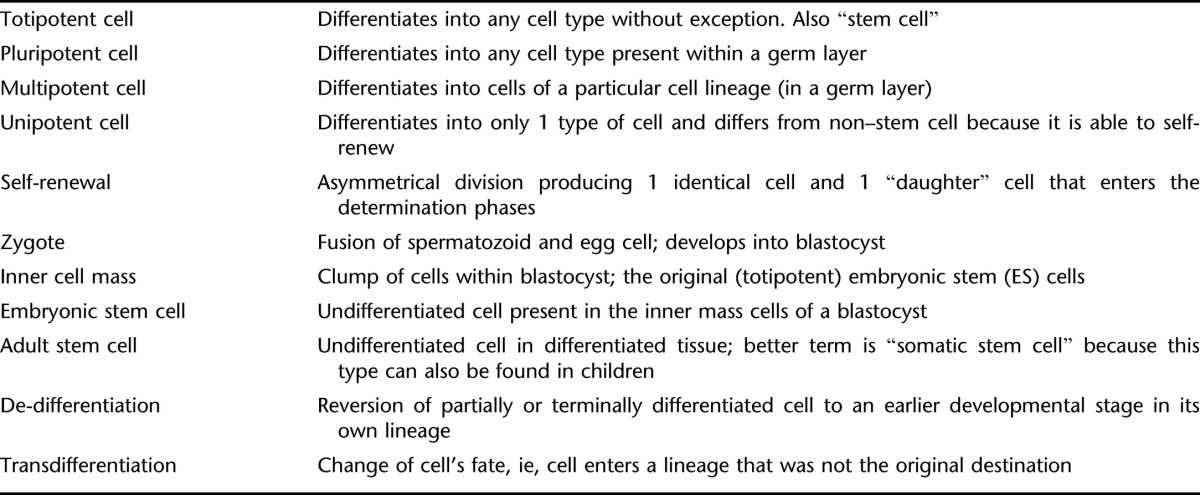
A stem cell is defined by its ability of self-renewal and its totipotency. Self-renewal is characterized by the ability to undergo an asymmetric division in which one of the resulting cells remains a “stem cell,” without signs of aging, and the other (daughter) cell becomes restricted to one of the germ layers. A stem cell may become quiescent and at later stages re-enter the cycle of cell division (13,14) (Figure 1).
Figure 1. All tissues in an organism originate from the 3 germ layers: the ectoderm layer, endoderm layer, and the mesoderm layer. Neural cells that form the central and peripheral nervous system derive from the ectoderm.
A true stem cell is a totipotent cell; it can become any cell type present in an organism. Many consider the zygote to be the only true totipotent (stem) cell because it is able to differentiate into either a placenta cell or an embryonic cell. Others define the cells of the inner cell mass within the blastocyst as embryonic stem cells (ESCs). These cells are pluripotent because they can not become a placenta cell (Table 1). Besides ESCs, undifferentiated cells can be found among differentiated cells of a specific tissue after birth. These cells are known as adult stem cells, although a better term would be “somatic stem cell” because they are also present in children and umbilical cords. There is ample evidence that adult stem cells are not restricted to a particular germ layer and can transdifferentiate (15–19). An important advantage of adult stem cells over ESCs is that they can be harvested without destruction of an embryo. As a result, adult stem cells have gained ample interest for their application in a variety of disorders (see below).
Table 1.
Terms Most Frequently Used in Stem Cell Biology
Differentiation
The pluripotent stem cell differentiates into a multipotent cell of the 3 germ layers. These 3 layers are the ectodermal layer (from which skin and neural tissue originate), the mesodermal layer (connective tissue, muscle, bone, and blood cells), and the endodermal layer (gastrointestinal tract and internal glandular organs) (Figure 2).
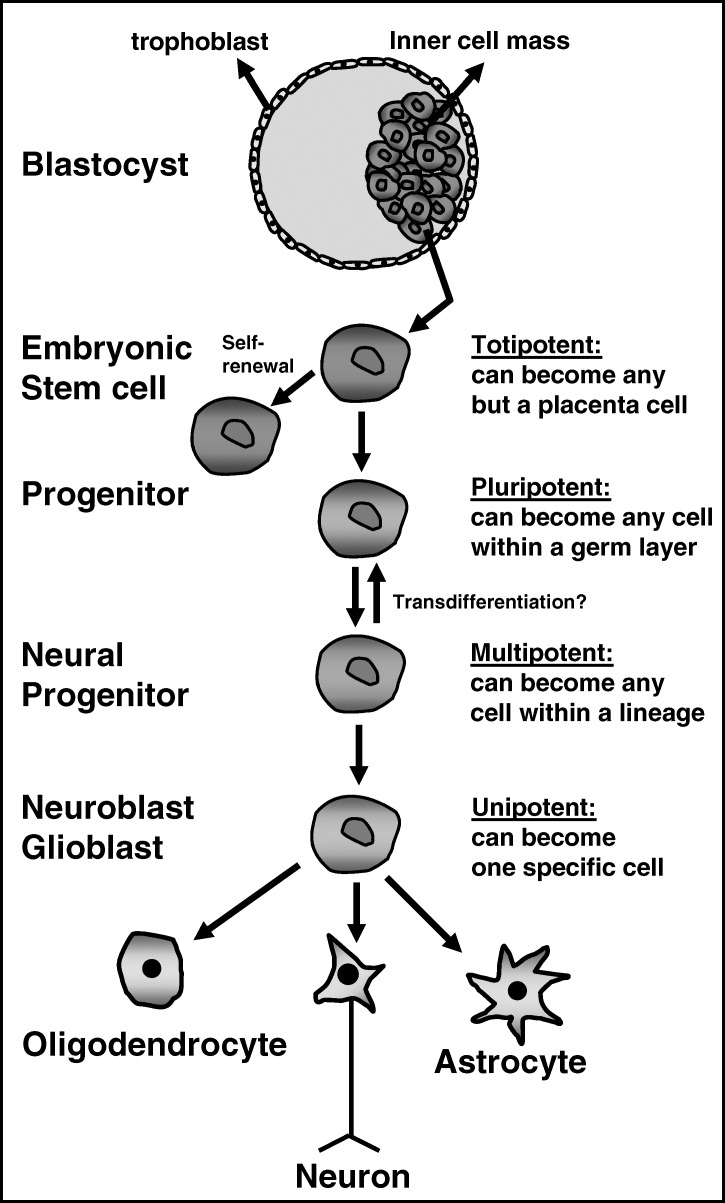
Figure 2. From embryonic stem cell to differentiated neural cell. Embryonic stem cells from the inner cell mass of the blastocyst are pluripotent and undergo phases of differentiation that change them into unipotent cells. This depicts the generation of neural cells; oligodendrocytes, neurons, and astrocytes.
The multipotent cell differentiates into a unipotent cell of a particular cell lineage within its own germ layer (Figure 2). The unipotent cell is capable of becoming a cell type within that particular cell lineage (Figure 2). At the successive phases of differentiation (or determination), the resulting progeny are known as progenitor cells; “stem cell-like” cells capable of self-renewal. Within the central nervous system, unipotent neural progenitors become the neurons and glial cells present in brain and spinal cord (Figure 2).
Transdifferentiation
In classic embryology, the totipotent stem cell becomes unipotent through successive phases of fate restriction. The steps in this process were thought to be irreversible. However, recently it was shown in vitro that the fate of multipotent cells can be changed to another germ layer (15–19). This process is known as transdifferentiation. The unlimited potential of transdifferentiation prompted many investigators to obtain cells that normally derive from stem cells that are more difficult to harvest from stem cells that are easier to harvest. For instance, it is less complicated to harvest stem cells from skin (20,21) or bone marrow (22,23) than from the brain (24,25). Thus, it would be more efficient to obtain neural cells from skin- or bone marrow–derived stem cells through transdifferentiation.
Transdifferentiation has often been shown using nonspecific markers and ignoring possible artifacts caused by culturing methods (26,27). Therefore, the existence of transdifferentiation is still debated (27,28). It should be kept in mind that forced differentiation into a cell from a lineage within an unnatural germ layer could result in abnormal phenotypes that, after grafting, could induce carcinogenesis (29).
POTENTIAL FOR SPINAL CORD REPAIR
After SCI, endogenous regenerative events occur, indicating that the spinal cord attempts to repair itself. Schwann cells, the myelinating and regeneration-promoting cell in the peripheral nervous system, migrate from spinal roots into the damaged tissue and myelinate spinal cord axons (30,31). The expression of regeneration-associated genes is increased in damaged neurons (32,33). There is a surge in proliferation of local adult stem cells and progenitor cells (34–36). However, axonal growth is thwarted by growth inhibitors present on oligodendrocyte myelin debris and on cells that form scar tissue (37–39). Also, the newborn stem cells and progenitor cells do not integrate functionally into the injured spinal cord tissue. Thus, the endogenous regenerative events that occur after injury fail to repair the spinal cord.
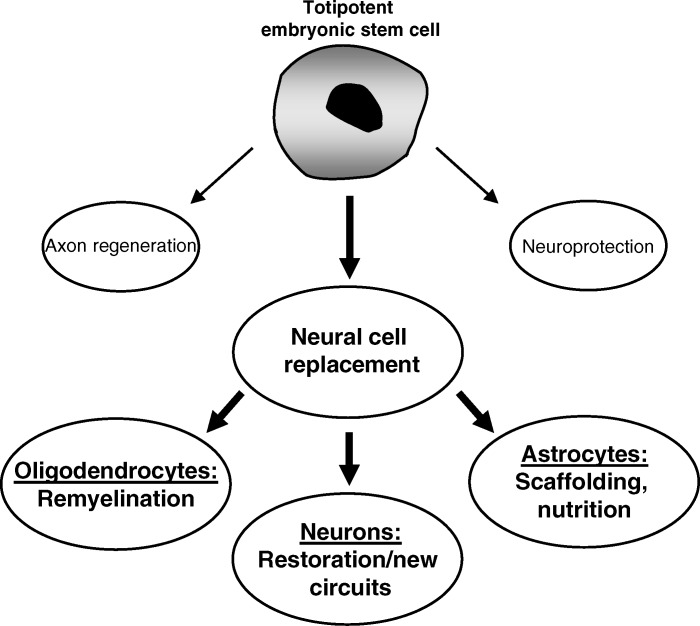
Improved functional outcome after SCI may be elicited by neuroprotective approaches that limit secondary tissue loss and thus the loss of function. Alternatively, functional recovery could be elicited by axon growth-promoting approaches that result in restoration of damaged and/or formation of new axon circuits that could become involved in function. There is little doubt that stem cells and neural progenitor cells could become invaluable components of repair strategies for the spinal cord. They can become neural cells that may support anatomical/functional recovery. Alternatively, they may secrete growth factors that could support neuroprotection and/or axon regeneration (Figure 3). The potential of stem cells or progenitor cells to support spinal cord repair has been studied extensively (40–42). Their shortcomings for repair are also understood (43,44). Over the last decade, stem cells have often been studied without implementing explicit criteria that would define the used cells as such. Consequently, the therapeutic potential of true stem/progenitor cells is still unknown. Other matters related to the use of stem/progenitor cells for SCI also need to be resolved before effective therapies can be developed. How can the cells be best obtained? Do they need to be differentiated in vitro before transplantation? How can survival of grafted stem/progenitor cells be improved and uncontrolled division and differentiation be prevented (45)? How can functional integration of the transplanted cells be improved?
Figure 3. Potential effects of stem cells on spinal cord repair. Although transplanted stem cells could elicit axon regeneration and/or neuroprotection through secretion of growth factors, the most logical contribution to repair could come from their ability to replace lost neural cells. This could result in remyelination of demyelinated axons if they become oligodendrocytes, restoration of (new) circuits if they become neurons, and providing scaffolding and nutrition of the injured area if they become astrocytes. Generally, the last is not preferred because astrocytes express a number of axon growth inhibitory molecules that could prevent axon regeneration and thus limit the overall restoration.
Cell Replacement in the Injured Spinal Cord
Considering the ability of stem cells to become any cell type, their potential use for cell replacement strategies is common sense. With the appropriate combination of (growth) factors (induction cocktail), ESCs can be used to obtain neurons and glial cells (46,47). ES-derived neurons can survive and integrate after injection into the injured rat spinal cord (48). It was shown that transplanted mouse ESCs myelinate axons in the myelin-deficient shiverer rat spinal cord (49). Also, mouse ESCs grafted into the injured (normal) rat spinal cord result in improved functional recovery (50). Importantly, ESCs were found to survive well within the injured spinal cord, suggesting that long-term treatments could be achieved using this approach (51).
Human ESC can be directed toward multipotent neural precursors (52), motor neurons (53,54), and oligodendrocyte progenitor cells (55). The latter were found to differentiate into mature oligodendrocytes in vitro and in vivo (56). Moreover, these cells are able to myelinate axons after transplantation into the spinal cord of myelin-deficient shiverer mice and adult rats (55).
Neural progenitor cells (ie, multipotent cells from which the cells of the central nervous system arise) often aggregate into neurospheres. Cao et al (57) showed that neural progenitor cells transplanted into the injured rat spinal cord favored differentiation into astrocytes. These results indicated the need for differentiation protocols before grafting (58). Fetal neural precursor cells genetically modified to express noggin, an antagonist of bone morphogenetic protein, differentiate preferably into neurons and oligodendrocytes (59). Transplantation of these cells into the injured mouse spinal cord resulted in improved functional outcome (59). However, this result could not be shown by others using the same approach (60).
Human neural progenitor cells can be harvested from blastocyst-stage embryos and manipulated to generate functional neurons and glia (61). When human neural progenitor cells were grafted into the injured rat spinal cord, some of them were found to differentiate into oligodendrocytes (62,63). Moreover, this finding was accompanied by improved functional outcome (62,63).
Mesenchymal stem cells from bone marrow may also have therapeutic promise for SCI (64,65). Although still debated (66), these particular adult stem cells have been shown to differentiate into bone, fat, tendon, and cartilage cells (67). It has been published that these cells can also transdifferentiate in vitro into liver (68), skeletal (69,70), and cardiac muscle (71,72) cells and into central nervous system cells (68,70,73–77). This makes mesenchymal bone marrow stromal stem cells interesting for strategies for repair of the injured spinal cord. Many medical fields are exploring mesenchymal stem cells, for instance, for repair of the heart after myocardial infarction (78,79), osteogenesis imperfecta in orthopedics (80,81), organogenesis in internal medicine (82,83), intervertebral disk disease in neurosurgery (84–87), and stroke/neurodegenerative diseases in neurology (88–90).
Neuroprotection
A neuroprotective strategy implemented soon after SCI would be the first line of defense against injury-induced tissue loss and could contribute to an improved neurological outcome. It has been shown that neural progenitor cells can protect against excitotoxicity (91,92). Also, neural progenitor cells secrete a variety of molecules that could protect neural cells from death mechanisms other than excitotoxicity (91,92). Thus, transplantation of these cells into the injured spinal cord could in fact exert neuroprotective effects. Bone marrow stromal cells have also been shown to elicit neuroprotective effects because grafting into the injured adult rat spinal cord resulted in tissue sparing (93,94). This may have resulted from the secretion of a number of growth factors (95–98).
Axon Regeneration
Promoting axon growth in the injured spinal cord could contribute to restoring function. The ability of neural progenitor cells to secrete a variety of neurotrophic factors indicates that they could promote growth of damaged axons (91,92). Adult neural progenitor cells were found to provide a permissive guiding substrate for corticospinal axon regeneration after spinal cord injury (99). The stem cell–like olfactory ensheathing cells assist axon regeneration in the injured spinal cord in a different manner. These cells are capable of preventing axons from recognizing growth inhibitory molecules thereby allowing them to elongate into otherwise inhibitory terrain (100,101).
CLINICAL APPLICATION TO SCI
The translation of approaches developed in the laboratory involving stem cells into the clinic is in progress. The use of stem cells harvested from tissue from an adult has facilitated the use of stem cells in the clinic because it has practically dismissed the moral objections surrounding the use of stem cells derived from an embryo. Nevertheless, for reasons described below, the use of ESCs is often preferred over that of adult stem cells. Use of human ESCs for spinal cord repair in the United States has been proposed by Geron, a California-based biotechnology company. Application of adult human stem cells for treatment of SCI is in progress in many countries around the world (102). For instance, autologous bone marrow–derived stem cells have been transplanted in the injured spinal cords of 25 patients in Guayaquil, Ecuador, a trial that is supported by a California-based biotechnology company, PrimeCell Therapeutics. Encouraging results have been reported such as improved walking and sensory perception. It has been suggested that surmounting the ethical hurdles (see below) could benefit the clinical application of ESCs (103).
EMBRYONIC VS ADULT STEM CELLS
ESCs can develop into more than 200 different cell types present in the human body (104) and under the appropriate circumstances into an entire organism (105). Human ESCs have been isolated from blastocyst-stage embryos (106). They have also been created using somatic cell nuclear transfer (107,108) or parthenogenetic activation of eggs (109,110). Isolated ESCs do not undergo senescence and retain high telomerase activity and normal cell cycle signaling, which explains their rapid proliferation in culture (111,112). These plastic characteristics make the ESC suitable for central nervous system repair strategies. However, transplantation of ESC can result in teratomas because of uncontrollable cell proliferation (113–115). Also, ESCs in culture may undergo genomic and epigenetic changes that could lead to transformation, although this can be prevented using proper culture techniques (116). Transplanted ESC are prone to be rejected after injection into adult tissue, and long-term treatment with immunosuppressive drugs may be required to prevent this loss (114). These findings have to some extent tempered the enthusiasm for application of ESC in repair strategies for the central nervous system, despite the fact that ESC possess by far the greatest potential and could be applied in a broad selection of reparative cell therapies.
An alternative for ESC are stem cells obtained from tissue after birth. For instance, neural progenitor cells have been harvested from adult brain (117,118) and spinal cord (119). However, adult stem cells are less plastic than ESCs and divide less frequently in culture (120). Also, their differentiation potential may decrease in time (121). This makes them a possible but somewhat limited alternative for ESCs. On the other hand, they offer the advantage that they can be transplanted without genetic modifications or pretreatments. Immune rejection would not be an issue with adult stem cells when the cells are isolated from the patient (autografting) (122). Also, adult stem cells show a high degree of genomic stability during culture (123,124) and usually do not result in tumor formation (124). Finally, there is much less moral concern surrounding the use of adult stem cells because they can be harvested from the patient. These latter features support the use of adult stem cells over ESCs for strategies aimed at repairing the central nervous system. This is certainly true if strategies can be developed that circumvent the potential drawbacks of using adult stem cells such as the lower plastic ability and lower rate of proliferation in vitro compared with ESCs.
ETHICAL AND SOCIAL CONCERNS
One of the issues that surround the use of ESCs is the time point at which an embryo is considered to be a person (125–127). According to the Roman Catholic Church and other religious institutions, an embryo “must be treated from conception as a living person” (128). This implies that a blastocyst cannot be used to harvest cells. Others consider an embryo to be a person only after the 20th week of gestation (125,126), implying that ESCs can be harvested from blastocysts. Also, in that case, ESCs could be harvested from embryos that were generated but not selected for in vitro fertilization. These would otherwise be discarded.
Discussions on what constitutes “life” and when does “life” start are often intense because they are driven by moral concerns fueled by religious and political ideas. These issues need to be addressed with respect to all opponents. Rules regarding the harvest and use of stem cells can only be set after full agreement by all groups within a society.
Ethical issues that surround the use of adult stem cells mostly involve their possible misuse (129). For instance, oocytes can be derived from stem cells of male origin, which allows the production of a child from one or two male biological parents (130–132). The potential biological problems and psychological effects on the child are unknown. It would also be possible that the offspring develops defects because of acquisition of pairs of (recessive) genes (130–132).
Therapeutic cloning and genetic manipulation are other issues that surround the use of stem cells. Cloning of cells, genetically matched for the host, could in theory be beneficial for organ transplantation because it may solve issues such as organ shortage and rejection. Genetic manipulation could convert ESCs into gametes, which would allow germ line gene therapy (GLGT) (131).
INDUCED PLURIPOTENT STEM CELLS
It is now possible to obtain pluripotent cells by reprogramming differentiated cells, such as fibroblasts, through the introduction of 4 transcription factors, OCT3/4 (octamer-4), SOX2 (sex-determining region Y-box2), KLF4 (Kruppel-like factor), and MYC (induced pluripotent stem (iPS) cells (133,134). This new technology was first described by Takahashi and Yamanaka (135) for mouse fibroblasts and has now been applied for other mouse cells (136) and for human somatic cells (137). Of the 4 transcription factors, MYC and KLF4 can be substituted by others (138,139). The underlying mechanisms for this typically straightforward and robust reprogramming procedure are still unknown and intensely debated. At present, it is still unclear in how closely iPS cells resemble conventional ESCs and whether application of iPS cells would result in similar functional results as can be obtained with ESCs. Comparative gene expression profiles of human ESCs and human iPS cells is now ongoing (137,140). Several hurdles need to be overcome before iPS cell technology can produce cells for clinical use (133), such as the use of retroviral vectors to introduce the transcription factors and the need for selection markers to identify the reprogrammed cells, as well as the use of the oncogene MYC and the integration of retroviral vectors into the genome. These needs are required for proper reprogramming, but they modify the cell genetically and modified cells face regulatory obstacles for therapeutic applications. Nevertheless, it is evident that iPS cell technology is promising and has opened exciting avenues for the clinical application of pluripotent cells without the ethical obstacles that go along with the use of ESCs.
CONCLUSIONS
Stem cells hold promise for spinal cord repair, but their true potential has not yet clearly been shown. At this time, stem cell–based therapies are at an early stage, and the associated risks are still unclear. When a patient has a disabling or life-threatening disease, a case might be made for surmounting the existing ethical and social barriers to enable treatment. Changing ethical barriers will not be accomplished overnight. Most likely, stem cell science will advance faster than the debate on ethical issues. Therefore, to enable future use of stem cells for therapeutic purposes, discussions on all related issues and especially the moral aspects need to be held today. As with any medical intervention, the questions to be asked are whether this approach is the most likely one to achieve success and whether the risks justify the benefits.
REFERENCES
- Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp Neurol. 1962;5:302–318. doi: 10.1016/0014-4886(62)90040-7. [DOI] [PubMed] [Google Scholar]
- The Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166(2):257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria in rat. J Neurocytol. 1980;9(2):145–162. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227(4690):1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- McKay JS, Blakemore WF, Franklin RJ. The effects of the growth factor-antagonist, trapidil, on remyelination in the CNS. Neuropathol Appl Neurobiol. 1997;23(1):50–58. [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Snyder EY. Neural transplantation: an approach to cellular plasticity in the developing central nervous system. Review. Semin Perinatol. 1992;16(2):106–121. [PubMed] [Google Scholar]
- Fisher LJ, Gage FH. Grafting in the mammalian central nervous system. Physiol Rev. 1993;73(3):583–616. doi: 10.1152/physrev.1993.73.3.583. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neuronal stem cells: their characterization and utilization. Neurobiol Aging. 1994;15((suppl 2)):S191. doi: 10.1016/0197-4580(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Macklis JD. Multipotent neural progenitor or stem-like cells may be uniquely suited for therapy for some neurodegenerative conditions. Clin Neurosci. 1995;3(5):310–316. [PubMed] [Google Scholar]
- Brüstle O, McKay RD. Neuronal progenitors as tools for cell replacement in the nervous system. Curr Opin Neurobiol. 1996;6(5):688–695. doi: 10.1016/s0959-4388(96)80104-8. [DOI] [PubMed] [Google Scholar]
- Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;Apr(1068):352–366. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls, and uncertainties. Lessons for and from the crypt. Development. 1990;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Koenig S, Krause P, Drabent B, et al. The expression of mesenchymal, neural and haematopoietic stem cell markers in adult hepatocytes proliferating in vitro. J Hepatol. 2006;44(6):1115–1124. doi: 10.1016/j.jhep.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Yoon J, Min BG, Kim YH, et al. Differentiation, engraftment and functional effects of pre-treated mesenchymal stem cells in a rat myocardial infarct model. Acta Cardiol. 2005;60(3):277–284. doi: 10.2143/AC.60.3.2005005. [DOI] [PubMed] [Google Scholar]
- Murrell W, Féron F, Wetzig A, et al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233(2):496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]
- Kruse C, Bodó E, Petschnik AE, Danner S, Tiede S, Paus R. Towards the development of a pragmatic technique for isolating and differentiating nestin-positive cells from human scalp skin into neuronal and glial cell populations: generating neurons from human skin. Exp Dermatol. 2006;15(10):794–800. doi: 10.1111/j.1600-0625.2006.00471.x. [DOI] [PubMed] [Google Scholar]
- Chou SH, Kuo TK, Liu M, Lee OK. In utero transplantation of human bone marrow-derived multipotent mesenchymal stem cells in mice. J Orthop Res. 2006;24(3):301–312. doi: 10.1002/jor.20047. [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26(24):6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CE, Paratore C, Dours-Zimmermann MT, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175(6):1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth S, Narang R, Bhargava B, et al. AIIMS Cardiovascular Stem Cell Study Group. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: clinical and histopathological results: the first-in-man ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial. J Am Coll Cardiol. 2006;48(11):2350–2351. doi: 10.1016/j.jacc.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Tang Y, Yasuhara T, Hara K, et al. Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Review. Cell Transplant. 2007;16(2):159–169. [PubMed] [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisén J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253(2):733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- Pincus DW, Goodman RR, Fraser RA, Nedergaard M, Goldman SA. Neural stem and progenitor cells: a strategy for gene therapy and brain repair. Neurosurgery. 1998;42(4):858–867. doi: 10.1097/00006123-199804000-00103. [DOI] [PubMed] [Google Scholar]
- Enzmann GU, Benton RL, Talbott JF, Cao Q, Whittemore SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma. 2006;23((3–4)):479–495. doi: 10.1089/neu.2006.23.479. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells. 2007;25(11):2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Tao H, Ma DD. Evidence for transdifferentiation of human bone marrow-derived stem cells: recent progress and controversies. Pathology. 2003;35(1):6–13. [PubMed] [Google Scholar]
- Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Review. Nat Rev Mol Cell Biol. 2007;8(5):369–378. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Blakemore WF. Requirements for Schwann cell migration within CNS environments: a viewpoint. Review. Int J Dev Neurosci. 1993;11(5):641–649. doi: 10.1016/0736-5748(93)90052-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22(15):6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4(9):703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Gardiner P, Beaumont E, Cormery B. Motoneurones “learn” and “forget” physical activity. Review. Can J Appl Physiol. 2005;30(3):352–370. doi: 10.1139/h05-127. [DOI] [PubMed] [Google Scholar]
- Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16(6):1045–1057. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131(1):177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Ke Y, Chi L, Xu R, Luo C, Gozal D, Liu R. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells. 2006;24(4):1011–1019. doi: 10.1634/stemcells.2005-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Millet JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Asher RA, Nothias F, Fawcett JW. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain. 2007;130((Pt 4)):926–939. doi: 10.1093/brain/awl372. [DOI] [PubMed] [Google Scholar]
- Tian DS, Dong Q, Pan DJ, et al. Attenuation of astrogliosis by suppressing of microglial proliferation with the cell cycle inhibitor olomoucine in rat spinal cord injury model. Brain Res. 2007;1154:206–214. doi: 10.1016/j.brainres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Teng YD, Liao WL, Choi H, et al. Physical activity-mediated functional recovery after spinal cord injury: potential roles of neural stem cells. Regen Med. 2006;1(6):763–776. doi: 10.2217/17460751.1.6.763. [DOI] [PubMed] [Google Scholar]
- Coutts M, Keirstead HS. Stem cells for the treatment of spinal cord injury. Exp Neurol. 2008;209(2):368–377. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Hardy SA, Maltman DJ, Przyborski SA. Mesenchymal stem cells as mediators of neural differentiation. Review. Curr Stem Cell Res Ther. 2008;3(1):43–52. doi: 10.2174/157488808783489471. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kiel ME, Sadowski D, McKinnon RD. From stem cells to oligodendrocytes: prospects for brain therapy. Stem Cell Rev. 2007;3(4):280–288. doi: 10.1007/s12015-007-9006-9. [DOI] [PubMed] [Google Scholar]
- Zietlow R, Lane EL, Dunnett SB, Rosser AE. Human stem cells for CNS repair. Cell Tissue Res. 2008;331(1):301–322. doi: 10.1007/s00441-007-0488-1. [DOI] [PubMed] [Google Scholar]
- Keirstead HS. Stem cell transplantation into the central nervous system and the control of differentiation. Review. J Neurosci Res. 2001;63(3):233–236. doi: 10.1002/1097-4547(20010201)63:3<233::AID-JNR1016>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Liu S, Qu Y, Stewart TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97(11):6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Jolicoeur C, Raff M. Generation and characterization of oligodendrocytes from lineage-selectable embryonic stem cells in vitro. Methods Mol Biol. 2006;330:15–32. doi: 10.1385/1-59745-036-7:015. [DOI] [PubMed] [Google Scholar]
- Deshpande DM, Kim YS, Martinez T, et al. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006;60(1):32–44. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- Brüstle O. Building brains: neural chimeras in the study of nervous system development and repair. Review. Brain Pathol. 1999;9(3):527–545. doi: 10.1111/j.1750-3639.1999.tb00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5(12):1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- Jendelová P, Herynek V, Urdzíková L, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76(2):232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172(2):383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23(2):215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25(8):1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49(3):385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, et al. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167(1):48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Cao QL, Onifer SM, Whittemore SR. Labeling stem cells in vitro for identification of their differentiated phenotypes after grafting into the CNS. Methods Mol Biol. 2002;198:307–318. doi: 10.1385/1-59259-186-8:307. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Nakashima K, Takizawa T, et al. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp Neurol. 2004;189(1):33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Enzmann GU, Benton RL, Woock JP, Howard RM, Tsoulfas P, Whittemore SR. Consequences of noggin expression by neural stem, glial, and neuronal precursor cells engrafted into the injured spinal cord. Exp Neurol. 2005;195(2):293–304. doi: 10.1016/j.expneurol.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9(4):439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102(39):14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Anderson AJ. Human neural stem cell differentiation following transplantation into spinal cord injured mice: association with recovery of locomotor function. Neurol Res. 2006;28(5):474–481. doi: 10.1179/016164106X115116. [DOI] [PubMed] [Google Scholar]
- Nandoe Tewarie RD, Hurtado A, Levi AD, Grotenhuis JA, Oudega M. Bone marrow stromal cells for repair of the spinal cord: towards clinical application. Cell Transplant. 2006;15(7):563–577. doi: 10.3727/000000006783981602. [DOI] [PubMed] [Google Scholar]
- Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24(5):835–845. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297(5585):1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284(5417):1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18(12):1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1995;279(5256):1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann Acad Sci. 2001;Jun(938):221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats-similarities to astrocyte grafts. PNAS. 1998;95(7):3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290(5497):1779–1180. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Kohyama J, Abe H, Shimazaki T, et al. Brain from bone: Efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation. 2001;68((4–5)):235–244. doi: 10.1046/j.1432-0436.2001.680411.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Kuang JQ, Lin CC, Chiu RC. Transcoronary implantation of bone marrow stromal cells ameliorates cardiac function after myocardial infarction. J Thorac Cardiovasc Surg. 2003;126(1):114–123. doi: 10.1016/s0022-5223(03)00118-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Burch JB, Eisenberg LM. Bone marrow cells transdifferentiate to cardiomyocytes when introduced into the embryonic heart. Stem Cells. 2006;24(5):1236–1245. doi: 10.1634/stemcells.2005-0128. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Chamberlain JR, Schwarze U, Wang PR, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303(5661):1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- Olsson F, Denham M, Cole TJ, Hooper SB, Mollard R. Deriving respiratory cell types from stem cells. Curr Stem Cell Res Ther. 2007;2(3):197–208. doi: 10.2174/157488807781696203. [DOI] [PubMed] [Google Scholar]
- Masaka T, Miyazaki M, Du G, et al. Derivation of hepato-pancreatic intermediate progenitor cells from a clonal mesenchymal stem cell line of rat bone marrow origin. Int J Mol Med. 2008;22(4):447–452. [PubMed] [Google Scholar]
- Sakai D, Mochida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24(20):3531–3541. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- Sakai D, Mochida J, Iwashina T, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27(3):335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30(21):2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- Helm GA, Gazit Z. Future uses of mesenchymal stem cells in spine surgery. Neurosurg Focus. 2005;19(6):E13. doi: 10.3171/foc.2005.19.6.14. Review. [DOI] [PubMed] [Google Scholar]
- Lee J, Kuroda S, Shichinohe H, et al. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology. 2003;23(3):169–180. doi: 10.1046/j.1440-1789.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Sugaya K. Possible use of autologous stem cell therapies for Alzheimer's disease. Curr Alzheimer Res. 2005;2(3):367–376. doi: 10.2174/1567205054367919. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Lladó J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27(3):322–331. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187(2):266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Labouyrie E, Dubus P, Groppi A, et al. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol. 1999;154(2):405–415. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García R, Aguiar J, Alberti E, de la Cuétara K, Pavón N. Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors. Biochem Biophys Res Commun. 2004;316(3):753–754. doi: 10.1016/j.bbrc.2004.02.111. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21(1):33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- Ye M, Chen S, Wang X, et al. Glial cell line-derived neurotrophic factor in bone marrow stromal cells of rat. Neuroreport. 2005;16(6):581–584. doi: 10.1097/00001756-200504250-00013. [DOI] [PubMed] [Google Scholar]
- Pfeifer K, Vroemen M, Blesch A, Weidner N. Adult neural progenitor cells provide a permissive guiding substrate for corticospinal axon growth following spinal cord injury. Eur J Neurosci. 2004;20(7):1695–1704. doi: 10.1111/j.1460-9568.2004.03657.x. [DOI] [PubMed] [Google Scholar]
- Raisman G, Li Y. Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci. 2007;8(4):312–319. doi: 10.1038/nrn2099. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A. Olfactory ensheathing glia transplantation into the injured spinal cord. Prog Brain Res. 2000;128:265–272. doi: 10.1016/S0079-6123(00)28024-2. [DOI] [PubMed] [Google Scholar]
- Mathews DJ, Sugarman J, Bok H, et al. Cell-based interventions for neurologic conditions. Ethical challenges for early human trials. Neurology. 2008;71(4):288–293. doi: 10.1212/01.wnl.0000316436.13659.80. [DOI] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217–233. doi: 10.1016/S0079-6123(06)61015-7. [DOI] [PubMed] [Google Scholar]
- Sell S. Stem Cells Handbook. Totowa, NJ: Humana Press; 2004. [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Bakken AM. Cryopreserving human peripheral blood progenitor cells. Review. Curr Stem Cell Res Ther. 2006;1(1):47–54. doi: 10.2174/157488806775269179. [DOI] [PubMed] [Google Scholar]
- Ballen KK, Haley NR, Kurtzberg J, et al. Outcomes of 122 diverse adult and pediatric cord blood transplant recipients from a large cord blood bank. Transfusion. 2006;46(12):2063–2070. doi: 10.1111/j.1537-2995.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Grant KA, Chapman KB, et al. Parthenogenetic stem cells in nonhuman primates. Science. 2002;295(5556):819. doi: 10.1126/science.1065637. [DOI] [PubMed] [Google Scholar]
- Vrana KE, Hipp JD, Goss AM, et al. Nonhuman primate parthenogenetic stem cells. Proc Natl Acad Sci U S A. 2003;100((suppl 1)):11911–11916. doi: 10.1073/pnas.2034195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Park YB, Kim YY, Oh SK, et al. Alterations of proliferative and differentiation potentials of human embryonic stem cells during long-term culture. Exp Mol Med. 2008;40(1):98–108. doi: 10.3858/emm.2008.40.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Atkuri KR, Deb-Basu D, et al. MYC can induce DNA breaks in vivo and in vitro independent of reactive oxygen species. Cancer Res. 2006;66(13):6598–6605. doi: 10.1158/0008-5472.CAN-05-3115. [DOI] [PubMed] [Google Scholar]
- Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21(7):1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- Shiras A, Chettiar ST, Shepal V, Rajendran G, Prasad GR, Shastry P. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25(6):1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- Zeng X, Rao MS. Human embryonic stem cells: long term stability, absence of senescence and a potential cell source for neural replacement. Neuroscience. 2007;145(4):1348–1358. doi: 10.1016/j.neuroscience.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90(5):2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19(4):773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res. 2006;312(11):2107–2120. doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Gorin NC, Piantadosi S, Stull M, Bonte H, Wingard JR, Civin C. Increased risk of lethal graft-versus-host disease-like syndrome after transplantation into NOD/SCID mice of human mobilized peripheral blood stem cells, as compared to bone marrow or cord blood. J Hematother Stem Cell Res. 2002;11(2):277–292. doi: 10.1089/152581602753658466. [DOI] [PubMed] [Google Scholar]
- Vats A, Bielby RC, Tolley NS, Nerem R, Polak JM. Review. Stem cells. Lancet. 2005;366(9485):592–602. doi: 10.1016/S0140-6736(05)66879-1. [DOI] [PubMed] [Google Scholar]
- Foroni C, Galli R, Cipelletti B, et al. Resilience to transformation and inherent genetic and functional stability of adult neural stem cells ex vivo. Cancer Res. 2007;67(8):3725–3733. doi: 10.1158/0008-5472.CAN-06-4577. [DOI] [PubMed] [Google Scholar]
- Goldenring JM. The brain-life theory: towards a consistent biological definition of humanness. J Med Ethics. 1985;11(4):198–204. doi: 10.1136/jme.11.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterfy A. Fetal viability as a threshold to personhood. A legal analysis. J Leg Med. 1995;16(4):607–636. doi: 10.1080/01947649509510995. [DOI] [PubMed] [Google Scholar]
- Robertson JA. Ethics and policy in embryonic stem cell research. Kennedy Inst Ethics J. 1999;9(2):109–136. doi: 10.1353/ken.1999.0013. [DOI] [PubMed] [Google Scholar]
- Roman Catholic Church. Catechism of the Catholic Church. 2nd English translation. Vatican City: Libreria Editrice Vaticana; 1994. [Google Scholar]
- Westpal SP. The next IVF revolution. New Scientist. 2003;178(2394):4–5. [PubMed] [Google Scholar]
- Hübner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- Newson AJ, Smajdor AC. Artificial gametes: new paths to parenthood. J Med Ethics. 2005;31(3):184–186. doi: 10.1136/jme.2003.004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker P. Stem cells to gametes: how far should we go. Hum Fertil Camb. 2007;10(1):1–5. doi: 10.1080/14647270600883234. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Review. Nat Rev Mol Cell Biol. 2008;9(9):725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;25;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22(15):1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blelloch R, Venere M, Yen J, Ramalho-Santos M. Generation of induced pluripotent stem cells in the absence of drug selection. Cell Stem Cell. 2007;1(3):245–247. doi: 10.1016/j.stem.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry WE, Richter L, Yachechko R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]