Abstract
Background/Objective:
To investigate inter-rater and intra-rater reliability of electrical perceptual threshold (EPT) testing in assessing somatosensory function in healthy volunteers.
Study Design:
Prospective experimental.
Setting:
Hospital-based spinal cord injuries unit.
Methods:
Cutaneous electrical stimulation of 4 dermatomes at ASIA sensory key points (C3, T1, L3, and S2) was performed on 40 control subjects. The lowest ascending stimulus intensity at which sensation was perceived was recorded as the EPT. Mean EPT values for each dermatome, as determined by 2 testers at 2 time points, were examined and plotted against a normative template. Differences and associations between intra- and inter-rater measurements and left-right measurements were studied. EPT results for 2 people with spinal cord injuries were also examined.
Results:
EPT measurements from left and right sides, obtained from the 2 time points and 2 testers, were found to be strongly associated, with the exception of left and right side measurements at the S2 dermatome. No significant differences in the mean EPT for tester or time period were found. The intra- and inter-rater reliability was good for all dermatomes tested. Mean EPT measurements fell within the range of a normative template at each of the 4 dermatomes tested.
Conclusion:
EPT is an objective, reproducible, and quantifiable method of assessing sensation in a control group. However, caution should be applied in certain dermatomes such as S2, where there was large variation between left and right side measurements.
Keywords: Spinal cord injuries, Sensory testing, Electrical stimulation, Electrical perceptual thresholds, Quantitative sensory testing, Reliability
INTRODUCTION
There are many clinical methods for assessing somatic sensation in people with spinal cord injury (SCI), including the common bedside tests of light touch, pinprick, temperature, vibration, 2-point discrimination, and position sense. The recommended neurologic examination for patients with SCI is the American Spinal Injury Association score (1). However, concerns have been raised regarding the reliability of the ASIA sensory assessment, such as limited inter-rater reliability and marked differences in reliability among different regions of the body (eg, trunk, extremities) or among dermatomes, with those at the zone of injury showing the least reliable scores (2). Jones et al (3) computed that, for individual examiner intraclass reliabilities (across 3 patients), coefficients were 0.62 to 1.00 for light touch and 0.56 to 0.99 for pinprick. Furthermore, the grading of posterior column function is ignored in this system, and the simple addition of vibration and position sense may not accurately reflect posterior column function (4).
New methods for assessing sensation need to be sensitive in detecting change over time, quantify the degree of improvement or dysfunction, and be valid, reliable, and thorough. Furthermore, the assessment should be repeatable with different testers, quick and easy to administer, noninvasive, and objective. Quantitative sensory testing (QST) is a promising method that has been used primarily to evaluate peripheral nerve disorders (5). Davey et al (6) found that repeatability was better for electrical perceptual threshold (EPT) testing, a form of QST, compared with 2-point discrimination ability. Ellaway et al (7) and Savic et al (8) built on the study of Davey et al, testing EPT in control subjects and subjects with SCI. In controls, EPT depended on the dermatome tested, with strong correlation between right and left sides and at repeat assessments. EPT results from dermatomes C3 to S2 plotted against a representation of the vertebral column produced a normative template or a normogram against which the EPT of subjects with SCI could be compared. It is, however, uncertain which pathway/s EPT testing stimulates in the noninjured population and whether this differs from that of the SCI population.
Our objective was to examine inter- and intra-rater reliability of EPT measurements in assessing somatosensory function in control subjects. We also plotted the EPT results for 2 individuals with SCI against a normative template, which sheds some light on the pathways that EPT testing may stimulate.
MATERIALS AND METHODS
Approval for the study was given by the Northern Sydney Health Human Research Ethics Committee in Sydney, Australia. Volunteers were recruited from within Royal North Shore Hospital by email and posters. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Subjects
Consent was obtained from 40 healthy volunteers (15 men and 25 women ranging from 20 to 64 years in age; mean age, 39.5 years). All denied a history of peripheral neuropathy, skin disorders, or systemic disease such as diabetes mellitus or renal failure.
Technique
Subjects were tested in a quiet, warm room in the supine position. A triggering unit drove a stimulator (Digitimer DS7A; Digitimer, Hertfordshire, UK), which produced constant current square wave electrical pulses (0.5-ms width, 3 Hz) using a disposable, self-adhesive rectangular cathode (3M Red Dot Repositionable Monitoring Electrode 1.56 × 1.25 inches in area; 3M Canada, London, Ontario). The electrode was selected for its low impedance, affordability, and availability. The stimulus intensity is usually increased manually on the Digitimer; however, doing so can introduce considerable variability in the rate of current change. Hence, a motor was designed to turn the dial of the Digitimer to increase the stimulus intensity at a constant rate, thus reducing inter-rater variability. After skin preparation with an alcohol swab, electrodes were attached to 4 ASIA sensory points (C3, T1, L3, S2) bilaterally, with an inactive anode attached to the subjects' posterior right lumbar quadrant. These dermatomes were selected because they have clear reference to anatomical landmarks to reduce the variability of electrode placement.
Each subject was familiarized with the process in a trial run. The stimulus current, set at a maximum of 10 mA, was increased from 0 at a constant rate of 0.24 mA until the volunteer indicated that he first felt sensation at the ASIA sensory point. This current was recorded as the “ascending” EPT. Current was increased further to slightly exceed the threshold and decreased until the stimulus was no longer sensed. This process was repeated 3 times on each dermatome. All subjects perceived a tapping sensation when EPT was reached, with sensations of “stinging” or “prickling” at higher stimulus intensities. Throughout testing, subjects were blind to amplitude of stimulus current. Testers were instructed not to look at the amplitude of the stimulus current, turning off the motor and recording the EPT value only when the subject indicated, thus reducing tester bias. The same 2 testers repeated this process within a few days or the same day in the same room. Training of the technique required only 10 minutes, with an additional 0.5 hours for explanation of equipment set up and trouble shooting. The procedure, including set up of equipment, explanation of the procedure to the volunteer, application of electrodes, and EPT testing of 4 dermatomes by 2 testers, took 30 minutes from start to finish. The repeated procedure took 20 minutes. Apart from the initial set-up cost of purchasing the Digitimer and building the triggering unit and motor, each test was cheap to run, because the only consumables were the electrodes.
Statistical Analysis
The lowest ascending stimulus intensity of the 3 trials was taken as the EPT. The mean EPT and SD were determined from all measurements for the 40 volunteers (2 sides, 2 trials with 2 testers). Analysis was performed for each dermatome using 3 statistical tests. The intraclass correlation coefficient (ICC) is the preferred technique to measure intra- and inter-rater reliability, and we used the method described by Hayen et al (9). Pearson's correlation coefficients were used to assess the association between EPT values obtained from the left and right side, the two testers, and at repeated trials by the same tester. Paired t tests (left-right) and independent t tests (testers, and trials) were used to test the difference of the mean for EPT values obtained for different sides, testers, and trials. The limitations of each method in assessing the reproducibility of data are discussed by Bedard (10). For each dermatome, the mean EPT was plotted against data from Savic et al (8), obtained with permission for comparison. Values obtained from 2 individuals with SCI were also plotted and compared.
RESULTS
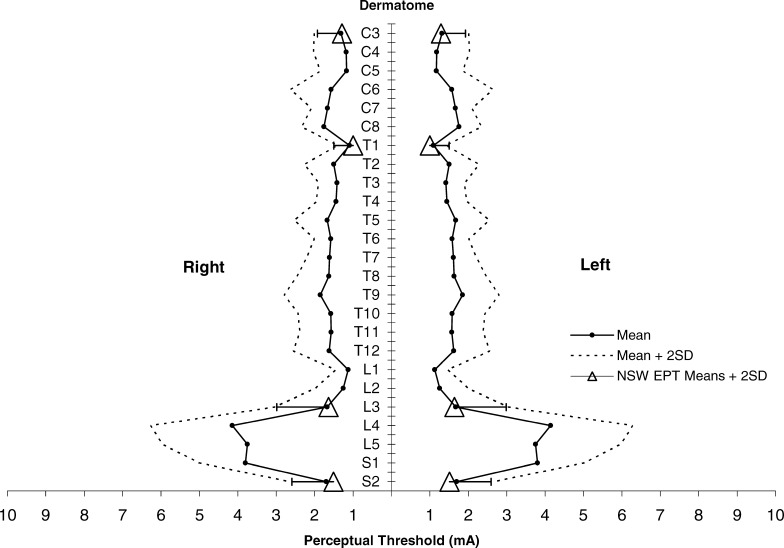
For each dermatome, C3, T1, L3, and S2, eight EPT measurements were obtained for each of the 40 participants (320 measurements in total for each dermatome). This includes left and right sides, Testers 1 and 2, and Trial A and Trial B. EPT means, SD, and range of values across all conditions and patients for the 4 dermatomes are shown (Table 1). EPT values at L3 showed the lowest sensitivity and the largest SD and those at T1 showed the highest sensitivity and the smallest SD. Our EPT measurements were consistently lower than, but similar to, the results of Savic et al (8), falling within the normal range of their template at each dermatome tested (Figure 1).
Table 1.
Descriptive Statistics of EPT Values by Spinal Level (N = 320)
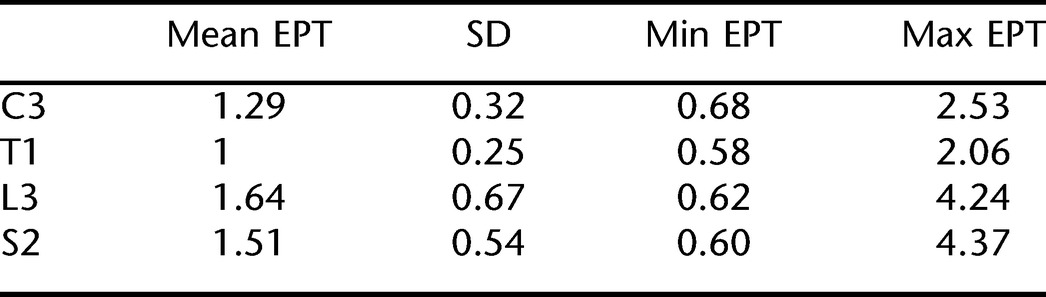
Figure 1. NSW EPT means ± 2 SD for 4 dermatomes plotted against a previously published normative template for perceptual threshold to electrical stimulation (mean ± 2 SD) by ASIA dermatomes (C3–S2). Adapted with permission from reference (8); Savic et al. Spinal Cord. 2006;44(9):560–566.
Reliability and Repeatability
Left and Right Side.
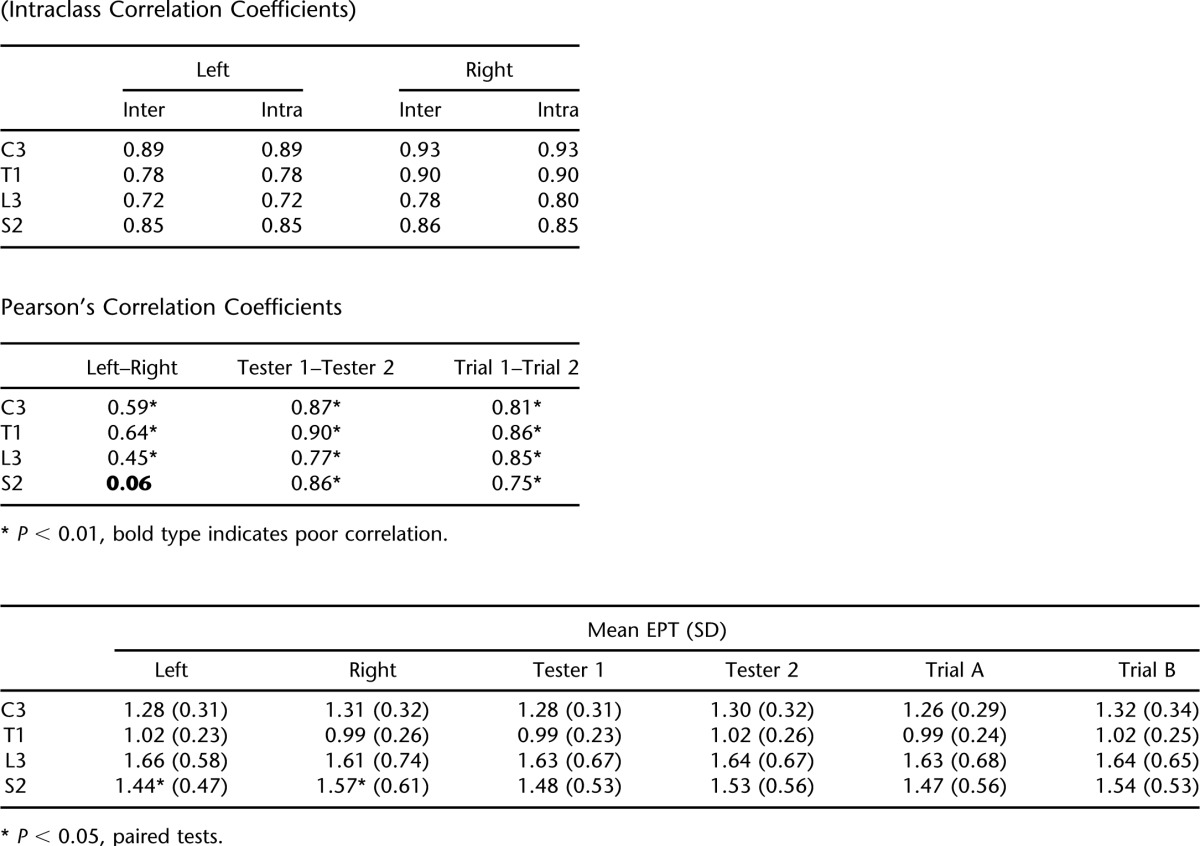
EPT values for left-right corresponding dermatomes were significantly associated for C3, T1, and L3 (r = 0.45–0.64, P < 0.01), and there was no difference in the mean EPT values for these dermatomes using paired t tests. The exception was at dermatome S2, which showed poor correlation (r = 0.06, P = 0.45) and a significant difference of the mean EPT (P < 0.05), with the left side more sensitive (Table 2).
Table 2.
Associations Between EPT Measurements by Side, Tester, and Trial (Intraclass Correlation Coefficients)
Inter- and Intra-Rater Reliability.
The inter- and intra-rater reliability was shown to be good using ICCs (ICC = 0.72–0.93). Correlations between testers' measurements were also strong for all dermatomes, as were measurements from the same tester between trials (r = 0.75–0.90, P < 0.001). There were no differences in the mean EPT values determined by different testers or at different trials. Table 2 summarizes these findings.
Case Studies.
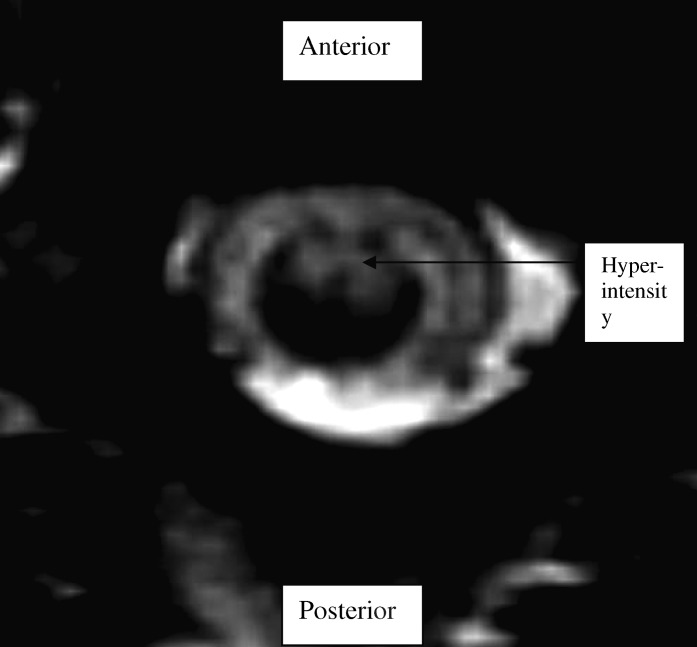
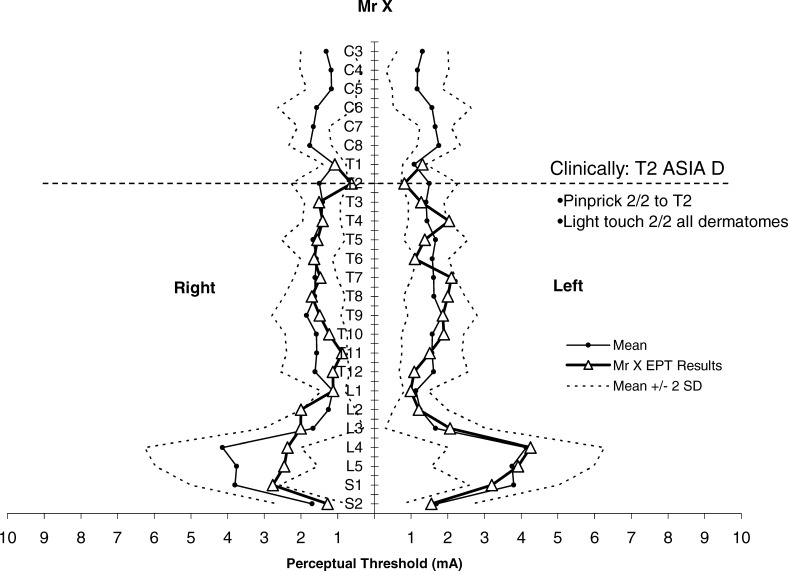
We applied EPT testing to Mr X, a 48-year-old man with anterior spinal artery infarction from a cardio-embolic event, resulting in T2 ASIA D paraplegia. MRI showed ischemic changes in the anterior portion of the spinal cord from T1 to T4 (Figure 2). On clinical testing, Mr X had preserved light touch sensation and proprioception with altered pinprick discrimination, consistent with an anterior cord syndrome. An assessor blinded to the ASIA examination conducted EPT testing within 24 hours of the ASIA examination. EPT values fell within the normal range of the normative template, which includes means ± 2 SD (Figure 3). Thus, despite altered pinprick sensation, EPTs were not abnormal. This raises the possibility that EPT testing might be activating nerve fibers that travel predominantly through the posterior columns.
Figure 2. T2-weighted MRI of Mr X showing hyperintensity in the anterior aspect of the spinal cord.
Figure 3. EPT results of Mr X plotted against normative template for perceptual threshold to electrical stimulation (mean ± 2 SD) by ASIA dermatomes (C3–S2). Adapted with permission from reference (8); Savic et al. Spinal Cord. 2006;44(9):560–566.
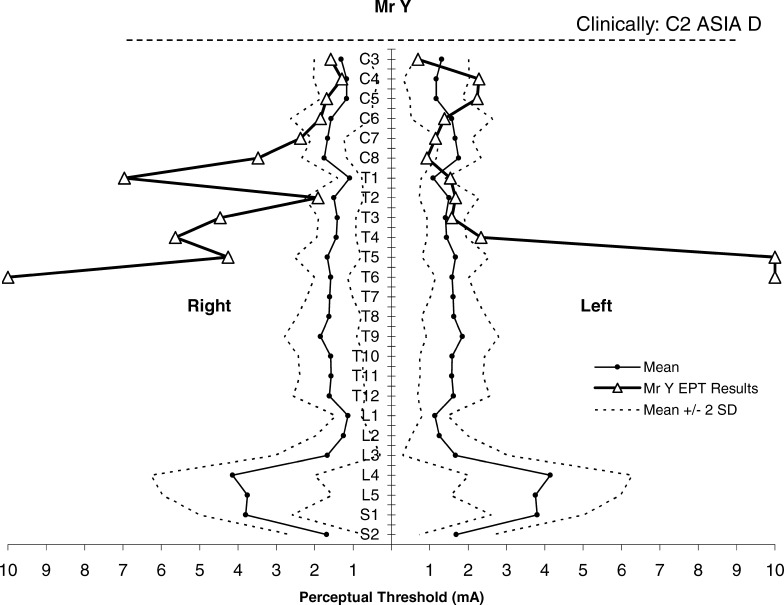
We contrasted this with the results of Mr Y, a 58-year-old man with C2 ASIA D tetraplegia, central cord syndrome. His clinical course was complicated by the development of a syrinx within the posterior cord at the C3-C4 level. On clinical testing, Mr Y had patchy sensory loss, worse for light touch than for pinprick discrimination, and severely impaired proprioception. He had good preservation of motor power, with more power in his lower limbs than his upper limbs. Mr Y's EPT results were abnormal when plotted against the normative template (Figure 4). Caudal to the T4 dermatome, he reported tightening and pressure rather than a tapping to EPT testing, making it difficult for him to indicate the start or end of sensation. Testing was discontinued at a dermatome when the stimulus intensity exceeded our preset maximum current of 10 mA.
Figure 4. EPT results of Mr Y plotted against normative template for perceptual threshold to electrical stimulation (mean ± 2 SD) by ASIA dermatomes (C3–S2). Adapted with permission from reference (8); Savic et al. Spinal Cord. 2006;44(9):560–566.
DISCUSSION
Sensitivity
EPT testing has mostly been used for monitoring peripheral nerve function, where it can differentiate among several levels of clinically observable severity (11), correlates better with clinical measures than vibration perception thresholds or nerve conduction velocities (12), is more sensitive than thermal perception threshold testing (13), and can be used to quantitatively map sensory neuropathy (14). EPT has only recently been developed for use in patients with SCI, stimulating standardized ASIA sensory key points (5,6). It is well known that QST requires the cooperation and concentration of the examined subject, which results in higher variation (15). Our EPT results were reproducible, showing good inter- and intra-rater reliability. The addition of a constant rate motor has probably made a significant contribution to reducing operator variability. We found that EPT varies with the dermatome tested and that variability is dermatome dependent. This is a similar finding to previously published normative data by Savic et al (8). A notable exception is at S2, where we found significant within-subject variation in EPT results between the left and right side. The S2 dermatome is situated over the popliteal fossa, and many of our volunteers noted that the tapping sensation produced by EPT testing was difficult to perceive compared with the other 3 dermatomes. This may be caused by several factors, such as skin temperature variations between the dermatomes, the position of S2 in a skin fold, or its situation over a major neurovascular bundle. This finding highlights that large individual variations in EPT results may occur in locations not yet tested, and caution should be applied when interpreting changes in EPT at certain dermatomes.
Neuroselectivity
To decide on the best EPT frequency to use, we tested square-wave frequencies of 1, 3, 10, 20, 50, and 100 Hz to ascertain subjectively the type of sensations perceived. At 1 and 3 Hz, tapping was perceived, compared with “fluttering” at 10 and 20 Hz, and “tightness” and “buzzing” at 50 and 100 Hz. Of the 6 frequencies tested, the volunteer found it easiest to indicate threshold at 3 Hz.
It has been suggested that stimulation may be preferentially activating small-diameter fibers (4). This is based on work using constant current sinusoidal electrical stimulation with a Neurometer, which claims a selective activation of C fibers at 5 Hz, Aδ fibers at 250 Hz, and Aβ fibers at 2,000 Hz (16). Although useful for assessing neuropathies, such selectivity for different fiber groups is only partial (correlation coefficients: 0.42 for large fibers, 0.34 for small fibers) (17). Importantly, the relative selectivity seen with sinusoidal stimulation is not applicable to the square wave stimulation used in our study.
Using square wave pulses, many studies (18,19) have shown successive recruitment of fibers of decreasing conduction velocity with increasing stimulus strength (Aβ activated at lower currents than Aδ, with highest currents required for C fibers), which is relatively independent of stimulus repetition rate. McAllister et al (20) confirmed this pattern of recruitment in animal studies and used the same stimulation technique in humans to show that activation of the larger fibers was associated with a tingling sensation. A sharp pricking sensation did not occur until higher stimulation of Aδ and possibly some C fibers occurred. This correlates with our control subjects' sensation of tapping at threshold, followed by sharp, tingling sensations when threshold was exceeded.
Valbo et al (21) correlated electrical activation with mechanical stimulation of different classes of slowly and rapidly adapting tactile afferents. The fastest, lowest threshold skin afferents (A beta) are known to innervate hair follicle afferents (in hairy skin), Meissner's corpuscles (in glabrous skin), slowly adapting Type I fibers (Merkel cells), slowly adapting Type II fibers (Ruffini endings), and Pacinian corpuscles. Electrical stimulation does not distinguish between the thresholds for these inputs. Moreover, all of these afferent inputs project to the dorsal column nuclei, and single impulses in single hair follicle afferents, SAI fibers, and Pacinian corpuscles afferents can give rise to sensations (22).
We can thus conclude that the square wave stimulation used here will have activated the largest, lowest threshold afferent fibers near the site of stimulation. These fibers are likely to project into the posterior column pathway to give rise to the tapping sensations reported by our subjects. This would be consistent with the normal EPTs found in patients like Mr X with anterior cord syndrome, in whom posterior columns are preserved.
Somatotopic Variations
Our results show somatotopic variations in EPT, with higher values for the L3 dermatome. Several factors have been suggested for this variation, including anatomical parameters such as thickness of the subcutis, sweat gland density, and arm diameter, as shown by computer simulations of intracorporeal current distribution between 2 electrodes (23). Other authors have postulated that the application of electric current to the skin stimulates nerve fibers directly rather than through stimulation of the sensory receptors, and threshold variability may be related to concentration of nerve fibers in the area tested (24). This is supported by a recent study showing that the density of epidermal nerve fibers decreases with age (25). However, Leitgeb et al (26) did not find significant differences between the results of elderly people (aged ≥ 61 years) and adults (17–60 years), and Savic et al (8) found only 1 dermatome (L5) with increased threshold values with age (although their control group had no subjects > 55 years of age). These discrepancies suggest that a normative template for the elderly population could provide a more accurate comparison between the spinal cord–injured and able-bodied populations.
Concerning sex differences, Leitgeb et al (26) found that, although there were statistically significant differences in current sensitivity between boys and adult men and between girls and boys, this difference decreased with adulthood such that there were no differences between adults. Davey et al (6) found that women had lower mean perceptual thresholds than men on the knee and foot. Savic et al (8) found a statistically significant difference in only 1 dermatome (L3) between men and women. Because sex differences are minimal, it is reasonable to use the same normative template for males and females for comparison.
CONCLUSION
EPT testing is an objective and quantifiable method of assessing sensation, with good inter-rater and intra-rater reliability found in all 4 dermatomes tested. In the dermatome S2, significant within-subject variation occurred between the left and the right side, and caution is required before attributing changes in EPT results at this dermatome to any therapy. Unlike electrophysiologic studies, the equipment required for EPT assessment is affordable and portable, and the technique requires minimal training; therefore, it can be used by junior medical staff or allied health professionals. Further study is needed to assess the reproducibility of EPT in persons with SCI and to evaluate EPT in patients recovering from acute SCI.
Acknowledgments
We thank Professor Peter Ellaway for his guidance in this study and article, Edward Crawford for building the trigger unit, Joan McClelland and Julia Lai Kwon for help in testing, Anthony Honeyfield for building a constant rate motor, Clare Ringland for advice on statistical analysis, and the OSMR for funding the Program Grant and Exchange Fellowship.
Footnotes
This study was sponsored by New South Wales Office of Science and Medical Research.
REFERENCES
- American Spinal Injury Association. Reference Manual for International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients (Revised 2002) Chicago, IL: American Spinal Injury Association; 2002. [Google Scholar]
- Cohen ME, Sheehan TP, Herbison GJ. Content validity and reliability of the International Standards for Neurological Classification of Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 1996;1:15–31. [Google Scholar]
- Jones L, Marino RJ, Kirshblum SK, Tal J. Reliability levels of the ASIA motor and sensory examination in physician versus physical therapist examiners; International Spinal Cord Society Hellenic Society Physical Medicine and Rehabilitation Outcome Measures; Athens, Greece,. September 26–29,; 2004. [Google Scholar]
- Martinez-Arizala A. Methods to measure sensory function in humans versus animals. J Rehabil Res Dev. 2003;40((Suppl 1)):35–40. doi: 10.1682/jrrd.2003.08.0035. [DOI] [PubMed] [Google Scholar]
- Quantitative sensory testing: a consensus report from the Peripheral Neuropathy Association. Neurology. 1993;43:1050–1052. doi: 10.1212/wnl.43.5.1050. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Nowicky AV, Zaman R. Somatotopy of perceptual threshold to cutaneous electrical stimulation in man. Exp Physiol. 2001;86(1):127–130. doi: 10.1113/eph8602086. [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Anand P, Bergstrom EMK, et al. Towards improved clinical and physiological assessments of recovery in spinal cord injury: a clinical initiative. Spinal Cord. 2004;42(6):325–337. doi: 10.1038/sj.sc.3101596. [DOI] [PubMed] [Google Scholar]
- Savic G, Bergstrom EMK, Frankel HL, Jamous MA, Ellaway PH, Davey NJ. Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord. 2006;44(9):560–566. doi: 10.1038/sj.sc.3101921. [DOI] [PubMed] [Google Scholar]
- Hayen A, Dennis RJ, Finch CF. Determining the intra- and inter-rater reliability of screening tools used in sports injury research. J Sci Med Sport. 2007;10(4):201–210. doi: 10.1016/j.jsams.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Begard M. Assessing reproducibility of data obtained with instruments based on continuous measurements. Exp Aging Res. 2000;26(4):353–365. doi: 10.1080/036107300750015741. [DOI] [PubMed] [Google Scholar]
- Katims JJ, Naviasky EH, Rendell MS, Ng LKY, Bleecker ML. Constant current sine wave transcutaneous nerve stimulation for the evaluation of peripheral neuropathy. Arch Phys Med Rehabil. 1987;68(4):210–213. [PubMed] [Google Scholar]
- Rendell MS, Katims JJ, Richter R, Rowland F. A comparison of nerve conduction velocities and current perception thresholds as correlates of clinical severity of diabetic sensory neuropathy. J Neurol Neurosurg Psychiatry. 1989;52(4):502–511. doi: 10.1136/jnnp.52.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitei DL, Watkins PJ, Stevens MJ, Edmonds ME. The value of the neurometer in assessing diabetic neuropathy by measurement of the current perception threshold. Diabetic Med. 1994;11(9):872–876. doi: 10.1111/j.1464-5491.1994.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Rendell MS, Dovgan DJ, Bergman TF, O'Donnell GP, Drobny EP, Katims JJ. Mapping diabetic sensory neuropathy by current perception threshold testing. Diabetes Care. 1989;12(9):636–640. doi: 10.2337/diacare.12.9.636. [DOI] [PubMed] [Google Scholar]
- Donaghue VM, Giurini JM, Rosenblum BI, Weissman PN, Veves A. Variability in function measurements of three sensory foot nerves in neuropathic diabetic patients. Diabetes Res Clin Pract. 1995;29(1):37–42. doi: 10.1016/0168-8227(95)01107-o. [DOI] [PubMed] [Google Scholar]
- Katims JJ, Naviasky EH, Rendell MS, Ng LK, Bleecker ML. Constant current sine wave transcutaneous nerve stimulation for the evaluation of peripheral neuropathy. Arch Phys Med Rehabil. 1987;68(4):210–213. [PubMed] [Google Scholar]
- Masson EA, Veves A, Fernando D, Boulton AJM. Current perception threshold: a new, quick, reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia, 1989;32(10):724–728. doi: 10.1007/BF00274531. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Torebjork HE. Electrically induced A and C fibre responses in intact human skin nerves. Exp Brain Res. 1973;16(3):309–320. doi: 10.1007/BF00233333. [DOI] [PubMed] [Google Scholar]
- Pfeiffer EA. Electrical stimulation of sensory nerves with skin electrodes for research, diagnosis, communication and behavioural conditioning: a survey. Med Biol Eng. 1968;6(6):637–651. doi: 10.1007/BF02474726. [DOI] [PubMed] [Google Scholar]
- McAllister RMR, Urban LA, Dray A, Smith PJ. Comparison of the sensory threshold in healthy human volunteers with the sensory nerve response of the rat in vitro hindlimb skin and saphenous nerve preparation on cutaneous electrical stimulation. J Hand Surg. 1995;20B(4):437–443. doi: 10.1016/s0266-7681(05)80149-4. [DOI] [PubMed] [Google Scholar]
- Valbo AB, Olsson KA, Westburgh KG, Clarke FJ. Microstimulation of single tactile afferents from the human hand. Brain. 1984;107:727–749. doi: 10.1093/brain/107.3.727. [DOI] [PubMed] [Google Scholar]
- Rowe MJ. Synaptic transmission between single tactile and kinaesthetic sensory nerve fibres and their central target neurones. Behav Brain Res. 2002;135((1–2)):197–212. doi: 10.1016/s0166-4328(02)00166-3. [DOI] [PubMed] [Google Scholar]
- Fluhr H, Leitgeb N. Consideration of anatomical factors at the electric current perception. Proc 20th Annu Int Conf IEEE-EMBS. 1998;20(6):3269–3271. [Google Scholar]
- Bostock H, Sears TA, Sheratt RM. The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. J Physiol (Lond). 1983;341:41–58. doi: 10.1113/jphysiol.1983.sp014791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gøransson LG, Mellgren SI, Lindal S, Omdal R. The effect of age and gender on epidermal nerve fiber density. Neurology. 2004;62(5):774–777. doi: 10.1212/01.wnl.0000113732.41127.8f. [DOI] [PubMed] [Google Scholar]
- Leitgeb N, Schroettner J, Cech R. Electric current perception of the general population including children and the elderly. J Med Eng Technol. 2005;29(5):215–218. doi: 10.1080/03091900412331291705. [DOI] [PubMed] [Google Scholar]