Abstract
Background/Objective:
To develop a latent behavioral model by identifying and confirming the factor structure of health behaviors of people with spinal cord injury (SCI) and their relationships with biographic, injury, and educational characteristics.
Research Design:
Survey data were collected from 1,388 adults with traumatic SCI of at least 1 year duration.
Main Outcome Measures:
Selection of health behaviors was based on a bidimensional behavioral risk model. Behaviors were measured by core item sets from the Behavioral Risk Factor Surveillance System and supplemented by an alcohol screening measure, select fitness proxies, and the SCI Health Survey.
Results:
Latent variable structural equation modeling was used to identify underlying factors and their relationship with participant characteristics. Seven specific factors were identified by exploratory factor analysis and were cross-validated using confirmatory factor analysis. They included: (a) healthy nutrition, (b) unhealthy nutrition, (c) fitness, (d) smoking, (e) alcohol use, (f) psychotropic prescription medications, and (g) SCI healthy activities. Two higher-order dimensions were also identified, including a risk dimension (b, d, e) and a protective dimension (a, c, g). Participant characteristics were associated with the domains. For instance, participants with the most severe injuries scored lower on smoking and alcohol but higher on psychotropic medications; age was positively correlated with healthy nutrition and negatively correlated with alcohol and tobacco use but also negatively correlated with fitness.
Conclusion:
Behaviors can be meaningfully combined into underlying dimensions to more efficiently use them as predictors of secondary conditions.
Keywords: Spinal cord injuries, Rehabilitation, Disability, Health behaviors, Smoking, Alcohol abuse, Prevention, Health promotion, Fitness, Nutrition, Outcomes
INTRODUCTION
The onset of spinal cord injury (SCI) is associated with a lifelong heightened risk of secondary conditions, such as pressure ulcers (1) and urinary tract infections (2), and diminished life expectancy (3). The increased susceptibility to health problems magnifies the importance of vigilance in performing healthy behaviors and avoiding risky behaviors. An almost limitless number of behaviors could be investigated in relation to outcomes after disabling conditions, including SCI. Selection of behaviors for investigation should be guided by both theoretical or empirical research models as well as those behaviors most widely used in previous research among participants with and without SCI. We have selected behaviors for this study based on 2 empirical models and prominent behaviors in both the general and SCI literature.
Krause (4) developed 2 empirical models in the investigation of risk and protective factors for secondary conditions after SCI. The first model is a general empirical risk model that identifies a series of hypothetical links between 5 levels of variables, including (a) biographic and injury factors, (b) psychological and environmental factors, (c) behavioral factors, (d) secondary conditions, and (e) mortality. With the exception of variables on the extreme ends of the model (a, e), each level in the model is predicted by the previous level of variables and predicts the subsequent level of variables. For instance, according to the model, health behaviors are both predicted by psychological (eg, personality) and environmental (eg, access to care) factors and are predictive of secondary conditions (eg, pressure ulcers). The first level of the model is used to predict all subsequent levels, often as statistical controls; therefore, the relationship of these variables with other variables in the model is important to define. For the model to be fully elaborated, it is also necessary for each level of the model to be defined by the major dimensions that summarize its content. In this study, we define the behavioral level of the model by identifying its underlying dimensions and relating these to the first set of variables (biographic and injury factors).
The second empirical model is the bidimensional behavioral model, which solely defines the behavioral domains in the larger general empirical risk model (4). This model mandates that behaviors include those that lead to an elevated risk of a secondary condition and those that relate to a diminished risk of a secondary condition. In public health models, these are referred to as risk and protective factors. According to the bidimensional behavioral model, risk and protective behaviors should be relatively independent of each other (minimally correlated). Analysis of behaviors should result in 2 independent risk and protective domains, each of which may include several subdomains, such as distinctive factors comprising multiple behaviors. The intersection of 2 behavioral dimensions results in 4 independent quadrants defined by the combination of the 2 dimensions, including: (a) high risk/high protective, (b) high risk/low protective, (c) low risk/high protective, (d) low risk/low protective. Therefore, what differentiates the bidimensional behavioral model from the general public health model is the hypothesized relationship between the dimensions and the implications for the combination of risk and protective variables in predicting secondary conditions. The public health model does not necessarily assume any particular relationship between risk and protective factors, such that behaviors could be plotted on a single continuum, with protective behaviors on one end of the continuum and risk behaviors on the other (1.00 correlation).
Investigators have focused on many behaviors that are of importance in the general population, as reflected by their prominence in surveillance and prevention activities by the Centers for Disease Control (CDC). For example, researchers have reported the protective effects of physical activity for individuals with SCI for preventing cardiovascular disease (5–7), hyperlipidemia (8,9), adiposity (10,11), diabetes (12,13), metabolic syndrome (14), depression (15), orthopedic abnormalities (16,17), urinary tract disease (18), pressure sores (19), and poor general health (20). Conversely, among individuals with SCI, researchers have reported risks associated with smoking (1,18,21,22), taking prescription medicine (1,15), lack of physical activity (23–27), alcohol misuse (28–30), and inadequate nutrition (31).
The identification of multiple types of risk and protective behaviors is important, because not all risk and protective behaviors will have an equally immediate impact. Whereas some behaviors, such as alcohol abuse, may produce an immediate elevated risk for secondary complications such as subsequent injuries (32), other behaviors (eg, tobacco use, poor nutrition) may have a cumulative effect that increases over time (15,18). It is also likely that a particular set of behaviors will be associated with an increased risk for particular conditions but not others. For example, excessive risk taking may lead to an elevated risk for events that lead to a need for hospitalization (32), but it may not be associated (or only indirectly associated) with other health conditions.
Because persons with SCI have an increased likelihood of developing secondary conditions, rehabilitation training includes education on behaviors to promote general health (33) as well as to avoid secondary conditions (34). Health maintenance behaviors specific to SCI include weight shifts to reduce pressure, daily skin checks for redness or breakdown of skin, range-of-motion exercises to preserve joint flexibility, muscle-strengthening exercises, drinking extra water, and checking urine regularly for any abnormalities (34,35). Engaging in health maintenance behaviors is intended to minimize the risk of developing secondary impairments such as pressure sores, loss of joint flexibility, spasticity, deep vein thrombosis, pneumonia, and urinary tract infections (4).
SUMMARY AND PURPOSE
Most investigations of health behaviors after SCI focus on a single variable or a small number of health variables. To more fully utilize predictive models such as the bidimensional model, it is first necessary to evaluate the extent to which different behaviors represent similar content areas or underlying factors, the relationship between factors, and their relationship to basic individual characteristics. Whereas individual risk and protective behaviors are manifestly observable variables, model building requires us to identify clusters of behaviors that may form underlying latent dimensions representative of broader constructs that are not tied to specific observations. Latent variables represent general tendencies to perform risk and protective behaviors.
The purpose of this study was to evaluate the dimensionality of health behaviors among individuals with SCI, the relationship between the underlying dimensions, their fit with the bidimensional behavioral model, and how they relate to individual characteristics (biographic, injury, and educational). There are 4 related objectives: (a) to establish an initial measurement model using exploratory factor analysis (EFA), (b) to cross-validate the measurement model using confirmatory factor analysis (CFA), (c) to determine with CFA whether the observed factors reflect higher-order risk and protective dimensions, and (d) to develop a complete model by identifying any associations of individual characteristics (exogenous observable variables) with the behavioral dimensions identified in the measurement model (latent dimensions). No particular relationships are assumed between biographic and injury factors and model dimensions, but identification of their relationship is a necessary step in building the general empirical model.
METHODS
After receiving approval from the Institutional Review Board, participants were selected from outpatient records of a large specialty hospital in the Southeastern United States that has been designated as a model system of care for SCI. Participants were selected from both system and registry cases, as well as general outpatient records. Participants had to meet 3 inclusion criteria: (a) traumatic SCI, (b) 18 years of age or older, and (c) at least 1 year postinjury. All those with disease-related–onset SCI were eliminated, although the sample does include surgical onset, consistent with Model SCI System guidelines. A total of 1,388 participants returned usable materials (72% response rate).
Cover letters were sent to participants 4 to 6 weeks prior to the mailing of the questionnaires. A second set of materials was sent to all nonrespondents, followed by a phone call. A third mailing was initiated only to nonrespondents who had verbally stated a willingness to participate and who requested additional materials. Participants were offered $20 to participate in the study and were made eligible for drawings totaling $1,500. When responses were incomplete because of substantial amounts of missing data (related to either printing errors or pages sticking together and thus being inadvertently skipped), we contacted participants by phone or by mail to elicit the missing information. However, we did not attempt to obtain responses to individual items that were left blank.
Measures
The 2 primary considerations in selecting the behavioral domains included use of the bidimensional behavioral model (ie, intersecting risk and protective dimensions) and those most frequently reported in the literature.
The Behavioral Risk Factor Surveillance System (BRFSS) (36) was used to measure the majority of content domains. These included alcohol use, tobacco use, and nutrition. Because no single measure is comprehensive enough to cover all primary domains relevant to special populations like SCI, we used additional measures, including the CAGE (37), a 4-item alcohol screening measure based on 4 symptoms (cutting back, people angry, guilty feelings, eye opener), and the Spinal Cord Injury Health Survey (32), which measures psychotropic prescription medication use, proxy variables for fitness activities, and SCI-specific health-maintenance behaviors (eg, weight shifts, skin checks).
The BRFSS (36) is a standardized instrument used by the CDC to monitor relevant basic health behaviors within the general population and in specific regions of the country. The survey addresses core issues, including smoking, alcohol use, and nutrition. We used the BRFSS to measure alcohol behaviors and smoking behaviors, as well as nutritional indicators, which were modified for survey data collection. We used 2 alcohol items: number of days consuming alcohol in the last month, and number of occasions in the past month consuming 5 or more drinks (ie, binge drinking). Participants were asked 3 questions regarding tobacco use: (a) Have you ever been a regular smoker? (b) How many cigarettes do you currently smoke per day? and (c) Do you smoke in bed? The nutritional indicators were supplemented with items to identify risk elements not incorporated in the BRFSS based on the recommendations of a CDC fitness specialist. There were 11 items: drink juice; eat fruit, salad, carrots, vegetables, breakfast, potatoes, fried foods, red meat, or junk food; and add salt to food. Participants were asked to report frequency of consumption using a 5-point scale: (a) never, (b) less than once a month, (c) less than once a week but at least once a month, (d) at least once a week but not every day, and (e) once a day or more.
The CAGE (37) is a widely used 4-question screening tool, with yes/no dichotomous items, designed by primary care physicians to detect alcoholism in the general population. It was used as a proxy measure for alcohol misuse behaviors. The tool asks the patient/participant whether he/she has thought about cutting down on drinking, felt annoyance at others' concern about their drinking, had guilty feelings about drinking, or used alcohol as an “eye-opener” in the morning. It was used in this study to triangulate the measurement of alcohol use behaviors with a proxy measure of misuse. Internal consistency of the CAGE is 0.80 over the lifetime and 0.78 for the past 12 months. While Cherpitel (38) reported excellent sensitivity (0.68–0.89) and specificity (0.85–0.91) for the CAGE, Cooney et al (39) and Kinney (40) noted that sensitivity generally ranged from 60% to 95% and specificity ranged from 40% to 95%. Inciardi (41) noted, however, that two “yes” answers will correctly identify 75% of individuals with alcohol dependence and accurately eliminate 96% of people without alcohol misuse problems; thus the 2-question cutoff has been recommended. Reliability information has not been reported (42). Hays et al (43) investigated internal consistency and reported a Cronbach alpha of 0.69.
The Spinal Cord Injury Health Survey (32) was developed to measure relevant behavioral and secondary condition domains after SCI. The behavioral items surveyed were prescription medication use, exercise and healthy lifestyle, and SCI-specific health-promotion behaviors. Prescription medication usage was measured to identify how frequently participants used prescription medications that might have psychotropic effects. Participants were asked how frequently they used medications for pain, spasticity, depression, and sleep (all frequently prescribed after SCI). Each item had 4 response categories: never, sometimes, weekly, and daily. Four health items were used to measure exercise and healthy lifestyle, the first 2 of which (overall lifestyle and overall fitness as a proxy for healthy behaviors) were measured on a 5-point, self-report scale consistent with the format of the self-reported general health items from the BRFSS (ie, poor, fair, good, very good, excellent). Two exercise items followed somewhat different formats. The amount of exercise was compared to others with similar SCI using a 5-point rating scale (much less, less, about the same, more, much more), and the second asked about the frequency of planned exercise grouped into 6 categories (rarely, once per month, 2 to 3 times per month, 1 to 2 times per week, 3 to 4 times per week, and 5 or more times per week). Finally, a set of items asked participants how frequently they performed certain behaviors in an effort to maintain their health. These items included skin checks, weight shifts, drinking extra water, turning in bed, range-of-motion exercises, bathing, or checking urine. Participants were presented with 4 response choices for each item, depending on how frequently they did the activity: (a) never; (b) sometimes—only when a problem is starting; (c) regularly—to prevent problems, but not every day; or (d) daily—1 or more times every day to prevent problems.
At present, although the behaviors were grouped into domains on an a priori basis, they do not represent homogeneous content and have not been converted into summative scales. By identifying and confirming the underlying factor structure, we can identify the latent dimensions that will serve as the building blocks for the development of summative scales.
Analyses
Mplus, specialized software for a wide range of structural equation models, was used to analyze the data (44). The program offers a diverse selection of models, estimators, and algorithms and has explicit features for missing data, complex survey data, and multilevel data. Our ability to estimate missing data was particularly important, because it allowed us to keep the maximum number of participants using sophisticated imputation of missing values. Mplus has special features for performing factor analysis on items that are in different metrics, such as a combination of dichotomous, multiple-category, and continuous variables. We used the Mplus categorical option that allows for skewness and kurtosis (44), since the ability to analyze variables in different metrics was central to development of our measurement model.
We used a combination of EFA and CFA consistent with the procedures outlined for health status measures by de Vet and colleagues (45). We also evaluated higher-order dimensions after the preliminary analyses. There were 4 stages in this process.
Stage 1.
Prior to the first stage of analysis, the sample was randomly split into 2 groups of approximately equal sizes. EFA using a weighted least-square parameter estimate was used to initially evaluate the factor structure of the behavioral variables (46). Chi-square and root mean square error of approximation (RMSEA) were used to evaluate the fit of the model with varying numbers of factors. RMSEA is a function of N, the chi-square, and the degrees of freedom; it is determined by the discrepancy per degrees of freedom and corrects for model complexity. RMSEA of less than .05 represents an excellent fit (47). The chi-square statistic measures deviation from a perfect fit. In reality, it will always be significant, as no model will represent a perfect fit, and the statistic will be inflated with larger sample sizes, such as that used in this study. Factor rotation was carried out on these common factors using a Promax approach (Varimax followed by Procrustean targets) so the resultant factors could have a simple structure and be correlated (48). The solution with the lowest RMSEA that maintains a minimum of 3 items of loading for each factor was used. A minimum of 3 items is necessary to produce stable factors that can be evaluated for their internal consistency.
Stage 2.
Techniques in CFA with rigid constraints (defined by the EFA) were then used to cross-validate the factor structure using the second half of the sample (ie, no overlapping cases). In contrast to the EFA, which has no preset number of factors or pattern of loadings, with CFA, the number of factors and the items that load with each factor are specified a priori.
Stage 3.
Higher-order factors were also produced from confirmatory procedures that grouped factors into risk and protective behavioral dimensions. This would allow us to determine whether the bidimensional behavioral model appeared to be a reasonable fit to the data. This was done with the full sample.
Stage 4.
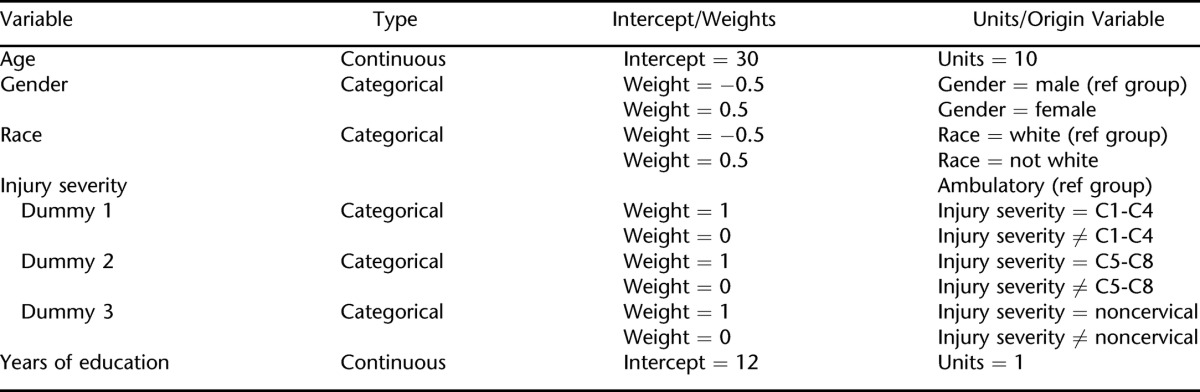
The final step was to develop the full structural model linking the latent variables to the measurement model. It uses the full participant sample. In the structural model presented here, the following biographic, injury, and educational variables were used: (a) gender, (b) race, (c) age, (d) injury severity, and (e) years of education. Table 1 summarizes the weights for these variables. The reference groups for gender and race were male and white (all nonwhites were grouped together). There were 4 groups for injury severity; these were based on a combination of injury level and ambulatory status. The ambulatory group served as the reference group and included all ambulatory participants, regardless of neurological level of injury. The other 3 nonambulatory groups were broken down according to level of injury as follows: C1-C4, C5-C8, or noncervical. Therefore, from most to least severe, injury severity was broken down into the 4 following groups: (a) C1-C4, nonambulatory; (b) C5-C8, nonambulatory; (c) noncervical, nonambulatory; and (d) ambulatory, all levels. This scheme is based upon a widely used convention that has been applied to employment (49) and mortality (50). Age and years of education are continuous variables. No specific relationships are hypothesized between these variables and the factors that were identified in the measurement model.
Table 1.
Biographic and Injury Variables That Comprise the Latent Model
The structural equation model (SEM) approach used here allows for correlation of exogenous (observable) variables (eg, age, injury severity, years of education) with the endogenous factors defined from the measurement model (ie, the confirmed factors). Use of factor analysis reduces the number of variables by defining dimensions of combinations of behavioral variables (ie, the measurement model). This addresses collinearity between behaviors by combining multiple indicators into a single dimension using unique information from each variable to the factor. The use of SEM also preserves degrees of freedom, making for a more powerful analysis. Because the observable data are used to identify a latent dimension, the newly defined latent dimension, in theory, is not restricted to the behaviors assessed in any given study. Therefore, similar predictors measuring the same latent variable may be introduced in lieu of those reported in a given study, although the new variables must still be validated with empirical data. Finally, SEM minimizes problems related to missing data by virtue of computing estimated values from available data using far more sophisticated techniques than simple mean replacement.
RESULTS
Participants
Seventy-four percent of the sample was male, and 74.8% was white, with another 22.2% African-American. Cervical injuries were reported by 54.2% of the participants. The primary etiology was motor vehicle crashes (51%), followed by falls or flying objects (17.2%), acts of violence (12.7%), and sporting events (11.9%). Just over 21% of participants retained some ability to walk. Participants were an average of 31.8 years of age at the time of injury and 41.6 years of age at the time of the study (an average of just under 10 years had passed since SCI onset). The average duration of education was 13.1 years.
Missing Data
Overall, 1,075 participants (77.3%) reported complete data on all items. The average portion of missing observations was approximately 1 of every 50 items skipped (2.04%). We used a full-information estimation algorithm in which all available information on all cases is used under typical assumptions of informative missing data (44,51). In essence, this means that each missing value was estimated for a participant using all available information from other variables that would predict the missing value for that particular individual. Although highly complex, such imputation is more accurate for predicting an individual's missing values than using more general procedures such as mean replacement.
Exploratory Factor Analysis
Table 2 presents a summary of the EFA, detailing the number of factors, RMSEA, the chi-square statistic, and the number of factors with fewer than 3 items. Table 2 summarizes comparisons with up to 8 factors, as the solution with 8 factors was the first to produce a factor with fewer than 3 items. Therefore, the solution with 7 factors was retained, as it had the lowest RMSEA (0.036) (chi [293, n = 700] = 561.3, P < 0.001) of the 7 solutions with no factor of fewer than 3 items. This is the solution that is used throughout the remainder of the manuscript.
Table 2.
Exploratory Factor Analysis: Number of Factors, RMSEA, Chi-Square Statistic, and Number of Factors With < 3 Items
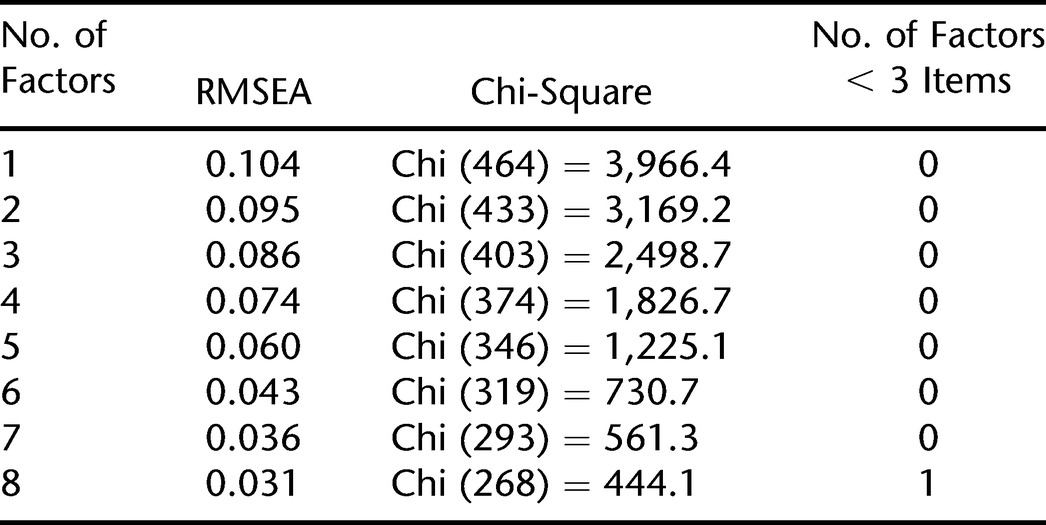
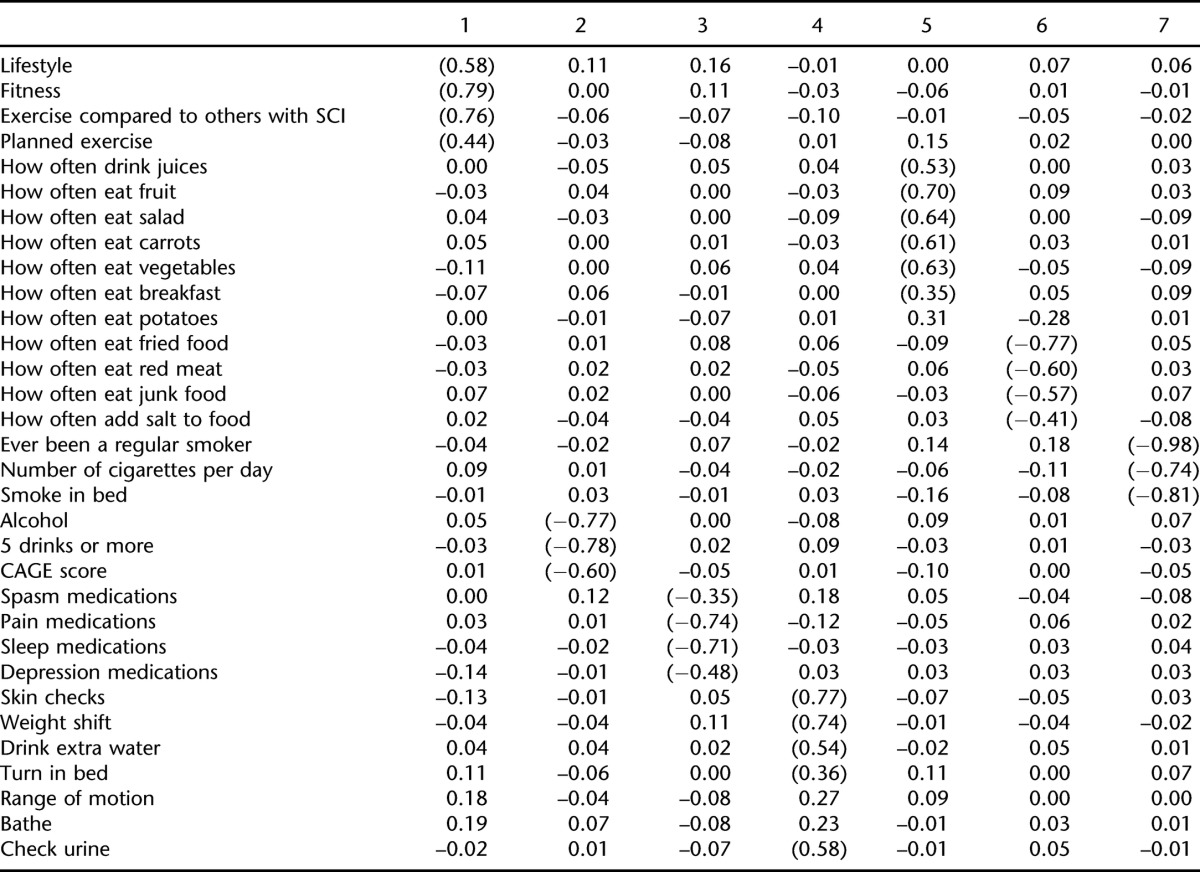
Table 3 is a summary of the factor loadings for the EFA using the first half of the random sample. There are no hard and fast rules regarding the size of the factor loading needed to be included with the factor. We used the more liberal 0.35 in the preliminary EFA, provided that the item did not also load heavily with another factor. The analysis resulted in identification of 7 dimensions.
Table 3.
Promax Rotated Loadings for Health Behaviors from Exploratory Factor Analysis of Split Sample
Confirmatory Factor Analysis
Based on the pattern of loadings, 7 factors were created for the CFA. They included prescription medication use (pain, spasticity, sleep, depression); alcohol use (days consuming alcohol, binge drinking, CAGE score); healthy nutrition (drink juices, eat fruit, eat salad, eat carrots, eat vegetables, eat breakfast); unhealthy nutrition (eat fried foods, eat red meat, eat junk food, add salt to food); smoking (smoke regularly, smoke in bed, number of cigarettes per day); fitness (lifestyle, fitness, exercise compared to others with SCI, planned exercise); and SCI healthy activities (skin checks, weight shifts, drink extra water, turn in bed, check urine). Table 4 is a summary of the subsequent restricted confirmatory solution with 7 factors. The RMSEA was 0.049 (chi [140, n = 688] = 372.7, P < 0.001), indicating an excellent fit (47). All items from the EFA were retained except for eating breakfast, which was dropped since its loading was only > 0.3 during the CFA.
Table 4.
Confirmatory Factor Analysis from Split Sample
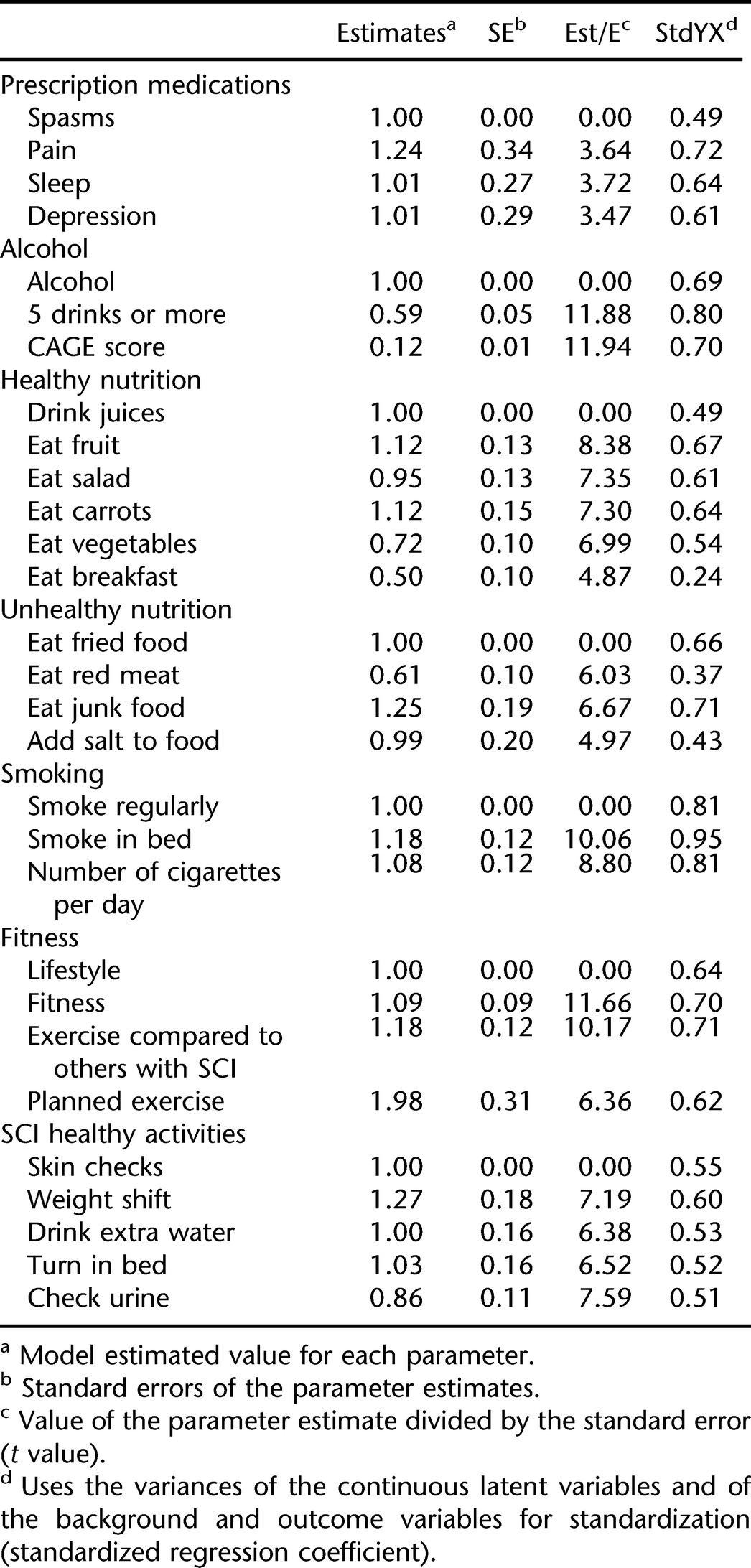
The alpha coefficients for the 7 dimensions ranged from a low of 0.58 for alcohol misuse to a high of 0.71 for healthy nutrition, with an average of 0.66. In general, higher coefficients were observed for protective dimensions (eg, SCI behaviors = 0.70, fitness = 0.68) and lower coefficients for risk dimensions (unhealthy nutrition = 0.62, smoking = 0.64, prescription medication use = 0.67). Because the dimensions represent behaviors, as opposed to self-reported ratings (eg, satisfaction, attitudes), somewhat lower coefficients may be expected.
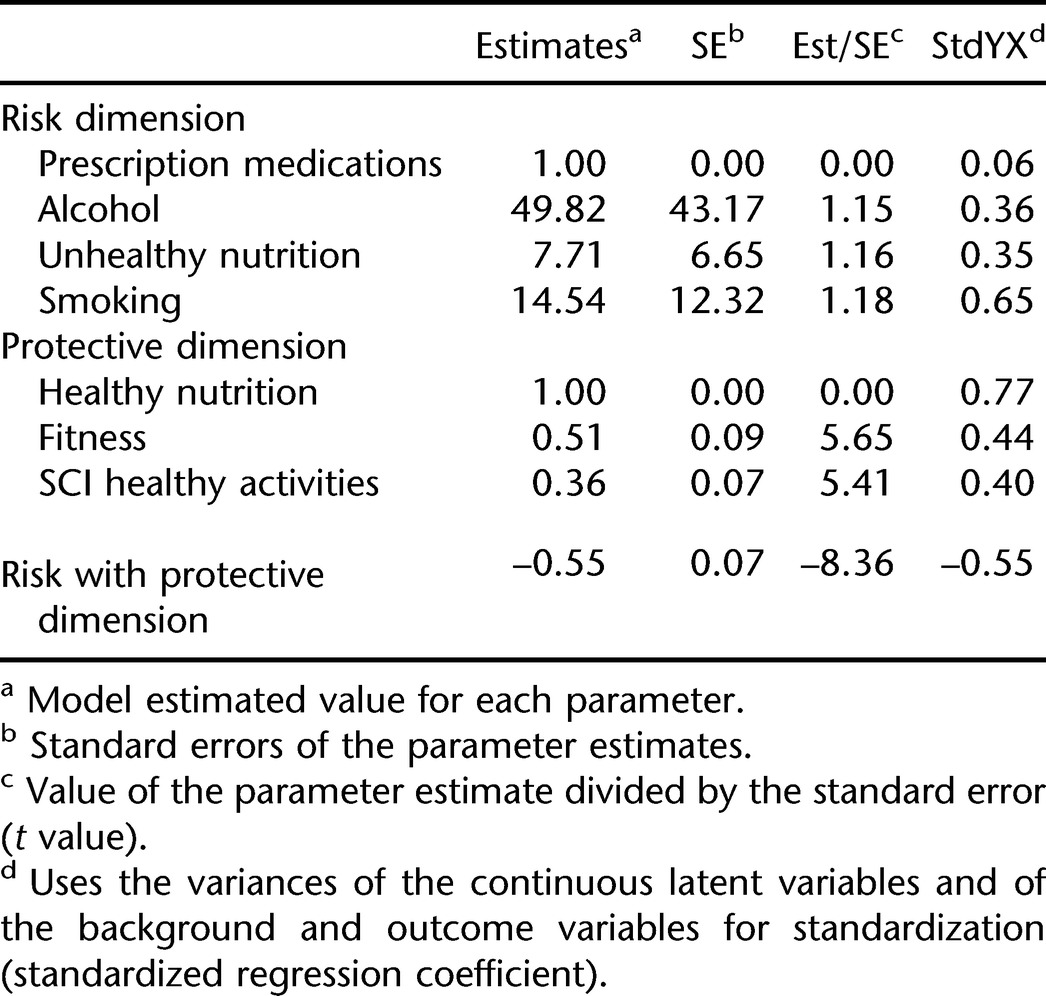
Higher-Order Dimensions
To test for the presence of higher-order dimensions, we added protective and risk dimensions to the CFA on the full participant sample. Four factors were included in the risk dimension on an a priori basis: smoking, alcohol, prescription medication use, and unhealthy nutrition. The remaining 3 factors were included in the protective dimensions: healthy nutrition, fitness, and SCI-specific behaviors. RMSEA for the CFA with the addition of analysis of higher-order dimensions was 0.057 (chi [139, n = 1,388] = 761.1, P < 0.001). Table 5 summarizes the results. Correlation of the 3 factors with the protective dimension ranged from 0.40 to 0.77, with an average coefficient of 0.54. On the risk dimension, the correlations were lower, ranging from 0.06 to 0.65, as the prescription medication use factor clearly did not fit the dimension. The average correlation of the other 3 factors was 0.45. The correlation between the risk and protective dimensions was −0.55.
Table 5.
Higher-Order Dimensions
Latent Variable Structural Path Model
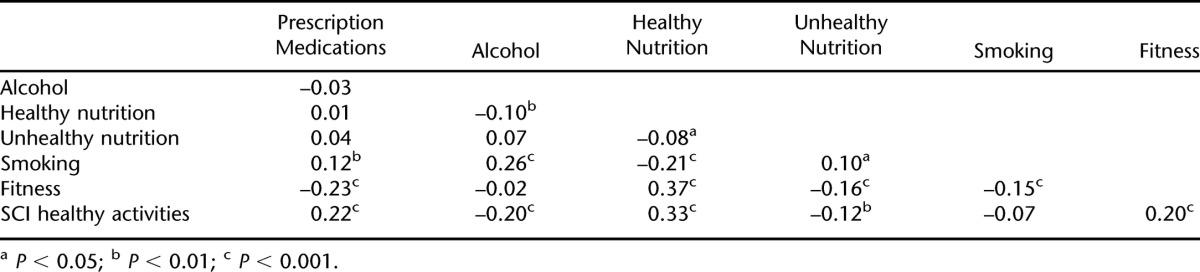
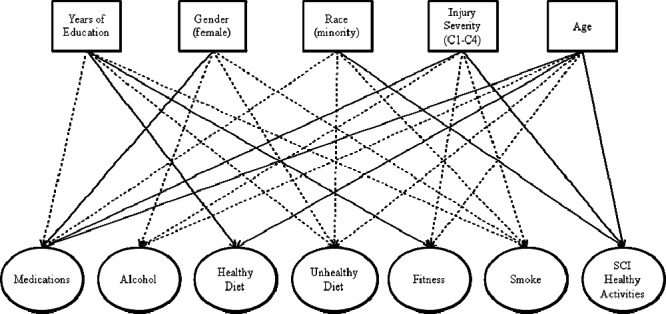
Table 6 is a summary of the full latent path model, including the items with the factors and the relationship between the demographic, injury, and educational factors and each of the observed factors (chi [248, n = 1,374] = 1,135.2, P < 0.001; RMSEA = 0.051). Table 7 summarizes the correlations between the factors, which ranged from −0.23 to 0.37. Figure 1 graphically depicts the primary information summarized in Table 6. However, because of the number of significant relationships between the exogenous variables (eg, age, injury severity) and endogenous variables (ie, the latent dimensions), only those significant at P < 0.001 are summarized in the figure.
Table 6.
Full Latent Model: Association of Exogenous and Endogenous Variables (Latent Portion of the Model)
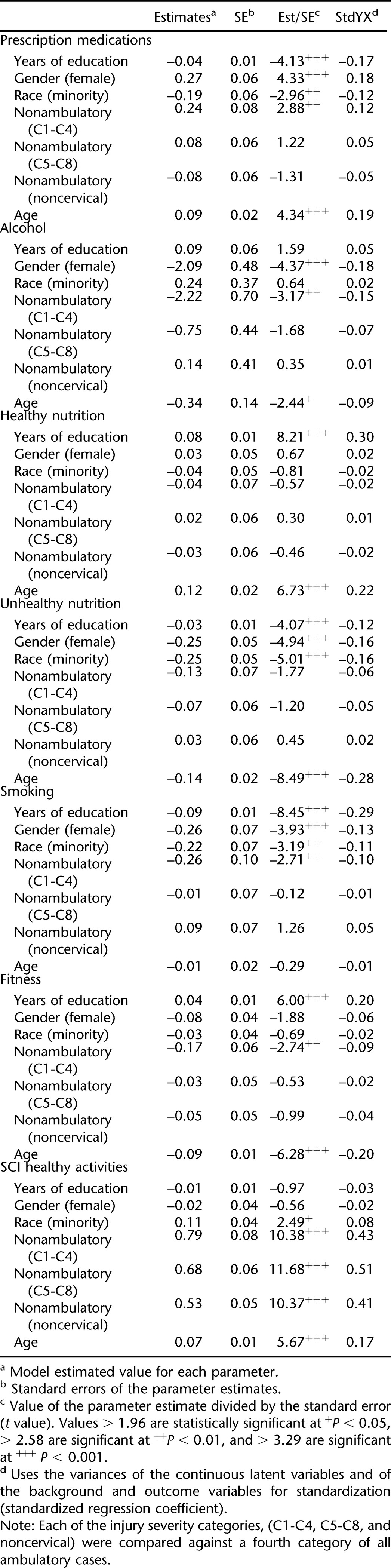
Table 7.
Correlation Coefficients Between Factor Scores
Figure 1. Graphic depiction of the full latent path model, including the items with the factors and the relationship between the biographic, injury, and educational factors with each of the observed factors. Note: Only correlations significant at P < 0.001 are shown. A solid line indicates a positive correlation, and a dashed line indicates a negative correlation.
Injury Factors
Injury severity was significantly related to 5 behavioral domains. The participants with cervical injury (C1-C4) (nonambulatory) were significantly different from the ambulatory participants, with 1 exception. They were more likely than ambulatory participants to report higher levels of prescription medication use but reported lower scores for alcohol use, smoking, and fitness. The exception to the pattern was for SCI healthy activities, where each of the 3 nonambulatory groups scored significantly higher than those who were ambulatory (some of these behaviors, such as weight shifts, are not relevant to ambulatory). No other comparisons were significant.
Biographic Characteristics
Each of the 3 biographic variables was significantly related to at least 2 of the behavioral domains. Women were more likely than men to report higher levels of prescription medication use but were less likely to report alcohol use, smoking, or unhealthy nutrition. They were not significantly different from men with regard to either healthy nutrition or fitness.
Minority participants were less likely to report prescription medication use and less likely to report unhealthy nutritional behaviors. They were no different from white participants in alcohol use or smoking domains; nor were they significantly different on either healthy nutrition or fitness. They were more likely to report SCI healthy activities.
Chronologic age was significantly related to each of the behavioral domains. The older the participants, the less likely they were to report alcohol use, smoking, or unhealthy nutritional habits. They were also less likely to report fitness behaviors. Older participants were more likely to report healthy nutritional practices, SCI healthy activities, and prescription medication use.
Education
Duration of education was significantly related to all of the behavioral domains, except for alcohol use and SCI healthy activities. Those with more education were more likely to report healthy nutritional patterns and fitness. Higher education levels were negatively correlated with prescription medication use, unhealthy nutritional patterns, and smoking.
DISCUSSION
The purpose of this study was to identify and confirm the underlying dimensions of health behaviors and develop a latent model of their relationships to biographic, injury, and educational characteristics. The unique contributions of this study include the diversity of the health behaviors under investigation, the size of the participant sample, and the use of SEM to develop, test, and validate the latent model. The combination of these factors allowed us to perform the analyses required to identify the underlying dimensions, account for multicollinearity between predictors, and successfully compensate for missing data.
These findings suggest that there is merit in identifying and differentiating behavioral domains, as behavioral factors were clearly identified. There was a great deal of consistency between the exploratory and confirmatory analyses, with the minor exception of 1 item dropping out during the CFA. The fit of the model was exceptional. Taken together, this suggests that the modeling was successful in identifying 7 relatively independent domains (since no correlations exceeded 0.37), and these resulting factor scales may now be used as predictor variables for health outcomes and future research.
Our success in identifying higher-order dimensions was more modest. Two clear dimensions were identified that were correlated just less than 0.60. This indicates they are not fully orthogonal. This is not particularly problematic, because they will not be used as a basis for prediction (the 7 domains with more narrowly defined content will be better in this respect). There was ambiguity regarding the extent to which the use of psychotropic prescription medications represented a risk dimension, and this will need to be clarified in future research.
Multiple biographic, injury, and educational factors were related to the behavioral factors. Typically, general biographic and injury characteristics are not highly correlated with outcomes, and the relationships observed in the current study with health behaviors, although statistically significant, were of modest strength. However, the pattern of relationships that emerged was quite interesting and informative.
Implications
There are several important implications of this study. First, there were variations in patterns of behaviors related to individual characteristics, suggesting that interventions and prevention strategies to increase protective behaviors and decrease risk behaviors can be targeted to the individuals at highest risk for a particular domain. For instance, with regard to prescription medication use, those with high cervical injuries, women, and older participants were particularly likely to report higher use.
Similarly, the findings that minority participants engaged less frequently in 2 key risk behaviors—prescription medication use and poor nutritional practices—suggest that other factors account for observed health disparities among minority participants. The lower levels of prescription medication use may be related to the affordability of the medications. The majority of effects were not particularly large, but they were consistent. Of course, until the protective and risk behaviors are linked to specific patterns of secondary conditions, we can only speculate as to how changing these behaviors would change the likelihood of secondary conditions developing.
Second, patterns of risk and protective behaviors must be monitored closely with variations in injury severity, as it appears that individuals with the most severe high cervical injuries are more likely to use prescription medications but less likely to use alcohol or tobacco. This may represent an intentional attempt to limit alcohol use that may have dangerous interactions with prescription medication. Prescription medication use should be closely monitored among those with cervical injuries.
Third, in general, women reported lower scores on the risk dimensions (alcohol use, tobacco use, unhealthy nutritional practices), with the exception of prescription medication use. Prescription medication use has previously been linked to a greater risk of injuries (32), so clearly there are instances when it represents a risk domain, but it may not represent a risk domain in all cases. Women were no more likely to perform positive protective behaviors, such as fitness or healthy nutritional practices. This suggests that interventions targeted toward risk behaviors, with the exception of prescription medication use, may best be targeted toward men.
Fourth, there appear to be greater variations in the risk domains, rather than the protective domains, as a function of the characteristics under investigation. Certainly, attention needs to be focused on both a reduction in risk behavioral domains and an increase in protective domains. It may be that, currently, less attention is focused on the latter during inpatient rehabilitation and at follow-up, at least in terms of diet and exercise. This would not be surprising since a medical model is still largely followed in rehabilitation, rather than the more preventative public health–type model.
Fifth, because we are identifying latent domains, behavioral domains are not as closely tied to observables, particularly individual behaviors. Rather, the observable variables (ie, individual behaviors) were used to identify the underlying dimensions that, in theory, would encompass other behaviors falling within the latent domain (not just those used in the current study). Therefore, additional behaviors may be introduced into these domains or serve as alternatives (eg, other types of foods that would fall within 1 of the 2 nutritional domains).
Limitations
There were several noteworthy limitations to the study. First, although they were objectively verifiable, all data were self-reported. We did not anticipate wide variations in self-reported and actual behaviors for items not generally considered to be sensitive or highly personal (eg, diet), but there could be underreporting of more sensitive items (eg, smoking and alcohol use). The reason for focusing on prescription medication use, rather than the use of illicit drugs, was specifically to avoid this problem. Although not exempt from these concerns, tobacco and alcohol use are frequently used in assessments, such that any underreporting is likely to be limited.
Second, all data were cross-sectional and correlational. Longitudinal data would greatly facilitate our understanding of the behavioral factors, behavioral changes, and the relationship of each to the characteristics under study (52). The use of correlational data precludes causal interpretations, so the observed relationships between the exogenous and endogenous variables can only be interpreted as associations.
Third, although we assessed multiple behavioral domains, other domains could be identified (this is a question for future research). It would also be possible to identify additional items within the domains identified. In fact, refining the measurement of each dimension and identifying and developing measurement models for additional dimensions will be central to the value of research on health behaviors and secondary conditions after SCI and other disabling conditions.
Fourth, the current study represents only the beginning of the development of a full latent model and testing of empirical models relating to secondary health conditions. Identifying the behavioral domains and individual factors associated with these domains is a worthy first step in this process. However, it is simply that—the first step. Additional research will be required to determine the interrelationships of the health behavior domains and secondary health conditions and mortality.
Future Research
This is only the first stage in a program of research with the ultimate goal of a design of behavioral interventions to prevent secondary conditions. Although this study establishes a necessary foundation for understanding the interrelationships between health behaviors and their association with individual characteristics, a much larger question relates to the relationships of behavioral domains and secondary conditions. It is necessary to continue to build or operationalize the risk model relating health behaviors to secondary conditions and mortality, including precursors of health behaviors. Linking personality and other psychological factors, as well as environmental precursors to the propensity to perform health behaviors, will help to interpret the circumstances under which the behaviors are performed and which prevention strategies could be implemented. Identifying the relationship between the health behaviors and specific secondary conditions will similarly help to target interventions to the behaviors that are most detrimental to well-being. Longitudinal research will then be needed to identify the relationship between health behaviors and future secondary health conditions.
Additional research may also clarify the differences in behaviors, such as prescription medication use, as a function of biographic characteristics. This research should consider issues of medication compliance, as well as potential misuse, because there is the potential to abuse prescription medications that are used to treat pain, spasticity, depression, and sleep.
Finally, we need to use information from this and future studies for the development of interventions to promote healthy behaviors and discourage risk behaviors. Targeting those individuals at high risk for each domain based on the current study findings will assist in this endeavor.
It is only through continued research efforts that we will understand who is at greatest risk for secondary health conditions, the behaviors that place them at risk for these conditions, and strategies that may successfully prevent these conditions.
Acknowledgments
This research was supported by funding from the National Institutes of Health Grant #1R01 NS 48117–01 A1; the US Department of Education, National Institute on Disability and Rehabilitation Research Grant #H133G050165; and the Model Spinal Cord Injury Systems Grant #H133N000005. The opinions here are those of the grantee and do not necessarily reflect those of the funding agencies.
REFERENCES
- Krause JS, Broderick L. Patterns of recurrent pressure ulcers after spinal cord injury: identification of risk and protective factors 5 or more years after onset. Arch Phys Med Rehabil. 2004;85:1257–1264. doi: 10.1016/j.apmr.2003.08.108. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center (NSCISC). Annual Statistical Report. Birmingham, AL: University of Alabama; 2006. [Google Scholar]
- Strauss DJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Krause JS. Secondary conditions and spinal cord injury: a model for prediction and prevention. Top Spinal Cord Inj Rehabil. 1996;2(2):217–227. [Google Scholar]
- Demirel S, Demirel G, Tukek T, Erk O, Yilmaz H. Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord. 2001;39(3):134–138. doi: 10.1038/sj.sc.3101135. [DOI] [PubMed] [Google Scholar]
- Krum H, Howes LG, Brown DJ, et al. Risk factors for cardiovascular disease in chronic spinal cord injury patients. Paraplegia. 1992;30(6):381–388. doi: 10.1038/sc.1992.87. [DOI] [PubMed] [Google Scholar]
- Noreau L, Shepherd R, Simard C, Pare G, Pomerleau P. Relationship of impairment and functional ability to habitual activity and fitness following spinal cord injury. Int J Rehabil Res. 1993;16:265–275. doi: 10.1097/00004356-199312000-00002. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266–277. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- Dallmeijer A, Hopman M, van der Woude L. Lipid, lipoprotein, and apolipoprotein profiles in active and sedentary men with tetraplegia. Arch Phys Med Rehabil. 1997;78(11):1173–1176. doi: 10.1016/s0003-9993(97)90327-0. [DOI] [PubMed] [Google Scholar]
- Hooker S, Wells C. Effects of low- and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc. 1989;21(1):18–22. doi: 10.1249/00005768-198902000-00004. [DOI] [PubMed] [Google Scholar]
- Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34(11):727–751. doi: 10.2165/00007256-200434110-00003. [DOI] [PubMed] [Google Scholar]
- Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil. 2005;86(6):1176–1181. doi: 10.1016/j.apmr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- McColl MA, Rosenthal C. A model of resource needs of aging spinal cord injured men. Paraplegia. 1994;32:261–270. doi: 10.1038/sc.1994.46. [DOI] [PubMed] [Google Scholar]
- Lee MY, Myers J, Hayes A, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med. 2005;28(1):20–25. doi: 10.1080/10790268.2005.11753794. [DOI] [PubMed] [Google Scholar]
- McColl M, Howland C. Spinal cord injury and lifestyle health risks. Can J Rehabil. 1996;9:69–82. [Google Scholar]
- Castro M, Apple D, Hillegass E, Dudley G. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- Shields RK. Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther. 2002;32(2):65–74. doi: 10.2519/jospt.2002.32.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DS, McColl MA. Lifestyle risks for three disease outcomes in spinal cord injury. Clin Rehabil. 2002;16(1):96–108. doi: 10.1191/0269215502cr443oa. [DOI] [PubMed] [Google Scholar]
- Stotts K. Health maintenance: paraplegic athletes and non-athletes. Arch Phys Med Rehabil. 1989;67:109–114. doi: 10.1016/0003-9993(86)90116-4. [DOI] [PubMed] [Google Scholar]
- Figoni S. Perspectives on cardiovascular fitness and SCI. J Am Paraplegia Soc. 1991;13(4):63–71. doi: 10.1080/01952307.1990.11735822. [DOI] [PubMed] [Google Scholar]
- Almenoff P, Spungen A, Lesser M, Bauman W. Pulmonary function survey in spinal cord injury: influences of smoking and level and completeness of injury. Lung. 1995;173(5):297–306. doi: 10.1007/BF00176893. [DOI] [PubMed] [Google Scholar]
- Linn WS, Spungen AM, Gong H, Jr, Bauman WA, Adkins RH, Waters RL. Smoking and obstructive lung dysfunction in persons with chronic spinal cord injury. J Spinal Cord Med. 2003;26(1):28–35. doi: 10.1080/10790268.2003.11753657. [DOI] [PubMed] [Google Scholar]
- Blackmer J, Marshall S. Obesity and spinal cord injury: an observational study. Spinal Cord. 1997;35(4):245–247. doi: 10.1038/sj.sc.3100392. [DOI] [PubMed] [Google Scholar]
- Buchholz A, McGillivray C, Pencharz P. Physical activity levels are low in free-living adults with chronic paraplegia. Obes Res. 2003;11(4):563–570. doi: 10.1038/oby.2003.79. [DOI] [PubMed] [Google Scholar]
- Groah S, Stiens S, Gittler M, Kirshblum S, McKinley W. Spinal cord injury medicine. 5. Preserving wellness and independence of the aging patient with spinal cord injury: a primary care approach for the rehabilitation medicine specialist. Arch Phys Med Rehabil. 2002;83((3 suppl 1)):S82–S98. doi: 10.1053/apmr.2002.32182. [DOI] [PubMed] [Google Scholar]
- Kocina P. Body composition of spinal cord injured adults. Sports Med. 1997;23(1):48–60. doi: 10.2165/00007256-199723010-00005. [DOI] [PubMed] [Google Scholar]
- Maki KC, Briones ER, Langbein WE, et al. Associations between serum lipids and indicators of adiposity in men with spinal cord injury. Paraplegia. 1995;33:102–109. doi: 10.1038/sc.1995.24. [DOI] [PubMed] [Google Scholar]
- Kolakowsky-Hayner SA, Gourley EV, III, Kreutzer JS, Marwitz JH, Meade MA, Cifu DX. Post-injury substance abuse among persons with brain injury and persons with spinal cord injury. Brain Inj. 2002;16(7):583–592. doi: 10.1080/02699050110119475. [DOI] [PubMed] [Google Scholar]
- Lidal I, Snekkevik H, Aamodt G, Hjeltnes N, Biering-Sorensen F, Stanghelle J. Mortality after spinal cord injury in Norway. J Rehabil Med. 2007;39(2):145–151. doi: 10.2340/16501977-0017. [DOI] [PubMed] [Google Scholar]
- Tate DG, Forchheimer M, Krause JS, Meade M, Bombardier CH. Patterns of alcohol and substance use and abuse in persons with spinal cord injury risk factors and correlates. Arch Phys Med Rehabil. 2004;85:1837–1847. doi: 10.1016/j.apmr.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Tomey KM, Chen DM, Wang X, Braunschweig Cl. Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Arch Phys Med Rehabil. 2005;4:664–671. doi: 10.1016/j.apmr.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Krause JS. Factors associated with risk for subsequent injuries after the onset of traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1503–1508. doi: 10.1016/j.apmr.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Chiodo AE, Scelza WM, Kirshblum SC, Wuermser LA, Ho CH, Priebe M. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76–S83. doi: 10.1016/j.apmr.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Pruitt SD, Wahlgren DR, Epping-Jordan JE, Rossi AL. Health behavior in persons with spinal cord injury: development and initial validation of an outcome measure. Spinal Cord. 1998;36:724–731. doi: 10.1038/sj.sc.3100649. [DOI] [PubMed] [Google Scholar]
- Bloemen-Vrencken JH, de Witte LP, Post MW, van den Heuvel WJ. Health behaviour of persons with spinal cord injury. Spinal Cord. 2007;45(3):243–249. doi: 10.1038/sj.sc.3101967. [DOI] [PubMed] [Google Scholar]
- Powell-Griner E, Anderson JE, Murphy W. State-and sex-specific prevalence of selected characteristics—behavioral risk factor surveillance system, 1994 and 1995. MMWR CDC Surveill Summ. 1997;46(3):1–31. [PubMed] [Google Scholar]
- Rumpf HJ, Hapke U, Hill A, John U. Development of a screening questionnaire for the general hospital and general practices. Alcohol Clin Exp Res. 1997;21:894–898. [PubMed] [Google Scholar]
- Cherpitel CJ. Comparison of screening instruments for alcohol problems between black and white emergency room patients from two regions of the country. Alcohol Clin Exp Res. 1997;21:1391–1397. [PubMed] [Google Scholar]
- Cooney NL, Zweben A, Fleming MF. Screening for alcohol problems and at-risk drinking in health-care settings. In: Hester RK, Miller WR, editors. Handbook of Alcoholism Treatment Approaches: Effective Alternatives. 2nd ed. Boston, MA: Allyn and Bacon; 1995. pp. 45–60. [Google Scholar]
- Kinney J. Clinical Manual of Substance Abuse. 2nd ed. St Louis: Mosby-YearBook; 1991. [Google Scholar]
- Inciardi JA. Screening and Assessment for Alcohol and Other Drug Abuse Among Adults in the Criminal Justice System. Rockville, MC: Center for Substance Abuse Treatment; 1994. [PubMed] [Google Scholar]
- Kitchens JM. Does this patient have an alcohol problem. JAMA. 1994;272:1782–1787. [PubMed] [Google Scholar]
- Hays RD, Merz JF, Nicholas R. Response burden, reliability and validity of the CAGE, short MAST, and AUDIT alcohol screening measures. Behav Res Meths Instruments Computers. 1995;27:277–280. [Google Scholar]
- Muthen L, Muthen B. Mplus, The Comprehensive Modeling Program for Applied Researchers User's Guide. 4th ed. Los Angeles, CA: Muthen & Muthen; 2006. [Google Scholar]
- de Vet HC, Ader HJ, Terwee CB, Pouwer F. Are factor analytical techniques used appropriately in the validation of health status questionnaires? A systematic review on the quality of factor analysis of the SF-36. Qual Life Res. 2005;14:1203–1218. doi: 10.1007/s11136-004-5742-3. [DOI] [PubMed] [Google Scholar]
- McDonald R. Factor Analysis and Related Methods. Mahwah, NJ: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen K, Long S, editors. Testing Structural Equation Models. Beverly Hills, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Browne M. An overview of analytic rotationin exploratory factor analysis. Multivariate Behavioral Res. 2001;36:111–150. [Google Scholar]
- Krause J, Kewman D, DeVivo M, et al. Employment after spinal cord injury: an analysis of cases from the model spinal cord injury systems. Arch Phys Med Rehabil. 1999;80(11):1492–1500. doi: 10.1016/s0003-9993(99)90263-0. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Stover SL. Long term survival and causes of death. In: Stover SL, DeLisa JA, Whiteneck GG, editors. Spinal Cord Injury: Clinical Outcomes From the Model Systems. Gaithersburg, MD: Aspen Publications; 1995. pp. 289–316. [Google Scholar]
- McArdle JJ. Structural factor analysis experiments with incomplete data. Multivariate Behavioral Res. 1994;29(4):409–454. doi: 10.1207/s15327906mbr2904_5. [DOI] [PubMed] [Google Scholar]
- McArdle J. Five steps in the structural factor analysis of longitudinal data. In: Cudeck R, MacCallum R, editors. Factor Analysis at 100 Years. Mahwah, NJ: Lawrence Erlbaum Associates; 2007. pp. 99–130. [Google Scholar]